Phytoextraction of Lead Using a Hedge Plant [Alternanthera bettzickiana (Regel) G. Nicholson]: Physiological and Biochemical Alterations through Bioresource Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design and Treatments
2.3. Harvesting of the Plants
2.4. Determination of Pigment Contents
2.5. Determination of Electrolyte Leakage
2.6. Determination of Antioxidant Enzymes and ROS
2.7. Determination of Catalase
2.8. Determination of Ascorbate Peroxidase
2.9. Determination of Hydrogen Peroxide and Lead Contents
2.10. Statistical Analysis
3. Results
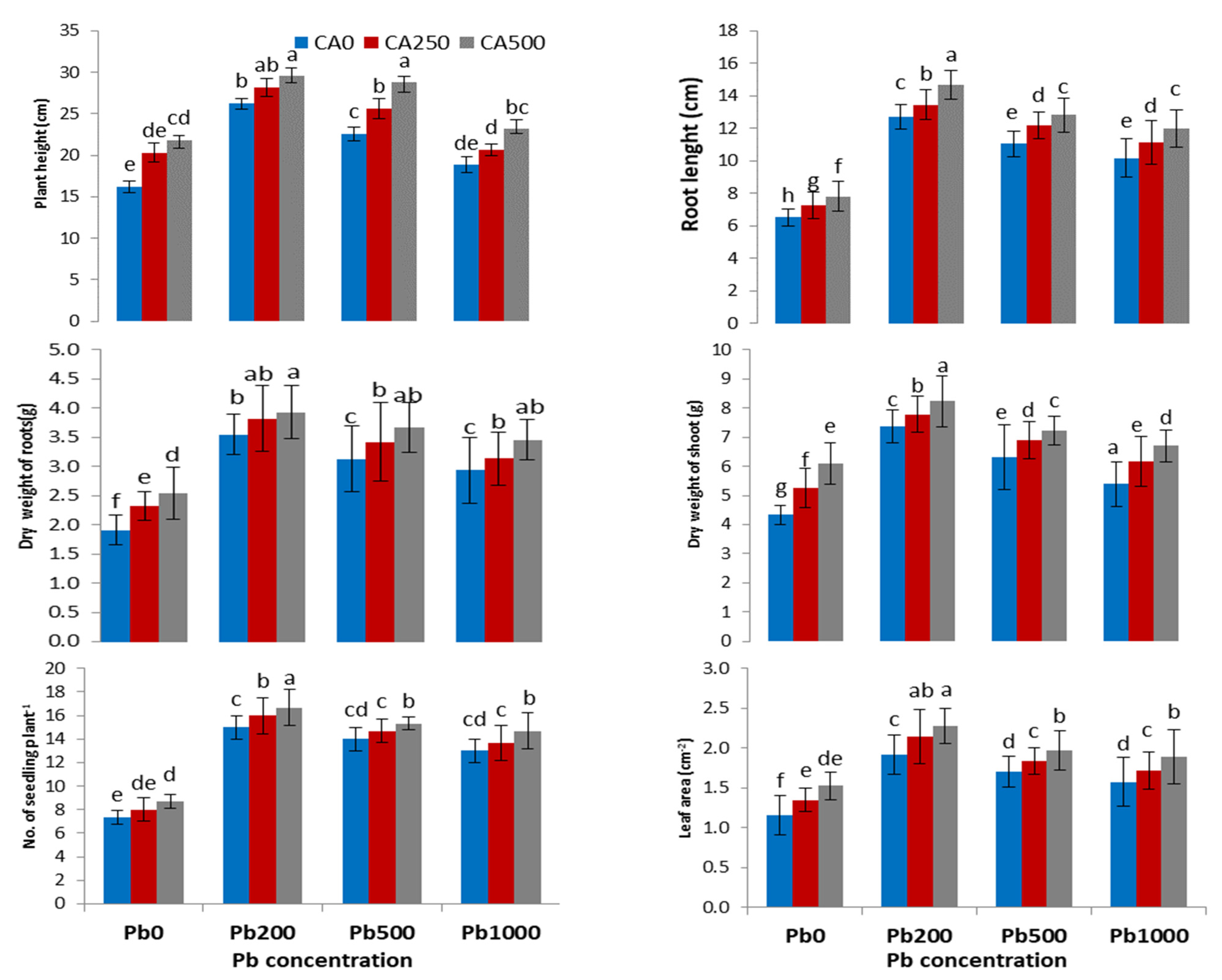
3.1. Plant Growth and Biomass
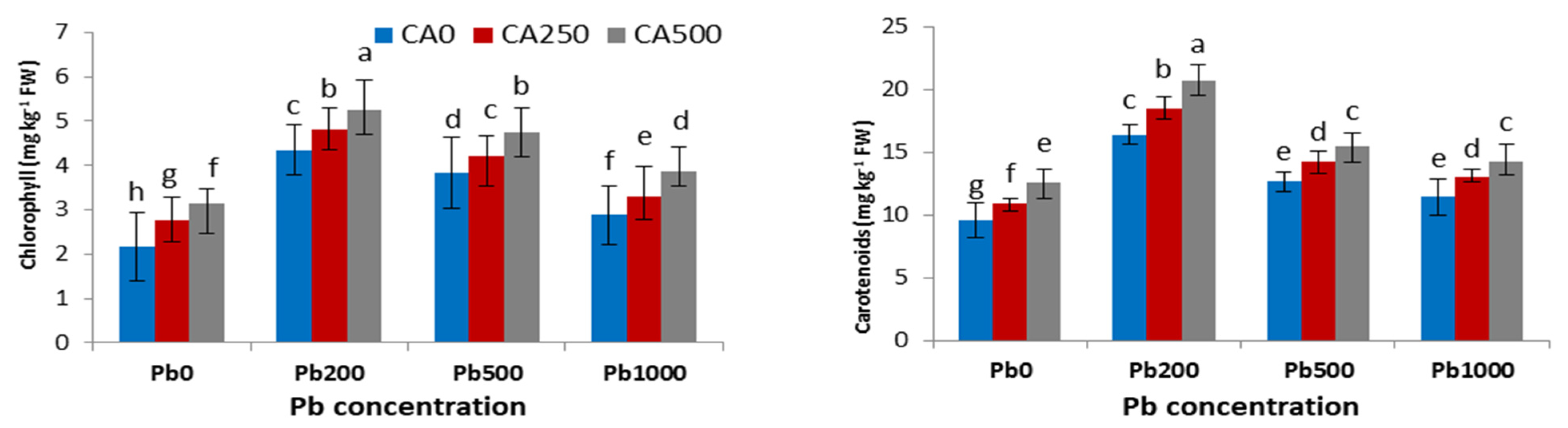
3.2. Photosynthetic Pigment
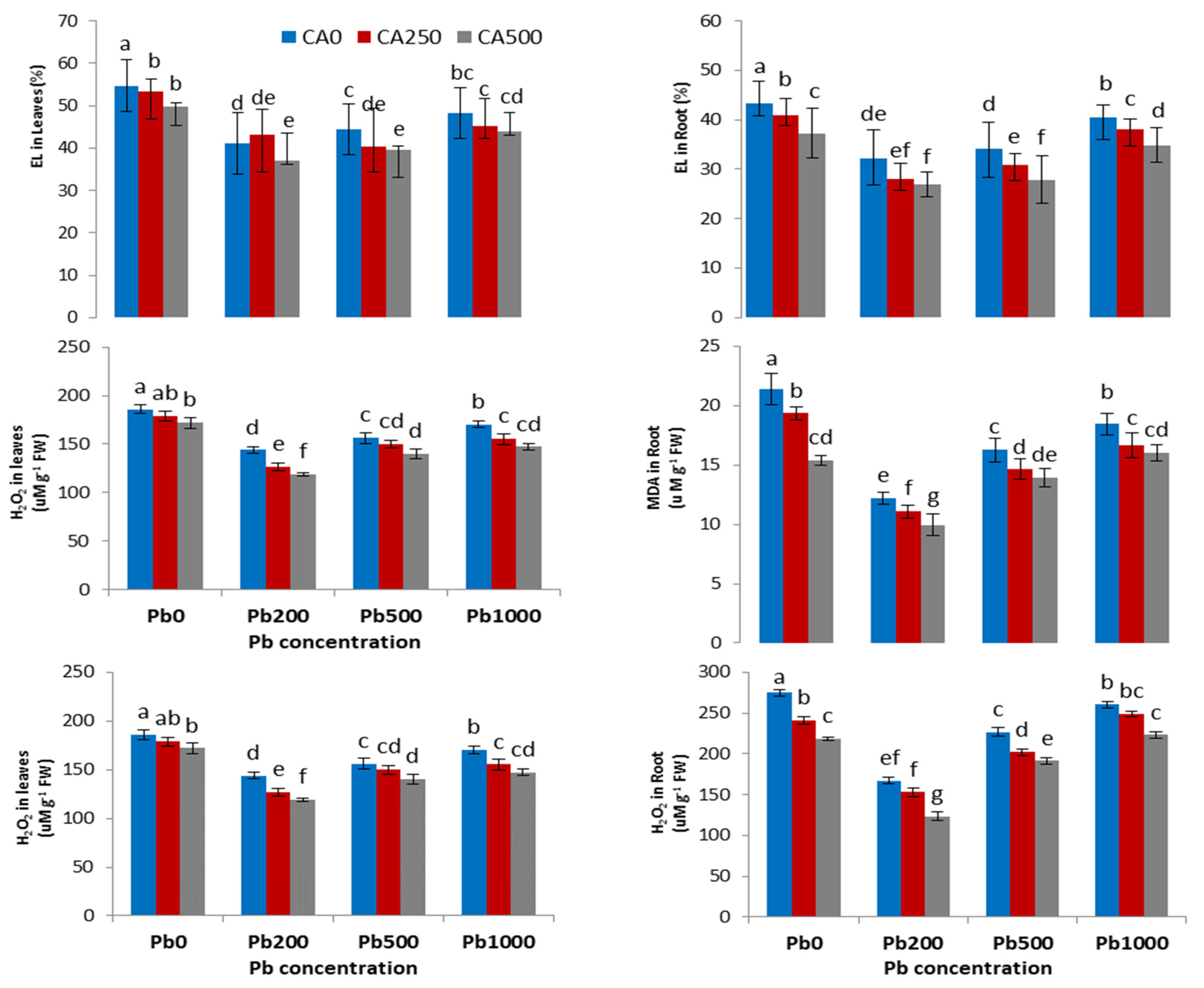
3.3. Plant Physiological Parameters (Concentration of H2O2 and MDA) and Electrolyte Leakage
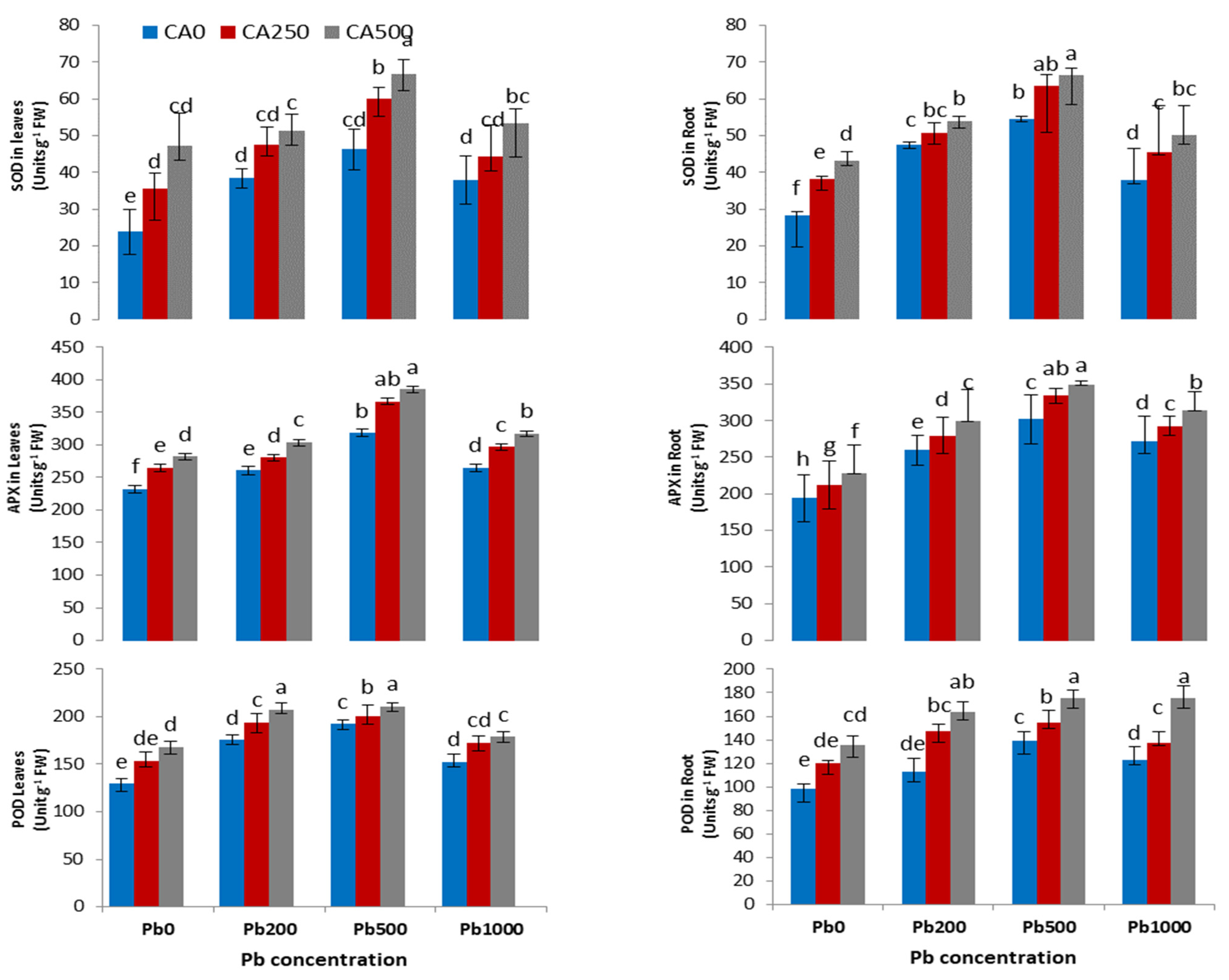
3.4. Activities of Antioxidant Enzymes and ROS
3.5. Metal Uptake
4. Discussion
4.1. Growth Parameters
4.2. Chlorophyll Contents
4.3. Antioxidant Enzyme Parameters and Reactive Oxygen Species
4.4. Antioxidant Enzyme Parameters and Reactive Oxygen Species
4.5. Lead Uptake
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mühlbachová, G. Soil microbial activities and heavy metal mobility in long-term contaminated soils after addition of EDTA and EDDS. Ecol. Eng. 2011, 37, 1064–1071. [Google Scholar] [CrossRef]
- Sharma, P.; Dubey, R.S. Lead toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 35–52. [Google Scholar] [CrossRef]
- Khopkar, S. Environmental Pollution Monitoring and Control; New Age International: New Delhi, India, 2007. [Google Scholar]
- Zhang, R.; Zhou, Y.; Yue, Z.; Chen, X.; Cao, X.; Xu, X.; Xing, Y.; Jiang, B.; Ai, X.; Huang, R. Changes in photosynthesis, chloroplast ultrastructure, and antioxidant metabolism in leaves of sorghum under waterlogging stress. Photosynth. 2019, 57, 1076–1083. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Corpuz, R.D.; Igarashi, T.; Villacorte-Tabelin, M.; Alorro, R.D.; Yoo, K.; Raval, S.; Ito, M.; Hiroyoshi, N. Acid mine drainage formation and arsenic mobility under strongly acidic conditions: Importance of soluble phases, iron oxyhydroxides/oxides and nature of oxidation layer on pyrite. J. Hazard. Mater. 2020, 399, 122844. [Google Scholar] [CrossRef] [PubMed]
- Afshan, S.; Ali, S.; Bharwana, S.A.; Rizwan, M.; Farid, M.; Abbas, F.; Ibrahim, M.; Mehmood, M.A.; Abbasi, G.H. Citric acid enhances the phytoextraction of chromium, plant growth, and photosynthesis by alleviating the oxidative damages in Brassica napus L. Environ. Sci. Pollut. Res. 2015, 22, 11679–11689. [Google Scholar] [CrossRef] [PubMed]
- Pourrut, B.; Jean, S.; Silvestre, J.; Pinelli, E. Lead-induced DNA damage in Vicia faba root cells: Potential involvement of oxidative stress. Mutat. Res. Toxicol. Environ. Mutagen. 2011, 726, 123–128. [Google Scholar] [CrossRef]
- Tauqeer, H.M.; Ali, S.; Rizwan, M.; Ali, Q.; Saeed, R.; Iftikhar, U.; Ahmad, R.; Farid, M.; Abbasi, G.H. Phytoremediation of heavy metals by Alternanthera bettzickiana: Growth and physiological response. Ecotoxicol. Environ. Saf. 2016, 126, 138–146. [Google Scholar] [CrossRef]
- Shahid, M.; Ferrand, E.; Schreck, E.; Dumat, C. Behavior and impact of zirconium in the soil–plant system: Plant uptake and phytotoxicity. Rev. Environ. Contam. Toxicol. 2013, 221, 107–127. [Google Scholar]
- Shakoor, M.B.; Ali, S.; Hameed, A.; Farid, M.; Hussain, S.; Yasmeen, T.; Najeeb, U.; Bharwana, S.A.; Abbasi, G.H. Citric acid improves lead (pb) phytoextraction in brassica napus L. by mitigating pb-induced morphological and biochemical damages. Ecotoxicol. Environ. Saf. 2014, 109, 38–47. [Google Scholar] [CrossRef]
- Jing, Y.-D.; He, Z.-L.; Yang, X.-E. Role of soil rhizobacteria in phytoremediation of heavy metal contaminated soils. J. Zhejiang Univ. Sci. B 2007, 8, 192–207. [Google Scholar] [CrossRef]
- Kambhampati, M.S.; Vu, V.T. EDTA Enhanced Phytoremediation of Copper Contaminated Soils Using Chickpea (Cicer aeritinum L.). Bull. Environ. Contam. Toxicol. 2013, 91, 310–313. [Google Scholar] [CrossRef]
- Pilon-Smits, E. Phytoremediation. Annu. Rev. Plant Biol. 2005, 56, 15–39. [Google Scholar] [CrossRef]
- Ahmad, K.; Khan, Z.I.; Ibrahim, M.; Ashraf, M.; Hussain, M. Evaluation of Nutritional Composition of Plant Species of Soone Valley in Punjab, Pakistan. J. Plant Nutr. 2010, 33, 496–517. [Google Scholar] [CrossRef]
- Wang, D.; Guo, W.; Zhang, G.; Zhou, L.; Wang, M.; Lu, Y.; Cai, D.; Wu, Z. Remediation of Cr(VI)-Contaminated Acid Soil Using a Nanocomposite. ACS Sustain. Chem. Eng. 2017, 5, 2246–2254. [Google Scholar] [CrossRef]
- Xu, X.; Chen, Q.; Mo, S.; Qian, Y.; Wu, X.; Jin, Y.; Ding, H. Transcriptome -wide modulation combined with morpho-physiological analyses of Typha orientalis roots in response to lead challenge. J. Hazard. Mater. 2020, 384, 121405. [Google Scholar] [CrossRef]
- Mehmood, S.; Saeed, D.A.; Rizwan, M.; Khan, M.N.; Aziz, O.; Bashir, S.; Ibrahim, M.; Ditta, A.; Akmal, M.; Mumtaz, M.A.; et al. Impact of different amendments on biochemical responses of sesame (Sesamum indicum L.) plants grown in lead-cadmium contaminated soil. Plant Physiol. Biochem. 2018, 132, 345–355. [Google Scholar] [CrossRef]
- Maroušek, J.; Kolář, L.; Strunecký, O.; Kopecký, M.; Bartoš, P.; Maroušková, A.; Cudlínová, E.; Konvalina, P.; Šoch, M.; Moudrý Jr, J.; et al. Modified biochars present an economic challenge to phosphate management in wastewater treatment plants. J. Clean. Prod. 2020, 272, 123015. [Google Scholar] [CrossRef]
- Kumar, A.; Prasad, M.N.V. Plant-lead interactions: Transport, toxicity, tolerance, and detoxification mechanisms. Ecotoxicol. Environ. Saf. 2018, 166, 401–418. [Google Scholar] [CrossRef] [PubMed]
- Anwer, S.; Ashraf, M.Y.; Hussain, M.; Ashraf, M.; Jamil, A. Citric acid mediated phytoextraction of cadmium by maize (Zea mays L.). Pak. J. Bot. 2012, 44, 1831–1836. [Google Scholar]
- Freitas, E.V.; Nascimento, C.W.; Silva, W.M. Citric Acid-Assisted Phytoextraction of Lead in the Field: The Use of Soil Amendments. Water Air Soil Pollut. 2013, 225, 1–9. [Google Scholar] [CrossRef]
- Farid, M.; Ali, S.; Saeed, R.; Rizwan, M.; Bukhari, S.A.H.; Abbasi, G.H.; Hussain, A.; Ali, B.; Zamir, M.S.I.; Ahmad, I. Combined application of citric acid and 5-aminolevulinic acid improved biomass, photosynthesis and gas exchange attributes of sunflower (Helianthus annuus L.) grown on chromium contaminated soil. Int. J. Phytoremediation 2019, 21, 760–767. [Google Scholar] [CrossRef]
- Ding, Y.Z.; Li, Z.A.; Zou, B. Low-molecular weight organic acids and their ecological roles in soil. Soils 2005, 37, 243–250. [Google Scholar]
- Stávková, J.; Maroušek, J. Novel sorbent shows promising financial results on P recovery from sludge water. Chemosphere 2021, 276, 130097. [Google Scholar] [CrossRef]
- Luo, C.; Shen, Z.; Li, X. Enhanced phytoextraction of Cu, Pb, Zn and Cd with EDTA and EDDS. Chemosphere 2005, 59, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sinhal, V.K.; Srivastava, A.; Singh, V.P. EDTA and citric acid mediated phytoextraction of Zn, Cu, Pb and Cd through marigold (Tagetes erecta). J. Environ. Biol. 2010, 31, 255. [Google Scholar] [PubMed]
- Haider, M.Z.; Hussain, S.; Ramzani, P.M.A.; Iqbal, M.; Iqbal, M.; Shahzad, T.; Fatima, M.; Khan, S.A.; Khan, I.; Shahid, M.; et al. Bentonite and Biochar Mitigate Pb Toxicity in Pisum sativum by Reducing Plant Oxidative Stress and Pb Translocation. Plants 2019, 8, 571. [Google Scholar] [CrossRef] [PubMed]
- Irshad, M.K.; Chen, C.; Noman, A.; Ibrahim, M.; Adeel, M.; Shang, J. Goethite-modified biochar restricts the mobility and transfer of cadmium in soil-rice system. Chemosphere 2020, 242, 125152. [Google Scholar] [CrossRef]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H. Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; John Wiley & Sons: Hoboken, NJ, USA, 1965. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Academic Press: Orlando, FL, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen Peroxide is Scavenged by Ascorbate-specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Marrugo-Negrete, J.; Durango-Hernández, J.; Pinedo-Hernández, J.; Olivero-Verbel, J.; Díez, S. Phytoremediation of mercury-contaminated soils by Jatropha curcas. Chemosphere 2015, 127, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Vymazal, J. Removal of nutrients in various types of constructed wetlands. Sci. Total. Environ. 2007, 380, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Maroušek, J.; Bartoš, P.; Filip, M.; Kolář, L.; Konvalina, P.; Maroušková, A.; Moudrý, J.; Peterka, J.; Šál, J.; Šoch, M.; et al. Advances in the agrochemical utilization of fermentation residues reduce the cost of purpose-grown phytomass for biogas production. Energy Sources, Part A: Recover. Util. Environ. Eff. 2020, 1–11. [Google Scholar] [CrossRef]
- Gao, Y.; Miao, C.; Mao, L.; Zhou, P.; Jin, Z.; Shi, W. Improvement of phytoextraction and antioxidative defense in Solanum nigrum L. under cadmium stress by application of cadmium-resistant strain and citric acid. J. Hazard. Mater. 2010, 181, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Sallah-Ud-Din, R.; Farid, M.; Saeed, R.; Ali, S.; Rizwan, M.; Tauqeer, H.M.; Bukhari, S.A.H. Citric acid enhanced the antioxidant defense system and chromium uptake by Lemna minor L. grown in hydroponics under Cr stress. Environ. Sci. Pollut. Res. 2017, 24, 17669–17678. [Google Scholar] [CrossRef]
- Baker, A.J.; Walker, P.L. Ecophysiology of metal uptake by tolerant plants. Heavy Met. Toler. Plants Evol. Asp. 1990, 2, 155–165. [Google Scholar]
- Saradhi, P.P. Proline Accumulation Under Heavy Metal Stress. J. Plant Physiol. 1991, 138, 554–558. [Google Scholar] [CrossRef]
- Maroušek, J.; Rowland, Z.; Valášková, K.; Král, P. Techno-economic assessment of potato waste management in developing economies. Clean Technol. Environ. Policy 2020, 22, 937–944. [Google Scholar] [CrossRef]
- Najeeb, U.; Jilani, G.; Ali, S.; Sarwar, M.; Xu, L.; Zhou, W. Insights into cadmium induced physiological and ultra-structural disorders in Juncus effusus L. and its remediation through exogenous citric acid. J. Hazard. Mater. 2011, 186, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Habiba, U.; Ali, S.; Farid, M.; Shakoor, M.B.; Rizwan, M.; Ibrahim, M.; Abbasi, G.H.; Hayat, T.; Ali, B. EDTA enhanced plant growth, antioxidant defense system, and phytoextraction of copper by Brassica napus L. Environ. Sci. Pollut. Res. 2015, 22, 1534–1544. [Google Scholar] [CrossRef] [PubMed]
- Hegedüs, A.; Erdei, S.; Horváth, G. Comparative studies of H2O2 detoxifying enzymes in green and greening barley seedlings under cadmium stress. Plant Sci. 2001, 160, 1085–1093. [Google Scholar] [CrossRef]
- Ji, X.; Liu, S.; Huang, J.; Bocharnikova, E.; Matichenkov, V. Monosilicic acid potential in phytoremediation of the contaminated areas. Chemosphere 2016, 157, 132–136. [Google Scholar] [CrossRef]
- Kanwal, U. Potential of Alternanthera Bettzickiana (Regel) G. Nicholson For Remediation of Cadmium-Contaminated Soil Using Citric Acid. Pak. J. Agric. Sci. 2019, 56. [Google Scholar] [CrossRef]
- Varun, M.; D’Souza, R.; Pratas, J.; Paul, M.S. Metal contamination of soils and plants associated with the glass industry in North Central India: Prospects of phytoremediation. Environ. Sci. Pollut. Res. 2012, 19, 269–281. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Ali, S.; Rizwan, M.; Dawood, M.; Farid, M.; Hussain, A.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Hydrogen sulfide alleviates chromium stress on cauliflower by restricting its uptake and enhancing antioxidative system. Physiol. Plant. 2019, 168, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Abbas, T.; Adrees, M.; Zia-Ur-Rehman, M.; Ibrahim, M.; Abbas, F.; Qayyum, M.F.; Nawaz, R. Residual effects of biochar on growth, photosynthesis and cadmium uptake in rice (Oryza sativa L.) under Cd stress with different water conditions. J. Environ. Manag. 2018, 206, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Adrees, M.; Ibrahim, M.; Tsang, D.C.; Zia-Ur-Rehman, M.; Zahir, Z.A.; Rinklebe, J.; Tack, F.M.; Ok, Y.S. A critical review on effects, tolerance mechanisms and management of cadmium in vegetables. Chemosphere 2017, 182, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Agnello, A.C.; Huguenot, D.; Van Hullebusch, E.D.; Esposito, G. Enhanced phytoremediation: A review of low molecular weight organic acids and surfactants used as amendments. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2531–2576. [Google Scholar] [CrossRef]
- Labra, M.; Gianazza, E.; Waitt, R.; Eberini, I.; Sozzi, A.; Regondi, S.; Grassi, F.; Agradi, E. Zea mays L. protein changes in response to potassium dichromate treatments. Chemosphere 2006, 62, 1234–1244. [Google Scholar] [CrossRef]

| Texture | Clay Soil |
|---|---|
| Sand (%) | 21.0 |
| Silt (%) | 16.0 |
| Clay (%) | 63.0 |
| ECe (dS m−1) | 2.79 |
| pH (1: 2.5 soil to water ratio) | 7.86 |
| Organic matter (%) | 0.54 |
| SAR (mmolc−1)1/2 | 6.27 |
| HCO3 (mmol L−1) | 3.33 |
| Available P (mg kg−1) | 2.21 |
| SO42− (mmol L−1) | 6.38 |
| Cl- (mmol L−1) | 2.29 |
| K+ (mmol L−1) | 0.04 |
| Na2+ (mmol L−1) | 3.52 |
| Ca2+ + Mg2+ (mmol L−1) | 3.61 |
| Available Zn2+ (mg kg−1) | 0.76 |
| Available Cu2+ (mg kg−1) | 0.21 |
| Available Pb2+ (mg kg−1) | 0.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanwal, U.; Ibrahim, M.; Abbas, F.; Yamin, M.; Jabeen, F.; Shahzadi, A.; Farooque, A.A.; Imtiaz, M.; Ditta, A.; Ali, S. Phytoextraction of Lead Using a Hedge Plant [Alternanthera bettzickiana (Regel) G. Nicholson]: Physiological and Biochemical Alterations through Bioresource Management. Sustainability 2021, 13, 5074. https://doi.org/10.3390/su13095074
Kanwal U, Ibrahim M, Abbas F, Yamin M, Jabeen F, Shahzadi A, Farooque AA, Imtiaz M, Ditta A, Ali S. Phytoextraction of Lead Using a Hedge Plant [Alternanthera bettzickiana (Regel) G. Nicholson]: Physiological and Biochemical Alterations through Bioresource Management. Sustainability. 2021; 13(9):5074. https://doi.org/10.3390/su13095074
Chicago/Turabian StyleKanwal, Urooj, Muhammad Ibrahim, Farhat Abbas, Muhammad Yamin, Fariha Jabeen, Anam Shahzadi, Aitazaz A. Farooque, Muhammad Imtiaz, Allah Ditta, and Shafaqat Ali. 2021. "Phytoextraction of Lead Using a Hedge Plant [Alternanthera bettzickiana (Regel) G. Nicholson]: Physiological and Biochemical Alterations through Bioresource Management" Sustainability 13, no. 9: 5074. https://doi.org/10.3390/su13095074
APA StyleKanwal, U., Ibrahim, M., Abbas, F., Yamin, M., Jabeen, F., Shahzadi, A., Farooque, A. A., Imtiaz, M., Ditta, A., & Ali, S. (2021). Phytoextraction of Lead Using a Hedge Plant [Alternanthera bettzickiana (Regel) G. Nicholson]: Physiological and Biochemical Alterations through Bioresource Management. Sustainability, 13(9), 5074. https://doi.org/10.3390/su13095074









