Effects of Selection Regime on Invasive Characteristics in an Emerging Biomass Crop, Switchgrass (Panicum virgatum L.)
Abstract
1. Introduction
2. Materials and Methods
3. Results
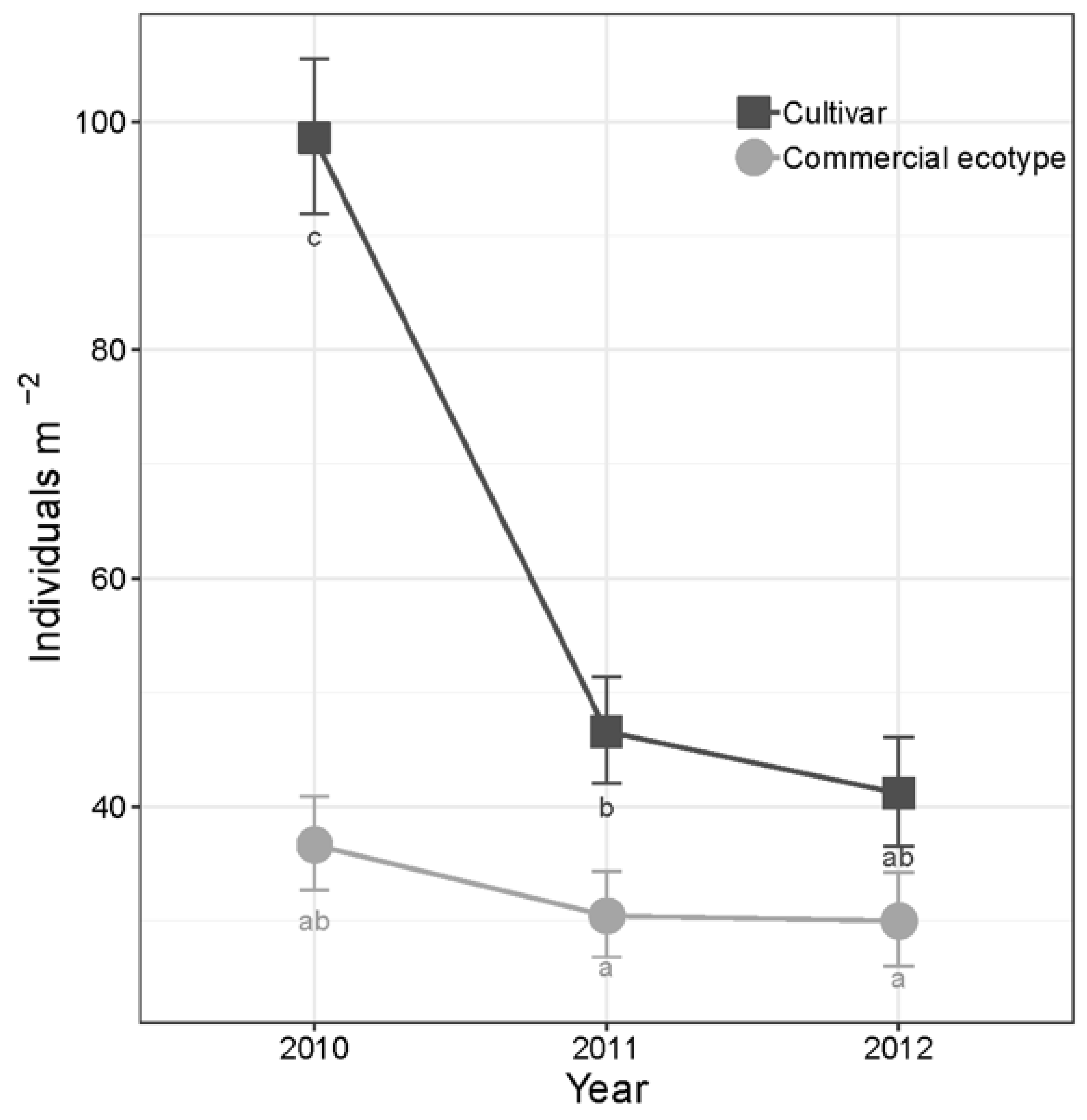
3.1. Effects of Selection Regime
3.2. Effects of Strain within Selection Regime
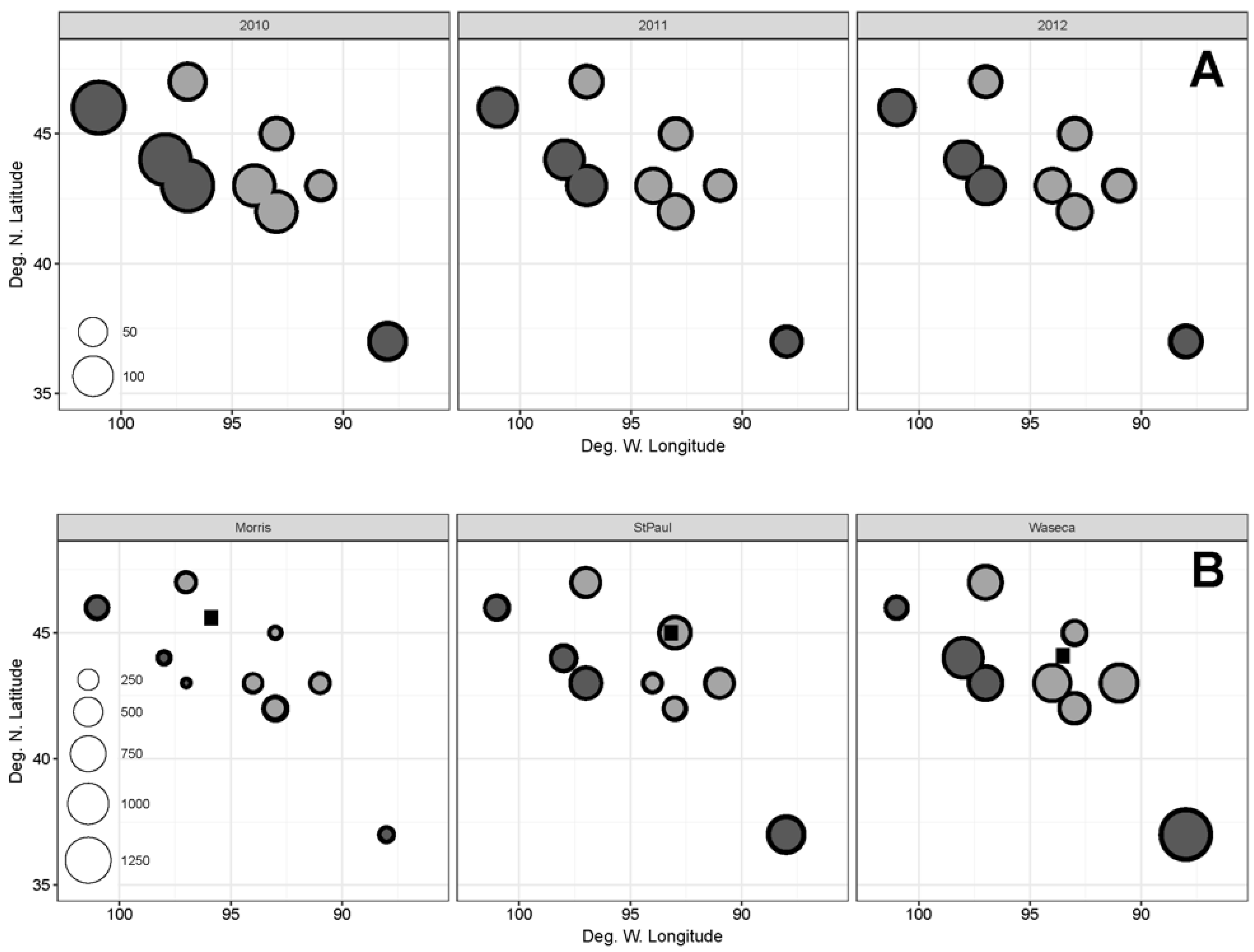
3.3. Effects of Geographic Origin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pfau, S.F.; Hagens, J.E.; Dankbaar, B.; Smits, A.J.M. Visions of sustainability in bioeconomy research. Sustainability 2014, 6, 1222–1249. [Google Scholar] [CrossRef]
- Kwit, C.; Stewart, C.N. Gene flow matters in switchgrass (Panicum virgatum L.), a potential widespread biofuel feedstock. Ecol. Appl. 2012, 22, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Vilà, M.; Espinar, J.L.; Hejda, M.; Hulme, P.E.; Jaroŝík, V.; Maron, J.L.; Pergl, J.; Schaffner, U.; Sun, Y.; Pyŝek, P. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 2011, 14, 702–708. [Google Scholar] [CrossRef]
- Hautier, Y.; Isbell, F.; Borer, E.T.; Seabloom, E.W.; Harpole, W.S.; Lind, E.M.; MacDougall, A.S.; Stevens, C.J.; Adler, P.B.; Alberti, J.; et al. Local loss and spatial homogenization of plant diversity reduce ecosystem multifunctionality. Nat. Ecol. Evol. 2018, 2, 50–56. [Google Scholar] [CrossRef]
- Barney, J.N.; DiTomaso, J.M. Nonnative species and bioenergy: Are we cultivating the next invader? BioScience 2008, 58, 64–70. [Google Scholar] [CrossRef]
- Davis, A.S.; Cousens, R.D.; Hill, J.; Mack, R.N.; Simberloff, D.; Raghu, S. Screening bioenergy feedstock crops to mitigate invasion risk. Front. Ecol. Environ. 2010, 8, 533–539. [Google Scholar] [CrossRef]
- Quinn, L.D.; Barney, J.N.; Matlaga, D.P. The bioenergy landscape: Sustainable resources or the next great invasion? In Bioenergy and Biological Invasions: Ecological, Agronomic, and Policy Perspectives on Minimizing Risk; Quinn, L.D., Matlaga, D.P., Barney, J.N., Eds.; CABI: Oxfordshire, UK, 2015. [Google Scholar]
- Ridley, C.E.; Jager, H.I.; Clark, C.M.; Efroymson, R.A.; Kwit, C.; Landis, D.A.; Leggett, Z.H.; Miller, D.A. Debate: Can bioenergy be produced in a sustainable manner that protects biodiversity and avoids the risk of invaders? Bull. Ecol. Soc. Am. 2013, 94, 277–290. [Google Scholar] [CrossRef]
- Pheloung, P.C.; Williams, P.A.; Halloy, S.R. A weed risk assessment model for use as a biosecurity tool evaluating plant introductions. J. Environ. Manag. 1999, 57, 239–251. [Google Scholar] [CrossRef]
- Daehler, C.C.; Denslow, J.S.; Ansari, S.; Kuo, H.-C. A risk-assessment system for screening out invasive pest plants from Hawaii and other Pacific islands. Conserv. Biol. 2004, 18, 360–368. [Google Scholar] [CrossRef]
- Gordon, D.R.; Onderdonk, D.A.; Fox, A.M.; Stocker, R.K. Consistent accuracy of the Australian weed risk assessment system across varied geographies. Divers. Distrib. 2008, 14, 234–242. [Google Scholar] [CrossRef]
- Buddenhagen, C.E.; Chimera, C.; Clifford, P. Assessing biofuel crop invasiveness: A case study. PLoS ONE 2009, 4, e5261. [Google Scholar] [CrossRef]
- Koop, A.L.; Fowler, L.; Newton, L.P.; Caton, B.P. Development and validation of a weed screening tool for the United States. Biol. Invasions 2012, 14, 273–294. [Google Scholar] [CrossRef]
- McGregor, K.F.; Watt, M.S.; Hulme, P.E.; Duncan, R.P. How robust is the Australian Weed Risk Assessment protocol? A test using pine invasions in the Northern and Southern hemispheres. Biol. Invasions 2012, 14, 987–998. [Google Scholar] [CrossRef]
- Flory, S.L.; Lorentz, K.A.; Gordon, D.R.; Sollenberger, L.E. Experimental approaches for evaluating the invasion risk of biofuel crops. Environ. Res. Lett. 2012, 7, 045904. [Google Scholar] [CrossRef]
- Barney, J.N. Bioenergy and invasive plants: Quantifying and mitigating future risks. Invasive Plant Sci. Manag. 2014, 7, 199–209. [Google Scholar] [CrossRef]
- Smith, L.L.; Tekiela, D.R.; Barney, J.N. Predicting biofuel invasiveness: A relative comparison to crops and weeds. Invasive Plant Sci. Manag. 2015, 8, 323–333. [Google Scholar] [CrossRef]
- Ellstrand, N.C.; Schierenbeck, K.A. Hybridization as a stimulus for the evolution of invasiveness in plants? Proc. Natl. Acad. Sci. USA 2000, 97, 7043. [Google Scholar] [CrossRef]
- Saltonstall, K. Cryptic invasion by a non-native genotype of the common reed, Phragmites australis, into North America. Proc. Natl. Acad. Sci. USA 2002, 99, 2445. [Google Scholar] [CrossRef]
- Lavergne, S.; Molofsky, J. Increased genetic variation and evolutionary potential drive the success of an invasive grass. Proc. Natl. Acad. Sci. USA 2007, 104, 3883. [Google Scholar] [CrossRef] [PubMed]
- Simberloff, D.; Souza, L.; Nuñez, M.A.; Barrios-Garcia, M.N.; Bunn, W. The natives are restless, but not often and mostly when disturbed. Ecology 2012, 93, 598–607. [Google Scholar] [CrossRef]
- Simberloff, D. Invasion biologists and the biofuels boom: Cassandras or colleagues? Weed Sci. 2008, 56, 867–872. [Google Scholar] [CrossRef]
- Anderson, N.O.; Galatowitsch, S.M.; Gomez, N. Selection strategies to reduce invasive potential in introduced plants. Euphytica 2006, 148, 203–216. [Google Scholar] [CrossRef]
- Parrish, D.J.; Fike, J.H. The biology and agronomy of switchgrass for biofuels. Crit. Rev. Plant Sci. 2005, 24, 423–459. [Google Scholar] [CrossRef]
- Sala, O.E.; Sax, D.; Leslie, H. Biodiversity consequences of increased biofuel production. In Biofuels: Environmental Consequences and Interactions with Changing Land Use; Howarth, R.W., Bringezu, S., Eds.; Cornell University Library’s Initiatives in Publishing (CIP): Ithaca, NY, USA, 2009. [Google Scholar]
- Ge, Y.; Fu, C.; Bhandari, H.; Bouton, J.; Brummer, E.C.; Wang, Z.Y. Pollen viability and longevity of switchgrass (Panicum virgatum L.). Crop Sci. 2011, 51, 2698–2705. [Google Scholar] [CrossRef]
- Vogel, K.P.; Mitchell, R.B.; Casler, M.D.; Sarath, G. Registration of ‘Liberty’ switchgrass. J. Plant Regist. 2014, 8, 242–247. [Google Scholar] [CrossRef]
- Millwood, R.; Nageswara-Rao, M.; Ye, R.; Terry-Emert, E.; Johnson, C.R.; Hanson, M.; Burris, J.N.; Kwit, C.; Stewart, C.N. Pollen-mediated gene flow from transgenic to non-transgenic switchgrass (Panicum virgatum L.) in the field. BMC Biotech. 2017, 17, 40. [Google Scholar] [CrossRef]
- Hager, H.A.; Quinn, L.D.; Barney, J.N.; Voigt, T.B.; Newman, J.A. Germination and establishment of bioenergy grasses outside cultivation: A multi-region seed addition experiment. Plant Ecol. 2015, 216, 1385–1399. [Google Scholar] [CrossRef]
- Mitchell, R.B.; Vogel, K.P. Grass invasion into switchgrass managed for biomass energy. Bioenergy Res. 2016, 9, 50–56. [Google Scholar] [CrossRef]
- Smith, L.L.; Allen, D.J.; Barney, J. The thin green line: Sustainable bioenergy feedstocks or invaders in waiting. Neobiota 2015, 25, 47–71. [Google Scholar] [CrossRef]
- Meyer, M.H.; Paul, J.; Anderson, N.O. Competive ability of invasive Miscanthus biotypes with aggressive switchgrass. Biol. Invasions 2010, 12, 3809–3816. [Google Scholar] [CrossRef]
- Wilsey, B.J. Productivity and subordinate species response to dominant grass species and seed source during restoration. Restor. Ecol. 2010, 18, 628–637. [Google Scholar] [CrossRef]
- Palik, D.J.; Snow, A.A.; Stottlemyer, A.L.; Miriti, M.N.; Heaton, E.A. Relative performance of non-local cultivars and local, wild populations of switchgrass (Panicum virgatum) in competition experiments. PLoS ONE 2016, 11, e0154444. [Google Scholar] [CrossRef]
- Eckberg, J.O.; Johnson, G.A.; Seefeldt, L.L.; Felton, A.J.; Casler, M.D.; Shaw, R.G. Competitive effects of cultivar and wild switchgrass on other native grasses. Biol. Invasions 2018, 20, 2439–2449. [Google Scholar] [CrossRef]
- Barney, J.N.; DiTomaso, J.M. Bioclimatic predictions of habitat suitability for the biofuel switchgrass in North America under current and future climate scenarios. Biomass Bioenergy 2010, 34, 124–133. [Google Scholar] [CrossRef]
- Barney, J.N.; DiTomaso, J.M. Global climate niche estimates for bioenergy crops and invasive species of agronomic origin: Potential problems and opportunities. PLoS ONE 2011, 6, e17222. [Google Scholar] [CrossRef]
- Barney, J.N.; Mann, J.J.; Kyser, G.B.; DiTomaso, J.M. Assessing habitat susceptibility and resistance to invasion by the bioenergy crops switchgrass and Miscanthus × giganteus in California. Biomass Bioenergy 2012, 40, 143–154. [Google Scholar] [CrossRef]
- DiTomaso, J.; Barney, J.; Mann, J.; Kyser, G. For switchgrass cultivated as biofuel in California, invasiveness limited by several steps. Calif. Agric. 2013, 67, 96–103. [Google Scholar] [CrossRef][Green Version]
- Barney, J.N.; DiTomaso, J.M. Invasive Species Biology, Ecology, Management and Risk Assessment: Evaluating and Mitigating the Invasion Risk of Biofuel Crops. In Plant Biotechnology for Sustainable Production of Energy and Co-Products; Mascia, P.N., Scheffran, J., Widholm, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 263–284. [Google Scholar]
- Ridley, C.E.; Mallory-Smith, C. Gene flow and invasiveness in bioenergy systems. In Bioenergy and Biological Invasions: Ecological, Agronomic and Policy Perspectives on Minimising Risk; Quinn, L.D., Matlaga, D.P., Barney, J.N., Eds.; CABI: Oxfordshire, UK, 2015; pp. 52–66. [Google Scholar]
- Barker, R.E.; Haas, R.J.; Jacobson, E.T.; Berdahl, J.D. Registration of ‘Forestburg’ switchgrass. Crop Sci. 1988, 28, 192. [Google Scholar] [CrossRef]
- Boe, A.; Ross, J.G. Registration of ‘Sunburst’ switchgrass. Crop Sci. 1998, 38, 540. [Google Scholar] [CrossRef]
- Shaidaee, G.; Dahl, B.E.; Hansen, R.M. Germination and emergence of different age seeds of six grasses. J. Range Manag. 1969, 22, 240–243. [Google Scholar] [CrossRef]
- Sladden, S.E.; Bransby, D.I.; Aiken, G.E. Biomass yield, composition and production costs for eight switchgrass varieties in Alabama. Biomass Bioenergy 1991, 1, 119–122. [Google Scholar] [CrossRef]
- Sanderson, M.A.; Reed, R.L.; Ocumpaugh, W.R.; Van Esbroeck, G.; Read, J.C.; Tischler, C.R.; Hons, F.M. Switchgrass cultivars and germplasm for biomass feedstock production in Texas. Bioresour. Technol. 1999, 67, 209–219. [Google Scholar] [CrossRef]
- Casler, M.D.; Vogel, K.P.; Taliaferro, C.M.; Wynia, R.L. Latitudinal adaptation of switchgrass populations. Crop Sci. 2004, 44, 293–303. [Google Scholar] [CrossRef]
- Casler, M.D.; Vogel, K.P.; Taliaferro, C.M.; Ehlke, N.J.; Berdahl, J.D.; Brummer, E.C.; Kallenback, R.L.; West, C.P.; Mitchell, R.B. Latitudinal and longitudinal adaptation of switchgrass populations. Crop Sci. 2007, 47, 2249–2260. [Google Scholar] [CrossRef]
- Martínez-Reyna, J.M.; Vogel, K.P. Heterosis in switchgrass: Spaced plants. Crop Sci. 2008, 48, 1312–1320. [Google Scholar] [CrossRef]
- Yang, J.; Worley, E.; Wang, M.; Lahner, B.; Salt, D.E.; Saha, M.; Udvardi, M. Natural variation for nutrient use and remobilization efficiencies in switchgrass. Bioenergy Res. 2009, 2, 257–266. [Google Scholar] [CrossRef]
- Cortese, L.M.; Honig, J.; Miller, C.; Bonos, S.A. Genetic diversity of twelve switchgrass populations using molecular and morphological markers. Bioenergy Res. 2010, 3, 262–271. [Google Scholar] [CrossRef]
- Aspinwall, M.J.; Lowry, D.B.; Taylor, S.H.; Juenger, T.E.; Hawkes, C.V.; Johnson, M.-V.V.; Kiniry, J.R.; Fay, P.A. Genotypic variation in traits linked to climate and aboveground productivity in a widespread C4 grass: Evidence for a functional trait syndrome. New Phytol. 2013, 199, 966–980. [Google Scholar] [CrossRef]
- Morris, G.P.; Hu, Z.; Grabowski, P.P.; Borevitz, J.O.; de Graaff, M.-A.; Miller, R.M.; Jastrow, J.D. Genotypic diversity effects on biomass production in native perennial bioenergy cropping systems. GCB Bioenergy 2016, 8, 1000–1014. [Google Scholar] [CrossRef]
- Casler, M.D. Ecotypic variation among switchgrass populations from the northern USA. Crop Sci. 2005, 45, 388–398. [Google Scholar] [CrossRef]
- Kindscher, K.; Wells, P.V. Prairie plant guilds: A multivariate analysis of prairie species based on ecological and morphological traits. Vegetatio 1995, 117, 29–50. [Google Scholar] [CrossRef]
- Parrish, D.J.; Casler, M.D.; Monti, A. The evolution of switchgrass as an energy crop. In Switchgrass; Springer: London, UK, 2012; pp. 1–28. [Google Scholar]
- Alderson, J. Grass varieties in the United States. In Agriculture Handbook; Soil, C.S., Sharp, W.C., Eds.; United States Department of Agriculture: Washington, DC, USA, 1994. [Google Scholar]
- Casler, M.D.; Vogel, K.P.; Harrison, M. Switchgrass germplasm resources. Crop Sci. 2015, 55, 2463. [Google Scholar] [CrossRef]
- Barker, R.E.; Haas, R.J.; Berdahl, J.D.; Jacobson, E.T. Registration of ‘Dacotah’ switchgrass. Crop Sci. 1990, 30, 1158. [Google Scholar] [CrossRef]
- Aiken, G.E.; Springer, T.L. Seed size distribution, germination, and emergence of 6 switchgrass cultivars. J. Range Manag. 1995, 48, 455–458. [Google Scholar] [CrossRef]
- Boe, A. Genetic and environmental effects on seed weight and seed yield in switchgrass. Crop Sci. 2003, 43, 63–67. [Google Scholar] [CrossRef]
- Zalapa, J.E.; Price, D.L.; Kaeppler, S.M.; Tobias, C.M.; Okada, M.; Casler, M.D. Hierarchical classification of switchgrass genotypes using SSR and chloroplast sequences: Ecotypes, ploidies, gene pools, and cultivars. Theor. Appl. Genet. 2011, 122, 805–817. [Google Scholar] [CrossRef]
- Boe, A. Variation between two switchgrass cultivars for components of vegetative and seed biomass. Crop Sci. 2007, 47, 636–640. [Google Scholar] [CrossRef]
- Packard, S. Restoration options. In The Tallgrass Restoration Handbook: For Prairies, Savannas and Woodlands; Packard, S., Mutel, C.F., Eds.; Island Press: Washington, DC, USA, 1997; pp. 47–62. [Google Scholar]
- Moser, L.E.; Vogel, K.P. Switchgrass, big bluestem, and indiangrass. In Forages: An Introduction to Grassland Agriculture, 5th ed.; Barnes, R.F., Miller, D.A., Nelson, C.J., Eds.; Iowa State University Press: Ames, IA, USA, 1995; pp. 409–420. [Google Scholar]
- Beuselinck, P.R.; Peters, E.J.; McGraw, R.L. Cultivar and management effects on stand persistence of birdsfoot trefoil. Agron. J. 1984, 76, 490–492. [Google Scholar] [CrossRef]
- Schmer, M.; Mitchell, R.; Vogel, K.; Schacht, W.H.; Marx, D.B. Spatial and temporal effects on switchgrass stands and yield in the Great Plains. Bioenergy Res. 2010, 3, 159–171. [Google Scholar] [CrossRef][Green Version]
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 2nd ed.; Sage: Thousand Oaks, CA, USA, 2011. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: https://www.r-project.org/ (accessed on 1 September 2018).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Landau, S.; Everitt, B.S. A Handbook of Statistical Analyses Using SPSS; Chapman and Hall/CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in linear mixed effects models. J. Stat. Sofw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Rouder, J.N.; Engelhardt, C.R.; McCabe, S.; Morey, R.D. Model comparison in ANOVA. Psychon. Bull. Rev. 2016, 23, 1779–1786. [Google Scholar] [CrossRef]
- Jessup, R.W.; Dowling, C.D. “Seeded-yet-sterile” perennial grasses: Towards sustainable and non-invasive biofuel feedstocks. In Bioenergy and Biological Invasions: Ecological, Agronomic and Policy Perspectives on Minimising Risk; Quinn, L.D., Matlaga, D.P., Barney, J.N., Eds.; CABI: Oxfordshire, UK, 2015; pp. 97–112. [Google Scholar]
- Schmer, M.R.; Vogel, K.P.; Mitchell, R.B.; Moser, L.E.; Eskridge, K.M.; Perrin, R.K. Establishment stand thresholds for switchgrass grown as a bioenergy crop. Crop Sci. 2006, 46, 157–161. [Google Scholar] [CrossRef]
- Flint, S.A.; Jordan, N.R.; Shaw, R.G. Plant community response to switchgrass (Panicum virgatum) population source in establishing prairies. Ecol. Appl. 2018, 28, 1818–1829. [Google Scholar] [CrossRef] [PubMed]
- Fike, J.H.; Parrish, D.J.; Wolf, D.D.; Balasko, J.A.; Green, J.T.; Rasnake, M.; Reynolds, J.H. Long-term yield potential of switchgrass-for-biofuel systems. Biomass Bioenergy 2006, 30, 198–206. [Google Scholar] [CrossRef]
- Mitchell, R.B.; Vogel, K.P.; Sarath, G. Managing and enhancing switchgrass as a bioenergy feedstock. Biofuels Bioprod. Biorefin. 2008, 2, 530–539. [Google Scholar] [CrossRef]
- Yoda, K. Self-thinning in overcrowded pure stands under cultivated and natural conditions (Intraspecific competition among higher plants. XI). J. Inst. Polytech. Osaka City Univ. Ser. D 1963, 14, 107–129. [Google Scholar]
- Jacobson, E.T.; Tober, D.A.; Haas, R.J.; Darris, D.C. The performance of selected cultivars of warm season grasses in the northern prairie and plains states. In Proceedings of the 9th North American Prairie Conference; Clambey, G.K., Pemble, R.H., Eds.; Tri-College University Center for Environmental Studies: Fargo, ND, USA, 1984; pp. 215–221. [Google Scholar]
- Tober, D.A.; Kuckwitz, W.; Jensen, N.; Knudson, M. Switchgrass biomass trials in North Dakota, South Dakota, and Minnesota. USDA NRCS Plant Materials Center, Bismarck, North Dakota. 2007. Available online: http://www.plant-materials.nrcs.usda.gov/pubs/ndpmcpu7093.pdf (accessed on 25 March 2021).
- Lee, D.K.; Boe, A. Biomass production of switchgrass in central South Dakota. Crop Sci. 2005, 45, 2583–2590. [Google Scholar] [CrossRef]
- Casler, M.D.; Vogel, K.P.; Lee, D.; Mitchell, R.B.; Adler, P.R.; Sulc, R.M.; Johnson, K.D.; Kallenbach, R.L.; Boe, A.R.; Mathison, R.D. 30 years of progress toward increased biomass yield of switchgrass and big bluestem. Crop Sci 2018, 58, 1242–1254. [Google Scholar] [CrossRef]
- Berdahl, J.D.; Frank, A.B.; Krupinsky, J.M.; Carr, P.M.; Hanson, J.D.; Johnson, H.A. Biomass yield, phenology, and survival of diverse switchgrass cultivars and experimental strains in western North Dakota. Agron. J. 2005, 97, 549–555. [Google Scholar] [CrossRef]
- Wullschleger, S.D.; Davis, E.B.; Borsuk, M.E.; Gunderson, C.A.; Lynd, L.R. Biomass production in switchgrass across the United States: Database description and determinants of yield. Agron. J. 2010, 102, 1158. [Google Scholar] [CrossRef]
- Jefferson, P.G.; McCaughey, W.P. Switchgrass (Panicum virgatum L.) cultivar adaptation, biomass production, and cellulose concentration as affected by latitude of origin. ISRN Agron. 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Evers, G.W.; Parsons, M.J. Soil type and moisture level influence on Alamo switchgrass emergence and seedling growth. Crop Sci. 2003, 43, 288–294. [Google Scholar] [CrossRef]
- Jump, A.S.; Peñuelas, J. Running to stand still: Adaptation and the response of plants to rapid climate change. Ecol. Lett. 2005, 8, 1010–1020. [Google Scholar] [CrossRef]
- Hartman, J.C.; Nippert, J.B.; Orozco, R.A.; Springer, C.J. Potential ecological impacts of switchgrass (Panicum virgatum L.) biofuel cultivation in the Central Great Plains, USA. Biomass Bioenergy 2011, 35, 3415–3421. [Google Scholar] [CrossRef]
- Falconer, D.S.; Mackay, T.F.C. Introduction to Quantitative Genetics, 4th ed.; Addison, Wesley, Longman Ltd.: Harlow, UK, 1996. [Google Scholar]

| Strain | Ploidy [62] | Release Date, Site of Origin, and Development History | |
|---|---|---|---|
| ‘Dacotah’ (1) ‘Dacotah’ (2) | 4× | 1989. Progenies from a wild population (Morten Co., ND) were grown in open-pollinated nurseries with other accessions for three generations. Ten individuals were chosen for uniform type and color, leafiness, level of vigor, high seed yields. Progenies from their bulked seed underwent two years of natural selection for hardiness in an increase field; then, 300 random individuals were established as a permanent breeder seed block [59] | |
| ‘Summer’ | 4× | 1963. Seed collected from a wild population (Otoe Co., NE). Mass selections were made for earliness, leafiness, and rust resistance. Progenies of these plants were grown in nurseries; selected individuals were polycrossed. Resulting seed was used to establish the foundation field [57,63] | |
| ‘Cave-in-Rock’ | 8× | 1973. Collected from one site (Hardin Co., IL). Seeds from the accession were planted at an evaluation site in Missouri. Plants with phenotypes superior to other accessions were used to establish a breeder block; bulked seed was the basis of foundation field [57] | |
| ‘Sunburst’ | 8× | 1983. Accessions from native prairies (Union Co., SD) were grown in open-pollinated nurseries. Two cycles of phenotypic recurrent selection for level of vigor, leafiness, and seed weight resulted in ten superior plants. Eighty half-sibs from each family were reared in a common nursery (800 plants from ten families). Their bulked seed comprised the first generation of breeder seed [43] | |
| ‘Forestburg’ | 8× | 1987. Four accessions (Sanborn Co., SD) were composited and grown in increase fields. Random individuals were moved to a different site and evaluated for morphological characteristics; off-type individuals were rogued from the population. A total of 500 remaining plants comprised breeder seed block [42] | |
| Kossuth | Kossuth County, IA, USA | The commercial ecotypes were accessioned from one or more wild populations, then increased in open-pollinated fields with no deliberate selection. | |
| LaCrosse | LaCrosse County, WI, USA | ||
| Isanti (1) Isanti (2) | Isanti County, MN, USA | ||
| Iowa | Multiple counties, IA, USA | ||
| Clay | Clay County, MN, USA | ||
| Site | Location | Temp.1,2 (°C) | Precip.1,2 (cm) | Soil Series (Type) | Soil Chemistry | Soil Family and Subgroup | |||
|---|---|---|---|---|---|---|---|---|---|
| %OM | pH | P | K | ||||||
| Morris | 45.59° N 95.88° W | 12.1 {11.5} 24.2 {24.1} | 38 {37} | Doland (silt loam) | 5.4 | 7.5 | 10 | 157 | Fine-loamy mixed Udic Haploboroll |
| St. Paul | 44.99° N 93.17° W | 14.1 {13.8} 24.8 {24.4} | 51 {44} | Waukegan (silt loam) | 3.8 | 5.6 | 70 | 124 | Fine-silty over sandy or sandy-skeletal, mixed, superactive, mesic Typic Hapludolls |
| Staples | 46.38° N 94.81° W | 10.4 {10.7} 22.7 {23.2} | 43 {40} | Verndale & Oylen (sandy loams) | 1.6 | 7.3 | 43 | 88 | Coarse-loamy mixed Udic Argiborolls Coarse-loamy mixed Aquic Argiborolls |
| Waseca | 44.08° N 93.53° W | 13.3 {13.0} 25.2 {24.9} | 49 {47} | Webster (clay loam) | 4.9 | 6.9 | 38 | 207 | Fine-loamy, mixed, superactive, mesic Typic Endoaquolls |
| Selection Regime | Plant Density (Plants m−2) | Survival (Plants m−2) | Persistence (Plants m−2) | Absolute Biomass (g m−2) | Switchgrass Proportion of Biomass |
|---|---|---|---|---|---|
| Cultivar | 71.3 (3.21); 396 | −59.2 (6.32); 144 | −8.48 (2.92); 108 | 343 (43.8); 54 | 0.594 (0.0486); 54 |
| Ecotype | 39.2 (1.92); 396 | −11.8 (4.64); 144 | −0.111 (2.40); 108 | 264 (36.7); 54 | 0.499 (0.0518); 54 |
| Predictors * | Plant Density | Juvenile Survival | Persistence | Aboveground Biomass | |
|---|---|---|---|---|---|
| Absolute | Proportion | ||||
| SR | 71.91, 283 **** | 36.51 **** | 5.011 * | 4.131 * | 4.631 * |
| S | 19.93, 287 **** | 3.293 ns | 3.242 ns | 61.62 **** | 1352 **** |
| Y | 62.12, 491 **** | --- | --- | --- | --- |
| SR × S | 0.7193, 287 ns | 2.973 ns | 5.422 º | 1.092 ns | 0.7592 ns |
| SR × Y | 31.42, 491 **** | --- | --- | --- | --- |
| S × Y | 4.135, 491 ** | --- | --- | --- | --- |
| SR × S × Y | 2.375, 491 * | --- | --- | --- | --- |
| Str | 11.111, 264 **** | 68.611 **** | 8.7311 ns | 88.611 **** | 69.311 **** |
| S | 19.43, 251 **** | 3.583 ns | 3.192 ns | 1052 **** | 2022 **** |
| Y | 82.72, 83 **** | --- | --- | --- | --- |
| Str × S | 1.5233, 251 * | 38.533 ns | 28.522 ns | 22.422 ns | 18.922 ns |
| Str × Y | 5.5122, 483 **** | --- | --- | --- | --- |
| Lat | 18.71, 270 *** | 3.211 º | 0.0711 ns | 5.201 * | 10.11 ** |
| Long | 12.21, 270 *** | 6.241 * | 0.331 ns | 7.571 ** | 12.71 *** |
| SR | 11.01, 270 *** | ||||
| S | 0.3573, 281 ns | 0.7773 ns | 1.062 ns | 10.32 ** | 4.082 ns |
| Y | 5.532, 509 *** | --- | --- | --- | --- |
| Lat × Long | --- | 4.081 * | 0.1271 ns | 7.021 ** | 12.11 *** |
| Lat × SR | 7.641, 270 ** | --- | --- | --- | --- |
| Long × SR | 12.51, 270 *** | --- | --- | --- | --- |
| S × Lat | --- | 6.633 º | 8.522 * | 0.452 ns | 1.492 ns |
| S × Long | --- | 2.743 ns | 8.172 * | 2.912 ns | 0.272 ns |
| Y × Lat | 5.262, 509 ** | --- | --- | --- | --- |
| Y × Long | 5.062, 509 ** | --- | --- | --- | --- |
| Sr × Y | 4.982, 509 ** | --- | --- | --- | --- |
| Lat × Long × SR | 10.32, 272 **** | --- | --- | --- | --- |
| Lat × Long × Y | 4.942, 509 ** | --- | --- | --- | --- |
| Lat × S × Y | 3.178, 404 ** | --- | --- | --- | --- |
| Long × S × Y | 3.228, 420 ** | --- | --- | --- | --- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flint, S.A.; Shaw, R.G.; Jordan, N.R. Effects of Selection Regime on Invasive Characteristics in an Emerging Biomass Crop, Switchgrass (Panicum virgatum L.). Sustainability 2021, 13, 5045. https://doi.org/10.3390/su13095045
Flint SA, Shaw RG, Jordan NR. Effects of Selection Regime on Invasive Characteristics in an Emerging Biomass Crop, Switchgrass (Panicum virgatum L.). Sustainability. 2021; 13(9):5045. https://doi.org/10.3390/su13095045
Chicago/Turabian StyleFlint, Shelby A., Ruth G. Shaw, and Nicholas R. Jordan. 2021. "Effects of Selection Regime on Invasive Characteristics in an Emerging Biomass Crop, Switchgrass (Panicum virgatum L.)" Sustainability 13, no. 9: 5045. https://doi.org/10.3390/su13095045
APA StyleFlint, S. A., Shaw, R. G., & Jordan, N. R. (2021). Effects of Selection Regime on Invasive Characteristics in an Emerging Biomass Crop, Switchgrass (Panicum virgatum L.). Sustainability, 13(9), 5045. https://doi.org/10.3390/su13095045






