Interrelationships of Chemical, Physical and Biological Soil Health Indicators in Beef-Pastures of Southern Piedmont, Georgia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. In Situ Soil Respiration and Soil Sampling
2.3. Soil Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Summary Statistics of Soil Health Indicators
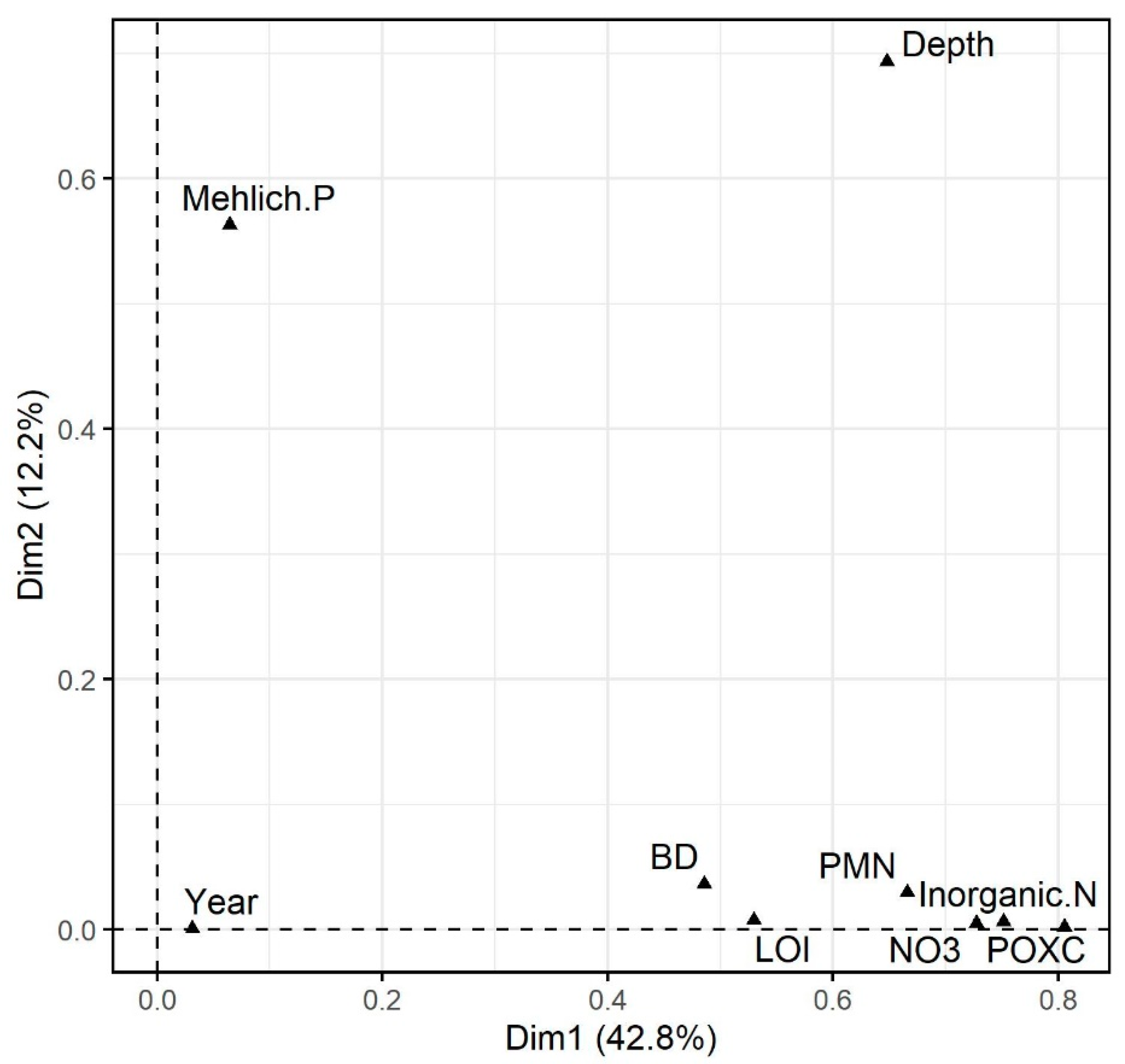
3.2. Exploratory Data Analysis
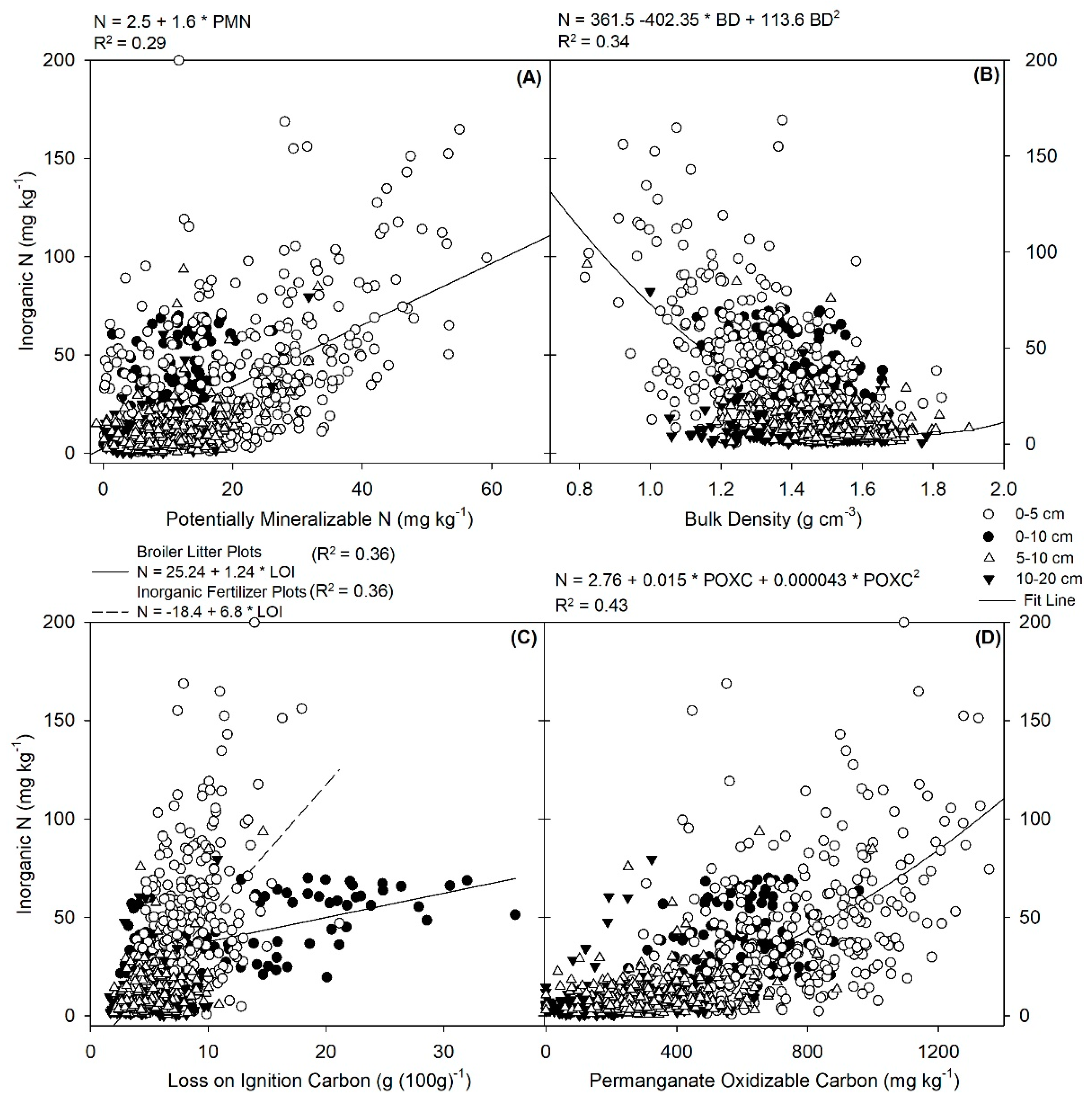
3.3. Relationship of Soil Nitrogen with Other Soil Health Indicators
3.4. Relationship of Soil Nitrogen with Other Soil Health Indicators
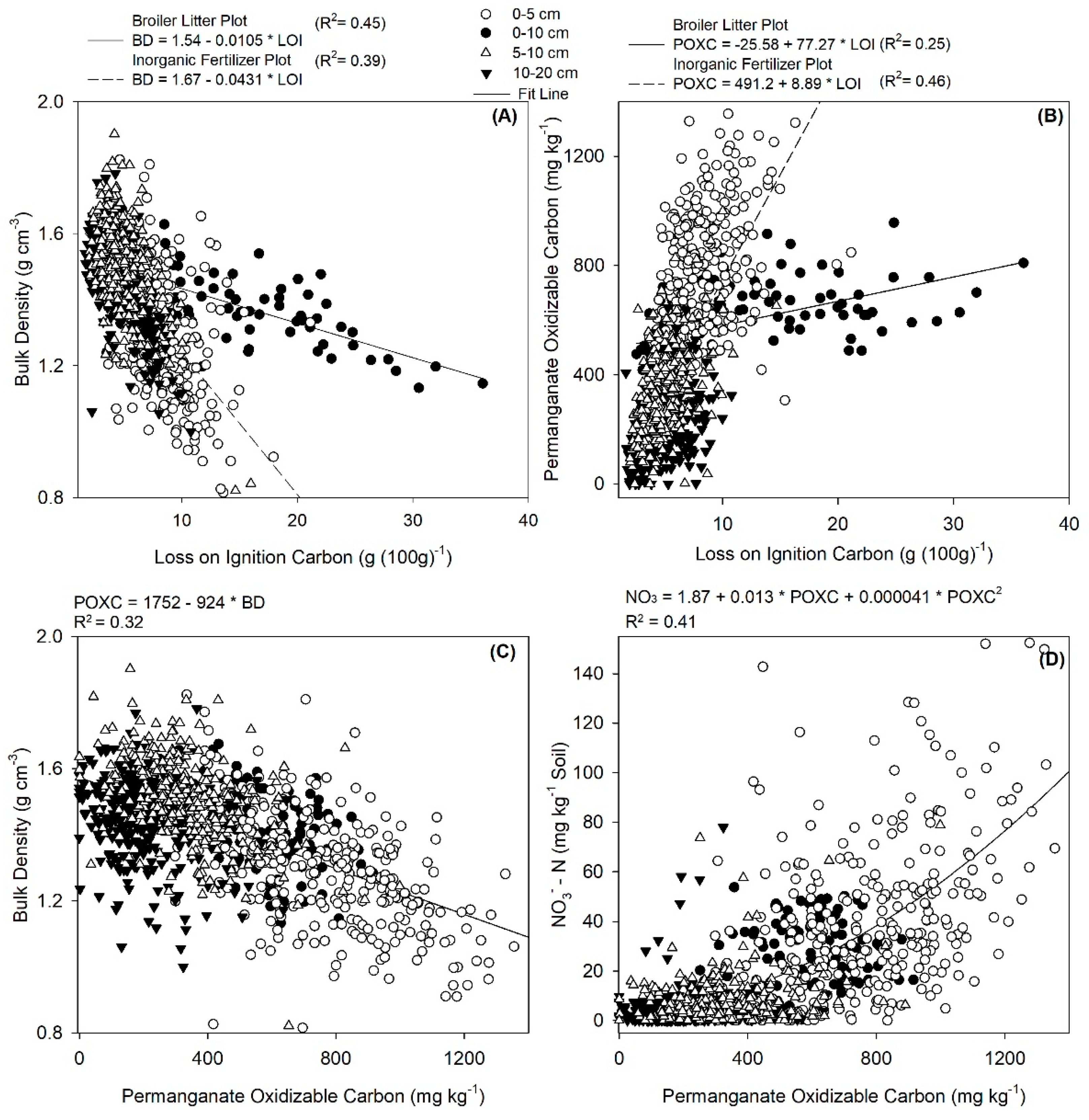
3.5. Soil Microbial Biomass vs. Other Soil Health Indicators
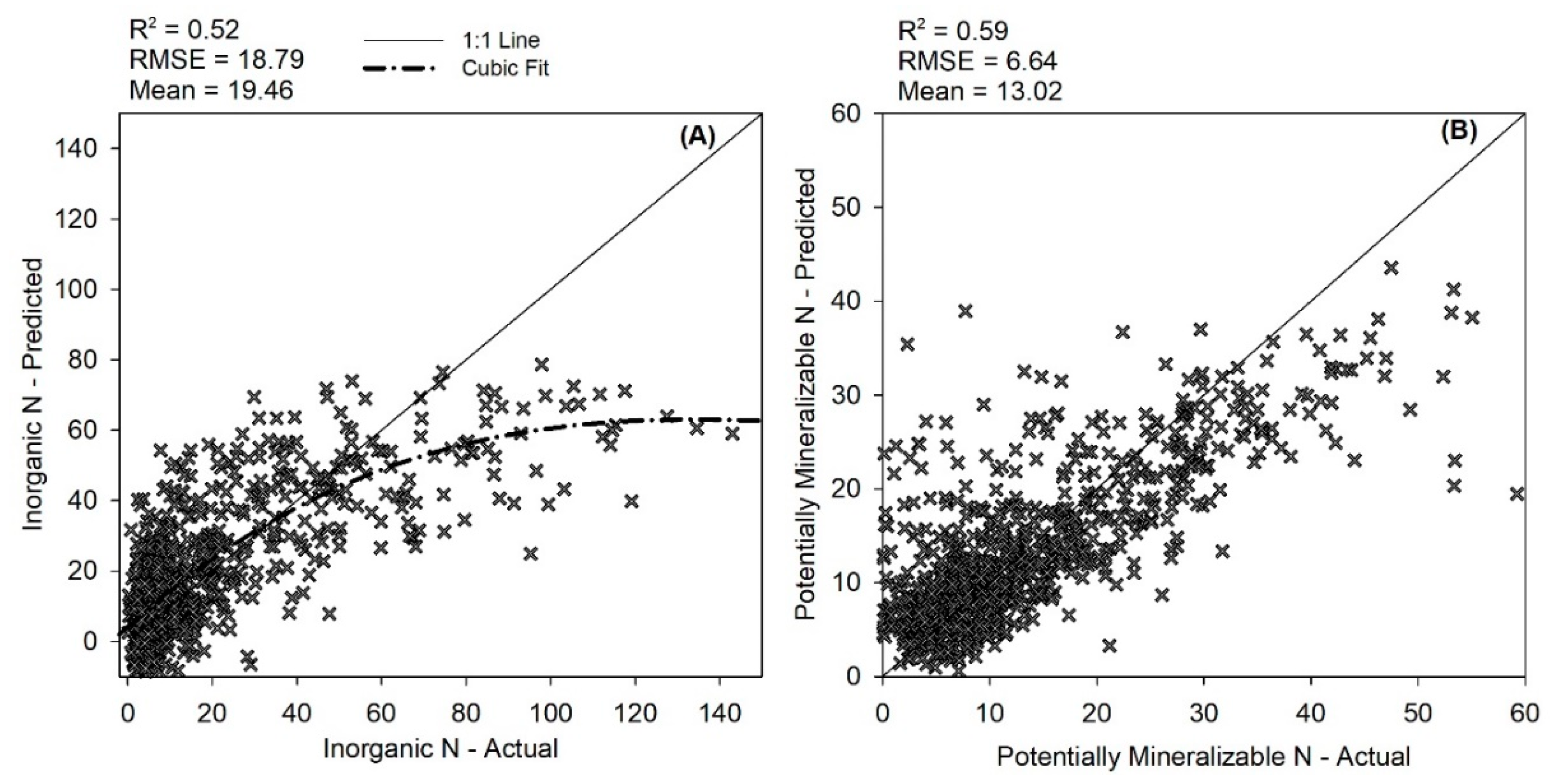
3.6. Inorganic N and PMN vs. Other Soil Health Indicators
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liebig, M.A.; Varvel, G.; Doran, J. A Simple Performance-Based Index for Assessing Multiple Agroecosystem Functions. Agron. J. 2001, 93, 313–318. [Google Scholar] [CrossRef]
- Roper, W.R.; Osmond, D.L.; Heitman, J.L.; Wagger, M.G.; Reberg-Horton, S.C. Soil Health Indicators Do Not Differentiate among Agronomic Management Systems in North Carolina Soils. Soil Sci. Soc. Am. J. 2017, 81, 828–843. [Google Scholar] [CrossRef]
- Moebius-Clune, B.N. Comprehensive Assessment of Soil Health: The Cornell Framework Manual; Cornell University: Ithaca, NY, USA, 2016. Available online: http://www.css.cornell.edu/extension/soil-health/manual.pdf (accessed on 11 February 2019).
- Bhandari, K.B.; West, C.P.; Acosta-Martinez, V.; Cotton, J.; Cano, A. Soil health indicators as affected by diverse forage species and mixtures in semi-arid pastures. Appl. Soil Ecol. 2018, 132, 179–186. [Google Scholar] [CrossRef]
- Bhowmik, A.; Fortuna, A.-M.; Cihacek, L.J.; Bary, A.I.; Cogger, C.G. Use of biological indicators of soil health to estimate reactive nitrogen dynamics in long-term organic vegetable and pasture systems. Soil Biol. Biochem. 2016, 103, 308–319. [Google Scholar] [CrossRef]
- Byrnes, R.C.; Eastburn, D.J.; Tate, K.W.; Roche, L.M. A Global Meta-Analysis of Grazing Impacts on Soil Health Indicators. J. Environ. Qual. 2018, 47, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, R.; Ghimire, B.; Mesbah, A.O.; Sainju, U.M.; Idowu, O.J. Soil Health Response of Cover Crops in Winter Wheat–Fallow System. Agron. J. 2019, 111, 2108–2115. [Google Scholar] [CrossRef]
- Dahal, S.; Franklin, D.H.; Cabrera, M.L.; Hancock, D.W.; Stewart, L.; Ney, L.C.; Subedi, A.; Mahmud, K. Spatial Distribution of Inorganic Nitrogen in Pastures as Affected by Management, Landscape, and Cattle Locus. J. Environ. Qual. 2018, 47, 1468–1477. [Google Scholar] [CrossRef]
- USDA. Soil Bulk Density: Soil Health Guide for Educators. Available online: https://www.nrcs.usda.gov/Internet/FSE_DOCUMENTS/nrcs142p2_050936.pdf (accessed on 10 December 2019).
- Haney, R.L.; Haney, E.B.; Smith, D.R.; Harmel, R.D.; White, M.J. The soil health tool—Theory and initial broad-scale application. Appl. Soil Ecol. 2018, 125, 162–168. [Google Scholar] [CrossRef]
- Parfitt, R.; Yeates, G.; Ross, D.; Mackay, A.; Budding, P. Relationships between soil biota, nitrogen and phosphorus availability, and pasture growth under organic and conventional management. Appl. Soil Ecol. 2005, 28, 1–13. [Google Scholar] [CrossRef]
- Schomberg, H.H.; Cabrera, M.L. Modeling in situ N mineralization in conservation tillage fields: Comparison of two versions of the CERES nitrogen submodel. Ecol. Model. 2001, 145, 1–15. [Google Scholar] [CrossRef]
- Veum, K.S.; Sudduth, K.A.; Kremer, R.J.; Kitchen, N.R. Sensor data fusion for soil health assessment. Geoderma 2017, 305, 53–61. [Google Scholar] [CrossRef]
- Anderson, T.-H.; Domsch, K. Application of eco-physiological quotients (qCO2 and qD) on microbial biomasses from soils of different cropping histories. Soil Biol. Biochem. 1990, 22, 251–255. [Google Scholar] [CrossRef]
- Staff, S.S.L. Soil survey laboratory methods manual. In Soil Survey Investigation Report 42; National Soil Survey Center: Lincoln, NE, USA, 1996. [Google Scholar]
- Islam, K.R.; Stine, M.A.; Gruver, J.B.; Samson-Liebig, S.E.; Weil, R.R. Estimating active carbon for soil quality assessment: A simplified method for laboratory and field use. Am. J. Altern. Agric. 2003, 18, 3–17. [Google Scholar] [CrossRef]
- Vance, E.; Brookes, P.; Jenkinson, D. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Maynard, D.; Kalra, Y.; Crumbaugh, J. Nitrate and Exchangeable Ammonium Nitrogen, 2nd ed.; CRC Press: Boca Raton, FL, USA; Taylor and Francis Group: Abingdon, UK, 1993; Volume 1, pp. 71–80. [Google Scholar]
- Kempers, A.; Zweers, A. Ammonium determination in soil extracts by the salicylate method. Commun. Soil Sci. Plant Anal. 1986, 17, 715–723. [Google Scholar] [CrossRef]
- Doane, T.A.; Horwáth, W.R. Spectrophotometric Determination of Nitrate with a Single Reagent. Anal. Lett. 2003, 36, 2713–2722. [Google Scholar] [CrossRef]
- Picone, L.I.; Cabrera, M.L.; Franzluebbers, A.J. A Rapid Method to Estimate Potentially Mineralizable Nitrogen in Soil. Soil Sci. Soc. Am. J. 2002, 66, 1843–1847. [Google Scholar] [CrossRef]
- Mehlich, A. Determination of P, Ca, Mg, K, Na, and NH4. In North Carolina Soil Test Division (Mimeo 1953); 1953; pp. 23–89. Available online: http://www.ncagr.gov/AGRONOMI/pdffiles/mehlich53.pdf (accessed on 10 December 2019).
- Soil Survey Staff, U. Web Soil Survey; US Department of Agriculture—Natural Resources Conservation Service. Available online: http://websoilsurvey.sc.egov.usda.gov/ (accessed on 10 December 2019).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Bozdogan, H. Model selection and Akaike’s information criterion (AIC): The general theory and its analytical extensions. Psychometrika 1987, 52, 345–370. [Google Scholar] [CrossRef]
- Kassambara, A. Practical guide to principal component methods in R: PCA, M (CA), FAMD, MFA, HCPC, Factoextra; Sthda. Available online: https://cloud.r-project.org/package=factoextra/factoextra.pdf (accessed on 12 August 2019).
- Saint-Fort, R.; Frank, K.; Schepers, J. Role of nitrogen mineralization in fertilizer recommendations. Commun. Soil Sci. Plant Anal. 1990, 21, 1945–1958. [Google Scholar] [CrossRef]
- Ros, G.H.; Hanegraaf, M.C.; Hoffland, E.; van Riemsdijk, W.H. Predicting soil N mineralization: Relevance of organic matter fractions and soil properties. Soil Biol. Biochem. 2011, 43, 1714–1722. [Google Scholar] [CrossRef]
- Kingery, W.; Wood, C.; Delaney, D.; Williams, J.; Mullins, G. Impact of Long-Term Land Application of Broiler Litter on Environmentally Related Soil Properties. J. Environ. Qual. 1994, 23, 139–147. [Google Scholar] [CrossRef]
- Chaudhari, P.R.; Ahire, D.V.; Ahire, V.D.; Chkravarty, M.; Maity, S. Soil bulk density as related to soil texture, organic matter content and available total nutrients of Coimbatore soil. Int. J. Sci. Res. Publ. 2013, 3, 1–8. [Google Scholar]
- Houlbrooke, D.J.; Thom, E.R.; Chapman, R.; McLay, C.D.A. A study of the effects of soil bulk density on root and shoot growth of different ryegrass lines. N. Z. J. Agric. Res. 1997, 40, 429–435. [Google Scholar] [CrossRef]
- Hu, C.; Li, F.; Xie, Y.-H.; Deng, Z.-M.; Chen, X.-S. Soil carbon, nitrogen, and phosphorus stoichiometry of three dominant plant communities distributed along a small-scale elevation gradient in the East Dongting Lake. Phys. Chem. Earth Parts A/B/C 2018, 103, 28–34. [Google Scholar] [CrossRef]
- Chapman, R.; Allbrook, R. The effects of subsoiling compacted soils under grass-a progress report. Available online: https://www.agronomysociety.nz/uploads/94803/files/1987_13._Subsoiling_compacted_soils_under_grass.pdf (accessed on 2 June 2012).
- Yang, Y.; Fang, J.; Guo, D.; Ji, C.; Ma, W. Vertical patterns of soil carbon, nitrogen and carbon: Nitrogen stoichiometry in Tibetan grasslands. Biogeosci. Discuss. 2010, 7. [Google Scholar] [CrossRef]
- Steffens, M.; Kölbl, A.; Totsche, K.U.; Kögel-Knabner, I. Grazing effects on soil chemical and physical properties in a semiarid steppe of Inner Mongolia (P.R. China). Geoderma 2008, 143, 63–72. [Google Scholar] [CrossRef]
- Dessureault-Rompré, J.; Zebarth, B.J.; Burton, D.L.; Sharifi, M.; Cooper, J.; Grant, C.A.; Drury, C.F. Relationships among Mineralizable Soil Nitrogen, Soil Properties, and Climatic Indices. Soil Sci. Soc. Am. J. 2010, 74, 1218–1227. [Google Scholar] [CrossRef]
- Schomberg, H.H.; Wietholter, S.; Griffin, T.S.; Reeves, D.W.; Cabrera, M.L.; Fisher, D.S.; Endale, D.M.; Novak, J.M.; Balkcom, K.S.; Raper, R.L.; et al. Assessing Indices for Predicting Potential Nitrogen Mineralization in Soils under Different Management Systems. Soil Sci. Soc. Am. J. 2009, 73, 1575–1586. [Google Scholar] [CrossRef]
- Wyngaard, N.; Cabrera, M.L.; Shober, A.; Kanwar, R. Fertilization strategy can affect the estimation of soil nitrogen mineralization potential with chemical methods. Plant Soil 2018, 432, 75–89. [Google Scholar] [CrossRef]
- Franklin, D.; Bender-Özenç, D.; Özenç, N.; Cabrera, M. Nitrogen mineralization and phosphorus release from composts and soil conditioners found in the Southeastern United States. Soil Sci. Soc. Am. J. 2015, 79, 1386–1395. [Google Scholar] [CrossRef]
- Riffaldi, R.; Saviozzi, A.; Levi-Minzi, R. Carbon mineralization kinetics as influenced by soil properties. Biol. Fertil. Soils 1996, 22, 293–298. [Google Scholar] [CrossRef]
- Melero, S.; López-Garrido, R.; Madejón, E.; Murillo, J.M.; Vanderlinden, K.; Ordóñez, R.; Moreno, F. Long-term effects of conservation tillage on organic fractions in two soils in southwest of Spain. Agric. Ecosyst. Environ. 2009, 133, 68–74. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nat. Cell Biol. 2015, 528, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.R.; Clay, D.E.; Smart, A.; Clay, S.A.; Kharel, T.P.; Mishra, U. Soil and land-use change sustainability in the Northern Great Plains of the USA. In Land Use Change and Sustainability; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Franzluebbers, A.J.; Pehim-Limbu, S.; Poore, M.H. Soil-Test Biological Activity with the Flush of CO2: IV. Fall-Stockpiled Tall Fescue Yield Response to Applied Nitrogen. Agron. J. 2018, 110, 2033–2049. [Google Scholar] [CrossRef]
- Hendricks, T.; Franklin, D.; Dahal, S.; Hancock, D.; Stewart, L.; Cabrera, M.; Hawkins, G. Soil carbon and bulk density distribution within 10 Southern Piedmont grazing systems. J. Soil Water Conserv. 2019, 74, 323–333. [Google Scholar] [CrossRef]
- Mendham, D.S.; O’Connell, A.M.; Grove, T.S. Organic matter characteristics under native forest, long-term pasture, and recent conversion to Eucalyptus plantations in Western Australia: Microbial biomass, soil respiration, and permanganate oxidation. Soil Res. 2002, 40, 859. [Google Scholar] [CrossRef]
- Plaza-Bonilla, D.; Álvaro-Fuentes, J.; Cantero-Martínez, C. Identifying soil organic carbon fractions sensitive to agricultural management practices. Soil Tillage Res. 2014, 139, 19–22. [Google Scholar] [CrossRef]
- Culman, S.W.; Snapp, S.S.; Freeman, M.A.; Schipanski, M.E.; Beniston, J.; Lal, R.; Drinkwater, L.E.; Franzluebbers, A.J.; Glover, J.D.; Grandy, A.S.; et al. Permanganate Oxidizable Carbon Reflects a Processed Soil Fraction that is Sensitive to Management. Soil Sci. Soc. Am. J. 2012, 76, 494–504. [Google Scholar] [CrossRef]
- Islam, K.; Weil, R. Land use effects on soil quality in a tropical forest ecosystem of Bangladesh. Agric. Ecosyst. Environ. 2000, 79, 9–16. [Google Scholar] [CrossRef]
- Franzluebbers, A.J.; Stuedemann, J.A. Bermudagrass management in the southern piedmont USA. III. Particulate and biologically active soil carbon. Soil Sci. Soc. Am. J. 2003, 67, 132–138. [Google Scholar] [CrossRef]
- Franzluebbers, A.J.; Haney, R.L.; Honeycutt, C.W.; Schomberg, H.H.; Hons, F.M. Flush of Carbon Dioxide Following Rewetting of Dried Soil Relates to Active Organic Pools. Soil Sci. Soc. Am. J. 2000, 64, 613–623. [Google Scholar] [CrossRef]
- Acosta-Martínez, V.; Harmel, R.D. Soil Microbial Communities and Enzyme Activities under Various Poultry Litter Application Rates. J. Environ. Qual. 2006, 35, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Franklin, D.; Truman, C.; Potter, T.; Bosch, D.; Strickland, T.; Jenkins, M.; Nuti, R. Nutrient losses in runoff from conventional and no-till pearl millet on pre-wetted Ultisols fertilized with broiler litter. Agric. Water Manag. 2012, 113, 38–44. [Google Scholar] [CrossRef]
- Brookes, P.C. The use of microbial parameters in monitoring soil pollution by heavy metals. Biol. Fertil. Soils 1995, 19, 269–279. [Google Scholar] [CrossRef]
- Francis, G.S.; Haynes, R.J.; Williams, P.H. Effects of the timing of ploughing-in temporary leguminous pastures and two winter cover crops on nitrogen mineralization, nitrate leaching and spring wheat growth. J. Agric. Sci. 1995, 124, 1–9. [Google Scholar] [CrossRef]
- Rustad, L.E.; News, G.-; Campbell, J.L.; Marion, G.M.; Norby, R.J.; Mitchell, M.J.; Hartley, A.E.; Cornelissen, J.H.C.; Gurevitch, J. A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 2001, 126, 543–562. [Google Scholar] [CrossRef] [PubMed]
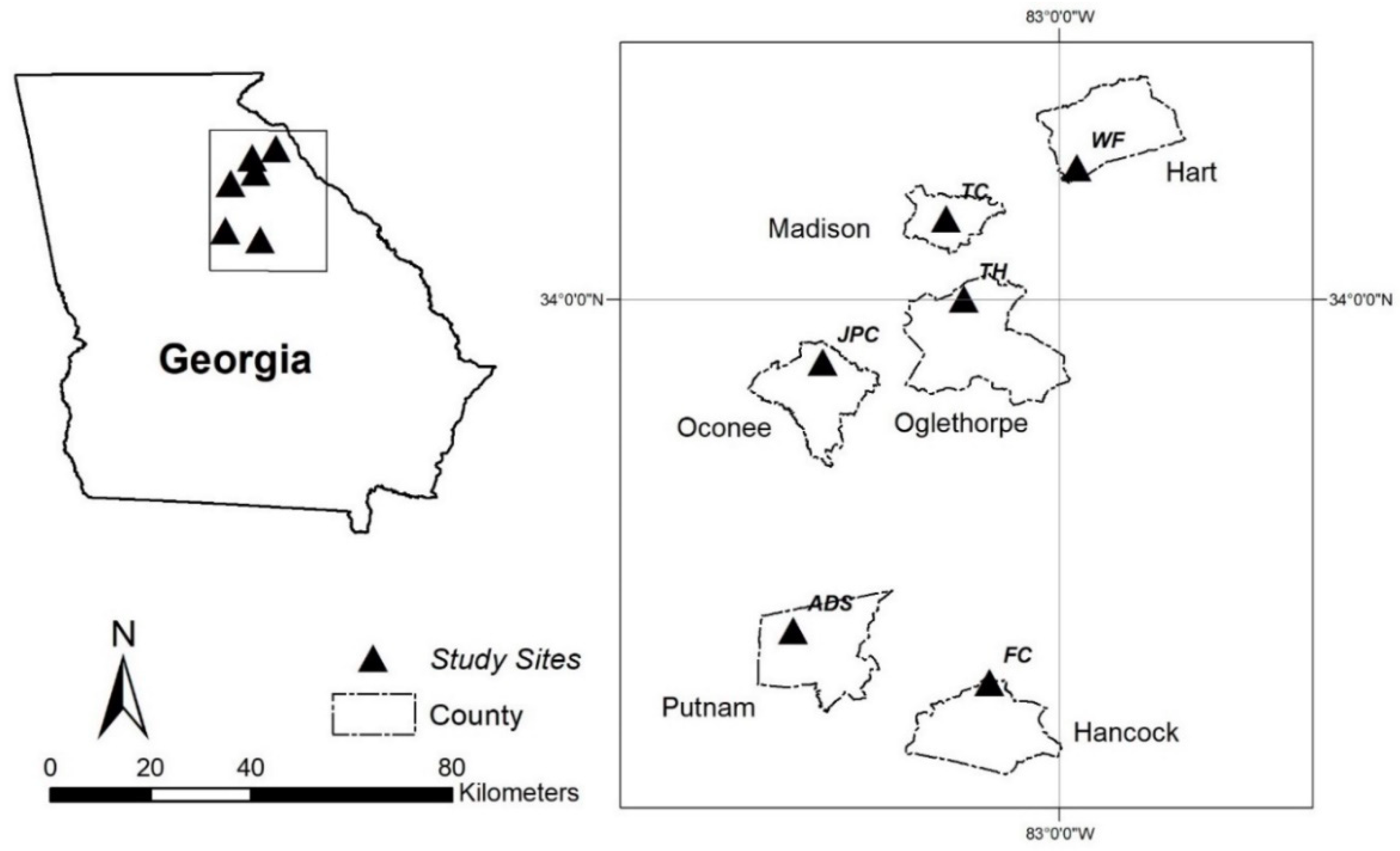


| Site | Location | Pasture Attributes | Management/Fertilization | Soil Type | Sampling Dates | Soil Samples |
|---|---|---|---|---|---|---|
| JPC | J. Phil Campbell Sr. Research and Education Center Watkinsville, GA | Four Tall Fescue-Bermudagrass mixed pastures, approx. 17 ha each. | Historically (more than 10 years before 2016) conventionally grazed. In May 2016, 2 pastures were strategically grazed and other 2 were continuously grazed with rolling out of hay. Inorganic fertilizer | i. Fine, kaolinitic, thermic Typic Kanhapludults (NRCS-First Order Soil Survey) | July 2017 July 2018 | 492 |
| ADS | Animal and Dairy Science Beef Cattle Farm, Eatonton, GA | Four Tall Fescue-Bermudagrass mixed pastures, approx. 17 ha each. | Same as JPC except no external fertilizer applied after May 2016. | i. Fine, kaolinitic, thermic Rhodic Kandiudults; ii. Loamy, mixed, active, thermic, shallow Typic Hapludalfs (NRCS-First Order Soil Survey) | July 2017 July 2018 | 528 |
| WF | Hartwell, GA | One Tall Fescue pasture (19.71 ha) and one Hay field (4.28 ha) | Rotationally grazed; Broiler litter fertilized | i. Fine, kaolinitic, thermic Typic Kanhapludults | May 2017 June 2018 | 52 |
| TC | Danielsville, GA | One Tall Fescue pasture (6.93 ha) | Rotationally grazed; Broiler litter fertilized | i. Fine, kaolinitic, thermic Rhodic Kandiudults; ii. Fine, kaolinitic, thermic Typic Kanhapludults | June 2018 | 25 |
| TH | Crawford, GA | One Tall Fescue pasture (2.27 ha) | Rotationally grazed; Broiler litter fertilized | i. Fine, kaolinitic, thermic Typic Kanhapludults | June 2018 | 13 |
| FC | Devereux, GA | One Tall Fescue pasture (11.26 ha) | Rotationally grazed; Broiler litter fertilized | i. Fine, kaolinitic, thermic Rhodic Kanhapludults; ii. Fine-loamy, mixed, active, nonacid, thermic Oxyaquic Udifluvents | May 2017 | 12 |
| Soil Health Indicator | Soil Depth | N | Median | Mean | SD | Min | Max |
|---|---|---|---|---|---|---|---|
| Research Pastures (Inorganic and/or No Fertilizer) | |||||||
| Loss on Ignition Carbon (LOI) (g 100 g−1) | 0–5 | 335 | 73 | 78 | 27 | 29 | 211 |
| 5–10 | 335 | 44 | 48 | 17 | 19 | 159 | |
| 10–20 | 323 | 41 | 45 | 17 | 16 | 107 | |
| Active Carbon (POXC) (mg kg −1) | 0–5 | 335 | 722 | 736 | 240 | 24.88 | 1355 |
| 5–10 | 334 | 299 | 317 | 143 | 0.1 | 9996 | |
| 10–20 | 321 | 180 | 192 | 110 | 0.1 | 645 | |
| Soil Microbial Biomass (SMBC) (mg CO2-C kg−1) | 0–5 | 27 | 168 | 172 | 85 | 16 | 409 |
| Soil Respiration (mg CO2-C m−2 24 h−1) | 0–5 | 316 | 844 | 1078 | 849 | 19 | 4136 |
| Inorganic Nitrogen (N) (mg kg−1) | 0–5 | 329 | 36.6 | 44.9 | 39.1 | 0.7 | 327.1 |
| 5–10 | 329 | 7.9 | 10.5 | 10.1 | 0.5 | 93.6 | |
| 10–20 | 316 | 4.7 | 6.3 | 7.6 | 0.1 | 79.5 | |
| Potentially mineralizable Nitrogen (PMN) (mg kg−1) | 0–5 | 330 | 20.7 | 21.6 | 12.2 | 0.1 | 59.2 |
| 5–10 | 329 | 9.6 | 10.1 | 5.1 | 1.0 | 33.1 | |
| 10–20 | 317 | 6.2 | 6.5 | 3.7 | 0.1 | 31.7 | |
| Nitrate-Nitrogen (NO3−-N) (mg kg−1) | 0–5 | 330 | 31.7 | 39.3 | 37.8 | 0.00 | 298.2 |
| 5–10 | 329 | 5.3 | 7.6 | 9.4 | 0.00 | 78.7 | |
| 10–20 | 317 | 2.5 | 4.3 | 7.5 | 0.00 | 77.9 | |
| Mehlich-I Phosphorus (P) (mg kg−1) | 0–5 | 302 | 13.3 | 18.3 | 27.5 | 0.2 | 383.5 |
| 5–10 | 328 | 6.7 | 17.0 | 33.1 | 0.0 | 257.9 | |
| 10–20 | 315 | 3.1 | 14.2 | 34.8 | 0.0 | 321.3 | |
| Bulk Density (BD) (g cm−3) | 0–5 | 333 | 1.32 | 1.31 | 0.19 | 0.68 | 2.41 |
| 5–10 | 330 | 1.51 | 1.51 | 0.15 | 0.82 | 2.41 | |
| 10–20 | 315 | 1.47 | 1.45 | 0.13 | 0.62 | 1.78 | |
| Clay% | 0–5 | 335 | 23.5 | 21.1 | 7.0 | 10 | 37.5 |
| 5–10 | 335 | 21 | 22.0 | 10.3 | 10 | 36.5 | |
| 10–20 | 323 | 21 | 26.6 | 14.9 | 10 | 50 | |
| A. Farmers’ Field (Broiler Litter Fertilized) | |||||||
| LOI (g 100 g−1) | 0–10 | 102 | 9.4 | 11.8 | 7.8 | 2.5 | 36.1 |
| POXC (mg kg−1) | 0–10 | 102 | 611 | 596 | 139 | 251 | 956 |
| SMBC (mg CO2-C kg−1) | 0–10 | 64 | 25.9 | 45.7 | 47.1 | 4.2 | 235.8 |
| N (mg kg−1) | 0–10 | 102 | 37.5 | 39.9 | 16.3 | 8.5 | 70.1 |
| PMN (mg kg−1) | 0–10 | 102 | 12.3 | 12.4 | 5.1 | 1.4 | 30.1 |
| NO3−-N (mg kg−1) | 0–10 | 102 | 28.2 | 28.1 | 12.9 | 5.1 | 53.8 |
| P (mg kg−1) | 0–10 | 64 | 64.1 | 62.0 | 44.9 | 1.5 | 246.7 |
| BD (g cm−3) | 0–10 | 102 | 1.41 | 1.41 | 0.62 | 1.13 | 1.67 |
| Clay% | 0–10 | 64 | 15 | 15.6 | 5.3 | 9.9 | 31 |
| Multiple Regression for SMBC | |||||
|---|---|---|---|---|---|
| Term | Estimate | SE | t-Value | p-Value | VIF |
| Intercept | 190.977 | 59.973 | 3.18 | 0.0021 | |
| Inorganic-Broiler | −42.586 | 6.563 | −6.49 | <0001 | 1.67 |
| POXC/PMN | 0.395 | 0.074 | 5.34 | <0001 | 1.51 |
| P | −0.564 | 0.118 | −4.79 | <0001 | 1.3 |
| PMN | 3.114 | 0.913 | 3.41 | 0.001 | 1.74 |
| LOI/P | −3.770 | 1.187 | −3.18 | 0.0021 | 1.16 |
| BD | −85.584 | 36.337 | −2.36 | 0.021 | 1.59 |
| Multiple Regression for N | Multiple Regression for PNM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Term | Estimate | SE | t-Value | p-Value | VIF | Term | Estimate | SE | t-Value | p-Value | VIF |
| Intercept | 40.134 | 7.598 | 5.28 | <0001 | Intercept | 9.057 | 2.559 | 3.54 | 0.0004 | ||
| POXC | 0.049 | 0.003 | 17.34 | <0001 | 1.60 | POXC | 0.022 | 0.001 | 19.64 | <0001 | 2.02 |
| BD | −32.034 | 4.693 | −6.83 | <0001 | 1.58 | N | 0.058 | 0.011 | 5.39 | <0001 | 1.88 |
| P | 0.136 | 0.021 | 6.5 | <0001 | 1.02 | Resp | −0.001 | 0.000 | −5.18 | <0001 | 1.02 |
| Resp | 0.002 | 0.001 | 2.77 | 0.0057 | 1.01 | BD | −3.399 | 1.599 | −2.13 | 0.0339 | 1.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahal, S.; Franklin, D.H.; Subedi, A.; Cabrera, M.L.; Ney, L.; Fatzinger, B.; Mahmud, K. Interrelationships of Chemical, Physical and Biological Soil Health Indicators in Beef-Pastures of Southern Piedmont, Georgia. Sustainability 2021, 13, 4844. https://doi.org/10.3390/su13094844
Dahal S, Franklin DH, Subedi A, Cabrera ML, Ney L, Fatzinger B, Mahmud K. Interrelationships of Chemical, Physical and Biological Soil Health Indicators in Beef-Pastures of Southern Piedmont, Georgia. Sustainability. 2021; 13(9):4844. https://doi.org/10.3390/su13094844
Chicago/Turabian StyleDahal, Subash, Dorcas H. Franklin, Anish Subedi, Miguel L. Cabrera, Laura Ney, Brendan Fatzinger, and Kishan Mahmud. 2021. "Interrelationships of Chemical, Physical and Biological Soil Health Indicators in Beef-Pastures of Southern Piedmont, Georgia" Sustainability 13, no. 9: 4844. https://doi.org/10.3390/su13094844
APA StyleDahal, S., Franklin, D. H., Subedi, A., Cabrera, M. L., Ney, L., Fatzinger, B., & Mahmud, K. (2021). Interrelationships of Chemical, Physical and Biological Soil Health Indicators in Beef-Pastures of Southern Piedmont, Georgia. Sustainability, 13(9), 4844. https://doi.org/10.3390/su13094844








