1. Introduction
The most abundant low-value co-product (used for animal feed) of the corn wet milling process is corn fibre [
1], which contains a significant fraction of valuable phenolic compounds, mainly ferulic acid. Corn fibre contains between 1.5 and 3% of ferulic acid (W
total ferulic acid/W
dry corn fibre) [
2,
3,
4,
5]. Ferulic acid presents several recognised benefits, namely antioxidant, anti-inflammatory, antiatherogenic, antidiabetic, antiaging, neuroprotective, radioprotective, hepatoprotective, and antiproliferative properties [
6,
7,
8], which make this compound widely used in the food, cosmetics, and pharmaceutical industries. It can also be used as a crosslinking agent for the preparation of films [
9,
10] and as precursor of bio-vanillin production [
11,
12]. Ferulic acid in corn fibre is mainly covalently linked by ester bonds to arabinoxylans. Arabinoxylans, also designated as corn fibre gum, are valuable non-starch cereal polysaccharides [
13], with many recognized health benefits, such as a prebiotic, for the control of diabetes, immunomodulatory activity, and a cholesterol lowering effect [
14,
15,
16]. They also have a functional application in edible film forming [
17,
18] and as an emulsifier for oil-in-water emulsion systems [
19].
Alkaline extraction is the most commonly reported method for releasing ferulic acid [
20,
21,
22,
23,
24] from agro-industrial by-products; however, alkaline extraction also releases arabinoxylans [
25,
26,
27]. It is not possible to use a sequential two-step extraction process, using different operating conditions, for the release of ferulic acid and then of arabinoxylans. Hence, in order to achieve an efficient recovery of these two compounds for their use in different applications, an efficient separation is required. This separation is quite challenging, due to the high viscosity of the alkaline extract caused by the presence of arabinoxylans [
22]. In fact, the high viscosity of arabinoxylans makes it difficult to use adsorption to purify ferulic acid in an alkaline extract [
22].
Therefore, the development of a process able to efficiently separate these two compounds is very important for the valorisation of corn fibre extracts and the future use of both compounds. Ultrafiltration seems to be a suitable option for the separation of ferulic acid from arabinoxylans, due to the high molecular mass difference between the two compounds. Although the molecular mass of arabinoxylans is not constant (it fluctuates depending on the raw material and on the alkaline extraction conditions [
25]), high molecular masses in the range of 244 to 491 kDa have been reported for arabinoxylans extracted from corn fibre [
28]. This range of masses makes possible the use of ultrafiltration to separate arabinoxylans from ferulic acid, which has a molecular mass of 194 Da. Ultrafiltration has already been reported as a method to separate arabinoxylans from ferulic acid and other compounds present in different extracts (e.g., alkaline-alcohol extract from corn bran [
22], alkaline-alcohol extract from wheat bran [
29], and alkaline extract from wheat bran [
26,
30,
31]).
However, the high viscosity of arabinoxylans can also influence the ultrafiltration process, leading to poor fluid dynamic conditions and, ultimately, causing membrane fouling, due to the formation of a gel layer on the membrane surface [
29]. In order to overcome the viscosity problem of arabinoxylans, strategies have been reported involving the addition of ethanol during alkaline extraction, which decreases the viscosity of the extracts [
22,
29], and the use of dia-ultrafiltration [
26]. However, the use of ethanol during the alkaline extraction decreases the ferulic acid released from the biomass [
22], so the use of this organic solvent should be avoided. Dia-ultrafiltration allows to maintain a constant viscosity in the retentate stream by addition of a solvent (at the same flow rate as the permeate is removed), but a diluted permeate stream is obtained requiring further concentration of ferulic acid, which is expensive (a reverse osmosis or thermal process will be required for that purpose). Aiming at recovering not only the arabinoxylans, but also the ferulic acid, and since ferulic acid is not expected to be rejected by the ultrafiltration membrane, an ultrafiltration process was selected instead of dia-ultrafiltration. The loss of ferulic acid in the retentate by processing the corn fibre extract by ultrafiltration is minimised by operating until achieving a high concentration factor. Although dia-ultrafiltration may minimise ferulic acid losses, it would also lead to very diluted permeates, requiring the removal of water, which would be costly. In fact, dia-ultrafiltration has only been reported in the literature for the purification of arabinoxylans without recovery of other valuable compounds, such as ferulic acid from the permeate [
26].
The purification of ferulic acid by ultrafiltration from alkaline extracts was reported, not using alkaline extracts from corn fibre, but from wheat bran [
29] and from corn bran [
22]. Also, in the reported cases, ethanol was used to decrease the viscosity of the extracts.
The purification of arabinoxylans from alkaline extracts (or from a steam explosion extract [
32]) was reported, using wheat bran [
26], wheat bran and wheat straw [
30], and barley husks [
32] as feedstocks. The purification of arabinoxylans was performed by ultrafiltration [
30,
33], by ultrafiltration operated in a diafiltration mode (dia-ultrafiltration) [
26], or by ultrafiltration followed by dia-ultrafiltration [
32].
The main innovation of the present work relies on the purification of both ferulic acid and of arabinoxylans using corn fibre alkaline extract, where no solvent, such as ethanol, was added (in order to decrease the viscosity of the extracts) and where the optimisation of the process involved the use of a design of experiments approach, considering the main operating conditions of ultrafiltration (operating temperature, initial crossflow velocity and transmembrane pressure). Consequently, increasing the overall sustainability of the corn wet milling process through the efficient, and also sustainable, valorisation of corn fibre.
Therefore, this work evaluates the use of ultrafiltration for the separation of ferulic acid from arabinoxylans in alkaline extracts from corn fibre rich in ferulic acid. In order to minimise the loss of membrane permeance induced by the high arabinoxylan viscosity, the most relevant ultrafiltration operating parameters were studied: membrane molecular weight cut-off, crossflow velocity of the feed stream, transmembrane pressure, and operating temperature. First, the type of membrane was evaluated by analysing the behaviour of each membrane in a dead-end configuration cell, in terms of ferulic acid rejection, arabinoxylans retention (assessed through the apparent viscosity of the retentate and rejection of the sugar constituents of arabinoxylans), and permeance.
The most suitable membrane was selected and was then used to set the other operating parameters (crossflow velocity, transmembrane pressure, and operating temperature), using a response surface methodology (RSM). Response surface methodology (RSM) is a multivariate regression tool based on statistical analysis, which allows the factors and response variables to be correlated using regression models that are optimised for each response studied [
34,
35]. RSM is combined with design of experiments (DoE) to find an optimal response. DoE introduces variation in the independent factors that directly affect the variation of responses and allows a reduction in the number of experiments required to develop a model [
35]. In this work, this statistical method was used to establish the correlation between the factors (operating temperature, crossflow velocity, and transmembrane pressure), and the responses (rejection of ferulic acid, retention of arabinoxylans, measured through the viscosity of retentate stream, and permeance), in order to define the best ultrafiltration conditions for an efficient separation of ferulic acid from arabinoxylans, while maintaining a good permeance.
3. Results and Discussion
3.1. Selection of Ultrafiltration Membranes
The aim of fractionation is to obtain a permeate rich in ferulic acid and a retentate with the maximum concentration of arabinoxylans. Membrane selection was accomplished with a first set of experiments, performed in a dead-end cell. Since corn fibre arabinoxylans have a molecular weight from 244 to 491 kDa [
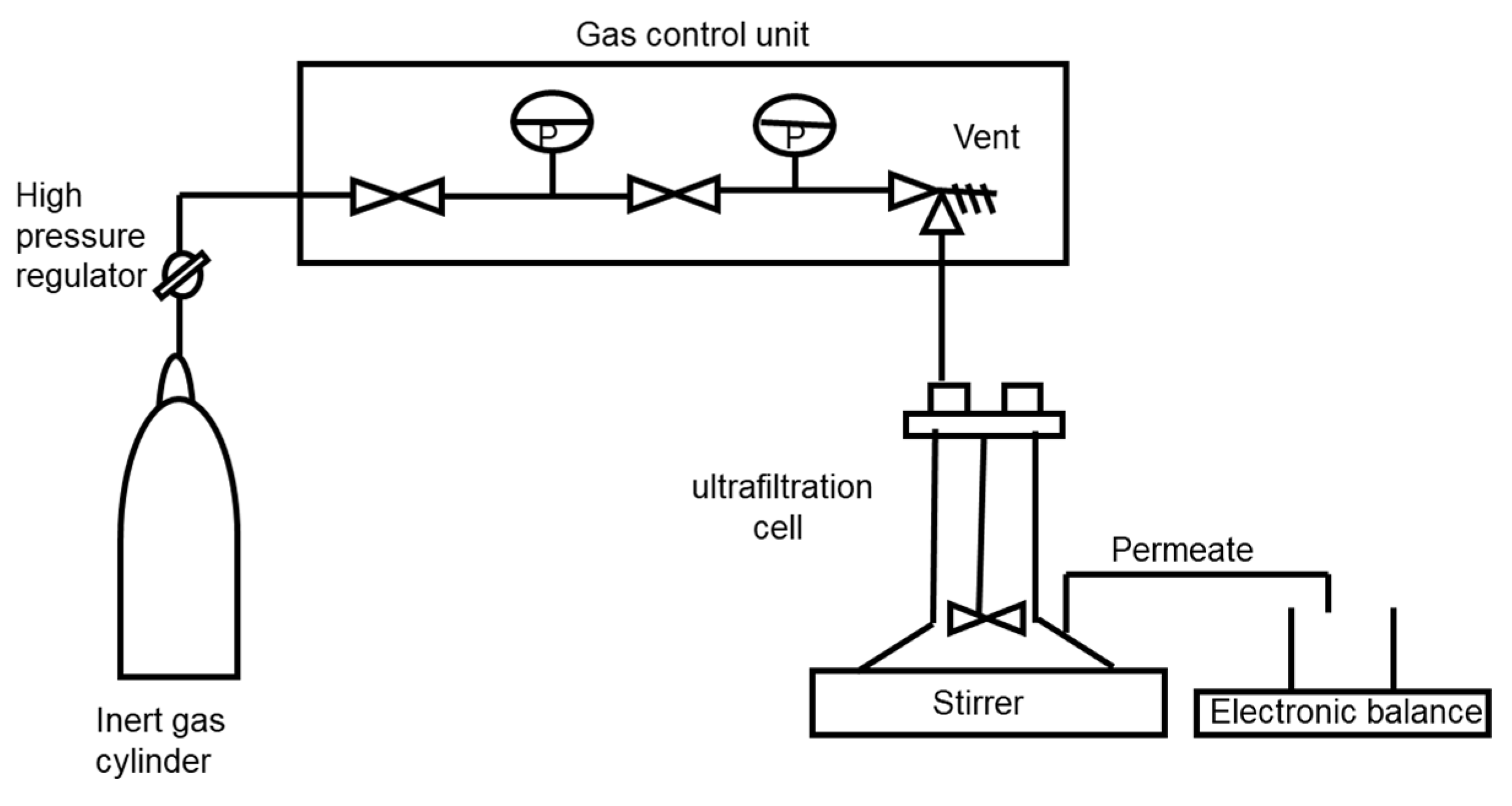
28], membranes of different materials with a molecular weight cut-off ranging from 50 to 150 kDa were evaluated in order to characterise their behaviour concerning the rejection of ferulic acid (targeting as low as possible values) and their permeance for ferulic acid (targeting high values). Regarding the arabinoxylans, the viscosity of the final retentate and the estimated rejection of arabinoxylans needed to be high, as the main objective was the effective separation between arabinoxylans and ferulic acid.
The first set of membrane experiments was performed at 0.7 bar and 50 °C using 230 mL of alkaline extract with an apparent viscosity of 3.13 ± 0.05 mPa.s at 20 °C. The main results obtained are shown in
Table 3,
Figure 3 and
Figure 4.
Table 3 shows the rejection of ferulic acid and of the four sugars that compose arabinoxylans and the estimated rejection of arabinoxylans; it also shows the viscosity of the permeate and the retentate at the end of each membrane experiment.
Under the acidic hydrolysis conditions, required to determine the neutral sugar content in the arabinoxylans, some sugars may be converted into hydroxymethylfurfural (HMF), which might lead to deviations in their measurements. Additionally, the arabinoxylans present in the feed may have different sugar compositions and be rejected slightly differently by the membrane, thus, an average rejection was calculated based on all monomers.
As shown in
Table 3, all membranes proved to be efficient in separating ferulic acid from arabinoxylans. For all membranes tested, no rejection of ferulic acid was observed (represented as 0% in this work), meaning that ferulic acid concentration in the final permeate and in final retentate is the same (Equation (2)). Ferulic acid passes freely through the membrane, due to its low molecular weight (194 Da). The rejection of arabinoxylans measured as sugar monomers and the estimated rejection of arabinoxylans (based on the average of the global rejection of neutral sugars) was higher than 94% and the viscosity of the retentates (85.54–158.54 mPa.s) was much higher than the permeates (1.30–1.37 mPa.s), indicating the efficient separation of the arabinoxylans from ferulic acid. The viscosity of the retentate (PL < Nadir US 100 < Nadir UP 150 < Nadir UH 50) follows the same order as the rejection of the 4 sugar monomers, indicating that high viscosity in the retentate means a high rejection of arabinoxylans, as expected.
More hydrophilic membranes, such as US (made of polysulfone with a hydrophilic treatment) and PL (made of regenerated cellulose), exhibited slightly lower rejection of arabinoxylans (which can be seen from the results obtained for the sugar monomers) and their retentates presented lower viscosities, as expected. On the other hand, the more hydrophobic polyethersulfone-based membranes, either with hydrophilic treatment (UH 50) or without (UP 150), presented a slightly better performance as they rejected the arabinoxylans, presenting an average rejection of these compounds of 98.7% ± 1.1% and 98.0% ± 1.7%, respectively.
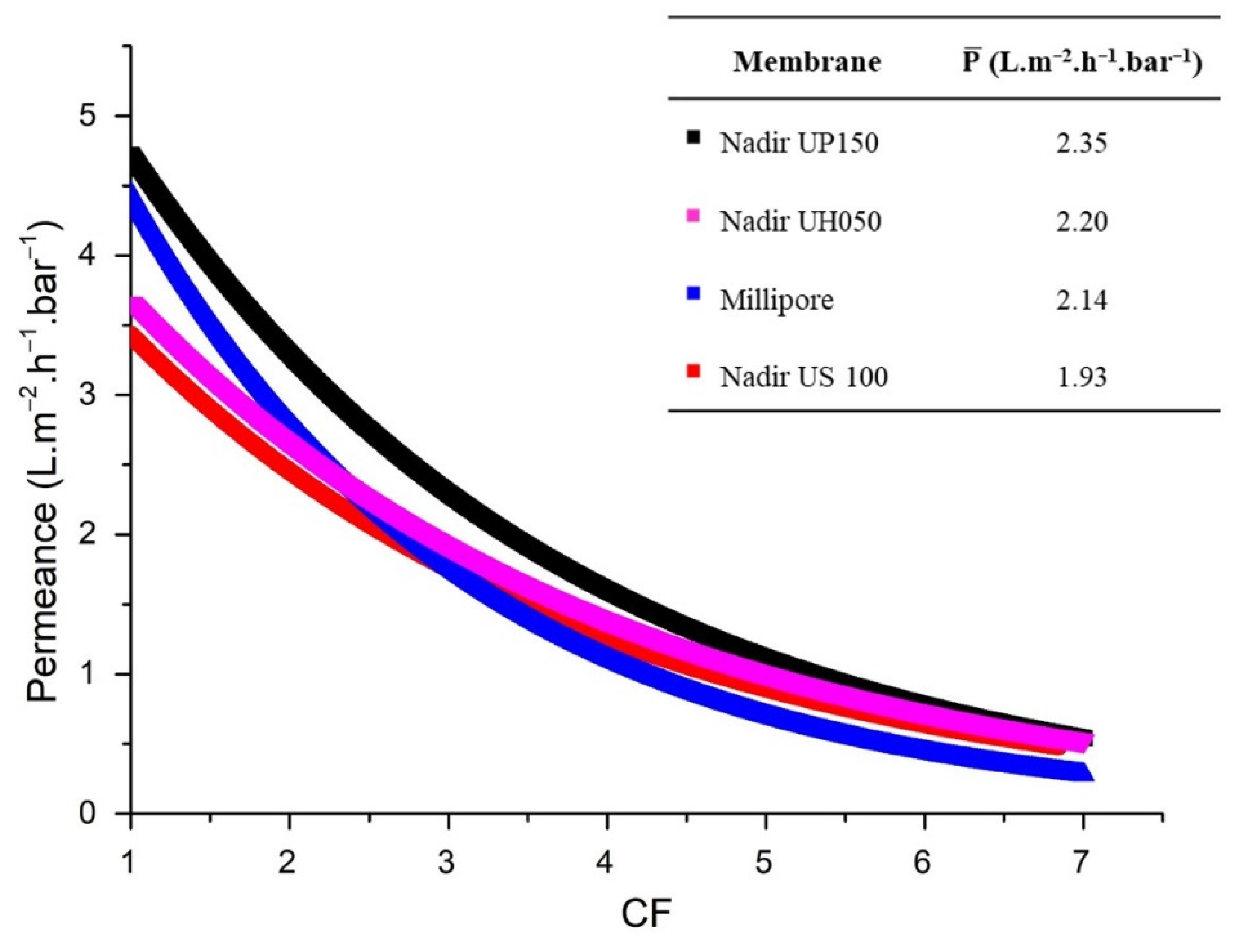
Figure 3 shows the permeance in relation to the concentration factor for each membrane, as well as the average permeance.
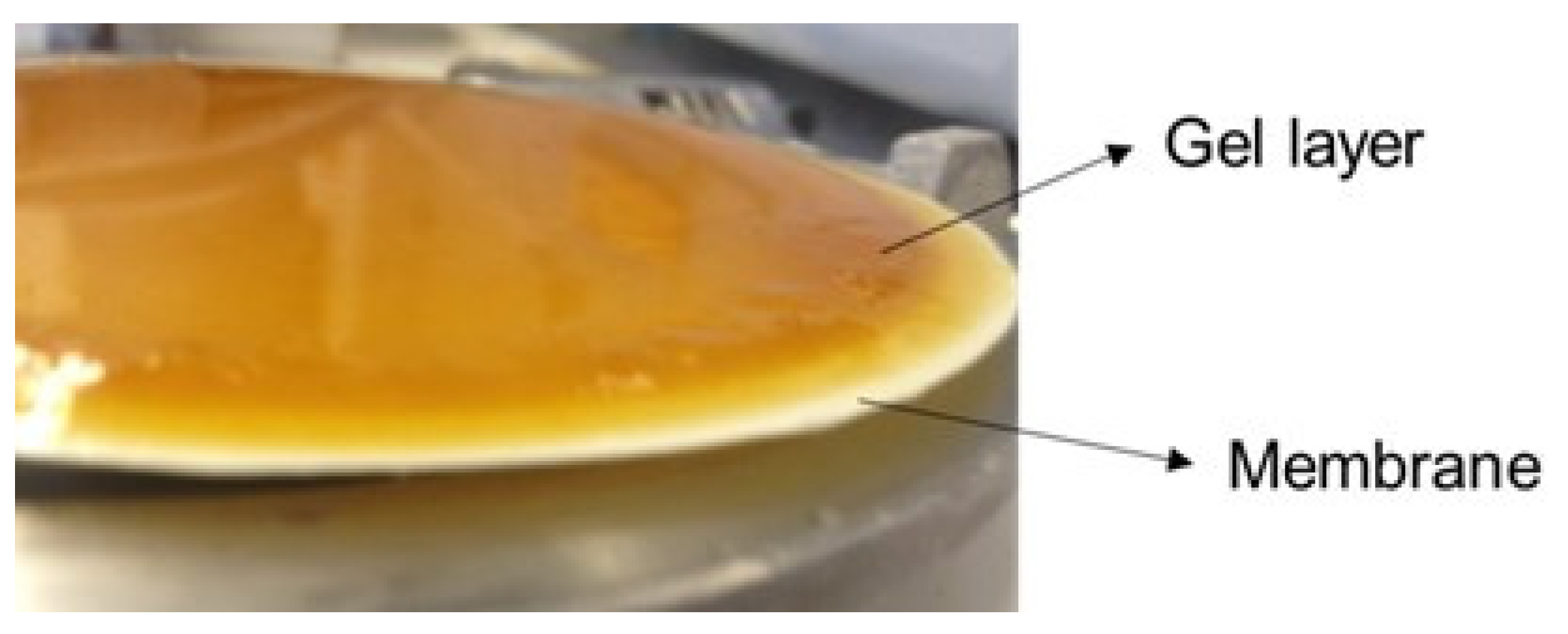
Figure 4 shows membrane UP150 after the experiments (the other membranes exhibited the same behaviour, data not shown).
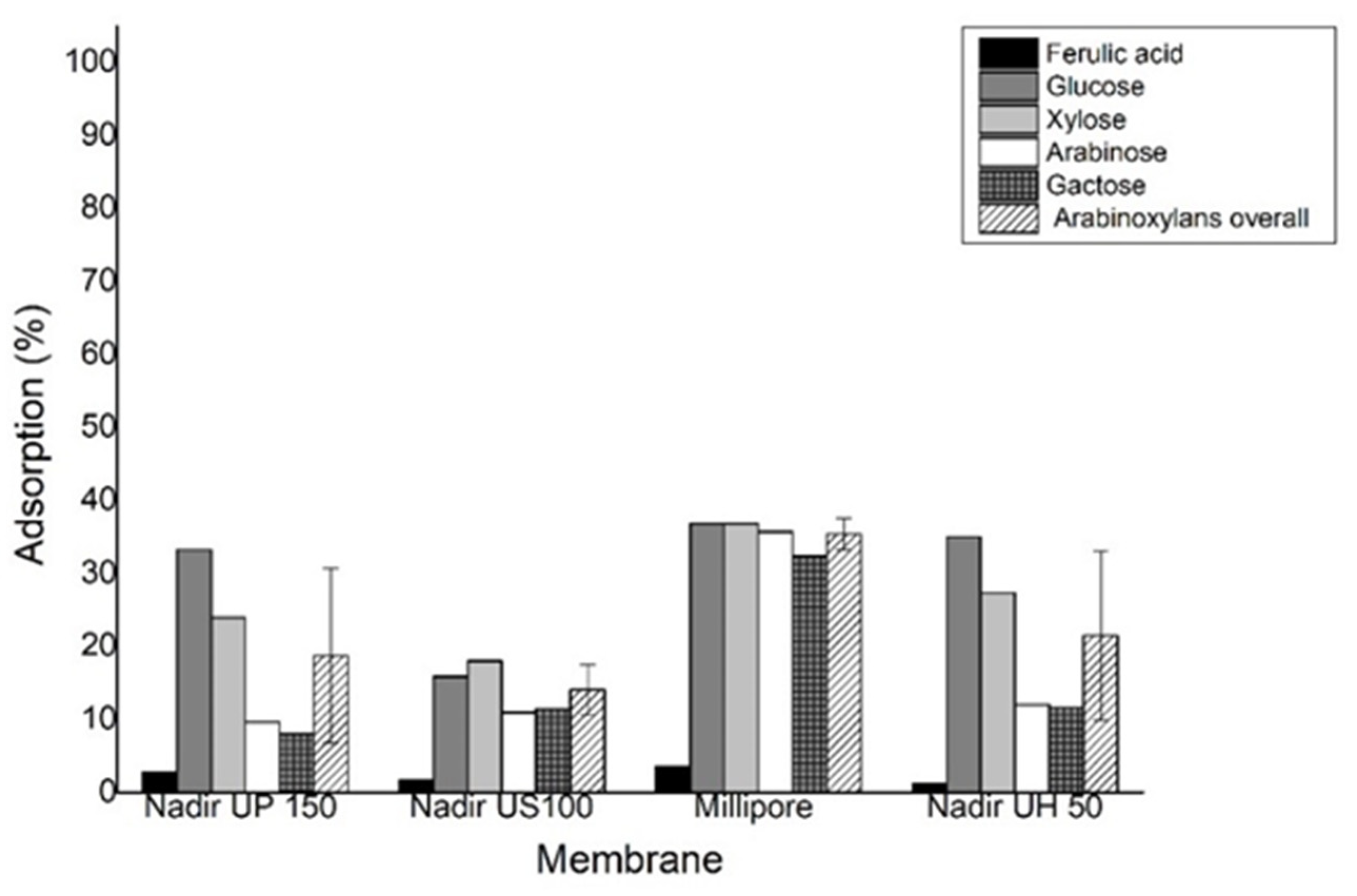
Figure 5 represents the adsorption of ferulic acid and arabinoxylans on the membrane.
As shown in
Figure 3, it was possible to achieve a concentration factor of 7, which corresponds to a rather good recovery value of 85% for ferulic acid in the permeate. The average permeance obtained for all membranes was modest for an ultrafiltration process. This can be explained by the formation of a gel layer on the membrane surface, induced by the relatively poor mass transfer conditions near the membrane surface, due to the high viscosity of the media containing arabinoxylan, which was observed at the end of each experiment. The formation of a gel layer was more pronounced, due to the use of a dead-end cell, where a process of frontal filtration takes place. The accumulation of the rejected arabinoxylans occurred near the membrane surface, promoting membrane fouling and affecting permeance. Relatively high values of arabinoxylan adsorption were detected for all membranes (
Figure 5), indicating the presence of membrane fouling phenomena. Nevertheless, the formation of a gel layer did not affect the rejection of ferulic acid, since all membranes presented no rejection of ferulic acid (0%) (
Figure 4 and
Table 3) and only a slight adsorption of ferulic acid was detected (
Figure 5).
All membranes allowed to achieve the main objective of this work—an efficient separation of ferulic acid from arabinoxylans (low ferulic acid rejection and high arabinoxylan rejection). The UP 150 membrane was selected as the best performing membrane, because it exhibits the second highest arabinoxylan rejection (98.0% ± 1.7%) and the highest average permeance (2.35 L.m2.h−1.bar−1).
3.2. Evaluation of the Effect of Operating Temperature, Crossflow Velocity and Transmembrane Pressure Using Design of Experiments (DoE)
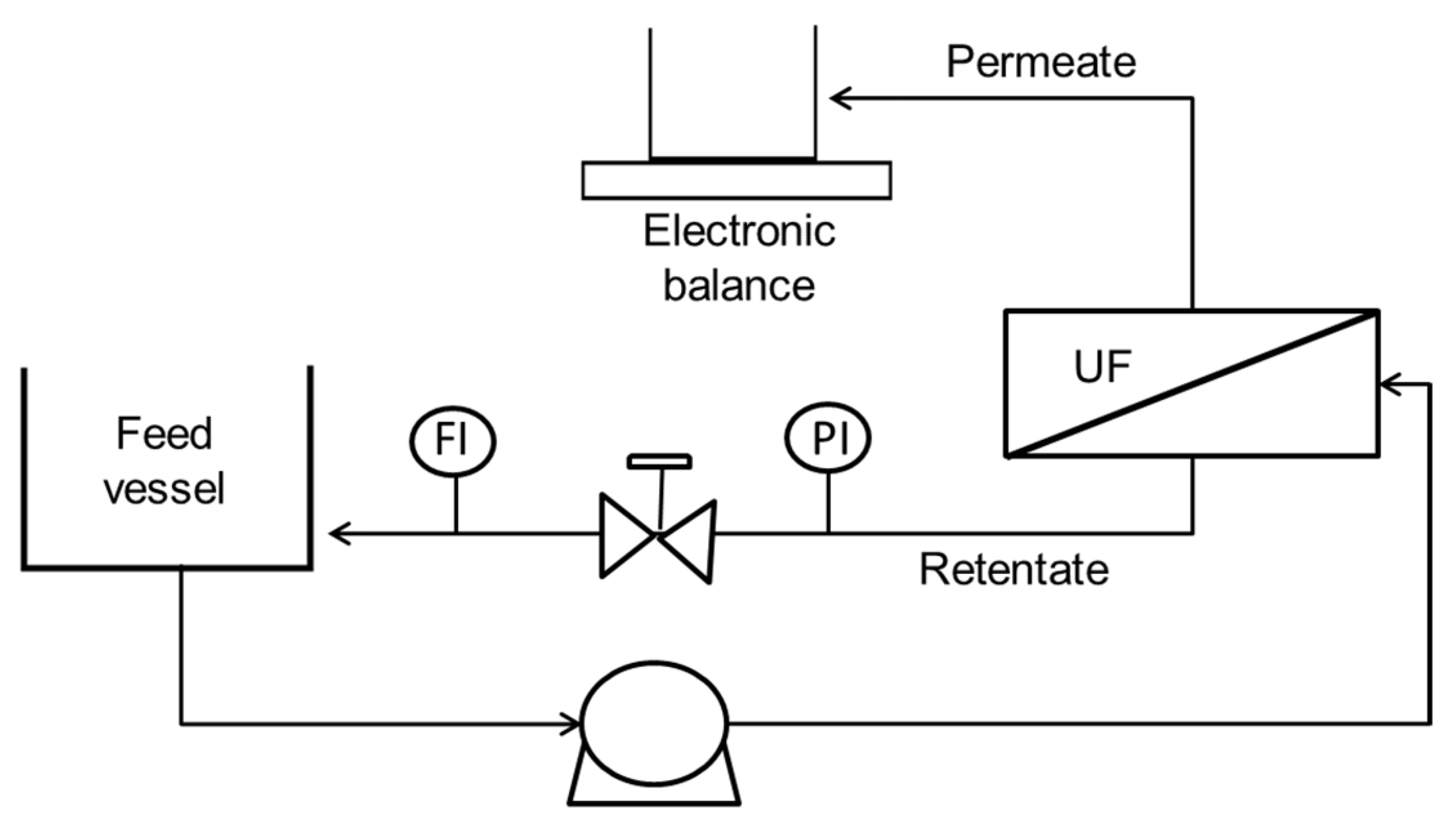
After selecting the membrane, the effect of the operating conditions (operating temperature, initial crossflow velocity, and transmembrane pressure) in the filtration performance was analysed in a crossflow cell. The 10 DoE experiments (
Table 2) were carried out, though with slight deviations between the values imposed and the ones selected to be set, due to practical operating circumstances. Temperature deviations were mainly due to differences between the set-point in the control and the temperature actually measured inside the feed vessel. The initial crossflow velocity and the transmembrane pressure were independent factors but when using a high crossflow velocity, the minimum achievable transmembrane pressure (with the valve totally open) was higher than 0.7 bar. Therefore, some adjustments were required to operate the equipment for the conditions selected.
Table 4 shows the value of the parameters actually used in the DoE, as well as that of the responses obtained (global rejection of ferulic acid (%), retention of arabinoxylans measured by retentate viscosity (mPa.s), and the average permeance (L.m
−2.h
−1.bar
−1). As discussed above, the retention of arabinoxylans was related with the retentates’ apparent viscosity, which was measured at the end of each experiment at 20 °C.
From the analysis of the responses obtained (
Table 4), it was found that there was no rejection of ferulic acid (0%) for all cases, indicating that the conditions tested had no influence (in the range of study) on the rejection of ferulic acid. Therefore, RSM was only applied to the other two responses, retention of arabinoxylans measured by apparent viscosity of retentate and average permeance, in order to find the models capable of correlating each of them with the factors.
An increase of the feed operating temperature and initial crossflow velocity, in spite of some negative impact on the energy needed for the separation process, had a positive significant impact on the performance of the process, reflected on an increase of the average permeance. Particularly, an increase of the operating temperature from 30 to 66 °C, led to an increase of the average permeance by 58% in experiments 1 and 2, and to an increase of the average permeance by 65% in experiments 7 and 8. Also, an increase of the initial crossflow velocity from 0.69 to 1.02 m.s−1, led to an increase of the average permeance of 17% in experiments 5 and 7.
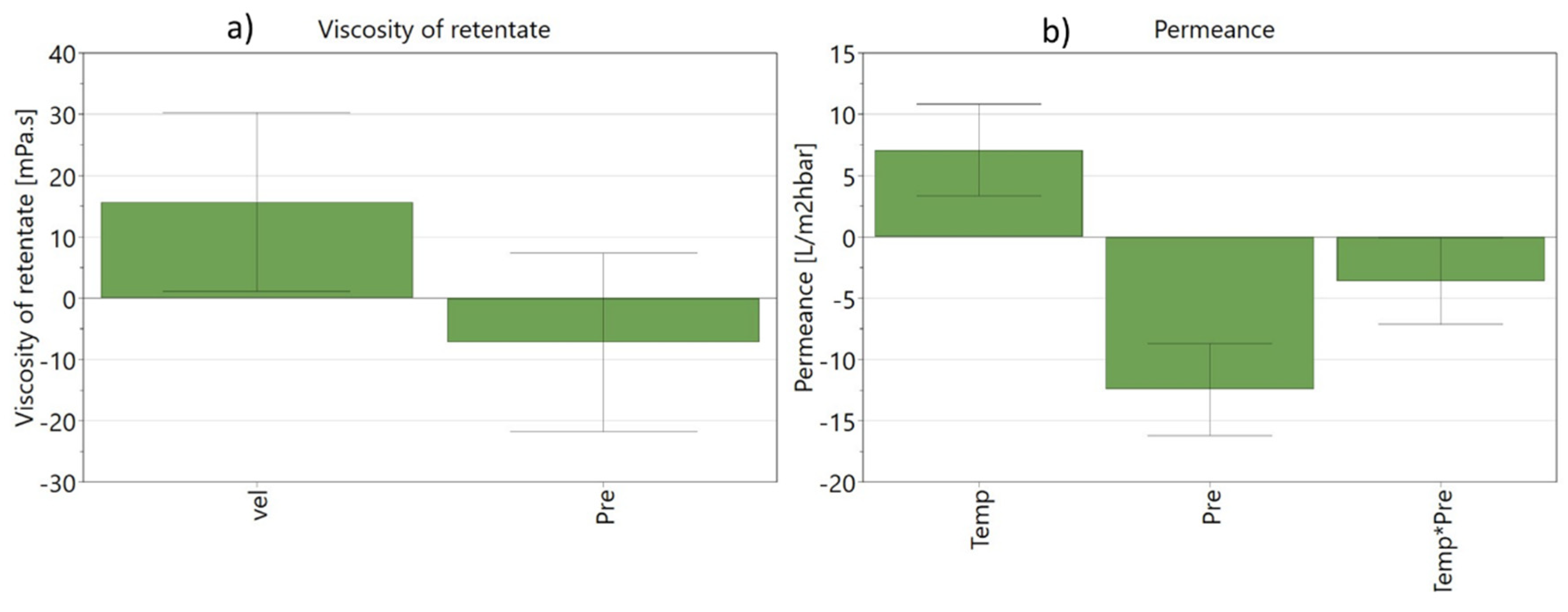
The multilinear models were optimised removing the factors and/or the interaction between factors that were insignificant for each response. The effect of the significant factors (and interactions between factors) on retention of arabinoxylans (assessed through the apparent viscosity of the retentate) and on permeance can be shown in
Figure 6, where the coefficients are normalised in order to compare the input weights.
The optimised models show that retention of arabinoxylans (measured by the viscosity of the retentate at 20 °C in the final of each experiment), is only affected by the initial crossflow velocity and by the transmembrane pressure. The operating temperature of the ultrafiltration (in the range tested) did not effluence the retention of the arabinoxylans.
The initial crossflow velocity is the most significant term, as it had a positive impact on fluid dynamics in the feed/retentate circuit, leading to better mass transfer conditions that improve the retention of arabinoxylans, which translates into an higher apparent viscosity of the retentate (high crossflow velocity increases the retentate’s apparent viscosity). In the opposite direction, operating with higher transmembrane pressure, increases the convective transport of solutes towards the membrane surface and, ultimately, facilitating fouling with a direct negative impact on the arabinoxylans present in the solution (
Figure 6a).
Regarding the permeance, the most significant term is the transmembrane pressure applied, which has a large negative impact, followed by operating temperature, which has a positive impact on permeance, and by the interaction between pressure and operating temperature (
Figure 6b). The negative impact of pressure has been reported by Lui and Wang (2002) in a study on the purification of ferulic acid from wheat bran alkaline-alcohol extract by ultrafiltration [
29], where an increase of transmembrane pressure led to the quicker formation of a gel layer, resulting in a faster membrane flux decline. The operating temperature, as expected, has a positive impact, since the viscosity of the retentate solution decreased during the experiment as the operating temperature increased and consequently increased the permeance.
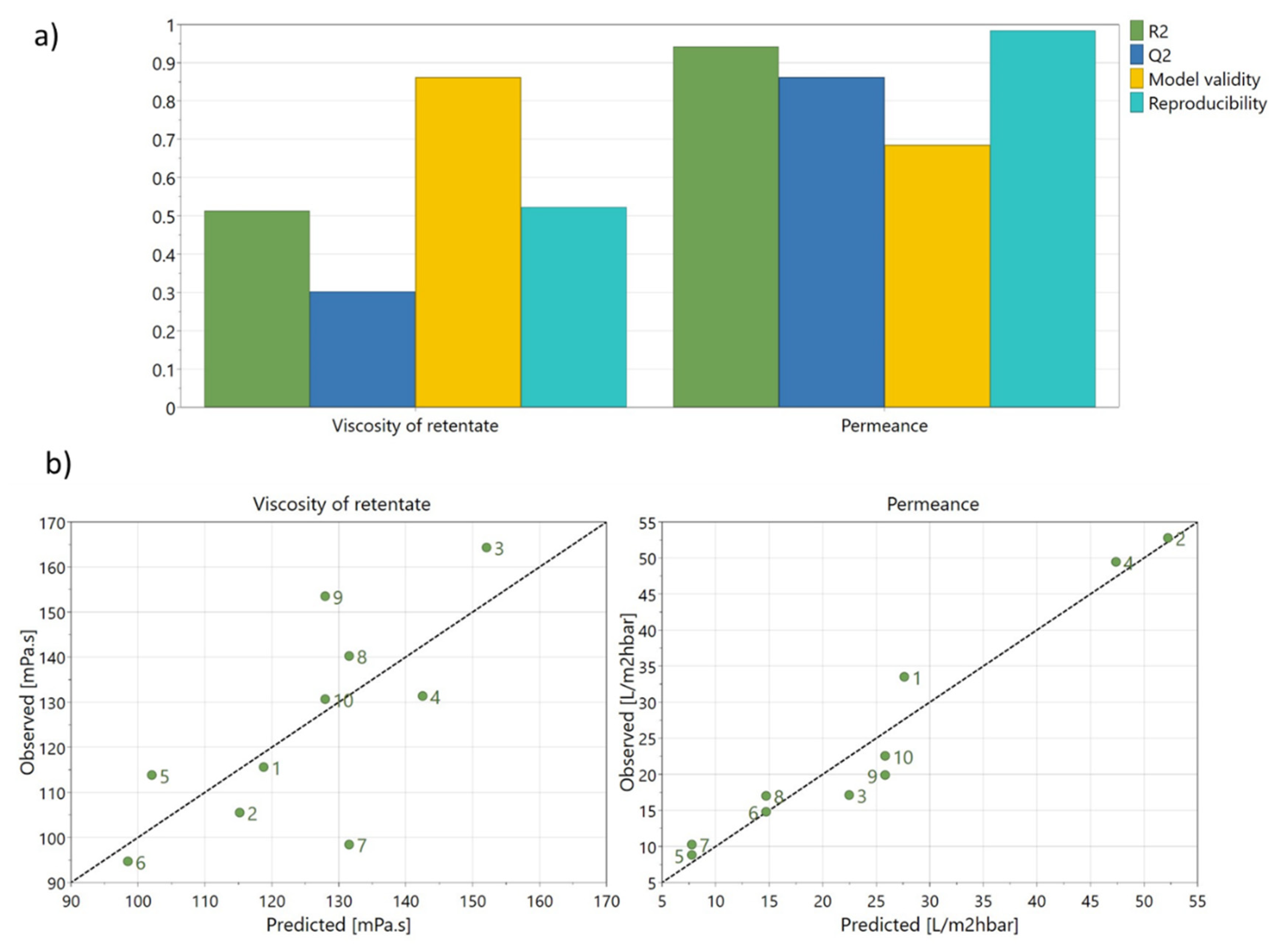
Figure 7a exhibits the statistical parameters of the model (Q
2, R
2, model validity, and reproducibility) provided by the software (MODDE Go 12.1).
Figure 7b represents the observed values for permeance and rejection of arabinoxylans, measured by the apparent viscosity of retentate versus the values predicted by the model. These two figures allow the model to be evaluated.
According to the MODDE software, parameter R
2 shows how the model fits the data and should be higher than 0.5 to be significant (showing a true correlation), Q
2 estimates the prediction precision, and should be higher than 0.1 for a significant model and higher than 0.5 for a good model [
39]. Model validity should be higher than 0.25, to exclude statistically significant problems, such as the high presence of outliers, an incorrect model, or a transformation problem [
39]. Reproducibility, defined as the variation of the replicated experience compared to overall variability, should be above 0.5 [
39]. Thus, by analysing the statistical parameters of each model individually (
Figure 7a), we can see that the permeance model can be considered a very good model (R
2 = 0.93, Q
2 = 0.86, reproducibility = 0.99, and model validity of 0.69). Furthermore, the fitting between predicted and observed values for the permeance model is good (
Figure 7b), also indicating a good model.
By analysing the statistical parameters for the viscosity model (
Figure 7a), we can see that the values of R
2, Q
2, and reproducibility were low (0.51, 0.32, and 0.52, respectively). However, the model can be considered a significant model since R
2 was higher than 0.5, Q
2 was higher than 0.1, and the reproducibility was higher than 0.5. Additionally, the model validity was much higher at 0.86, indicating there are no statistically significant problems. In
Figure 7b, showing predicted and observed values for the viscosity, it is possible to observe some data dispersion. The exclusion of experiment 7 from the model was assessed, and it resulted in a better model, with R
2 increasing from 0.51 to 0.80, Q
2 from 0.32 to 0.72, and model validity from 0.86 to 0.95. However, as it is not recommended to exclude experiments [
39], the initial model, with all data, was used.
Therefore, the final equations that translate the models achieved and that can be used for estimation of viscosity (
µretentate) and permeance (
) when varying the crossflow velocity (
), transmembrane pressure (Δ
p), and temperature (
T), within the range studied, are:
3.3. Optimal Operating Conditions
A visual evaluation of the model equations shows which ultrafiltration conditions lead to a higher permeance and a higher retentate viscosity (related to the concentration of arabinoxylans).
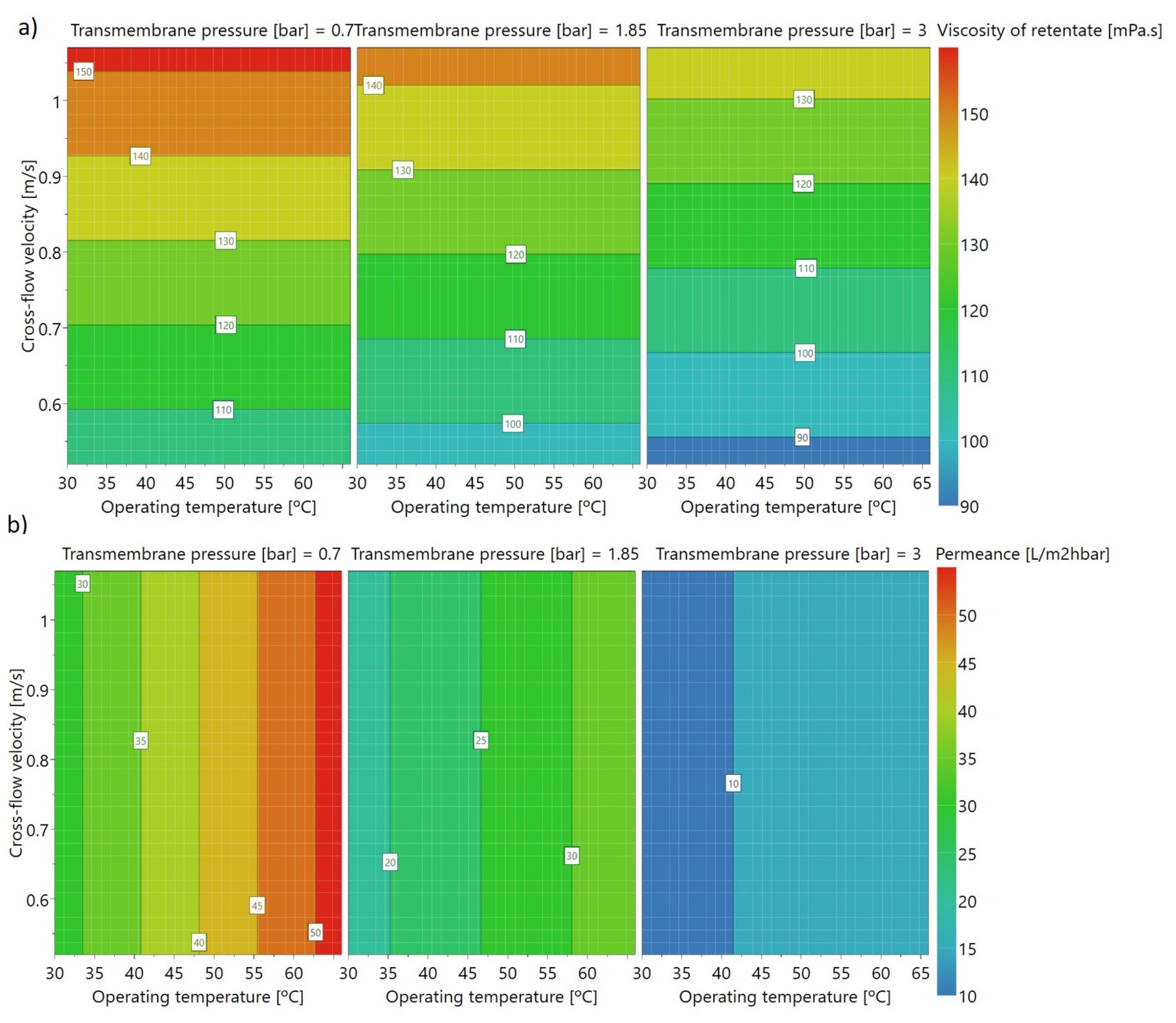
Figure 8 shows contour plots for each model for different pressures. The contour plots show the interaction between the factors in each model and the predicted response.
As shown in the contour plots for apparent viscosity (in
Figure 8a), a high final viscosity of the retentate (measured in the final of each experiment at 20 °C, and used as a measurement of the retention of arabinoxylans) is achieved with low transmembrane pressure and high initial crossflow velocity, independently of the operating temperature used during ultrafiltration (within the range of study), as also shown in
Figure 6a. The contour plot for permeance shows that high permeance is achieved with high operating temperature and low transmembrane pressure, and it is independent of velocity (in the study range). These graphs allow us to conclude that the best conditions (high permeance and high retentate viscosity) are: high operating temperature (66 °C), high initial crossflow velocity (1.06 m.s
−1), and low transmembrane pressure (0.7 bar). This result was confirmed by using the optimiser function of the MODDE software, set up to maximise these two factors, simultaneously. With this function it was possible to conclude that the transmembrane pressure is the main factor in achieving best conditions (46.3%), followed by the crossflow velocity (30.0%), and operating temperature (23.7%). As mentioned before, in this ultrafiltration system it was not possible to work in best conditions, as at high velocity the transmembrane pressure is higher than 0.7 bar. In the model, the transmembrane pressure had more impact than velocity, so a compromise has to be made between both the parameters, working at high velocity without much increase to the transmembrane pressure.
Furthermore, the same best conditions were shown using the two models assessed for viscosity (with and without experiment 7), showing that despite the dispersion of data obtained (which resulted in a lower quality model), the tendency is well described by the models, even when an experiment is excluded.
3.4. Model Validation
In order to validate the final model and ensure that its predictions were accurate, an additional experiment was performed at a temperature of 52 °C, an initial crossflow rate of 1.06 m.s
−1, and a transmembrane pressure of 1.4 bar.
Table 5 shows the model predictions, which were generated using the prediction function of the MODDE software, and values obtained for this experiment.
The models predict an apparent viscosity of retentate (
µretentate) of 146.8 mPa.s and an average permeance of 33.3 m
−2.h
−1.bar
−1 for the conditions used in this experiment. However, due to the errors associated with the experiments (shown through duplicates, experiments 9 and 10) plus the error associated with models fitting, the software used estimates each value within an interval (corresponding to an estimation error for each model), where the experimental observation should be (
Table 5). Therefore, the results obtained experimentally are within the range of values predicted by the model in
Table 5, proving the accuracy of the model.
4. Conclusions
With the aim of optimising the conditions needed to fractionate an alkaline extract from corn fibre, we assessed the most relevant operating parameters of an ultrafiltration process—membrane molecular weight cut-off, operating temperature, crossflow velocity, and transmembrane pressure. Out of the membranes tested, Nadir UP 150 (polyethersulfone with MWCO of 150 kDa) was found to be the best in terms of permeance, presenting no rejection of ferulic acid and a high estimated rejection of arabinoxylans (98.0% ± 1.7%). A response surface methodology (RSM) was used to infer the effect of temperature, crossflow velocity, and transmembrane pressure on the rejection of ferulic acid, retention of arabinoxylans (assessed through the retentate viscosity), and permeance. It was found that the ultrafiltration conditions (in the range tested) do not influence the global rejection of ferulic acid. The increase of transmembrane pressure showed a negative impact on the permeance and on the retention of arabinoxylans, while the temperature and crossflow velocity showed a positive impact on the permeance and on the retention of arabinoxylans retention, respectively.
Analysis of the two mathematical models developed to describe the retention of arabinoxylans (measured by apparent viscosity of retentate in the final of ultrafiltration) and permeance shows that it is possible to achieve the best conditions for ultrafiltration using the membrane Nadir UP 150, with an operating temperature of 66 °C, an initial crossflow velocity of 1.06 m.s
−1, and a transmembrane pressure of 0.7 bar. This study allowed us to determine the best conditions for an efficient separation of ferulic acid from arabinoxylans and obtain a fraction rich in ferulic acid with low viscosity, due to the removal of the arabinoxylans, facilitating a further step for purification and concentration of ferulic acid through adsorption or by nanofiltration, for use in bio-vanillin production. A fraction with a high concentration of arabinoxylans was also produced, which could be used, for example, as a material for edible films [
17,
18] and emulsifier for oil-in-waters emulsion systems [
19].
Therefore, the present work shows how to optimise an efficient separation method, based on ultrafiltration, for the recovery of both ferulic acid and arabinoxylans from a corn fibre alkaline extract, allowing an efficient valorisation of this co-product, increasing the overall sustainability of the corn wet milling process.