Life Cycle Assessment of Electrodialytic Technologies to Recover Raw Materials from Mine Tailings
Abstract
1. Introduction
2. Materials and Methods
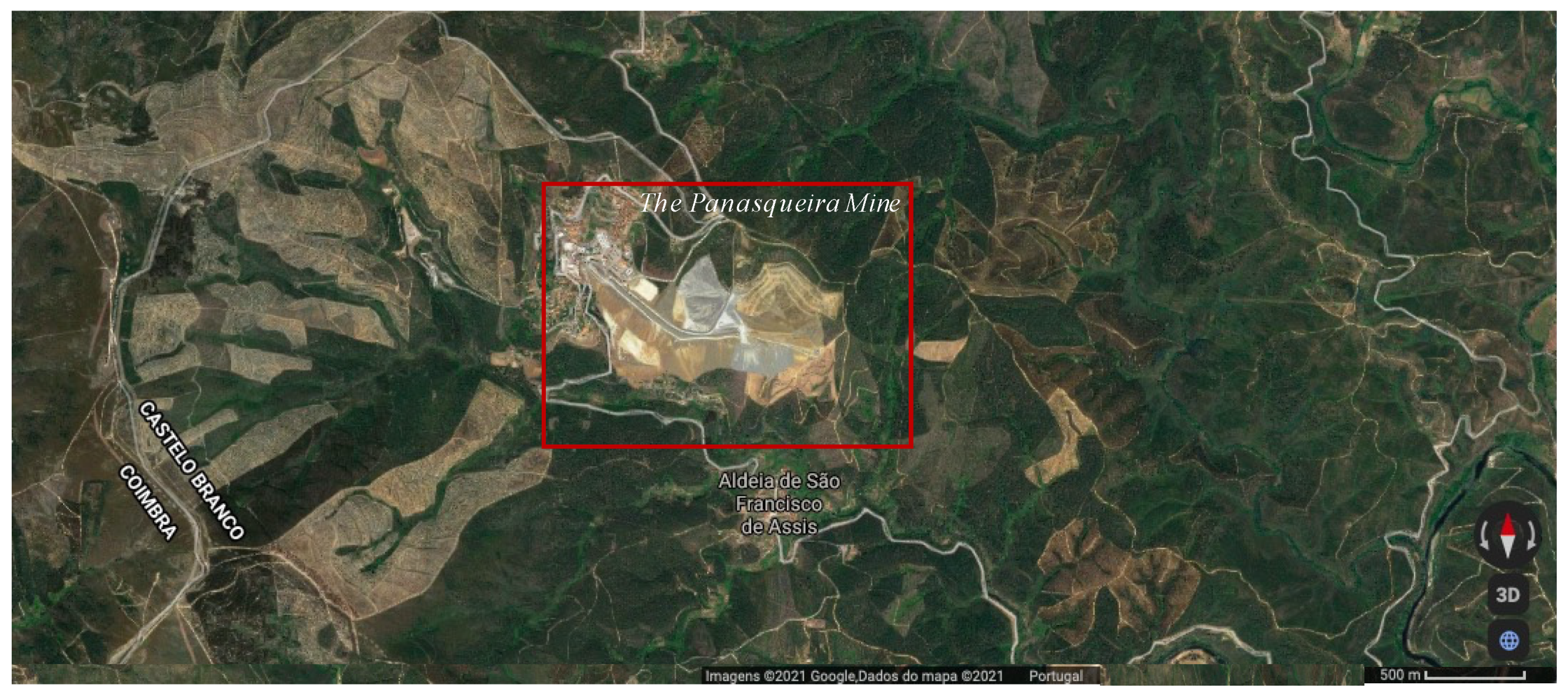
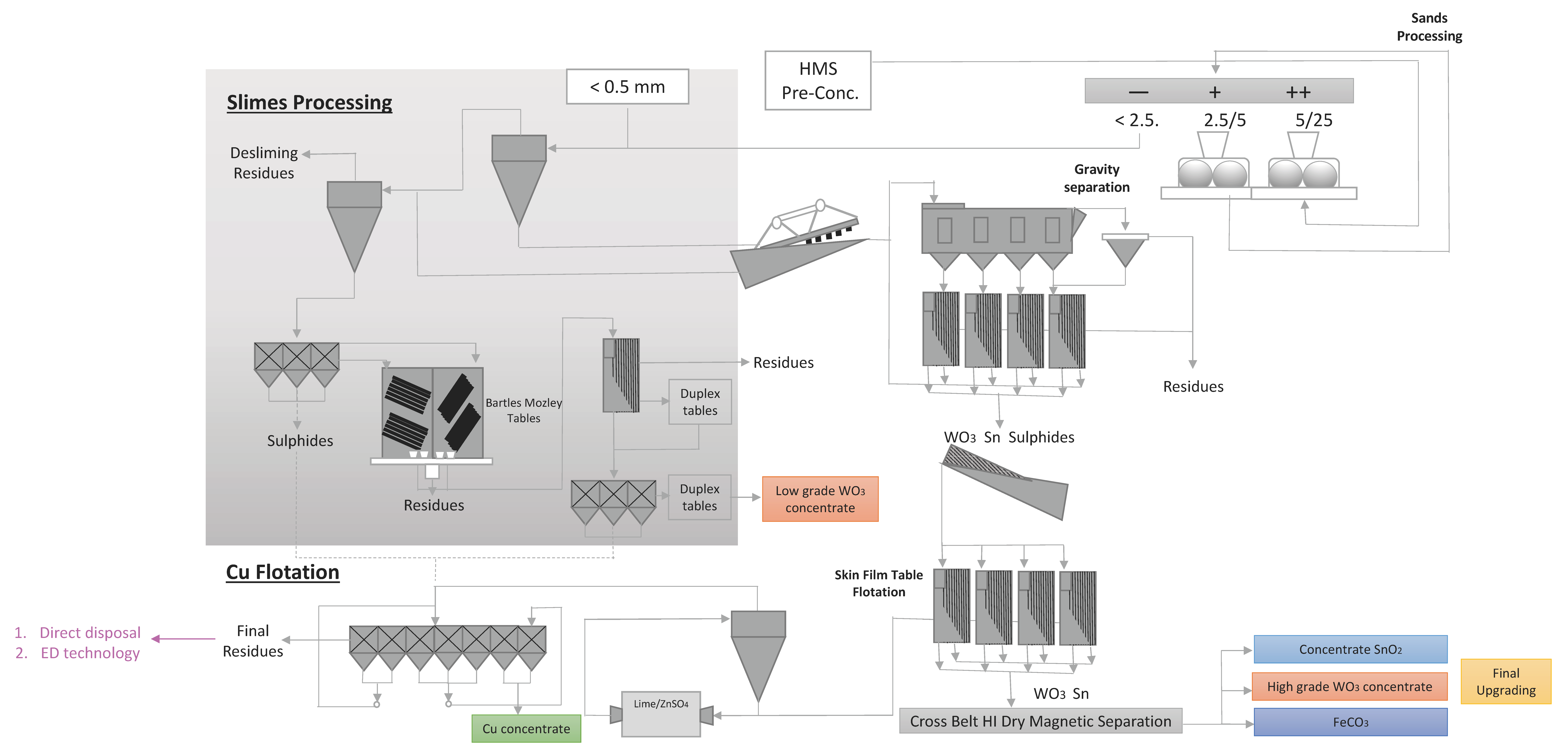
2.1. Case Description and Production System
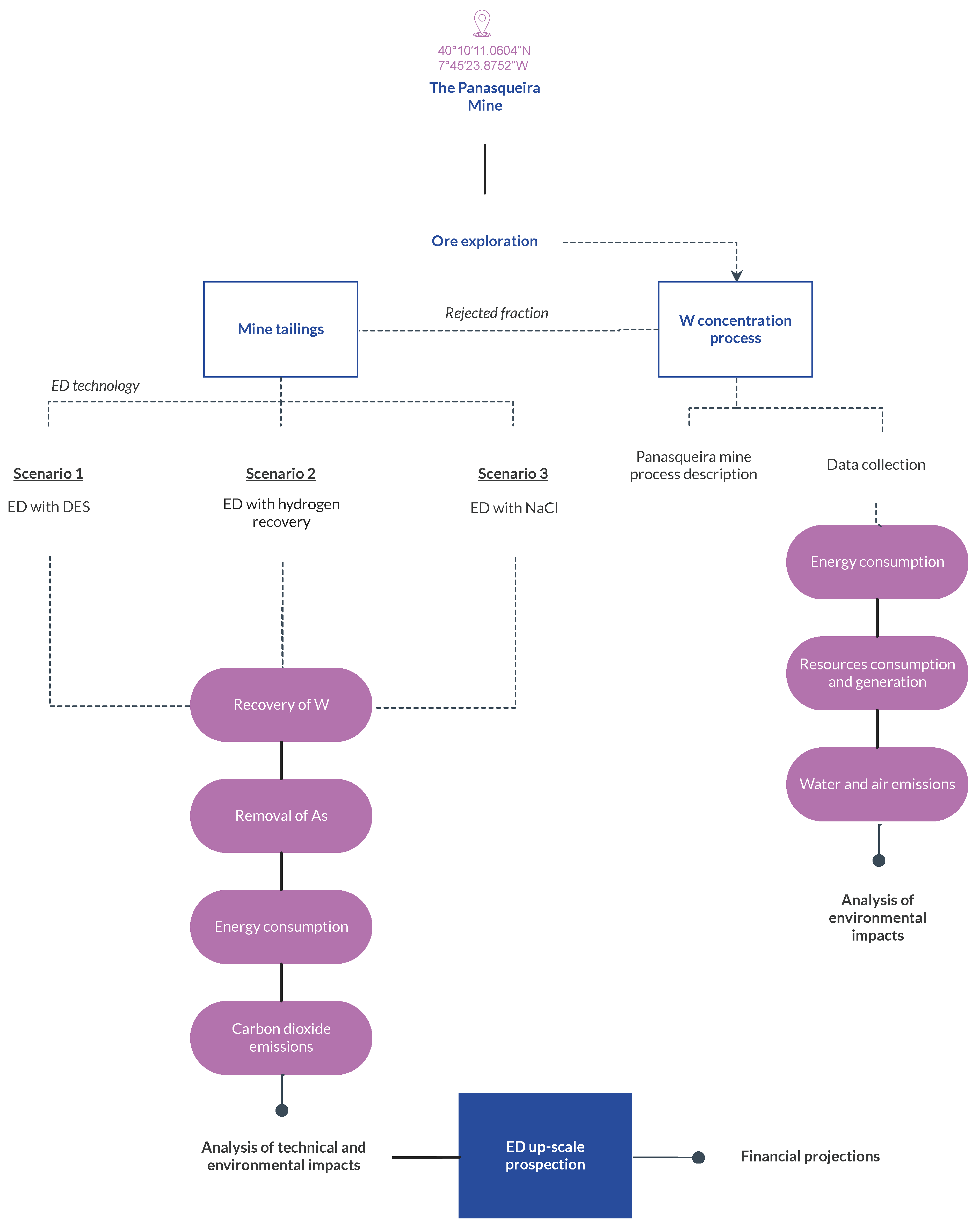
2.2. System Boundaries and LCA Road Map
2.3. Data Collection
2.4. Mine Tailings Characterization
2.5. Water and Air Emissions and Resources Consumed
2.6. Energy Consumption and CO2 Release
3. Results and Discussion
3.1. Tungsten Concentrate Production at the Panasqueira Mine: Environmental Impacts
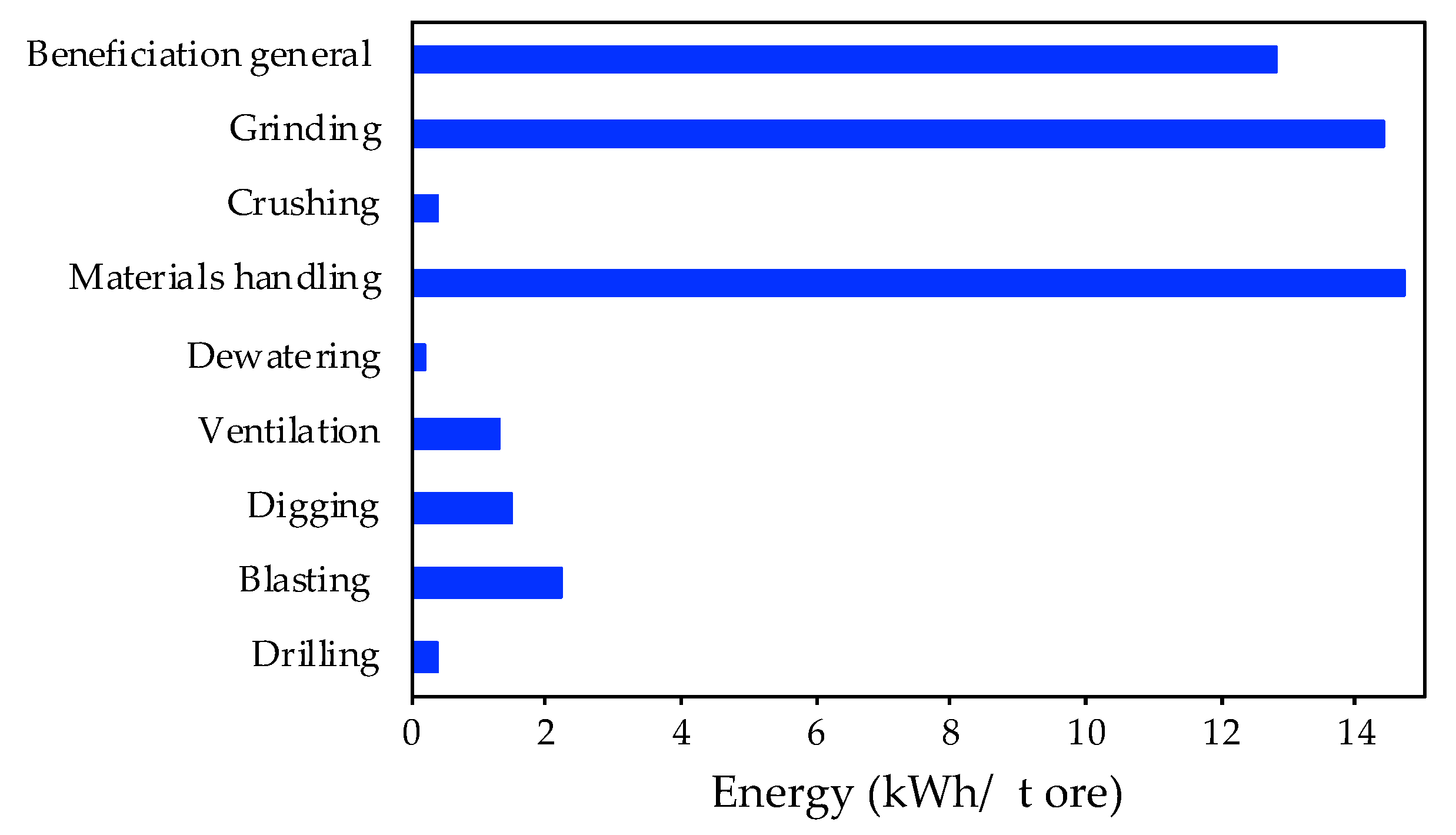
3.1.1. Energy Consumption
3.1.2. Air and Water Emissions
3.2. Mine Tailings Management
3.2.1. Electrodialytic Scenarios
3.2.2. Electrodialytic Treatment Upscale Prospection
- (1)
- two-compartment reactor design, which is easier to operate;
- (2)
- DES as enhancements, alleviating the consumption of strong acids and bases while incrementing the W recovery;
- (3)
- cation-exchange membrane, which allows H2 recovery for depreciation of implementation and maintenance costs, as well as flexibility in different market segments.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions—The European Green Deal. COM/2019/640 final; EC: Brussels, Belgium, 2019. [Google Scholar]
- European Commission. Going Climate-Neutral by 2050—A Strategic Long Term Vision for a Prosperous, Modern, Competitive and Climate-Neutral EU; EC: Brussels, Belgium, 2019. [Google Scholar]
- Mancini, L.; Vidal-Legaz, B.; Vizzarri, M.; Wittmer, D.; Grassi, G.; Pennington, D.W. Mapping the role of raw materials in sustainable development goals. In Sustainable Development Goals. A preliminary Analysis of Links, Monitoring Indicators and Related Policy Initiatives; P O of the European Union: Luxembourg, 2019; p. JRC112892. ISBN 978-92-76-08385. [Google Scholar]
- European Commission. Critical Raw Materials Resilience: Charting a Path towards Greater Security and Sustainability. COM/2020/474 Final; EC: Brussels, Belgium, 2020. [Google Scholar]
- Sharma, P.; Minakshi, M.; Whale, J.; Jean-Fulcrand, A.; Garnweitner, G. Effect of the anionic counterpart: Molybdate vs. tungstate in energy storage for pseudo-capacitor applications. Nanomaterials 2021, 11, 580. [Google Scholar] [CrossRef] [PubMed]
- Minakshi, M.; Mitchell, D.R.G.; Baur, C.; Chable, J.; Barlow, A.J.; Fichtner, M.; Banerjee, A.; Chakraborty, S.; Ahuja, R. Phase evolution in calcium molybdate nanoparticles as a function of synthesis temperature and its electrochemical effect on energy storage. Nanoscale Adv. 2019, 1, 565–580. [Google Scholar] [CrossRef]
- Yang, X. Beneficiation studies of tungsten ores—A review. Miner. Eng. 2018, 125, 111–119. [Google Scholar] [CrossRef]
- European Commission. Integrated Pollution Prevention and Control (IPPC): Reference Document on Best Available Techniques in the Non Ferrous Metals Industries; EC: Belgium, Brussels, 2001. [Google Scholar]
- Ávila, P.F.; Silva, E.F.; Salgueiro, A.R.; Farinha, J.A. Geochemistry and mineralogy of mill tailings impoundments from the Panasqueira Mine (Portugal): Implications for the surrounding environment. Mine Water Environ. 2008, 27, 210–224. [Google Scholar] [CrossRef]
- Candeias, C.; Ávila, P.F.; Silva, E.F.; Ferreira, A.; Salgueiro, A.R.; Teixeira, J.P. Acid mine drainage from the Panasqueira mine and its influence on Zêzere river (Central Portugal). J. Afr. Earth Sci. 2014, 99, 705–712. [Google Scholar] [CrossRef]
- Environmental Law Alliance. Worldwide Guidebook for Evaluating Mining Project EIAs, 1st ed; ELAW: Eugene, OR, USA, 2010. [Google Scholar]
- Agência Portuguesa do Ambiente. Environmental License No 347/0.1/2017—Extraction and Preparation of Non-Ferrous Metal Ores, Comprising Hazardous and Non-Hazardous Waste Disposal Activities; APDO: Lisbon, Portugal, 2017. (In Portuguese) [Google Scholar]
- Okvist, L.; Ye, G.; Hu, X. Innovation Potential in the Recovery of Refractory Metals from Secondary Resources—MSP-REFRAM H2020 project; D3.3; EC: Brussels, Belgium, 2016. [Google Scholar]
- Valderrama, C.; Granados, R.; Cortina, J.L.; Gasol, C.M.; Guillem, M.; Josa, A. Implementation of best available techniques in cement manufacturing: A life-cycle assessment study. J. Clean. Prod. 2012, 25, 60–67. [Google Scholar] [CrossRef]
- Garbarino, E.; Orveillon, G.; Saveyn, H.; Barthe, P.; Eder, P. Best Available Techniques (BAT) Reference Document for the Management of Waste from Extractive Industries in Accordance with Directive 2006/21/EC|EU Science Hub; European Commission: Brussels, Belgium, 2018. [Google Scholar]
- Almeida, J.; Magro, C.; Rosário, A.R.; Mateus, E.P.; Ribeiro, A.B. Electrodialytic treatment of secondary mining resources for raw materials extraction: Reactor design assessment. Sci. Total Environ. 2020, 752, 141822. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.B.; Rodríguez-Maroto, J.M. Electroremediation of heavy metal-contaminated soils -processes and applications. In Trace Elements in the Environment: Biogeochemistry, Biotechnology, and Bioremediation; Prasad, M.N.V., Sajwan, K.S., Naidu, R., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 341–368. ISBN 9781420032048. [Google Scholar]
- Almeida, J.; Craveiro, R.; Faria, P.; Silva, A.S.; Mateus, E.P.; Barreiros, S.; Paiva, A.; Ribeiro, A.B. Electrodialytic removal of tungsten and arsenic from secondary mine resources—deep eutectic solvents enhancement. Sci. Total Environ. 2020, 710, 136364. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.; Magro, C.; Mateus, E.P.; Ribeiro, A.B. Electrodialytic hydrogen production and critical raw materials recovery from secondary resources. Water 2020, 12, 1262. [Google Scholar] [CrossRef]
- Magro, C.; Almeida, J.; Paz-Garcia, J.M.; Mateus, E.P.; Ribeiro, A.B. Exploring hydrogen production for self-energy generation in electroremediation: A proof of concept. Appl. Energy 2019, 255, 113839. [Google Scholar] [CrossRef]
- Almeida, J.; Faria, P.; Ribeiro, A.B.; Silva, A.S. Effect of mining residues treated with an electrodialytic technology on cement-based mortars. Clean. Eng. Technol. 2020, 1, 100001. [Google Scholar] [CrossRef]
- European Commission. CEN EN 15804:2012+A2:2019—Sustainability of Construction Works. Environmental Product Declarations. Core Rules for the Product Category of Construction Products; EC: Brussels, Belgium, 2012. [Google Scholar]
- International Organization for Standardization. Environmental Management: Life Cycle Assessment. Principles and Framework; ISO: Geneva, Switzerland, 2006. [Google Scholar]
- International Organization for Standardization. Environmental Management: Life Cycle Assessment. Requirements and Guidelines; ISO: Geneva, Switzerland, 2006. [Google Scholar]
- Santos, T.; Almeida, J.; Silvestre, J.; Faria, P. Life cycle assessment of mortars: A review on technical potential and drawbacks. Constr. Build. Mater. 2021, 288, 123069. [Google Scholar] [CrossRef]
- Cuenca-Moyano, G.M.; Zanni, S.; Bonoli, A.; Valverde-Palacios, I. Development of the life cycle inventory of masonry mortar made of natural and recycled aggregates. J. Clean. Prod. 2017, 140, 1272–1286. [Google Scholar] [CrossRef]
- Almeida, J.; Ribeiro, A.B.; Silva, A.S.; Faria, P. Overview of mining residues incorporation in construction materials and barriers for full-scale application. J. Build. Eng. 2020, 29, 101215. [Google Scholar] [CrossRef]
- Leite, M. Reflection contributions on the topic of useful substances formation from extractive production residues. In Proceedings of the Mining Waste: Contributions to the Circular Economy Seminar, Lisbon, Portugal, 26 June 2017. (In Portuguese). [Google Scholar]
- Franco, A.; Vieira, R.; Bunting, R. The Panasqueira Mine at a Glance; International Tungsten Industry Association Newsletter: London, UK, 2014; Volume 3, pp. 1–12. [Google Scholar]
- Gonçalves, D. Recovery of Heavy Metals from Waste Water; Internship Report, The Panasqueira mine; University of Beira Interior: Covilhã, Portugal, 2015. (In Portuguese) [Google Scholar]
- Ecoinvent Tungsten Mine Operation and Beneficiation. Zurich, Switzerland. Available online: https://v371.ecoquery.ecoinvent.org/Details/UPR/c054cf42-f2c0-49ad-a431-a2ef7b4b13fe/8b738ea0-f89e-4627-8679-433616064e82 (accessed on 8 January 2021).
- Magro, C.; Almeida, J.; Paz-Garcia, J.; Mateus, E.; Ribeiro, A. Hydrogen recovery in electrodialytic-based technologies applied to environmental contaminated matrices. In Electrokinetic Remediation for Environmental Security and Sustainability; Ribeiro, A., Prasad, M., Eds.; John Wiley & Sons Ltd: Hoboken, NJ, USA, 2021; pp. 251–270. ISBN 978-1-119-67011-7. [Google Scholar]
- GOV.UK Streamlined Energy and Carbon Reporting (SECR) UK. Available online: https://www.gov.uk/government/publications/academy-trust-financial-management-good-practice-guides/streamlined-energy-and-carbon-reporting (accessed on 26 January 2021).
- Politis, A.; Paspaliaris, I.; Taxiarchou, M. Management of Wastes from Primary Resource Processing: Identification, Environmental Evaluations-MSP-REFRAM H2020 Project; EU: Luxembourg, 2017; Volume D2.4. [Google Scholar]
- Candeias, C.; Melo, R.; Ávila, P.F.; Ferreira da Silva, E.; Salgueiro, A.R.; Teixeira, J.P. Heavy metal pollution in mine-soil-plant system in S. Francisco de Assis—Panasqueira mine (Portugal). Appl. Geochem. 2014, 44, 12–26. [Google Scholar] [CrossRef]
- Metalary Tungsten Price 2020. Available online: https://www.metalary.com/tungsten-price/ (accessed on 11 January 2021).
- International Energy Agency Global Average Levelised Cost of Hydrogen Production by Energy Source and Technology, 2019 and 2050. Available online: https://www.iea.org/data-and-statistics/charts/global-average-levelised-cost-of-hydrogen-production-by-energy-source-and-technology-2019-and-2050 (accessed on 22 March 2021).
- Islam, M.M.M.; Shafi, S.; Bandh, S.A.; Shameem, N. Impact of environmental changes and human activities on bacterial diversity of lakes. In Freshwater Microbiology; Bandh, S.A., Shafi, S., Shameem, N., Eds.; Elsevier: London, UK, 2019; pp. 105–136. ISBN 9780128174951. [Google Scholar]
- Donoso, N.; Gobeyn, S.; Villa-Cox, G.; Boets, P.; Meers, E.; Goethals, P.L.M. Assessing the ecological relevance of organic discharge limits for constructed wetlands by means of a model-based analysis. Water 2018, 10, 63. [Google Scholar] [CrossRef]
- Antoniades, D. Paleolimnology—Lake chemistry. In Encyclopedia of Quaternary Science; Elias, S., Ed.; Elsevier: London, UK, 2007; pp. 2038–2046. ISBN 9780444527479. [Google Scholar]
- Lønborg, C.; Carreira, C.; Jickells, T.; Álvarez-Salgado, X.A. Impacts of global change on ocean dissolved organic carbon (DOC) cycling. Front. Mar. Sci. 2020, 7, 466. [Google Scholar] [CrossRef]
- Roudi, A.M.; Kamyab, H.; Chelliapan, S.; Ashokkumar, V.; Kumar, A.; Yadav, K.K.; Gupta, N. Application of response surface method for total organic carbon reduction in leachate treatment using Fenton process. Environ. Technol. Innov. 2020, 19, 101009. [Google Scholar] [CrossRef]
- Pacheco, M. Panasqueira. Atypical Mining Project. Master’s Thesis, University of Porto, Portugal, 2017. [Google Scholar]
- Schaeffer, N.; Martins, M.A.R.; Neves, C.M.S.S.; Pinho, S.P.; Coutinho, J.A.P. Sustainable hydrophobic terpene-based eutectic solvents for the extraction and separation of metals. Chem. Commun. 2018, 54, 8104–8107. [Google Scholar] [CrossRef]
- Abbott, A.P.; Frisch, G.; Hartley, J.; Ryder, K.S. Processing of metals and metal oxides using ionic liquids. Green Chem. 2011, 13, 471–481. [Google Scholar] [CrossRef]
- Miller de Melo Henrique, J.; Cañizares, P.; Saez, C.; Vieira dos Santos, E.; Rodrigo, M.A. Relevance of gaseous flows in electrochemically assisted soil thermal remediation. Curr. Opin. Electrochem. 2021, 100698. [Google Scholar] [CrossRef]
- Shalaby, A.; Nassef, E.; Mubark, A.; Hussein, M. Phosphate removal from wastewater by electrocoagulation using aluminium electrodes. Am. J. Environ. Eng. Sci. 2014, 1, 90–98. [Google Scholar]
- Greenice. Toledo, Spain. Available online: https://greenice.com/pt/816-economia-de-energia (accessed on 29 January 2021).
- Ganiyu, S.O.; Martínez-Huitle, C.A. The use of renewable energies driving electrochemical technologies for environmental applications. Curr. Opin. Electrochem. 2020, 22, 211–220. [Google Scholar] [CrossRef]
- Chave Vertical Lda. Aveiro, Portugal. Available online: http://www.chavevertical.pt/crbst_192.html (accessed on 29 January 2021).
- Christensen, I.V.; Pedersen, A.J.; Ottosen, L.M.; Ribeiro, A.B. Electrodialytic remediation of CCA-treated waste wood in a 2 m3 pilot plant. Sci. Total Environ. 2006, 364, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Castro-Gomes, J.; Magrinho, M.; Sedira, N.; Beghoura, I.; Humbert, P.; Manso, M.; Fernandes, A.; Silva, R. Alkali-activation of tungsten mining waste mud blended with waste glass: Reactivity, performance and innovative applications. In Proceedings of the ICEUBI 2017—International Conference on Engineering—A Vision for the Future, Covilhã, Portugal, 5–7 December 2017. [Google Scholar]
- Thompson Brewster, E.; Ward, A.J.; Mehta, C.M.; Radjenovic, J.; Batstone, D.J. Predicting scale formation during electrodialytic nutrient recovery. Water Res. 2017, 110, 202–210. [Google Scholar] [CrossRef] [PubMed]
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development. A/RES/70/1; UN: New York, NY, USA, 2015. [Google Scholar]
- Liu, W.; Huang, C.; Han, J.; Qin, W. Removal and reuse of arsenic from arsenic-bearing purified residue by alkaline pressure oxidative leaching and reduction of As (V). Hydrometallurgy 2021, 199, 105541. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Arsenic Treatment Technologies for Soil, Waste, and Water; U.S. Environmental Protection Agency: Washington, DC, USA, 2002.
- Denios online shop. Porto, Portugal. Available online: https://www.denios.pt/shop/ (accessed on 29 January 2020).
- ABC do Acrílico. Almada, Portugal. Available online: http://www.abcdoacrilico.com (accessed on 29 January 2021).
- Changsheng Titanium Co., Ltd. Baoji City, China. Available online: https://www.bjcstytitanium.com/products (accessed on 29 January 2021).
- Xion Water Solutions. London, UK. Available online: http://xionwater.com/products.html (accessed on 29 January 2021).
- Merck KGaA., Darmstadt, Germany. Available online: https://www.merckgroup.com/en/expertise.html (accessed on 29 January 2021).
- La Tienda del Apicultor. Valencia, Spain. Available online: https://www.latiendadelapicultor.com/pt/ (accessed on 29 January 2021).
- Chemical Book. China. Available online: https://www.chemicalbook.com/ProductList_En.aspx?kwd=cholinechloride (accessed on 29 January 2021).
- Ferramentas.pt. Seixal, Portugal. Available online: https://ferramentas.pt (accessed on 29 January 2021).
- Paipu Technology Co., Ltd. Changzhou, China. Available online: http://www.paiputech.com/EN/Productlist.Asp?SortID=48 (accessed on 29 January 2021).
- Expondo. Portugal. Available online: https://www.expondo.pt/artigos-industriais/ (accessed on 29 January 2021).
- PT Robotics. Rio de Mouro, Portugal. Available online: https://www.ptrobotics.com (accessed on 29 January 2021).
- European Commission. Guidelines on the Eligibility of Costs under the Connecting Europe Facility; EC: Brussels, Belgium, 2018. [Google Scholar]
- Andrade, L.H.; Mendes, F.D.S.; Espindola, J.C.; Amaral, M.C.S. Reuse of dairy wastewater treated by membrane bioreactor and nanofiltration: Technical and economic feasibility. Braz. J. Chem. Eng. 2015, 32, 735–747. [Google Scholar] [CrossRef]
- Samhaber, W.M.; Nguyen, M.T. Applicability and costs of nanofiltration in combination with photocatalysis for the treatment of dye house effluents. Beilstein J. Nanotechnol. 2014, 5, 476–484. [Google Scholar] [CrossRef]
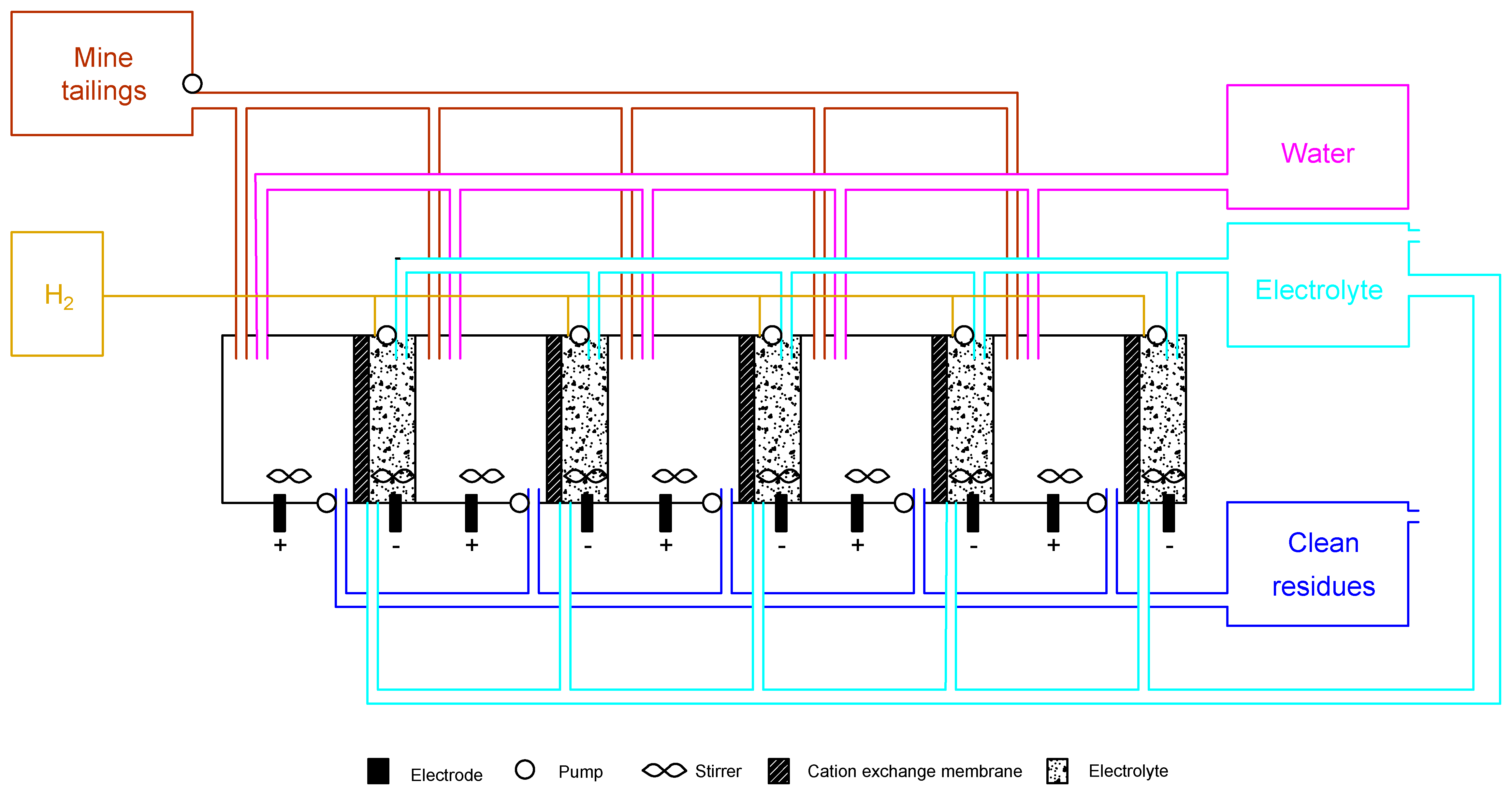
| Source | Topic | Documents Consulted |
|---|---|---|
| Ecoinvent version 3.7.1 | Tungsten concentrate production | - Tungsten mine operation and beneficiation [31] |
| European Commission technical reports | Mining industry operation | - Integrated Pollution Prevention and Control (IPPC) reference document on best available techniques in the nonferrous metals industries [8] |
| Research works published in international scientific journals | Electrodialytic process | - Exploring hydrogen production for self-energy generation in electroremediation: A proof of concept [20] - Electrodialytic hydrogen production and critical raw materials recovery from secondary resources [19] - Hydrogen recovery in electrodialytic-based technologies applied to environmental contaminated matrices [32] - Electrodialytic removal of tungsten and arsenic from secondary mine resources—deep eutectic solvent enhancement [18] |
| Item | Value per Functional Unit | Units | References |
|---|---|---|---|
| Panasqueira mine resources–ore | 1000 (functional unit) | kg | - |
| W content in Panasqueira mine resources | 3.0 | kg/t ore | 0.3% WO3 [33] |
| W concentrate after the concentration process | 2.3 | kg/t ore | 75% WO3 [29] |
| Mine tailings generation | 997.4 | kg/t ore | - |
| W in mine tailings | 0.8 | kg/t ore | [29,33] |
| As in mine tailings | 3.7 | kg/t ore | [16] |
| W price | 25,500 | EUR/t | [34] |
| H2 price | 2.7–6.5 | EUR/kg | [35] |
| Emissions | Value per Functional Unit | Units |
|---|---|---|
| Air | ||
| Carbon dioxide, nonfossil | 0.35 | kg/t ore |
| Carbon disulfide | 7.74 × 10−6 | kg/t ore |
| Particulates < 2.5 µm | 0.01 | kg/t ore |
| Particulates > 10 µm | 0.13 | kg/t ore |
| Particulates > 2.5 µm and < 10 µm | 0.11 | kg/t ore |
| Water | ||
| Aluminum | 9.26 × 10−6 | kg/t ore |
| Biological oxygen demand (BOD5) | 2.08 × 10−3 | kg/t ore |
| Chemical oxygen demand (COD) | 4.15 × 10−3 | kg/t ore |
| Dissolved organic carbon (DOC) | 1.54 × 10−3 | kg/t ore |
| Hydrocarbons | 1.29 × 10−5 | kg/t ore |
| Iron | 3.66 × 10−5 | kg/t ore |
| Nitrite | 1.29 × 10−5 | kg/t ore |
| Phosphorus | 1.29 × 10−5 | kg/t ore |
| Total organic carbon (TOC) | 1.54 × 10−3 | kg/t ore |
| Tungsten | 1.29 × 10−5 | kg/t ore |
| Water | 0.26 | m3/t ore |
| Extraction (%/kg t ore) | ||||||
|---|---|---|---|---|---|---|
| Scenario | ED Scheme | Duration (h) | Current Intensity (A) | W | As | H2 Purity/ Recovery (%) |
| 1. ED treatment with DES 1 |  | 96 | 0.05 | 22/0.2 | 16/0.6 | n.a. |
| 2. ED treatment with H2 recovery 2 |  | 1 | 0.1 | 7.5/0.06 | 48/1.8 | 74/50 |
| 3. ED treatment with NaCl 3 | 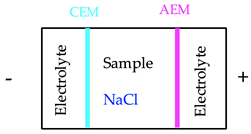 | 120 | 0.1 | 13/0.1 | 63/2.4 | n.a. |
| Scenario 1. ED Treatment with DES | ||||||
| Measure | Voltage (V) | Current intensity (A) | Energy consumed (kWh) | kWh/g W extracted | kWh/g As extracted | g CO2 |
| Day 0 | 32.30 | 0.05 | 2.0 × 10−3 | 1.0 × 10−2 | 4.2 × 10−5 | 0.38 |
| Day 1 | 13.20 | 1.0 × 10−3 | 5.0 × 10−3 | 2.1 × 10−5 | 0.15 | |
| Day 2 | 11.10 | 1.0 × 10−3 | 5.0 × 10−3 | 2.1 × 10−5 | 0.13 | |
| Day 3 | 10.80 | 1.0 × 10−3 | 5.0 × 10−3 | 2.1 × 10−5 | 0.13 | |
| Day 4 | 11.80 | 1.0 × 10−3 | 5.0 × 10−3 | 2.1 × 10−5 | 0.14 | |
| Average | 1.0 × 10−3 | 6.0 × 10−3 | 2.5 × 10−5 | 0.18 | ||
| Scenario 2. ED treatment with H2 recovery | ||||||
| Measure | Voltage (V) | Current intensity (A) | Energy consumed (kWh) | kWh/g W extracted | kWh/g As extracted | g CO2 |
| 0 min | 35.00 | 0.1 | 4.0 × 10−3 | 6.7 × 10−2 | 2.8 × 10−5 | 0.82 |
| 10 min | 27.50 | 3.0 × 10−3 | 5.0 × 10−2 | 2.1 × 10−5 | 0.64 | |
| 20 min | 25.80 | 3.0 × 10−3 | 5.0 × 10−2 | 2.1 × 10−5 | 0.60 | |
| 30 min | 26.10 | 3.0 × 10−3 | 5.0 × 10−2 | 2.1 × 10−5 | 0.61 | |
| 40 min | 28.00 | 3.0 × 10−3 | 5.0 × 10−2 | 2.1 × 10−5 | 0.65 | |
| 50 min | 38.80 | 4.0 × 10−3 | 6.7 × 10−2 | 2.8 × 10−5 | 0.90 | |
| 60 min | 59.50 | 6.0 × 10−3 | 1.0 × 10−1 | 4.2 × 10−5 | 1.39 | |
| Average | 3.0 × 10−3 | 6.2 × 10−2 | 2.6 × 10−5 | 0.80 | ||
| Scenario 3. ED treatment with NaCl | ||||||
| Measure | Voltage (V) | Current intensity (A) | Energy consumed (kWh) | kWh/g W extracted | kWh/g As extracted | g CO2 |
| Day 0 | 98.40 | 0.1 | 1.0 × 10−2 | 1.0 × 10−1 | 5.3 × 10−5 | 2.29 |
| Day 1 | 77.25 | 8.0 × 10−3 | 8.0 × 10−2 | 4.2 × 10−5 | 1.80 | |
| Day 2 | 46.80 | 5.0 × 10−3 | 5.0 × 10−2 | 2.7 × 10−5 | 1.09 | |
| Day 3 | 41.65 | 4.0 × 10−3 | 4.0 × 10−2 | 2.1 × 10−5 | 0.97 | |
| Day 4 | 27.85 | 3.0 × 10−3 | 3.0 × 10−2 | 1.6 × 10−5 | 0.65 | |
| Day 5 | 14.70 | 1.0 × 10−3 | 1.0 × 10−2 | 5.3 × 10−6 | 0.34 | |
| Average | 5.0 × 10−3 | 5.2 × 10−2 | 2.7 × 10−5 | 1.19 | ||
| Item | Quantity | Cost/Uni (EUR) | Initial Investment (EUR) | 1 Year of ED (EUR) | 5 Years of ED (EUR) |
|---|---|---|---|---|---|
| Stirring | 10 | 1986.00 | 19,860.00 | ||
| Reactor in polyethylene (diameter = 1.6 m; length = 1 m) | 5 | 650.00 | 3250.00 | ||
| Block compartments (diameter = 1.6 m; length = 1 m) | 20 | 149.00 | 2980.00 | ||
| Electrodes Ti/MMO (0.5 × 0.1 m; width = 3 mm) | 10 | 100.00 | 1000.00 | ||
| Membranes CEM–CR67, MKIII, Blank (diameter = 0.8 m) | 5 | 499.00 | 2495.00 | ||
| NaNO3 * (1 kg per unit) | 29 | 151.90 | 4405.10 | 1,101,275.0 | 5,506,375.0 |
| Natural deep eutectic solvents (choline chloride. 1 kg per unit + oxalic acid. 25 kg per unit) * | 6 | 143.00 | 858.00 | 214,500.00 | 1,072,500.00 |
| Pumps | 11 | 489.00 | 5379.00 | ||
| Tubes | 36 | 1.89 | 68.04 | ||
| Power boxes | 10 | 383.81 | 3838.10 | ||
| Crocodiles + wires | 20 | 0.99 | 19.70 | ||
| Solar Panels (2025 × 996 × 40 cm) | 5 | 476.00 | 2380.00 | ||
| Implementation | 10% of the total reactor price | 4653.29 | |||
| Maintenance | 5% of the initial investment (every 3 months) | 10,237.25 | 51,186.23 | ||
| Cleaning of Membranes | 15 EUR/m2 (twice per month for 2 m2 of membranes area) | 3600.00 | 18,000.00 | ||
| Replace of Membranes | Every 4 years | 2495.00 | |||
| Cleaning of Reactor | 2% of the initial investment (once per year) | 1023.72 | 5118.62 | ||
| Total investment | 51,186.23 | 1,330,635.97 | 6,655,674.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, J.; Magro, C.; P. Mateus, E.; Ribeiro, A.B. Life Cycle Assessment of Electrodialytic Technologies to Recover Raw Materials from Mine Tailings. Sustainability 2021, 13, 3915. https://doi.org/10.3390/su13073915
Almeida J, Magro C, P. Mateus E, Ribeiro AB. Life Cycle Assessment of Electrodialytic Technologies to Recover Raw Materials from Mine Tailings. Sustainability. 2021; 13(7):3915. https://doi.org/10.3390/su13073915
Chicago/Turabian StyleAlmeida, Joana, Cátia Magro, Eduardo P. Mateus, and Alexandra B. Ribeiro. 2021. "Life Cycle Assessment of Electrodialytic Technologies to Recover Raw Materials from Mine Tailings" Sustainability 13, no. 7: 3915. https://doi.org/10.3390/su13073915
APA StyleAlmeida, J., Magro, C., P. Mateus, E., & Ribeiro, A. B. (2021). Life Cycle Assessment of Electrodialytic Technologies to Recover Raw Materials from Mine Tailings. Sustainability, 13(7), 3915. https://doi.org/10.3390/su13073915









