Biotechnology for Metal Recovery from End-of-Life Printed Circuit Boards with Aspergillus niger
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Waste Printed Circuit Boards (PCBs)
2.2. Microorganisms and Inoculum
2.3. Bioleaching Experiments
2.4. Analytical Determination
2.5. Statistical Analysis
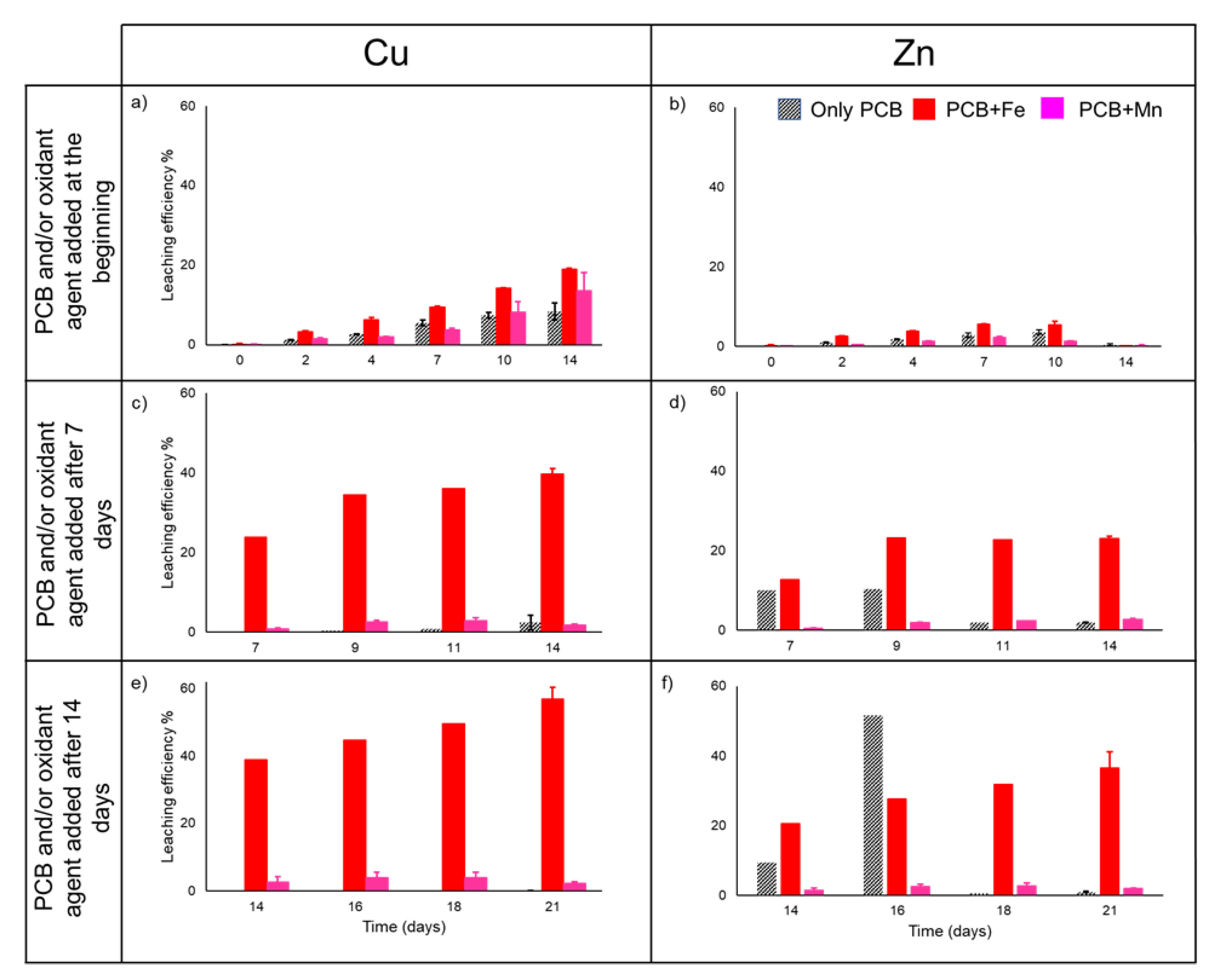
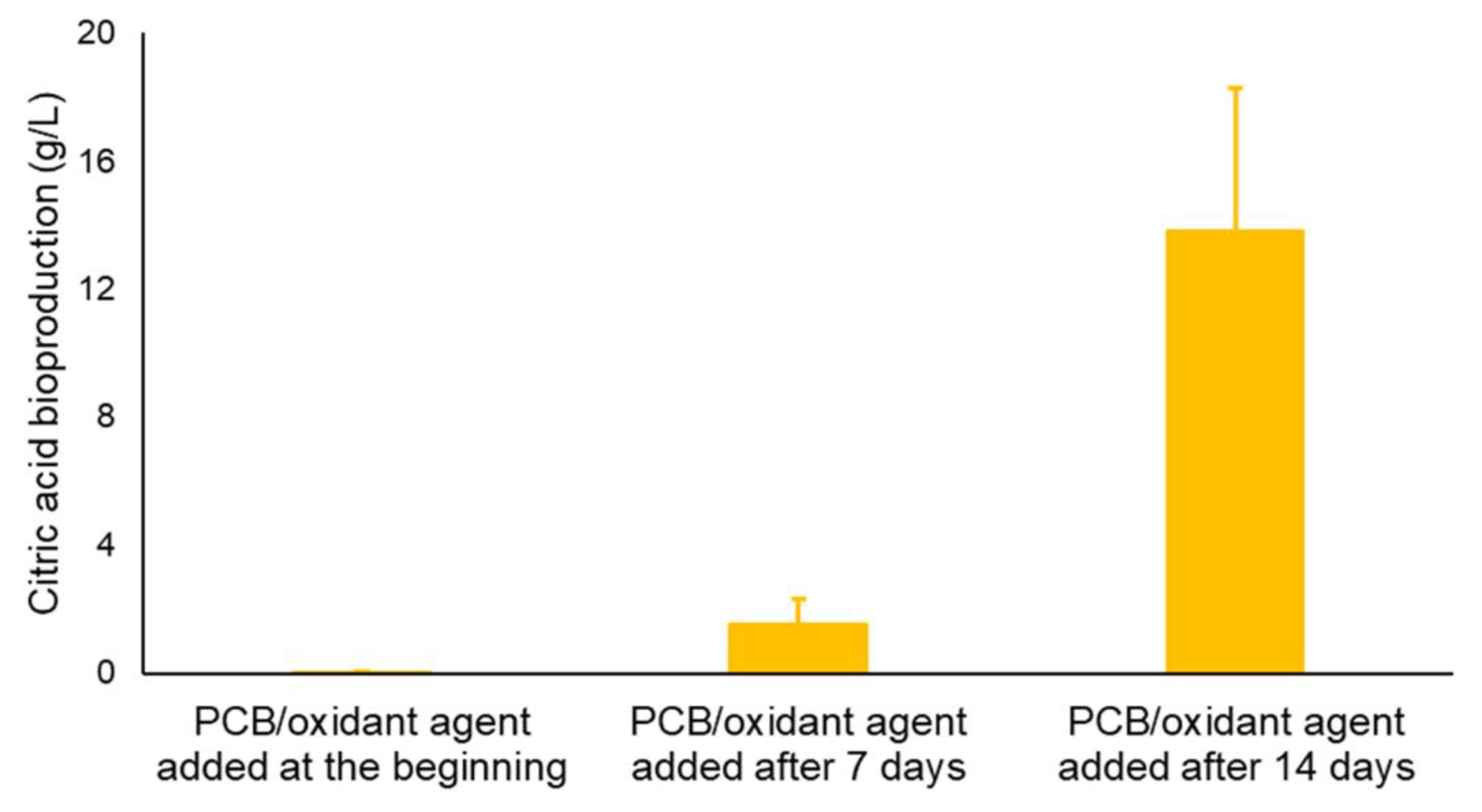
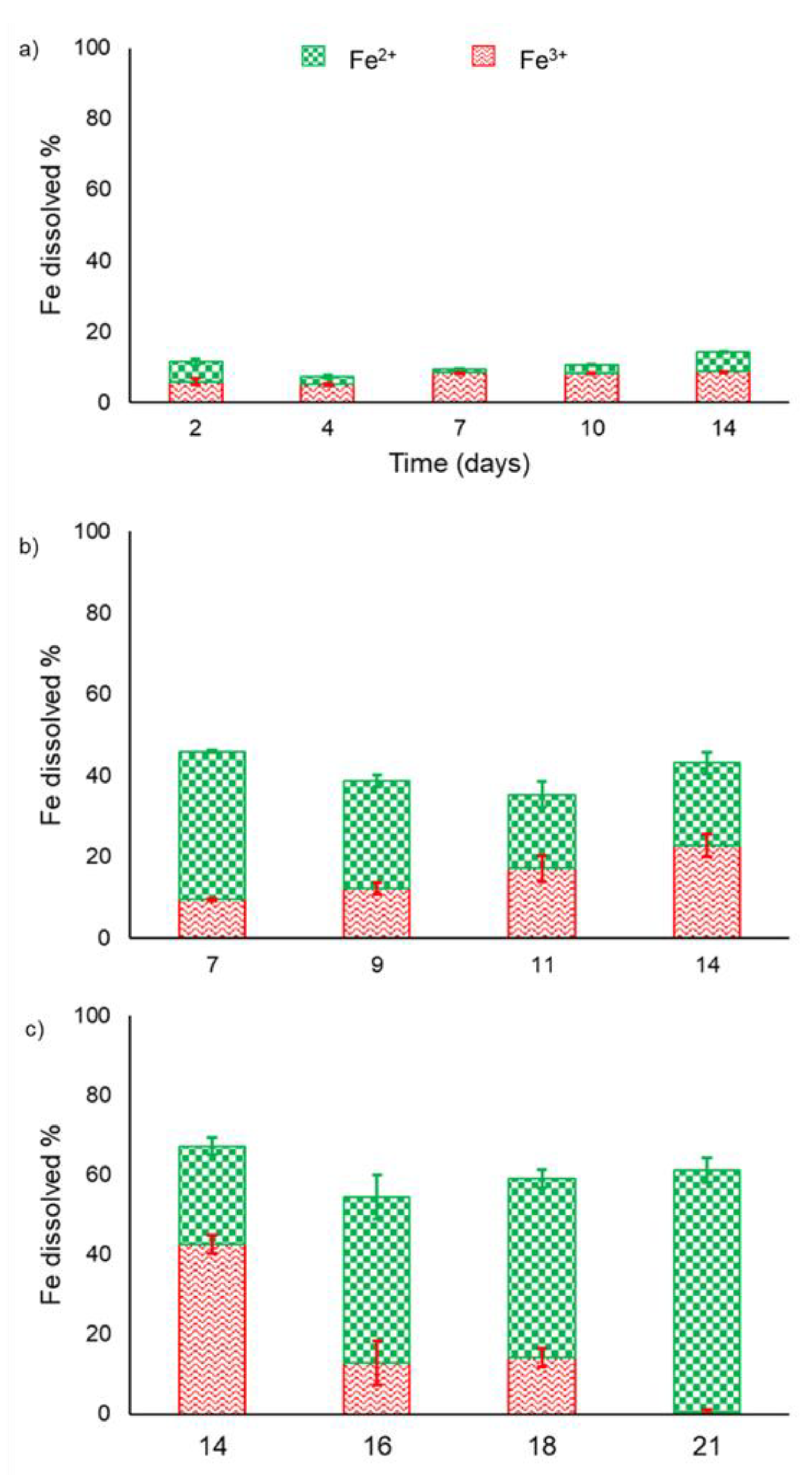
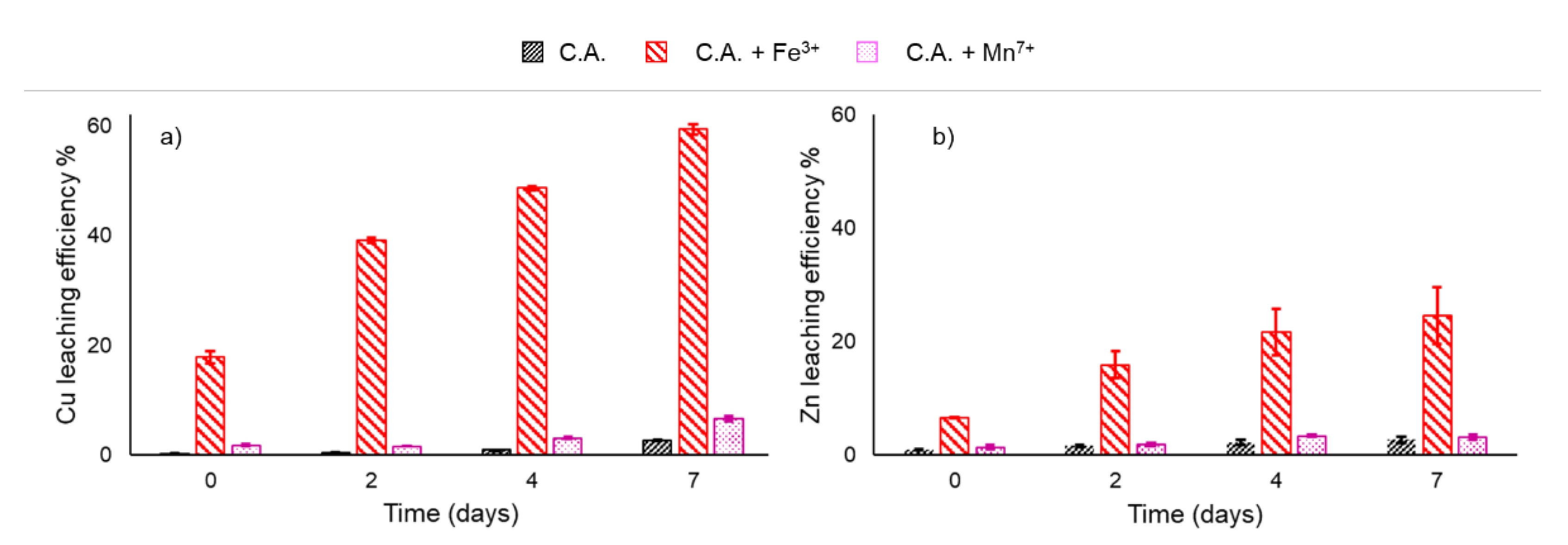
3. Results
3.1. Bioleaching Experiments
3.2. Chemical Controls and Statistical Analysis
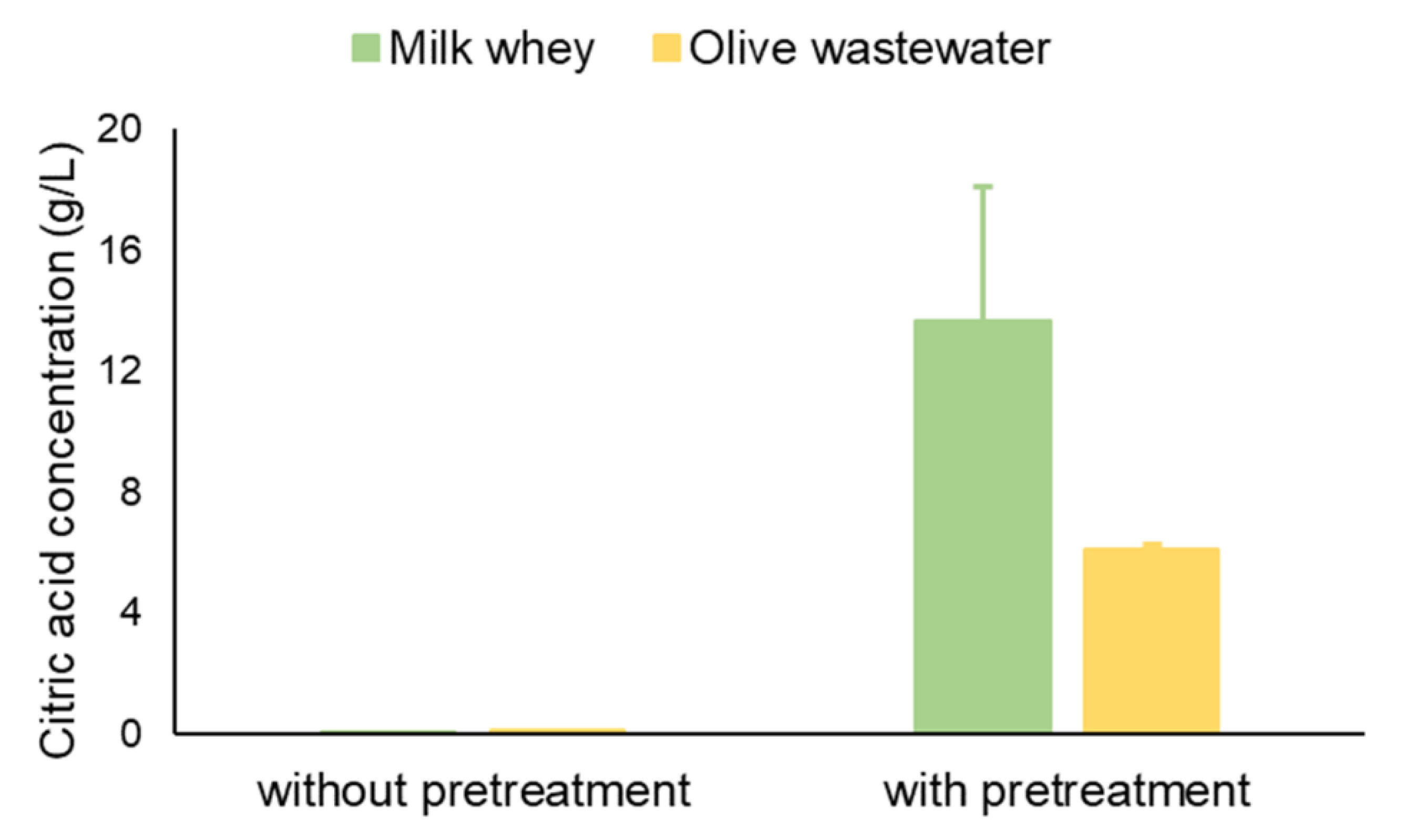
3.3. Citric Acid Production Using Alternative Carbon Sources
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arshadi, M.; Mousavi, S.M. Multi-objective optimization of heavy metals bioleaching from discarded mobile phone PCBs: Simultaneous Cu and Ni recovery using Acidithiobacillus ferrooxidans. Sep. Purif. Technol. 2015, 147, 210–219. [Google Scholar] [CrossRef]
- Baldé, C.P.; Forti, V.; Gray, V.; Kuehr, R.; Stegmann, P. The Global E-waste Monitor 2017. Available online: https://www.itu.int/en/ITU-D/Climate-Change/Pages/Global-E-waste-Monitor-2017.aspx (accessed on 11 August 2020).
- Jadhav, C.H.H.; Su, U. Waste Printed Circuit Boards recycling: An extensive assessment of current status. J. Clean. Prod. 2015, 94, 5–19. [Google Scholar] [CrossRef]
- Kaya, M. Electronic Waste and Printed Circuit Board Recycling Technologies; Springer: Berlin, Germany, 2019. [Google Scholar] [CrossRef]
- Jadhav, C.H.H.; Su, U. RSC Advances leaching of metals from printed circuit board powder by an Aspergillus niger culture supernatant and hydrogen peroxide. R. Soc. Chem. 2016, 6, 43442–43452. [Google Scholar] [CrossRef]
- Rocchetti, L.; Amato, A.; Beolchini, F. Printed circuit board recycling: A patent review. J. Clean. Prod. 2018, 178, 814–832. [Google Scholar] [CrossRef]
- Evangelopoulos, P.; Kantarelis, E.; Yang, W. Waste Electric and Electronic Equipment: Current Legislation, Waste Management, and Recycling of Energy, Materials, and Feedstocks. Available online: https://www.sciencedirect.com/science/article/pii/B9780444642004000177 (accessed on 11 August 2020).
- Zhang, L.; Xu, Z. A review of current progress of recycling technologies for metals from waste electrical and electronic equipment. J. Clean. Prod. 2016, 127, 19–36. [Google Scholar] [CrossRef]
- Vermeșan, H.; Tiuc, A.E.; Purcar, M. Advanced Recovery Techniques for Waste Materials from IT and Telecommunication Equipment Printed Circuit Boards. Sustainability 2020, 12, 74. [Google Scholar] [CrossRef]
- Faraji, F.; Golmohammadzadeh, R.; Rashchi, F. Fungal bioleaching of WPCBs using Aspergillus niger: Observation, optimization and kinetics. J. Environ. Manag. 2018, 217, 775–787. [Google Scholar] [CrossRef]
- Birloaga, I.; Vegliò, F. Study of multi-step hydrometallurgical methods to extract the valuable content of gold, silver and copper from waste printed circuit boards. J. Environ. Chem. Eng. 2016, 4, 20–29. [Google Scholar] [CrossRef]
- Xia, M.; Bao, P.; Liu, A.; Zhang, S.; Peng, T.; Shen, L.; Yu, R.; Wu, X.; Li, J.; Liu, Y.; et al. Isolation and identification of Penicillium chrysogenum strain Y5 and its copper extraction characterization from waste printed circuit boards. J. Biosci. Bioeng. 2018, 126, 78–87. [Google Scholar] [CrossRef]
- D’Adamo, I.; Ferella, F.; Gastaldi, M.; Maggiore, F.; Rosa, P.; Terzi, S. Towards sustainable recycling processes: Wasted printed circuit boards as a source of economic opportunities. Resour. Conserv. Recycl. 2019, 149, 455–467. [Google Scholar] [CrossRef]
- Ljunggren Söderman, M.; André, H. Effects of circular measures on scarce metals in complex products–Case studies of electrical and electronic equipment. Resour. Conserv. Recycl. 2019, 151, 104464. [Google Scholar] [CrossRef]
- Kim, M.; Seo, J.; Choi, Y.; Kim, G. Bioleaching of spent Zn–Mn or Ni–Cd batteries by Aspergillus species. Waste Manag. 2016, 51, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, U.; Hocheng, H. Use of Aspergillus niger 34770 culture supernatant for tin metal removal. Corros. Sci. 2014, 82, 248–254. [Google Scholar] [CrossRef]
- Beolchini, F.; Fonti, V.; Dell’Anno, A.; Rocchetti, L.; Vegliò, F. Assessment of biotechnological strategies for the valorization of metal bearing wastes. Waste Manag. 2012, 32, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Arshadi, M.; Mousavi, S.M. Simultaneous recovery of Ni and Cu from computer-printed circuit boards using bioleaching: Statistical evaluation and optimization. Bioresour. Technol. 2014, 174, 233–242. [Google Scholar] [CrossRef]
- Yang, T.; Xu, Z.; Wen, J.; Yang, L. Factors influencing bioleaching copper from waste printed circuit boards by Acidithiobacillus ferrooxidans. Hydrometallurgy 2009, 97, 29–32. [Google Scholar] [CrossRef]
- Brandl, H.; Bosshard, R.; Wegmann, M. Computer-munching microbes: Metal leaching from electronic scrap by bacteria and fungi. Hydrometallurgy 2001, 59, 319–326. [Google Scholar] [CrossRef]
- Wang, J.; Bai, J.; Xu, J.; Liang, B. Bioleaching of metals from printed wire boards by Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans and their mixture. J. Hazard. Mater. 2009, 172, 1100–1105. [Google Scholar] [CrossRef]
- Lee, J.; Dhar, B. Bio-processing of solid wastes and secondary resources for metal extraction–A review. Waste Manag. 2012, 32, 3–18. [Google Scholar] [CrossRef]
- Işıldar, A.; van de Vossenberg, J.; Rene, E.R.; van Hullebusch, E.D.; Lens, P.N. Two-step bioleaching of copper and gold from discarded printed circuit boards (PCB). Waste Manag. 2016, 57, 149–157. [Google Scholar] [CrossRef]
- Hong, Y.; Valix, M. Bioleaching of electronic waste using acidophilic sulfur oxidising bacteria. J. Clean. Prod. 2014, 65, 465–472. [Google Scholar] [CrossRef]
- Gu, W.; Bai, J.; Dai, J.; Zhang, C.; Yuan, W.; Wang, J.; Wang, P.; Zhao, X. Characterization of extreme acidophile bacteria (Acidithiobacillus ferrooxidans) bioleaching copper from flexible PCB by ICP-AES. J. Spectrosc. 2014, 2014, 269351. [Google Scholar] [CrossRef]
- Ilyas, S.; Lee, J.; Chi, R. Bioleaching of metals from electronic scrap and its potential for commercial exploitation. Hydrometallurgy 2013, 131, 138–143. [Google Scholar] [CrossRef]
- Rodrigues, M.L.M.; Leão, V.A.; Gomes, O.; Lambert, F.; Bastin, D.; Gaydardzhiev, S. Copper extraction from coarsely ground printed circuit boards using moderate thermophilic bacteria in a rotating-drum reactor. Waste Manag. 2015, 41, 148–158. [Google Scholar] [CrossRef]
- Priya, A.; Hait, S. Extraction of metals from high grade waste printed circuit board by conventional and hybrid bioleaching using Acidithiobacillus ferrooxidans. Hydrometallurgy 2018, 177, 132–139. [Google Scholar] [CrossRef]
- Liang, G.; Tang, J.; Liu, W.; Zhou, Q. Optimizing mixed culture of two acidophiles to improve copper recovery from printed circuit boards (PCBs). J. Hazard. Mater. 2013, 250, 238–245. [Google Scholar] [CrossRef]
- Becci, A.; Amato, A.; Fonti, V.; Karaj, D.; Beolchini, F. An innovative biotechnology for metal recovery from printed circuit boards. Resour. Conserv. Recycl. 2020, 153, 104549. [Google Scholar] [CrossRef]
- Arshadi, M.; Mousavi, S.M. Enhancement of simultaneous gold and copper extraction from computer printed circuit boards using Bacillus megaterium. Bioresour. Technol. 2015, 175, 315–324. [Google Scholar] [CrossRef]
- Pradhan, J.K.; Kumar, S. Metals bioleaching from electronic waste by Chromobacterium violaceum and Pseudomonads sp. Waste Manag. Res. 2012, 30, 1151–1159. [Google Scholar] [CrossRef]
- Tay, S.B.; Natarajan, G.; Bin, M.N.; Rahim, A.; Tan, H.T.; Chung, M.C.M.; Ting, Y.P.; Yew, W.S. Enhancing gold recovery from electronic waste via lixiviant metabolic engineering in Chromobacterium violaceum. Sci. Rep. 2013, 3, 2236. [Google Scholar] [CrossRef]
- Pham, V.A.; Ting, Y.P. Gold bioleaching of electronic waste by cyanogenic bacteria and its enhancement with Bio-Oxidation. Adv. Mater. Res. 2009, 71, 661–664. [Google Scholar] [CrossRef]
- Chi, T.D.; Lee, J.; Pandey, B.D.; Yoo, K.; Jeong, J. Bioleaching of gold and copper from waste mobile phone PCBs by using a cyanogenic bacterium. Miner. Eng. 2011, 24, 1219–1222. [Google Scholar] [CrossRef]
- Horeh, N.B.; Mousavi, S.M.; Shojaosadati, S.A. Bioleaching of valuable metals from spent lithium-ion mobile phone batteries using Aspergillus niger. J. Power Sources 2016, 320, 257–266. [Google Scholar] [CrossRef]
- Ren, W.X.; Li, P.J.; Geng, Y.; Li, X.J. Biological leaching of heavy metals from a contaminated soil by Aspergillus niger. J. Hazard. Mater. 2009, 167, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Aung, K.M.M.; Ting, Y.P. Bioleaching of spent fluid catalytic cracking catalyst using Aspergillus niger. J. Biotechnol. 2005, 116, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Santhiya, D.; Ting, Y. Bioleaching of spent refinery processing catalyst using Aspergillus niger with high-yield oxalic acid. J. Biotechnol. 2005, 116, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Santhiya, D.; Ting, Y. Use of adapted Aspergillus niger in the bioleaching of spent refinery processing catalyst. J. Biotechnol. 2006, 121, 62–74. [Google Scholar] [CrossRef]
- Rasoulnia, P.; Mousavi, S.M.; Rastegar, S.O.; Azargoshasb, H. Fungal leaching of valuable metals from a power plant residual ash using Penicillium simplicissimum: Evaluation of thermal pretreatment and different bioleaching methods. Waste Manag. 2016, 52, 309–317. [Google Scholar] [CrossRef]
- Xu, T.; Ramanathan, T.; Ting, Y. Bioleaching of incineration fly ash by Aspergillus niger–precipitation of metallic salt crystals and morphological alteration of the fungus. Biotechnol. Rep. 2014, 3, 8–14. [Google Scholar] [CrossRef]
- Rasoulnia, P.; Mousavi, S.M. Maximization of organic acids production by Aspergillus niger in a bubble column bioreactor for V and Ni recovery enhancement from power plant residual ash in spent-medium bioleaching experiments. Bioresour. Technol. 2016, 216, 729–736. [Google Scholar] [CrossRef]
- Vakilchap, F.; Mousavi, S.M.; Shojaosadati, S.A. Role of Aspergillus niger in recovery enhancement of valuable metals from produced red mud in Bayer process. Bioresour. Technol. 2016, 218, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Lian, B.; Mo, B.; Liu, C. Bioleaching of heavy metals from red mud using Aspergillus niger. Hydrometallurgy 2013, 136, 71–77. [Google Scholar] [CrossRef]
- Magnuson, J.K.; Lasure, L.L. Organic Acid Production by filamentous fungi. In Advances in Fungal Biotechnology for Industry, Agriculture, and Medicine; Springer: Boston, MA, USA, 2004; pp. 307–340. [Google Scholar] [CrossRef]
- Amato, A.; Becci, A.; Beolchini, F. Citric acid bioproduction: The technological innovation change. Crit. Rev. Biotechnol. 2020, 40, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Becci, A.; Amato, A.; Rodríguez Maroto, J.M.; Beolchini, F. Prediction model for Cu chemical leaching from printed circuit boards. Ind. Eng. Chem. Res. 2019, 58, 20585–20591. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, L. Metallurgical recovery of metals from electronic waste: A review. J. Hazard. Mater. 2008, 158, 228–256. [Google Scholar] [CrossRef]
- Boukhoubza, F.; Jail, A.; Korchi, F.; Idrissi, L.L.; Hannache, H.; Duarte, J.C.; Hassani, L.; Nejmeddine, A. Application of lime and calcium hypochlorite in the dephenolisation and discolouration of olive mill wastewater. J. Environ. Manag. 2009, 91, 124–132. [Google Scholar] [CrossRef]
- Hande Gursoy-Haksevenler, B.; Arslan-Alaton, I. Treatment of olive mill wastewater by chemical processes: Effect of acid cracking pretreatment. Water Sci. Technol. 2014, 69, 1453. [Google Scholar] [CrossRef][Green Version]
- Choi, M.; Cho, K.; Kim, D.S.; Kim, D. Microbial recovery of copper from printed circuit boards of waste computer by Acidithiobacillus ferrooxidans. J. Environ. Sci. Health Part A 2004, 39, 2973–2982. [Google Scholar] [CrossRef]
- Amato, A.; Becci, A.; Beolchini, F. Sustainable recovery of Cu, Fe and Zn from end-of-life printed circuit boards. Resour. Conserv. Recycl. 2020, 158, 104792. [Google Scholar] [CrossRef]
- De Michelis, I.; Ferella, F.; Karakaya, E.; Beolchini, F.; Vegliò, F. Recovery of zinc and manganese from alkaline and zinc-carbon spent batteries. J. Power Sources 2007, 172, 975–983. [Google Scholar] [CrossRef]
- Öngen, G.; Güngör, G.; Kanberoglu, B. Decolourisation and dephenolisation potential of selected Aspergillus section Nigri strains—Aspergillus tubingensis in olive mill wastewater. World J. Microbiol. Biotechnol. 2007, 23, 519–524. [Google Scholar] [CrossRef]
- Hamdi, M.; Khadir, A.; Garcia, J. The use of Aspergillus niger for the bioconversion of olive mill wastewaters. Appl. Microbiol. Biotechnol. 1991, 34, 828–831. [Google Scholar] [CrossRef]
- Andreozzi, R.; Longo, G.; Majone, M.; Modesti, G. Integrated treatment of olive oil mill effluents (OME): Study of ozonation coupled with anaerobic digestion. Water Res. 1998, 32, 2357–2364. [Google Scholar] [CrossRef]
- Xu, D.; Madrid, C.; Rohr, M.; Kubicek, C. The influence of type and concentration of the carbon source on production of citric acid by Aspergillus niger Ding-Bang. Appl. Microbiol. Biotechnol. 1989, 30, 553–558. [Google Scholar] [CrossRef]
- Rivas, B.; Torrado, A.; Torre, P.; Converti, A.; Domínguez, J.M. Submerged citric acid fermentation on orange peel autohydrolysate. J. Agric. Food Chem. 2008, 56, 2380–2387. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Kaur, S.; Verma, M. Utilization of different agro-industrial wastes for sustainable bioproduction of citric acid by Aspergillus niger. Biochem. Eng. J. 2011, 54, 83–92. [Google Scholar] [CrossRef]
- Murad, A.E.H.; Khalaf, S.A.D. Citric acid production from whey with sugars and additives by Aspergillus niger. Afr. J. Biotechnol. 2003, 2, 356–359. [Google Scholar] [CrossRef]
- Santoro, R.; Cameselle, C.; Rodríguez-Couto, S.; Sanromán, Á. Influence of milk whey, nitrogen and phosphorus concentration on oxalic acid production by Aspergillus niger. Bioprocess Eng. 1999, 20, 1–5. [Google Scholar] [CrossRef]
- Manjengwa, E.R. Evaluating the Economics of znd Business Models for Metal Recycling from Waste Printed Circuit Boards in a South African Context. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2019. [Google Scholar]
- Natarajan, G.; Ting, Y. Chemosphere gold biorecovery from e-waste: An improved strategy through spent medium leaching with pH modification. Chemosphere 2015, 136, 232–238. [Google Scholar] [CrossRef]
- European Commission. A New Circular Economy Action Plan for a Cleaner and More Competitive Europe. Available online: https://moderndiplomacy.eu/2020/03/17/a-new-circular-economy-action-plan-for-a-cleaner-and-more-competitive-europe/ (accessed on 11 August 2020).
| Treatment | Statistical Analysis (ANOVA) | ||||||
|---|---|---|---|---|---|---|---|
| df | Cu | Zn | |||||
| MS | F | P | MS | F | P | ||
| C.A. | 1 | 2.98 | 3.74 | 0.19 | 2.06 | 50.75 | 0.05 |
| C.A.+Fe3+ | 1 | 5.74 | 0.46 | 0.57 | 140.6 | 2.98 | 0.23 |
| C.A.+Mn7+ | 1 | 0.58 | 0.33 | 0.62 | 1.48 | 8.74 | 0.10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becci, A.; Karaj, D.; Merli, G.; Beolchini, F. Biotechnology for Metal Recovery from End-of-Life Printed Circuit Boards with Aspergillus niger. Sustainability 2020, 12, 6482. https://doi.org/10.3390/su12166482
Becci A, Karaj D, Merli G, Beolchini F. Biotechnology for Metal Recovery from End-of-Life Printed Circuit Boards with Aspergillus niger. Sustainability. 2020; 12(16):6482. https://doi.org/10.3390/su12166482
Chicago/Turabian StyleBecci, Alessandro, Dafina Karaj, Giulia Merli, and Francesca Beolchini. 2020. "Biotechnology for Metal Recovery from End-of-Life Printed Circuit Boards with Aspergillus niger" Sustainability 12, no. 16: 6482. https://doi.org/10.3390/su12166482
APA StyleBecci, A., Karaj, D., Merli, G., & Beolchini, F. (2020). Biotechnology for Metal Recovery from End-of-Life Printed Circuit Boards with Aspergillus niger. Sustainability, 12(16), 6482. https://doi.org/10.3390/su12166482





