Exposure to Dodecamethylcyclohexasiloxane (D6) Affects the Antioxidant Response and Gene Expression of Procambarus clarkii
Abstract
1. Introduction
2. Materials and Methods
2.1. Resources for the Experiment
2.2. Experimental Procedure
2.3. Preparation of Samples and Analysis
2.4. Determination of Enzyme and Nonenzyme Activities
2.5. Determination of Si Content
2.6. RNA Isolation, Preparation, and qRT-PCR
2.7. Data Analysis
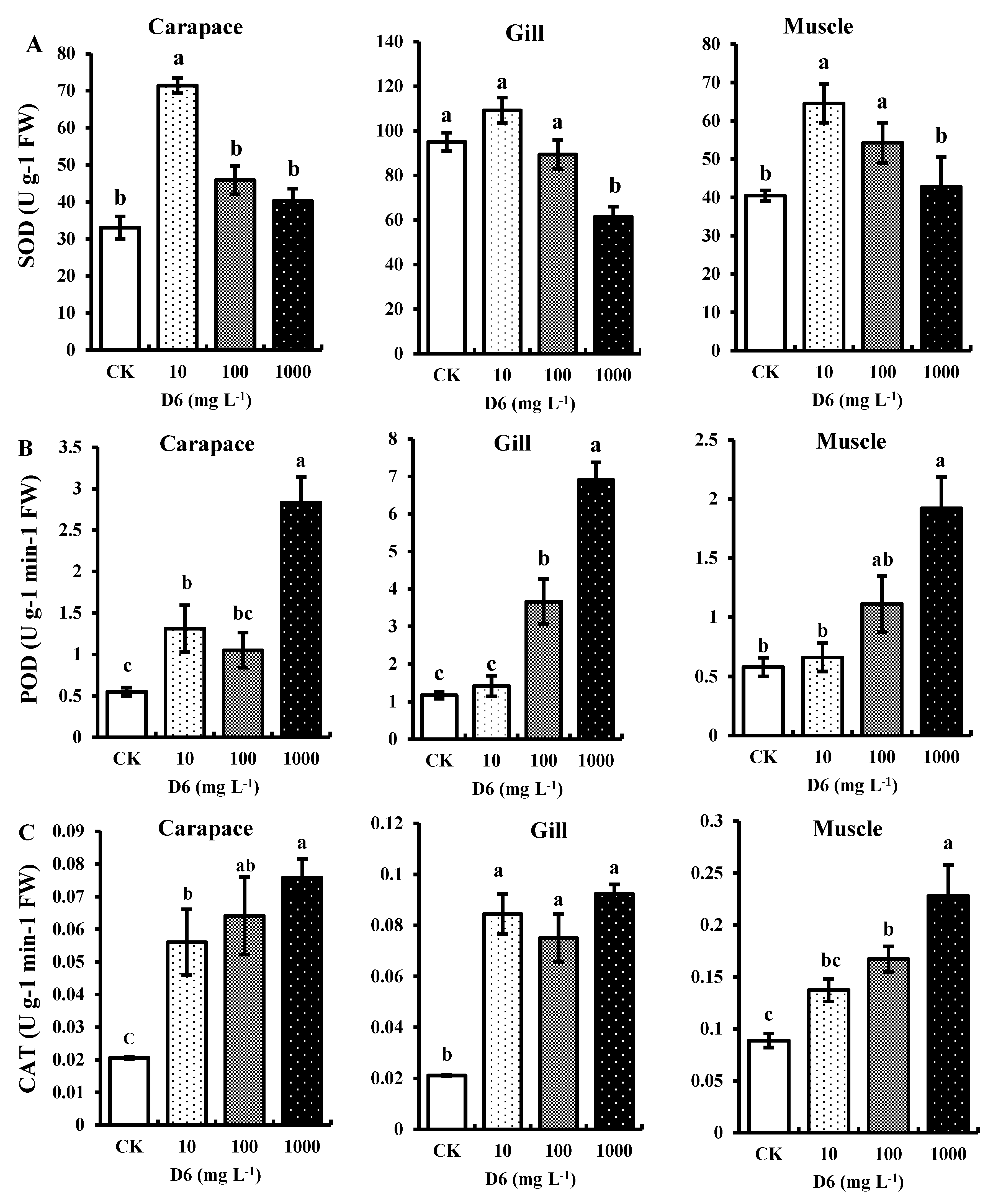
3. Results
3.1. Effect of D6 on the Activity of Antioxidant Enzymes
3.1.1. Superoxide Dismutase (SOD) Activity
3.1.2. Peroxidase (POD) Activity
3.1.3. Catalase (CAT) Activity
3.2. Effect of D6 on the Non-Enzymatic Antioxidant
3.2.1. Determination of Malondialdehyde (MDA) Content
3.2.2. Determination of Ascorbate (AsA) and Glutathione (GSH) Contents
3.3. Silicon (Si) Content and Survival Rate of Crayfish
3.4. Effects of D6 on Gene Expression in Muscle
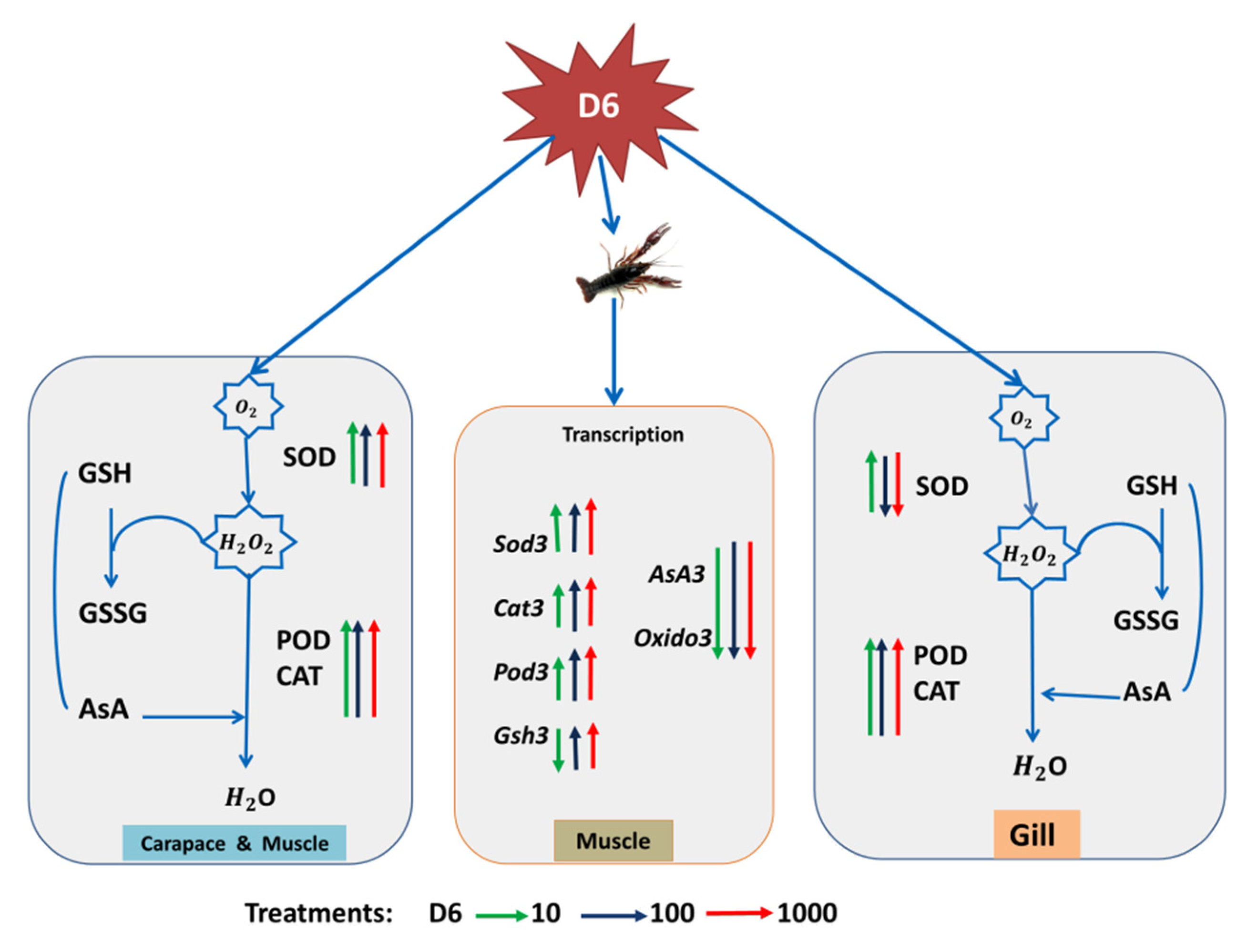
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Will, R.; Löchner, U.; Masahiro, Y. CEH Marketing Research Report Siloxanes; SRI Consulting: Menlo Park, CA, USA, 2007. [Google Scholar]
- Rücker, C.; Kümmerer, K. Environmental Chemistry of Organosiloxanes. Chem. Rev. 2015, 115, 466–524. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-G.; Norwood, W.; Alaee, M.; Byer, J.D.; Brimble, S. Review of recent advances in research on the toxicity, detection, occurrence and fate of cyclic volatile methyl siloxanes in the environment. Chemosphere 2013, 93, 711–725. [Google Scholar] [CrossRef]
- USEPA (United States Environmental Protection Agency). High Production Volume (HPV) Challenge Program. Sponsored Chemicals. Available online: http://www.epa.gov/hpv/pubs/update/spnchems.htm (accessed on 1 September 2007).
- OECD (Organisation for Economic Co-operation and Development). Manual for Investigation of HPV Chemicals. OECD Secretariat. Available online: http://www.oecd.org/document/7/0,3343,en_2649_34379_1947463_1_1_1_1,00.html (accessed on 1 July 2007).
- Dudzina, T.; von Goetz, N.; Bogdal, C.; Biesterbos, J.W.; Hungerbühler, K. Concentrations of cyclic volatile methylsiloxanes in European cosmetics and personal care products: Prerequisite for human and environmental exposure assessment. Environ. Int. 2014, 62, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.G.; Steer, H.; Tait, T.; Williams, Z.; Pacepavicius, G.; Young, T.; Ng, T.; Smyth, S.A.; Kinsman, L.; Alaee, M. Concentration of cyclic volatile methylsiloxanes in bio-solid amended soil, influent, effluent, receiving water, and sediment of wastewater treatment plants in Canada. Chemosphere 2013, 93, 766–773. [Google Scholar] [CrossRef]
- Mojsiewicz-Pienkowska, K.; Krenczkowska, D. Evolution of consciousness of exposure to siloxanes—Review of publications. Chemosphere 2018, 191, 204–221. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Non-Confidential Inventory Update Reporting Production Volume Information. Toxic Substances Control Act (TSCA) Inventory. Available online: http://www.epa.gov/oppt/iur/tools/data/2002-vol.htm (accessed on 16 March 2021).
- De Arespacochaga, N.; Valderrama, C.; Raich-Montiu, J.; Crest, M.; Mehta, S.C.; Cortina, J. Understanding the effects of the origin, occurrence, monitoring, control, fate and removal of siloxanes on the energetic valorization of sewage biogas—A review. Renew. Sustain. Energy Rev. 2015, 52, 366–381. [Google Scholar] [CrossRef]
- PMRA (Pest Management Regulatory Agency). Regulatory Note REG 2007-04: PMRA List of Formulants Ottawa (ON): Health Canada, Pest Management Regulatory Agency. Available online: http://www.pmra-arla.gc.ca/english/pdf/reg/reg2007-04-e.pdf (accessed on 1 July 2007).
- DPD (Drug Product Database). Ottawa (ON): Health Canada. 2007. Available online: http://www.hc-sc.gc.ca/dhp-mps/prodpharma/databasdon/index_e.html (accessed on 1 July 2007).
- Norden. Siloxanes in the Nordic Environment. TemaNord 2005, 593. Copenhagen (NO), Nordic Council of Ministers. Available online: http://www.norden.org/pub/miljo/miljo/uk/TN2005593.pdf (accessed on 16 March 2021).
- Kaj, L.; Andersson, J.; Palm Cousins, A.; Remberger, M.; Ekheden, Y.; Dusan, B.; Bror-ström-Lundén, E. Results from the Swedish National Screening Programme 2004: Subreport 4: Siloxanes, IVL. Swedish Environmental Research Institute. Available online: www.imm.ki.se/Datavard/PDF/B1643_siloxaner.pdf (accessed on 16 March 2021).
- Zhang, K.; Wong, J.W.; Begley, T.H.; Hayward, D.G.; Limm, W. Determination of siloxanes in silicone products and potential migration to milk, formula and liquid simulants. Food Addit. Contam. Part A 2012, 29, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Horii, Y.; Kannan, K. Survey of Organosilicone Compounds, Including Cyclic and Linear Siloxanes, in Personal-Care and Household Products. Arch. Environ. Contam. Toxicol. 2008, 55, 701–710. [Google Scholar] [CrossRef]
- Lucas, S.V.; Kopfler, F.C. GC/MS Analysis of Organics in Drinking Water Concentrates and Advanced Waste Treatment Concentrates. U.S. Environmental Protection Agency, Office of Research and Development, Health Effects Research Laboratory.Volume 1, EPA 600/1-84-020a; EPA 600/1-84-020b; EPA 600/1-84-020c; EPA-68-03-2548; PB85128221. Available online: https://nepis.epa.gov/Exe/ZyPDF.cgi?Dockey=9100A600.PDF (accessed on 16 March 2021).
- USEPA. Thirtieth Report of the Interagency Testing Committee to the Adminstrator, Receipt of Report and Request for Comments Regarding Priority Testing List of Chemicals; Federal Register, EPA: Washington, DC, USA, 1992; Volume 57.
- Allen, R.B.; Kochs, P.; Chandra, G. Industrial Organic Materials, Their Environmental Entry and Predicted Fate. In Organosilicon Materials; Hutzinger, O., Ed.; Part of the Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 1997; pp. 1–25. [Google Scholar] [CrossRef]
- Flynn, K.; Spellman, T. Environmental levels of atrazine decrease spatial aggregation in the fresh water mussel, Elliptio complanata. Ecotoxicol. Environ. Saf. 2009, 72, 1228–1233. [Google Scholar] [CrossRef]
- TemaNord. Siloxanes in the Nordic Environment; Nordic Council of Ministers, TemaNord: Copenhagen, Denmark, 2005; p. 593. [Google Scholar]
- Environment Canada. Data for Batch 2 Substances Collected under the Canadian Environmental Protection Act, 1999, Section 71: Notice with Respect to Certain Batch 2 Challenge Substances; Existing Substances Program: Toronto, ON, Canada, 2007.
- CEPA (Canadian Environmental Protection Act). Statutes of Canada. Ottawa: Public Works and Government Services Canada. Canada Gazette. Part III. 1999, 22, Chapter 33. Available online: http://canadagazette.gc.ca/partIII/1999/g3-02203.pdf (accessed on 16 March 2021).
- Sanchís, J.; Llorca, M.; Picó, Y.; Farré, M.; Barceló, D. Volatile dimethylsiloxanes inmarket seafood and freshwater fish from the Xúquer River. Spain. Sci. Total Environ. 2016, 545–546, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Annelin, R.; Frye, C. The piscine bioconcentration characteristics of cyclic and linear oligomeric permethylsiloxanes. Sci. Total. Environ. 1989, 83, 1–11. [Google Scholar] [CrossRef]
- Opperhuizen, A.; Damen, H.W.J.; Asyee, G.M.; Van der Steen, J.M.D. Uptake and Elimination by Fish of Polydime-thylsiloxanes (Silicones) after Dietary and Aqueous Exposure. Toxicol. Environ. Chem. 1987, 13, 265–285. [Google Scholar] [CrossRef]
- Drottar, K.R. 14C-Dodecamethylcyclohexasiloxane (14C-D6): Bioconcentration in the Fathead Minnow (Pimphales promelas) under Flow-Through Test Conditions. HES Study No. 9882-102; Health and Environmental Sciences, Dow Corning Corporation: Auburg, MI, USA, 2005. [Google Scholar]
- IUCLID. IUCLID Dataset for Dodecamethylcyclohexa siloxane; Epona Associates LLC.: Willington, CT, USA, 2005. [Google Scholar]
- Berres, M.-L.; Trautwein, C.; Zaldivar, M.M.; Schmitz, P.; Pauels, K.; Lira, S.A.; Tacke, F.; Wasmuth, H.E. The chemokine scavenging receptor D6 limits acute toxic liver injury in vivo. Biol. Chem. 2009, 390, 1039–1045. [Google Scholar] [CrossRef]
- Environment Canada. Draft Screening Assessment for Dodecamethylcyclohexasiloxane (CAS 540-97-6). Draft for Public Consultation, May 2008; Environment Canada and Health Canada: Ottawa, ON, Canada, 2008. Available online: https://www.ec.gc.ca/ese-ees/FC0D11E7-DB34-41AA-B1B3-E66EFD8813F1/batch2_540-97-6_en.pdf (accessed on 16 March 2021).
- Onnekink, C.; Kappel, R.M.; Boelens, W.C.; Pruijn, G.J.M. Low molecular weight silicones induce cell death in cultured cells. Sci. Rep. 2020, 10, 9558. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Kujala, H.; Bland, L.M.; Burgman, M.; Lahoz-Monfort, J.J. Assessing the impacts of uncertainty in climate-change vulnerability assessments. Divers. Distrib. 2019, 25, 1234–1245. [Google Scholar] [CrossRef]
- Schilderman, P.; Moonen, E.; Maas, L.; Welle, I.; Kleinjans, J. Use of Crayfish in Biomonitoring Studies of Environmental Pollution of the River Meuse. Ecotoxicol. Environ. Saf. 1999, 44, 241–252. [Google Scholar] [CrossRef][Green Version]
- Dhindsa, R.S.; Dhindsa, P.P.; Thorpe, T.A. Leaf senescence correlated with increased levels of membrane permeability and lipid-peroxidation and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1980, 32, 93–101. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Huang, W.; Bi, X.; Zhang, X.; Liao, X.; Hu, X.; Wu, J. Comparative study of enzymes, phenolics, carotenoids and color of apricot nectars treated by high hydrostatic pressure and high temperature short time. Innov. Food Sci. Emerg. Technol. 2013, 18, 74–82. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated Chloroplasts of Fatty Acid Peroxidation chlorophyll. Arch. Biochem. Biophisics. 1968, 126, 189–198. [Google Scholar] [CrossRef]
- Guo, T.R.; Zhang, G.P.; Zhang, Y.H. Physiological changes in barley plants under combined toxicity of aluminum, copper and cadmium. Colloids Surf. B Biointerfaces 2007, 57, 182–188. [Google Scholar] [CrossRef]
- Han, D.; Xiong, S.; Tu, S.; Liu, J.; Chen, C. Interactive effects of selenium and arsenic on growth, antioxidant system, arsenic and selenium species of Nicotiana tabacum L. Environ. Exp. Bot. 2015, 117, 12–19. [Google Scholar] [CrossRef]
- Huang, H.; Rizwan, M.; Li, M.; Song, F.; Zhou, S.; He, X.; Ding, R.; Dai, Z.; Yuan, M.; Cao, Y.; et al. Comparative efficacy of organic and inorganic silicon fertilisers on antioxidant response, Cd/Pb accumulation and health risk assessment in wheat (Triticum aestivum L.). Environ. Pollut. 2019, 255 Pt 1, 113146. [Google Scholar] [CrossRef] [PubMed]
- Thiel, T.; Michalek, W.; Varshney, R.; Graner, A. Exploiting EST databases for the development and characterisation of gene-derived SSR-markers in barley (Hordeum vulgare L.). Theor Appl Genet. 2003, 106, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Untergrasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3-New Capabilities and Interfaces. Nucl. Acids Res. 2012, 40, e115. Available online: http://bioinfo.ut.ee/primer3 (accessed on 16 March 2021). [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- STAR (Statistical Tool for Agricultural Research). Biometrics and Breeding Informatics, PBGB Division, R-packages 1.5, version 2.0.1. 2014; International Rice Research Institute: Los Baños, Philippines, 2014. [Google Scholar]
- Das, S.; Tseng, L.-C.; Chou, C.; Wang, L.; Souissi, S.; Hwang, J.-S. Effects of cadmium exposure on antioxidant enzymes and histological changes in the mud shrimp Austinogebia edulis (Crustacea: Decapoda). Environ. Sci. Pollut. Res. 2019, 26, 7752–7762. [Google Scholar] [CrossRef]
- Hossain, M.; Huang, H.; Yuan, Y.; Wan, T.; Jiang, C.; Dai, Z.; Xiong, S.; Cao, M.; Tu, S. Silicone stressed response of crayfish (Procambarus clarkii) in antioxidant enzyme activity and related gene expression. Environ. Pollut. 2021, 274, 115836. [Google Scholar] [CrossRef]
- Soegianto, A.; Winarni, D.; Handayani, U.S. Hartati Bioaccumulation, Elimination, and Toxic Effect of Cadmium on Structure of Gills and Hepatopancreas of Freshwater Prawn Macrobrachium sintangese (De Man, 1898). Water Air Soil Pollut. 2013, 224, 1–10. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, J.-J.; Dahms, H.-U.; Zhen, J.-J.; Ying, X.-P. Cell damage and apoptosis in the hepatopancreas of Eriocheir sinensis induced by cadmium. Aquat. Toxicol. 2017, 190, 190–198. [Google Scholar] [CrossRef]
- Pedrajas, J.R.; Gavilanes, F.; López-Barea, J.; Peinado, J. Incubation of superoxide dismutase with malondialdehyde and 4-hydroxy-2-nonenal forms new active isoforms and adducts. An evaluation of xenobiotics in fish. Chem. Interactions 1998, 116, 1–17. [Google Scholar] [CrossRef]
- Almroth, B.C.; Sturve, J.; Berglund, Å.; Förlin, L. Oxidative damage in eelpout (Zoarces viviparus), measured as protein carbonyls and TBARS, as biomarkers. Aquat. Toxicol. 2005, 73, 171–180. [Google Scholar] [CrossRef]
- Livingstone, D.R. Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar. Pollut. Bull. 2001, 42, 656–666. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef]
- Wei, K.; Yang, J. Oxidative damage of hepatopancreas induced by pollution depresses humoral immunity response in the freshwater crayfish Procambarus clarkii. Fish Shellfish. Immunol. 2015, 43, 510–519. [Google Scholar] [CrossRef]
- Carvalho, C.S.; Bernusso, V.A.; Araujo, H.S.S.; Espíndola, E.L.G.; Fernandes, M.N. Biomarker responses as indication of contaminant effects in Oreochromis niloticus. Chemosphere 2012, 89, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zheng, S.; Pu, Y.; Shu, L.; Sun, L.; Liu, W.; Fu, Z. Cypermethrin has the potential to induce hepatic oxidative stress, DNA damage and apoptosis in adult zebra fish (Danio rerio). Chemosphere 2011, 82, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Köprücü, K.; Yonar, S.M.; Şeker, E. Effects of cypermethrin on antioxidant status, oxidative stress biomarkers, behavior, and mortality in the freshwater mussel Unio elongatulus eucirrus. Fish. Sci. 2010, 76, 1007–1013. [Google Scholar] [CrossRef]
- Barim-Oz, O.; Şahin, S. Oxidative stress and some biochemical parameters during starvation and refeeding in Astacus leptodactylus (Esch., 1823). Cell. Mol. Biol. 2016, 62, 35–43. [Google Scholar] [CrossRef]
- Kovacevic, B.T.; Pavlovic, Z.S.; Borkovic, S.S.; Milosevic, M.S.; Kosanovic, K.; Stajn, S.A.; Petrovic, M.V.; Saicic, S.Z. Superoxide dismutase (SOD) and catalase (CAT) activities in some tissues of three freshwater crayfish species. Acta Physiol. Pharmacol. Serb. 2006, 42, 193–201. [Google Scholar]
- Chen, Y.F.; Ai, C.X.; Lin, Q.W.; Xu, H.; Shen, Y.H. Effect of salinity stress on the activities of phenol oxidase and superoxide dismutase of the serum, tissue and organ of mud crab, Scylla serrata. J. Oceanogr. Taiwan Strait 2007, 26, 569–575. [Google Scholar]
- Rameshthangam, P.; Ramasamy, P. Antioxidant and membrane bound enzymes activity in WSSV-infected Penaeus monodon Fabricius. Aquaculture 2006, 254, 32–39. [Google Scholar] [CrossRef]
- Liu, Y.; Jang, X.L.; Lv, Q.; Guan, H.S. Effects of mannuronate polysaccharide on enzymes of Penaeus chinensis related with immune and hemolysis. J. Fish. China 2000, 24, 549e53. [Google Scholar]
- Barim-Oz, O.; Yilmaz, S. Effects of dietary antioxidants on oxidative stress, antioxidant defence and growth of freshwater crayfish Astacus leptodactylus (Eschscholtz, 1823) during the reproductive period in females. Aquac. Res. 2016, 48, 2516–2527. [Google Scholar] [CrossRef]
- Thomas, P.; Juedes, M.J. Influence of lead on glutathione status of Atlantic croaker tissues. Aquat. Toxicol. 1992, 23, 11–30. [Google Scholar] [CrossRef]
- Sheader, D.L.; Williams, T.D.; Lyons, B.P.; Chipman, J.K. Oxidative stress response of European flounder (Platichthys flesus) to cadmium determined by acustom cDNA microarray. Mar. Environ. Res. 2006, 62, 33–44. [Google Scholar] [CrossRef]
- Woo, S.; Yum, S.; Kim, D.-W.; Park, H.-S. Transcripts level responses in a marine medaka (Oryzias javanicus) exposed to organophosphorus pesticide. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 149, 427–432. [Google Scholar] [CrossRef]
- Craig, P.M.; Wood, C.M.; McClelland, G.B. Oxidative stress response and gene expression with acute copper exposure in zebra fish (Danio rerio). Am. J. Physiol.Regul. Integr. Compar. Physiol. 2007, 293, 1882–1892. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.S.; Wei, Y.H.; Zhang, H.X.; Xu, M.Q.; Dai, J.Y. Induction of time dependent oxidative stress and related transcriptional effects of perfluorododecanoic acid in zebra fish liver. Aquat. Toxicol. 2008, 89, 242–250. [Google Scholar] [CrossRef]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free. Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Li, Y.T.; Liu, Y.; Lee, S.C.; Wang, L. Transcriptome assembly and expression profiling of molecular responses to cadmium toxicity in hepatopancreas of the freshwater crab Sinopotamon henanense. Sci. Rep. 2016, 6, 19405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Z.; Kholodkevich, S.; Sharov, A.; Feng, Y.; Ren, N.; Sun, K. Cadmium-induced oxidative stress, histopathology, and transcriptome changes in the hepatopancreas of freshwater crayfish (Procambarus clarkii). Sci. Total. Environ. 2019, 666, 944–955. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, M.M.; Yuan, Y.; Huang, H.; Wang, Z.; Baki, M.A.; Dai, Z.; Rizwan, M.; Xiong, S.; Cao, M.; Tu, S. Exposure to Dodecamethylcyclohexasiloxane (D6) Affects the Antioxidant Response and Gene Expression of Procambarus clarkii. Sustainability 2021, 13, 3495. https://doi.org/10.3390/su13063495
Hossain MM, Yuan Y, Huang H, Wang Z, Baki MA, Dai Z, Rizwan M, Xiong S, Cao M, Tu S. Exposure to Dodecamethylcyclohexasiloxane (D6) Affects the Antioxidant Response and Gene Expression of Procambarus clarkii. Sustainability. 2021; 13(6):3495. https://doi.org/10.3390/su13063495
Chicago/Turabian StyleHossain, Md Muzammel, Yuan Yuan, Hengliang Huang, Ziwei Wang, Mohammad Abdul Baki, Zhihua Dai, Muhammad Rizwan, Shuanglian Xiong, Menghua Cao, and Shuxin Tu. 2021. "Exposure to Dodecamethylcyclohexasiloxane (D6) Affects the Antioxidant Response and Gene Expression of Procambarus clarkii" Sustainability 13, no. 6: 3495. https://doi.org/10.3390/su13063495
APA StyleHossain, M. M., Yuan, Y., Huang, H., Wang, Z., Baki, M. A., Dai, Z., Rizwan, M., Xiong, S., Cao, M., & Tu, S. (2021). Exposure to Dodecamethylcyclohexasiloxane (D6) Affects the Antioxidant Response and Gene Expression of Procambarus clarkii. Sustainability, 13(6), 3495. https://doi.org/10.3390/su13063495






