Abstract
The Montado is an agro-silvo-pastoral ecosystem characteristic of the Mediterranean region. Pasture productivity and, consequently, the possibilities for intensifying livestock production depend on soil fertility. Soil organic matter (SOM) and phosphorus (P2O5) are two indicators of the evolution of soil fertility in this ecosystem. However, their conventional analytical determination by reference laboratory methods is costly, time consuming, and laborious and, thus, does not meet the needs of current production systems. The aim of this study was to evaluate an alternative approach to estimate SOM and soil P2O5 based on near infrared spectroscopy (NIRS) combined with multivariate data analysis. For this purpose, 242 topsoil samples were collected in 2019 in eleven fields. These samples were subjected to reference laboratory analysis and NIRS analysis. For NIRS, 165 samples were used during the calibration phase and 77 samples were used during the external validation phase. The results of this study showed significant correlation between NIRS calibration models and reference methods for quantification of these soil parameters. The coefficient of determination (R2, 0.85 for SOM and 0.76 for P2O5) and the residual predictive deviation (RPD, 2.7 for SOM and 2.2 for P2O5) obtained in external validation indicated the potential of NIRS to estimate SOM and P2O5, which can facilitate farm managers’ decision making in terms of dynamic management of animal grazing and differential fertilizer application.
1. Introduction
The Montado is an agro-silvo-pastoral ecosystem typical of the temperate Mediterranean climate (“Csa: hot-summer Mediterranean climate” according to Köppen–Geiger climate classification). This ecosystem covers 3.5–4.0 Mha in Portugal and Spain and is usually associated with poor soils [1]. Pasture productivity and, consequently, the possibilities for intensifying pasture-based livestock production depend on soil fertility [2]. Soil organic matter (SOM; [3,4]) and phosphorus (P2O5; [5,6]) are two indicators of the evolution of soil fertility in this ecosystem. SOM is an important source of soil nutrients, which are essential for the growth and development of agricultural crops [4]. In agro-ecosystems, such as pastures, mineral fertilization represents other important part of nutrient supplies to plants. In addition to the crucial role in nutrient cycles and soil physical structure, SOM also delivers multiple additional ecosystem services such as climate mitigation and adaptation, as well as helping with water and fertilizer retention [4]. Since low SOM (and, consequently, soil organic carbon, SOC) content may have negative impacts on soil physical properties and on nutrient cycling, SOM plays a vital role in determining the susceptibility to land degradation and efforts have been made to define soil degradation thresholds based on SOC or in SOM content [2]. It is estimated that 16% of cultivated land in Europe is vulnerable to desertification, although this proportion may be even higher in areas with harsh climates such as the Mediterranean, which experiences frequent summer droughts [2]. In this scenario, ensuring permanent land cover has been an effective strategy in management land degradation through the reduction of water, soil, and nutrient losses and increase of soil fertility [2]. The recommended procedure for recovering degraded soils and pastures in the Montado ecosystem is to increase soil fertility through application of chemical fertilizers in order to promote the development of a bio-diverse flora, especially legumes [2,5,6]. Soil phosphorus (P) is a crucial nutrient for plant growth and yield [7], particularly in biodiverse pastures in the Alentejo (Southern region of Portugal; [5]). Soil P dynamics in the Montado ecosystem are complex, since P returns to the soil through animal excreta (dung and urine), which is distributed in a discontinuous pattern in space and time and is influenced by animal density, grazing system, and management, as well as the presence of shade and rest areas, and land topography [8]). Knowledge of SOM and P content in the soil–pasture–tree–animal system is, therefore, essential for efficient and sustainable implementation of management practices in the Montado ecosystem [3,6].
Conventional analytical determinations of SOM and P by reference laboratory methods are relatively complex, costly, and laborious [4,9]. In addition to an initial stage of preparing soil samples (including air-drying, grinding, and sieving), they undergo a phase of chemical analysis. SOM is frequently measured by combustion and CO2 measured using an infrared detection cell, while P2O5 is extracted by the Egner–Riehm method and measured using the colorimetric method [10]. Given that soil fertility attributes have different scales and forms of spatial and temporal variations in agricultural fields [11], a representative soil survey requires the analysis of numerous soil samples in order to characterize SOM or P spatial variability in the field [9]). Hence, the results of these conventional analyses are only available after a few weeks or even months and, thus, do not meet the needs of current production management [4]. Therefore, the development of fast, inexpensive, environmentally friendly and sufficiently accurate methods to predict soil fertility and assess its spatial variability is indispensable [9,12]. Adequate spatiotemporal characterization of these soil attributes is fundamental to the successful development of strategies for variable rate application of fertilizers, enabling the classic benefits of the precision agriculture (PA) approach [11].
In this perspective, soil spectral information obtained through satellites, field and laboratory spectrometers have been used to infer soil properties by many researchers [4]. This process consists of measuring the reflectance response of the soil, that is, the percentage of incident electromagnetic radiation that is reflected by the soil at different wavelengths, which is represented by a spectrum [11]. For this purpose, remote sensing requires that the soil should not be covered by vegetation or a crop and, therefore, does not suit the Montado ecosystem (where a permanent cover of the soil with pasture is sought). Calibration models developed from processed samples cannot be utilized for field soil samples with near infrared spectroscopy (NIRS) due to the variability of external environmental factors, such as the moisture content, one of the most critical factors that degrades the prediction accuracy [12]. All of these problems can be overcome in the laboratory, after soil sample preparation [7,9]. Under these conditions, NIRS combined with the partial least squares regression (PLSR) method is considered to be an effective way of determining soil properties [4]. This widely used approach for evaluating the accuracy of sensing techniques is undertaken by comparing the prediction given by sensors with that provided by traditional reference laboratory methods, creating calibration models [11].
The aim of this study was thus to evaluate an alternative to the conventional approach to estimate SOM and soil P2O5 based on laboratory NIRS combined with multivariate data analysis (Figure 1).
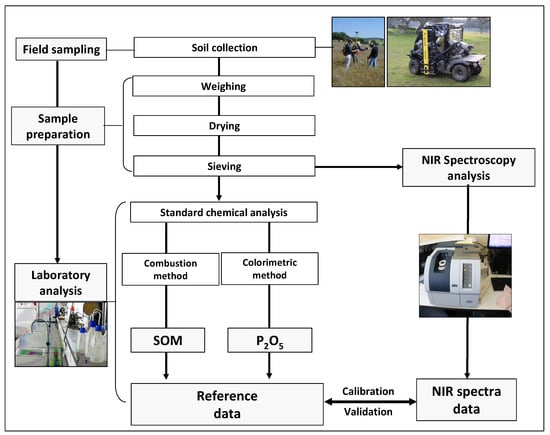
Figure 1.
Diagram of the proposed procedure.
2. Materials and Methods
2.1. Experimental Fields
This work is based on the results of several projects conducted by this research team to monitor the soil in the Montado ecosystem at eleven experimental fields (Figure 2). The main characteristics of the experimental fields used in this study are presented in Table 1. These are typical permanent sown biodiverse dryland pastures that usually grow under a low or moderate density plantation of Holm oak or Cork oak, and that are mainly used for grazing by cattle, sheep, horses, or pigs on a rotational or permanent basis. The soil type is Cambisol with a granite origin [13], characterized by slight or moderate weathering of parent material and by absence of appreciable quantities of illuviated clay, organic matter, aluminum, and/or iron compounds. These acid soils present medium to coarse texture (Table 1), are not very fertile, and are mainly used for mixed agro-silvo-pastoral systems [14]. The location of these fields is representative of the temperate climatic conditions of Portugal, with a temperature and precipitation gradient, with higher mean temperatures and smaller amounts of rainfall in the southern districts (“Beja”, “Évora”, and “Portalegre”, Figure 2, “Csa: hot-summer Mediterranean climate” according to Köppen–Geiger climate classification) and the reverse in the northern district (“Guarda”, Figure 2, “Csb: warm-summer Mediterranean climate”).
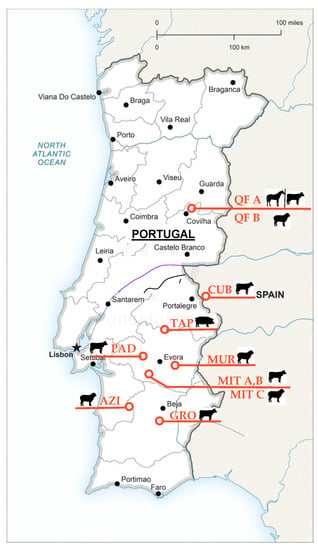
Figure 2.
Location of the experimental fields in Portugal and Spanish: graphical illustration of animal species in each field.

Table 1.
Main characteristics of the eleven experimental fields used in this work.
2.2. Soil Sample Collection and Reference Chemical Processing
Two hundred and forty two soil samples were collected between January and December 2019 in eleven different fields, eight located in the Portuguese Alentejo region (“AZI”, “GRO”, “MIT A”, “MIT B”, “MIT C”, “MUR”, “PAD”, and “TAP”), two in the Portuguese Beira Baixa region (“QF A” and “QF B”), and one in the Spanish Extremadura region (“CUB”) (Figure 2). In each field, 22 composite samples from the upper soil layer (at 0–0.30 m depth) were georeferenced and collected using a gouge auger and a hammer. Each composite sample was the result of the combination of five sub-samples collected in an area of 10 × 10 m. These soil samples were inserted in plastic bags and transported to the “MED- Soil Analysis Laboratory” at University of Évora. After weighing, air-drying, and sieving, the samples were analyzed for particle-size distribution, and the fine components of the soil (fraction with diameter < 2 mm) were characterized in terms of pH, organic matter (SOM), and phosphorus (P2O5). These fine components were analyzed using the following standard reference laboratory methods [10]: (i) pH was determined in 1:2.5 (soil:water) suspension, using the potentiometric method, because water pH responds more rapidly to lime applications; (ii) SOM was measured by combustion and CO2 measurement, using an infrared detection cell; (iii) P2O5 was extracted by the Egner–Riehm method and measured using the colorimetric method.
2.3. Sample Spectra Acquisition and Processing
Soil samples were transported to the “MED-Post Harvest Laboratory” at University of Évora for optical reflectance measurements and data analysis. Spectroscopic measurements were made on all samples using an FT-NIR spectrometer (MPA, Bruker Optik GmbH, Germany). Dried and sieved soil samples were placed in a small circular rotating cup of 90 mm diameter, and spectra were collected in an integrating sphere in diffuse reflectance mode at room temperature of 20 °C. Reflectance data (R) were measured as log 1/R (absorbance data), and NIR spectra data were recorded. An average spectrum was collected from each sample and was used for further mathematical processing and chemometrics analysis. Spectra data were obtained in the entire near infrared region of 12,500–3600 cm−1 (800–2777 nm) after a total of 32 scans with a scanner velocity of 10 kHz and an average resolution of 16 cm−1, with a receiver gain function with the lowest gain setting defined as 1 [15]. The first 70 values of each spectrum were discarded to eliminate the noise below 4065 cm−1 (2460 nm). The background measurement was performed using the MPA FT-NIR spectrometer by measuring the internal gold-coated diffuse reflector, mounted inside the integrating sphere. This procedure was repeated every 2 h.
2.4. Statistical Analysis
For evaluating the accuracy of this approach, SOM and P2O5 obtained by reference laboratory methods were compared with the data obtained by calibration models resulting from NIRS combined with multivariate data analysis.
The Opus v. 7.5 software (Bruker Optik GmbH, Ettlingen, Germany) was employed for spectral data collection, and FT-NIR spectra were exported to the Unscrambler software (version 10.5.1, Camo AS, Oslo, Norway) for chemometrics analysis, calibration, and external validation models. Prediction models were developed using the PLSR algorithm, considering an independent validation sample set for the chemometrics analysis [16]. In order to obtain the best predictive model, for PLSR, samples were random split in two sets: a training set (calibration) with two-thirds of samples (15 samples in each field, totaling 165 samples) and a test set, with the remaining one-third of samples (seven samples in each field, totaling 77 samples) used as an external and independent validation set of the NIRS calibration models.
To find the most accurate model to quantify SOM and P2O5, the calibration process was performed on the raw spectra data, and after the application of adequate pre-processing techniques, mathematical algorithms were used to remove any irrelevant information. In NIR spectroscopy, several pre-processing techniques are widely used to improve the subsequent analysis by removing unnecessary information. Two of the most used pre-processing methods are the multiplicative scatter correction (MSC) and the baseline offset correction (BOC), both scattering correction methods; the first reduces the variability among samples and the second, performed through subtraction of a linear from original spectra, adjusts baseline shifts between samples. Calibration and validation models were developed based on partial least squares regression. Model performance evaluation was assessed by three parameters [17,18]: the coefficient of determination (R2; an excellent indicator of the accuracy and robustness of a model), the root mean square error (RMSE; Equation (1)) for the calibration and external validation data sets, and the ratio of performance to deviation or residual predictive deviation (RPD; Equation (2). The model with the highest R2 and the lowest RMSE is considered as the best model [19]. RPD corresponds to the ratio between standard deviation (SD) of the results obtained by the reference method and the corrected mean error of the prediction of the validation (SEP bias) and is commonly used to investigate the prediction error. The value of RPD is usually used as an indicator of the quality of a calibration model.
where n is the number of observations and Ei and Mi are the estimated and observed (measured) values, respectively.
ArcGIS 10.8 (Esri, Redlands, CA, USA) software was used to produce the spatial maps for soil data (SOM and P2O5). All surfaces were produced with the ArcMap/Spatial Analyst module, using the inverse distance weighting (IDW) interpolator with a 1-m grid resolution.
3. Results
One of the characteristics common to soils where pastures are installed under Montado is the low pH. Table 1 shows that in nine of the 11 experimental fields, the average pH ranges between 5.4 and 5.8. This is a relevant aspect, since the availability of nutrients, particularly phosphorus, is conditioned by low pH [20].
Table 2 shows SOM and P2O5 results of soil samples at each location, determined with the reference method and used in the calibration and external validation models. These results show (i) relatively low values of SOM (on average, between 1.4 and 2.9%) and of phosphorus (on average, between 6 and 61 mg kg−1), (ii) an important variability between fields (with average values ranging from simple to double in SOM and up to ten times more in phosphorus), (iii) and an important spatial variability of both parameters within each experimental field (CV between 15–40% in SOM and CV between 25–90% in P2O5), as a result of the simultaneous effect of trees and animal grazing [21] and intrinsic soil heterogeneity (namely due to land morphology). The range of variation of these parameters showed that the properties of the soils used in this study are representative of the Montado ecosystem, a decisive factor in obtaining adequate calibration models [7].

Table 2.
Soil organic matter (SOM) and phosphorus (P2O5) reference values of each experimental field.
Table 3 shows the statistics for calibration and external validation of the prediction models developed using PLSR to correlate NIRS absorbance spectra with the reference values of SOM and P2O5 obtained by chemical reference processing. For each parameter, only the selected pre-treatment is shown based on the criteria presented above (for each parameter the pre-treatment with higher values of R2 and RPD and with lower values of RMSE and bias was selected; [17,18]). In this case, the best results were obtained using (i) the “baseline offset correction” (BOC) and the “multiplicative scatter correction” (MSC) pre-processing for SOM due to the highest R2 (0.85) and RPD (2.7) and the lowest RMSE (0.291) and bias (0.070) of the external validation model (Table 3) and (ii) the “raw spectra” procedure for the P2O5 due to the highest R2 (0.76) and RPD (2.2) and the lowest RMSE (13.97) and bias (2.640) of the external validation model (Table 3). BOC was the transformation used to correct the baseline of samples, and MSC method can handle both additive and multiplicative effects of light scattering.

Table 3.
Statistics for calibration and external validation models of soil organic matter (SOM) and soil phosphorous (P2O5) using near-infrared spectroscopy (NIRS) spectra and partial least squares regression (PLSR).
Figure 3 shows the optimized spectra of NIRS in the wavenumber region 4065 to 8817 cm−1 (wavelength region 2460 to 1134 nm) for SOM and phosphorus, considering the selected pre-processing methods. The NIR spectrum from soil varies because the spectral reflectance characteristics are related to soil mineral composition. The soils in this study showed the same sensitive spectral regions for SOM and phosphorus; both calibration models used the spectral bands between 7500–7000 cm−1 (1333–1429 nm) and 5501–4400 cm−1 (1818–2460 nm), with peaks of absorbance to wavenumbers 7000 cm−1 (wavelength 1400 nm), 5100 cm−1 (wavelength 1960 nm), and 4400 cm−1 (wavelength 2270 nm), which seems to indicate that these two soil parameters are related in agro-silvo-pastoral systems, such as the Montado.
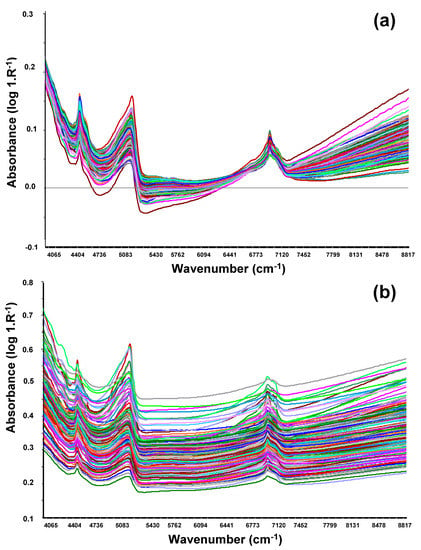
Figure 3.
Optimized near infrared (NIR) spectra in the wavenumber region 4065 to 8817 cm−1 (wavelength region 2460 to 1134 nm) for (a) soil organic matter (SOM) and (b) phosphorus (P2O5).
Figure 4 shows measured versus predicted values for SOM and phosphorus (P2O5). The similar R2 (0.85 and 0.86 to SOM and 0.78 and 0.76 to P2O5) and of range of calibration and validation phases in both parameters (SOM: 0.4–3.9% in calibration and validation sets; P2O5: 2–120 mg kg−1 in calibration set and 2–145 mg kg−1 in validation set) confirms the good representativeness of the whole group of samples and the accuracy of NIRS calibration models, especially for SOM. Figure 5, Figure 6 and Figure 7 present as an example the maps of the reference and the predicted values of SOM and P2O5 of three of the eleven experimental fields used in this study. All these maps confirm the similarity of the spatial patterns of the reference and predictive data, demonstrating that prediction models were able to successfully identify spatial SOM and P2O5 variability in the same field.
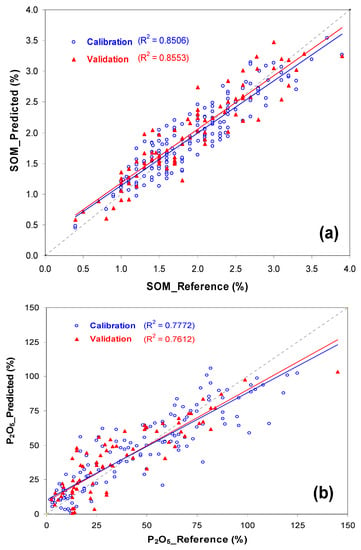
Figure 4.
Plot of reference values versus predicted values for calibration and validation phases: (a) soil organic matter (SOM) and (b) phosphorus (P2O5).
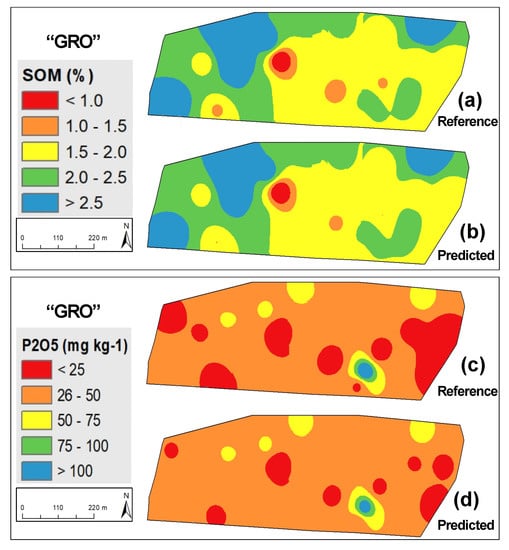
Figure 5.
Maps of reference and predicted parameters in “GRO” experimental field: (a) reference soil organic matter (SOM); (b) predicted SOM; (c) reference phosphorus (P2O5); (d) predicted P2O5.
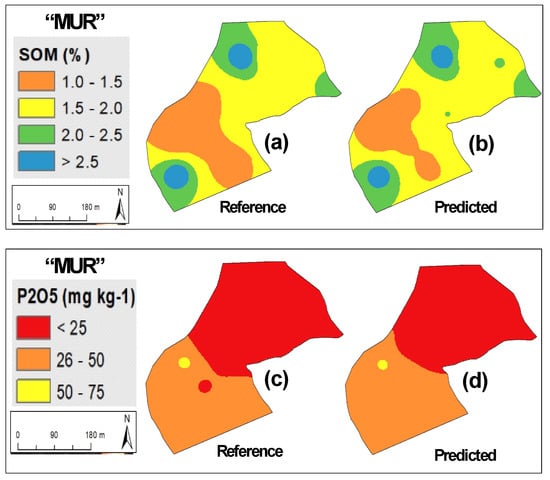
Figure 6.
Maps of reference and predicted parameters in “MUR” experimental field: (a) reference soil organic matter (SOM); (b) predicted SOM; (c) reference phosphorus (P2O5); (d) predicted P2O5.
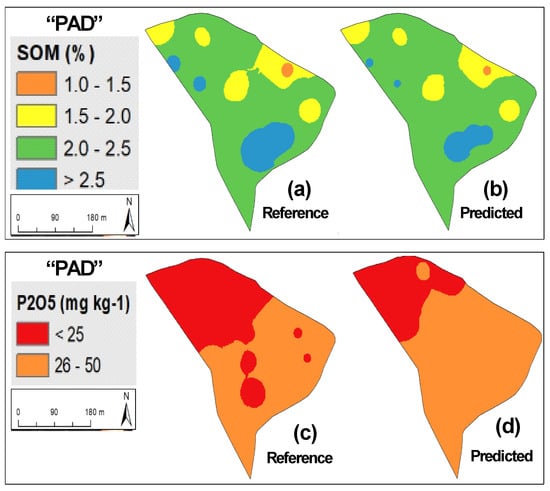
Figure 7.
Maps of reference and predicted parameters in “PAD” experimental field: (a) reference soil organic matter (SOM); (b) predicted SOM; (c) reference phosphorus (P2O5); (d) predicted P2O5.
4. Discussion
It has been estimated that in the coming decades the world will need 70–100% more food to feed an estimated nine billion people [11,22]. Therefore, pressure on land productivity—and thus on the environment—will continue to rise. Thus, it is crucial to increase our knowledge of how different production practices influence crop yields and the environment [22]. Improving agricultural management based on the understanding of soil processes is a first step in increasing the potential for SOM storage, improving site-specific carbon sequestration, and ensuring the sustainability of ecosystems [23,24].
In pastures, where a crop is produced within an agroforestry system, SOM is the largest reservoir of soil organic carbon (SOC), containing about 90% of the total SOC [24,25], whereby carefully designed pasture management strategies can change SOM dynamics and promote storing SOC content, reducing atmospheric CO2 levels [24], limiting the net flux of greenhouse gases towards the atmosphere, and thus contributing to climate change mitigation [3,23,26]. Small changes in the SOC pool can have a serious impact on climate and soil properties [3]. Thus, modelling SOM and other soil parameters such as phosphorus is invaluable for assessing the effect of physicochemical and biological factors on soil dynamics under various changes in land use and management practices [23].
The results of this study showed significant correlation between NIRS calibration models and reference methods for quantifying SOM and P2O5. The R2 (0.85 to SOM and 0.76 to P2O5) indicated the potential of NIRS to estimate SOM and P2O5. According to Viscara Rossel et al. [27], the value of RPD obtained for SOM (2.7) and for P2O5 (2.2) classify these models as adequate for screening purposes, allowing the definition of homogeneous management zones in precision agriculture projects, as is the case.
Several studies have shown the interest of NIRS to estimate SOM [4,7,9]. Kuang and Mouazen [7], for example, obtained and R2 between 0.85–0.93 and RPD between 2.61–3.96 for SOM. Jiang et al. [9] justified this potential by the direct spectral response of the overtones and combinations of C-H, C=O, or O-H. In general, calibration of SOM is more likely to succeed because the spectrally active soil constituents have well-known spectral features in the NIR region [11]. According to Shen et al. [4] in the NIR region, three absorbance peaks (as identified in our study; Figure 3) are common mainly caused by the absorbance of the O-H bonds existing in the soil. Additionally, Zizala et al. [28] report that spectral absorption regions that can be used to quantify SOC (and, indirectly, SOM) are located mainly between 6250 and 5250 cm−1 (between 1600 and 1900 nm) and between 4750 and 4150 cm−1 (between 2100 and 2400 nm). Molin and Tavares [11] registered important absorption features in the soil spectra between 11,900 and 10,600 cm−1 (between 840 and 940 nm), related to the presence of Fe oxides (e.g., hematite and goethite), between 7000 and 5250 cm−1 (between 1400 and 1900 nm) due to the presence of water and O-H functionalities, between 4535 and 4495 cm−1 (between 2205 and 2225 nm) related to the presence of kainite, and at 4415 cm−1 (2265 nm) in the presence of gibbsite.
The correlations of soil phosphorus with NIR spectra are generally weaker than those of SOM with NIR [11], which agrees with the results obtained in our study (R2 of 0.85 for SOM and R2 of 0.76 for phosphorus). Phosphates are hardly detected by infrared radiation due to the low dipole moment between phosphorous and oxygen, which inhibits the direct detection of orthophosphate [29]. Notwithstanding, several works have presented interesting results in the validation of soil phosphorus (P) content based on the NIRS approach, as are the cases of Hermansen et al. [19], Bogrekci and Lee [30], Maleki et al. [31], Mouazen and Kuang [32], and Kawamura et al. [33], with R2 of, respectively, 0.79–0.84, 0.78–0.99, 0.73–0.75, 0.75, and 0.80, similar to those obtained in this study (0.76). Additionally, the RPD in our study (2.2) fits within the range of values obtained in these works (between 1.8 and 2.7). It is widely referred to in the literature that P can be indirectly detected by NIR spectroscopy if organically bound [34,35,36], which we believe is the reason for P detection in these soils. According to Morón and Cozzolino [35], the success of NIR spectroscopy in the calibration of P fractions in the soil is dependent on the selected reference method. Phosphorous obtained by Egner–Riehm reference method, used in this work, is more correlated with P fraction bound to soil organic matter [37] and, consequently, SOM is usually punted as an indirect surrogate marker of a fraction of total P.
Figure 3 showed that peaks of absorbance have been verified in the same spectral bands of SOM and phosphorus, demonstrating that the most active spectral region is 4000–5500 cm−1 (2450–1800 nm), where the water absorption band (5100 cm−1 or 1950 nm) and clay minerals, C-H + C-H, C-H + C-C, OH + minerals, and N-H combinations (4350 cm−1 or 2300 nm) stand out [7]. These results seem to indicate that SOM and soil phosphorus are connected in agro-silvo-pastoral systems, such as the Montado, which underlines the need for further investigation of soils of this important ecosystem. This aspect gains particular importance because, on the one hand, the decrease of organic matter in agricultural soils is generally considered a major threat to sustainability [4], and, on the other hand, soil phosphorus is a crucial nutrient for pasture yield [5,7].
Based on the reference indications of Sims et al. [38] that identified four soil P2O5 categories for plant growth (low: 0–25 mg kg−1; medium: 26–50 mg kg−1; optimum: 51–100 mg kg−1; and excessive: >100 mg kg−1) and the mean values of soil P2O5 in the eleven experimental fields (Table 2), it is clear that six present low values (<25 mg kg−1) and all require phosphorus application. About 15 years ago, some pioneering works (for example, Bogrekci and Lee [30] or Maleki et al. [31]) sought to integrate this spectral analysis technique (NIRS) to predict soil phosphorus content as a preliminary step and provide the necessary information for the development of an automatic variable rate fertilizer applicator. This approach integrates the concept of PA, replacing the traditional fertilizer application at a single rate throughout the entire field by “the right amount at the right place” [39]. The results of our study confirm the potential of NIRS for this purpose (Figure 5, Figure 6, and Figure 7c,d), for example, indicating the need for application of P2O5 throughout the entire area. Simultaneously, Figure 6 and Figure 7 show clearly the existence of two zones of differentiated phosphorus application, respectively, in “MUR” and “PAD” experimental fields: greater quantities in areas with low concentrations (below 25 mg kg−1 [38]) and smaller quantities in areas with medium concentrations (between 25 and 50 mg kg−1 [38]) to achieve the objective of having 80–100 mg kg−1 of phosphorus in the topsoil [5].
Nevertheless, some challenges remain, because it requires technicians to travel to the field to collect soil samples and some preliminary processing. To overcome these limitations, some works are currently focused on the use of satellite hyperspectral data for accurate soil property prediction over large areas [4,28]. The continuous spatial and temporal monitoring of the SOM, and of other soil characteristics or soil cover and crop development provided by remote sensing, becomes extremely important, not only from the environmental perspective, but also in economic terms to ensure, in general, that the beneficiaries of Common Agricultural Policy (CAP) respect their cross-compliance obligations [40], and, particularly in the Montado ecosystem, it can facilitate decision making by the farm manager in dynamic management of animal grazing and differential fertilizer application [4].
5. Conclusions
Soil organic matter (SOM) and phosphorus (P2O5) are two indicators of the evolution of soil fertility in the Montado Mediterranean ecosystem. The results of this study showed significant correlation between NIRS calibration models and reference methods to quantify these soil parameters (R2, 0.85 for SOM and 0.76 for P2O5; RPD, 2.7 for SOM and 2.2 for P2O5), which can mean an important simplification of the time and costs involved, better suited to the needs of the current agricultural production management. To go further, in a perspective of PA, two research topics require further attention: (i) improvement of portable spectrometers in order to achieve a more accurate response in direct field measurements and (ii) further exploration of soil spectral information obtained through satellites (remote sensing). Future research along any of these lines will involve more advanced technologies, such as the use of methods drawn from the field of artificial intelligence.
Author Contributions
J.S. (≈30%): conceptualization, formal analysis, funding acquisition, investigation, methodology, supervision, and writing. S.S. (15%): conceptualization, review, and editing. J.M.d.S. (≈5%): conceptualization and formal analysis. L.P. (5%): methodology. M.d.C. (5%): investigation and supervision. F.M. (≈5%): investigation. J.N.-B. (5%): formal analysis. R.F.M.T. (5%): conceptualization and review. T.D. (5%): conceptualization and review. M.J. (5%): conceptualization. A.E.R. (≈15%): conceptualization, formal analysis, and methodology. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by National Funds through FCT (Foundation for Science and Technology; Projects UIDB/05183/2020 and CEECIND/00365/2018), by the projects PDR2020−101-030693 and PDR2020−101-031244 (“Programa 1.0.1-Grupos Operacionais”) and ERDF (European Regional Development Fund) and National funds through the Programa Operacional Regional ALENTEJO 2020 [ALT20-03-0246-FEDER-000064].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to express their sincere appreciation to the farmers who participated in this work and provided access to their farms (“Azinhal”, “Cubillos”, “Grous”, “Mitra”, “Murteiras”, “Padres”, “Quinta da França”, and “Tapada dos Números” farms).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Seddaiu, G.; Porcua, G.; Luigi, L.; Roggero, P.P.; Agnelli, A.; Cortic, G. Soil organic matter content and composition as influenced by soil management in a semi-arid Mediterranean agro-silvo-pastoral system. Agric. Ecosyst. Environ. 2013, 167, 1–11. [Google Scholar] [CrossRef]
- Serrano, J.M.; Shahidian, S.; da Silva, J.M.; Carvalho, M. Monitoring of soil organic carbon over 10 years in a Mediterranean silvo-pastoral system: Potential evaluation for differential management. Prec. Agric. 2016, 17, 274–295. [Google Scholar] [CrossRef]
- Pouladia, N.; Møllera, A.B.; Tabatabai, S.; Greve, M.H. Mapping soil organic matter contents at field level with Cubist, Random Forest and kriging. Geoderma 2006, 131, 59–75. [Google Scholar] [CrossRef]
- Shen, L.; Gao, M.; Yan, J.; Li, Z.-L.; Leng, P.; Yang, Q.; Duan, S.B. Hyperspectral estimation of soil organic matter content using different spectral preprocessing techniques and PLSR method. Remote Sens. 2020, 12, 1206. [Google Scholar] [CrossRef]
- Efe Serrano, J. Pastures in Alentejo: Technical Basis for Characterization, Grazing and Improvement; Universidade de Évora—ICAM, Ed.; Gráfica Eborense: Évora, Portugal, 2006; pp. 165–178. [Google Scholar]
- Serrano, J.M.; Peça, J.O.; da Silva, J.M.; Shahidian, S.; Carvalho, M. Phosphorus dynamics in permanent pastures: Differential fertilizing and the animal effect. Nutr. Cycl. Agroecosyst. 2011, 90, 63–74. [Google Scholar] [CrossRef]
- Kuang, B.; Mouazen, A.M. Calibration of visible and near infrared spectroscopy for soil analysis at the field scale on three European farms. Eur. J. Soil Sci. 2011, 62, 629–636. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Cecato, U.; Fukumoto, N.M.; Galbeiro, S.; Santos, G.T.; Barbero, L.M. Concentrations and amounts of macronutrients in the animal dung grazing Panicum maximum fertilized with phosphorous. R. Bras. Zoot. 2008, 37, 990–997. [Google Scholar] [CrossRef][Green Version]
- Jiang, Q.; Chen, Y.; Guo, L.; Fei, T.; Qi, K. Estimating soil organic carbon of Cropland soil at different levels of soil moisture using VIS-NIR spectroscopy. Remote Sens. 2016, 8, 755. [Google Scholar] [CrossRef]
- Egner, H.; Riehm, H.; Domingo, W.R. Utersuchungeniiber die chemische Bodenanalyse als Grudlagefir die Beurteilung des Nahrstof-zunstandes der Boden. II. K. Lantbr. Ann. 1960, 20, 199–216. [Google Scholar]
- Molin, J.P.; Tavares, T.R. Sensor systems for mapping soil fertility attributes: Challenges, advances, and perspectives in Brazilian tropical soils. Eng. Agric. 2019, 39, 126–147. [Google Scholar] [CrossRef]
- Nawar, S.; Munnaf, M.A.; Mouazen, A.M. Machine learning based on-line prediction of soil organic carbon after removal of soil moisture effect. Remote Sens. 2020, 12, 1308. [Google Scholar] [CrossRef]
- FAO. World Reference Base for Soil Resources; World Soil Resources Reports N Æ 103; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006. [Google Scholar]
- Serrano, J.; Shahidian, S.; da Silva, J.M.; Paixão, L.; Carreira, E.; Pereira, A.; Carvalho, M. Climate changes challenges to the management of Mediterranean Montado ecosystem: Perspectives for use of precision agriculture technologies. Agronomy 2020, 10, 218. [Google Scholar] [CrossRef]
- Milinovic, J.; Garcia, R.; Rato, A.E.; Cabrita, M.J. Rapid assessment of monovarietal portuguese Extra Virgin Olive Oil’s (EVOO’s) fatty acids by Fourier-Transform Near-Infrared Spectroscopy (FT-NIRS). Eur. J. Lipid Sci. Technol. 2020, 121, 1800392. [Google Scholar] [CrossRef]
- Wold, S.; Sjostrom, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemometr. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.L.; Nieuwoudt, H.; Olivieri, A.; Aleixandre, J.L.; Toit, W. Phenolic profiling of grapes, fermenting samples and wines using UV-Visible spectroscopy with chemometrics. Food Control. 2018, 85, 11–22. [Google Scholar] [CrossRef]
- Donis-González, I.R.; Valero, C.; Momin, M.A.; Kaur, A.; Slaughter, D.C. Performance evaluation of two commercially available portable spectrometers to non-invasively determine table grape and peach quality attributes. Agronomy 2020, 10, 148. [Google Scholar] [CrossRef]
- Hermansen, C.; Norgaard, T.; Jonge, L.W.; Moldrup, P.; Müller, K.; Knadel, M. Predicting glyphosate sorption across New Zealand pastoral soils using basic soil properties or Vis–NIR spectroscopy. Geoderma 2020, 360, 114009. [Google Scholar] [CrossRef]
- Carvalho, M.; Goss, M.J.; Teixeira, D. Manganese toxicity in Portuguese Cambisols derived from granitic rocks: Causes, limitations of soil analyses and possible solutions. Rev. Cienc. Agrárias 2015, 38, 518–527. [Google Scholar] [CrossRef]
- Serrano, J.; Shahidian, S.; da Silva, J.M.; Moral, F.; Carvajal-Ramirez, F.; Carreira, E.; Pereira, A.; Carvalho, M. Evaluation of the effect of dolomitic lime application on pastures—Case study in the Montado Mediterranean ecosystem. Sustainability 2020, 12, 3758. [Google Scholar] [CrossRef]
- Chen, Y.; Camps-Arbestain, M.; Shen, Q.; Singh, B.; Cayuela, M.L. The long-term role of organic amendments in building soil nutrient fertility: A meta-analysis and review. Nutr. Cycl. Agroecosyst. 2018, 111, 103–125. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R. Soil organic carbon sequestration in agroforestry systems. A review. Agron. Sustain. Dev. 2014, 34, 443–454. [Google Scholar] [CrossRef]
- Ordoñez, M.-C.; Olaya, J.F.C.; Galicia, L.; Figueroa, A. Soil Carbon Dynamics under Pastures in Andean Socio-Ecosystems of Colombia. Agronomy 2020, 10, 507. [Google Scholar] [CrossRef]
- Teixeira, R.F.M.; Domingos, T.; Costa, A.P.S.V.; Oliveira, R.; Farropas, L.; Calouro, F.; Carneiro, J.P.B.G. Soil organic matter dynamics in Portuguese natural and sown rainfed grasslands. Ecol. Modell. 2011, 222, 993–1001. [Google Scholar] [CrossRef]
- Vaudour, E.; Gomez, C.; Loiseau, T.; Baghdadi, N.; Loubet, B.; Arrouays, D.; Ali, L.; Lagacherie, P. The Impact of Acquisition Date on the Prediction Performance of Topsoil Organic Carbon from Sentinel-2 for Croplands. Remote Sens. 2019, 11, 2143. [Google Scholar] [CrossRef]
- Rossel, R.A.V.; Walvoort, D.J.J.; McBratney, A.B.; Janik, L.J.; Skjemstad, J.O. Visible, near infrared, mid infrared or combined diffuse reflectance spectroscopy for simultaneous assessment of various soil properties. Geoderma 2006, 131, 59–75. [Google Scholar] [CrossRef]
- Zizala, D.; Minank, R.; Zádorová, T. Soil organic carbon mapping using multispectral remote sensing data: Prediction ability of data with different spatial and spectral resolutions. Remote Sens. 2019, 11, 2947. [Google Scholar] [CrossRef]
- Malley, D.F.; Martin, P.D.; Ben-Dor, E. Application in analysis of soils. In Near-Infrared Spectroscopy in Agriculture; Agronomy Series; Roberts, C.A., Workmann, J.J., Reeves, J.B., III, Eds.; American Society of Agronomy; Crop Science Society of America Inc: Madison, WI, USA, 2004; Volume 44, pp. 729–784. [Google Scholar]
- Bogrekci, I.; Lee, W.S. Spectral phosphorus mapping using diffuse reflectance of soils and grass. Biosyst. Eng. 2005, 91, 305–312. [Google Scholar] [CrossRef]
- Maleki, M.R.; Holm, L.V.; Ramon, H.; Merckx, R.; De Baerdemaeker, J.; Mouazen, A.M. Phosphorus sensing for fresh soils using visible and near infrared spectroscopy. Biosyst. Eng. 2006, 95, 425–436. [Google Scholar] [CrossRef]
- Mouazen, A.M.; Kuang, B. On-line visible and near infrared spectroscopy for in-field phosphorous management. Soil Till. Res. 2016, 155, 471–477. [Google Scholar] [CrossRef]
- Kawamura, K.; Tsujimoto, Y.; Nishigaki, T.; Andriamananjara, A.; Rabenarivo, M.; Asai, H.; Razafimbelo, T. Laboratory visible and near-infrared spectroscopy with genetic algorithm-based partial least squares regression for assessing the soil phosphorus content of upland and lowland rice fields in Madagascar. Remote Sens. 2019, 11, 506. [Google Scholar] [CrossRef]
- Patzold, S.; Leenen, M.; Frizen, P.; Heggeman, T.; Wagner, P.; Rodionov, A. Predicting plant available phosphorus using infrared spectroscopy with consideration for future mobile sensing applications in precision farming. Prec. Agric. 2020, 21, 737–761. [Google Scholar] [CrossRef]
- Morón, A.; Cozzolino, D. Measurement of phosphorus in soils by near infrared reflectance spectroscopy: Effect of reference method on calibration. Commun. Soil Sci. Plant. Anal. 2007, 38, 1965–1974. [Google Scholar] [CrossRef]
- Niederberger, J.; Todt, B.; Boča, A.; Nitschke, R.; Kohler, M.; Kühn, P.; Bauhus, J. Use of near-infrared spectroscopy to assess phosphorus fractions of different plant availability in forest soils. Biogeosciences 2015, 12, 3415–3428. [Google Scholar] [CrossRef]
- Auxtero, E.; Madeira, M. Phosphorus desorbability in soils with andic properties from the Azores, Portugal. R. Ciênc. Agrár. 2009, 32, 423–433. [Google Scholar]
- Sims, J.T.; Leytem, A.B.; Gartley, K.L. Interpreting Soil Phosphorus Tests; Department of Plant and Soil Sciences, College of Agriculture and Natural Resources, University of Delaware: Newark, DE, USA, 2002; p. 5. [Google Scholar]
- Mallarino, A.P.; Wittry, D.J. Efficacy of grid and zone soil sampling approaches for site-specific assessment of phosphorus, potassium, pH, and organic matter. Prec. Agric. 2004, 5, 131–144. [Google Scholar] [CrossRef]
- Castaldi, F.; Chabrillat, S.; Don, A.; Van Wesemael, B. Soil organic carbon mapping using LUCAS topsoil database and Sentinel-2 data: An approach to reduce soil moisture and crop residue effects. Remote Sens. 2019, 11, 2121. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).