Isolation and Characterization of Phosphate Solubilizing Microbes from Rock Phosphate Mines and Their Potential Effect for Sustainable Agriculture
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Microbial Isolation
2.2. Characterization of Isolated Microbial Strains
2.3. Effect of Phosphate Solubilizing Microbes on Maize Growth (Pot Experiment)
2.4. Statistical Analysis
3. Results
3.1. Qualitative Measurement of Phosphate Solubilization
3.2. Quantitative Measurement of Phosphate Solubilization
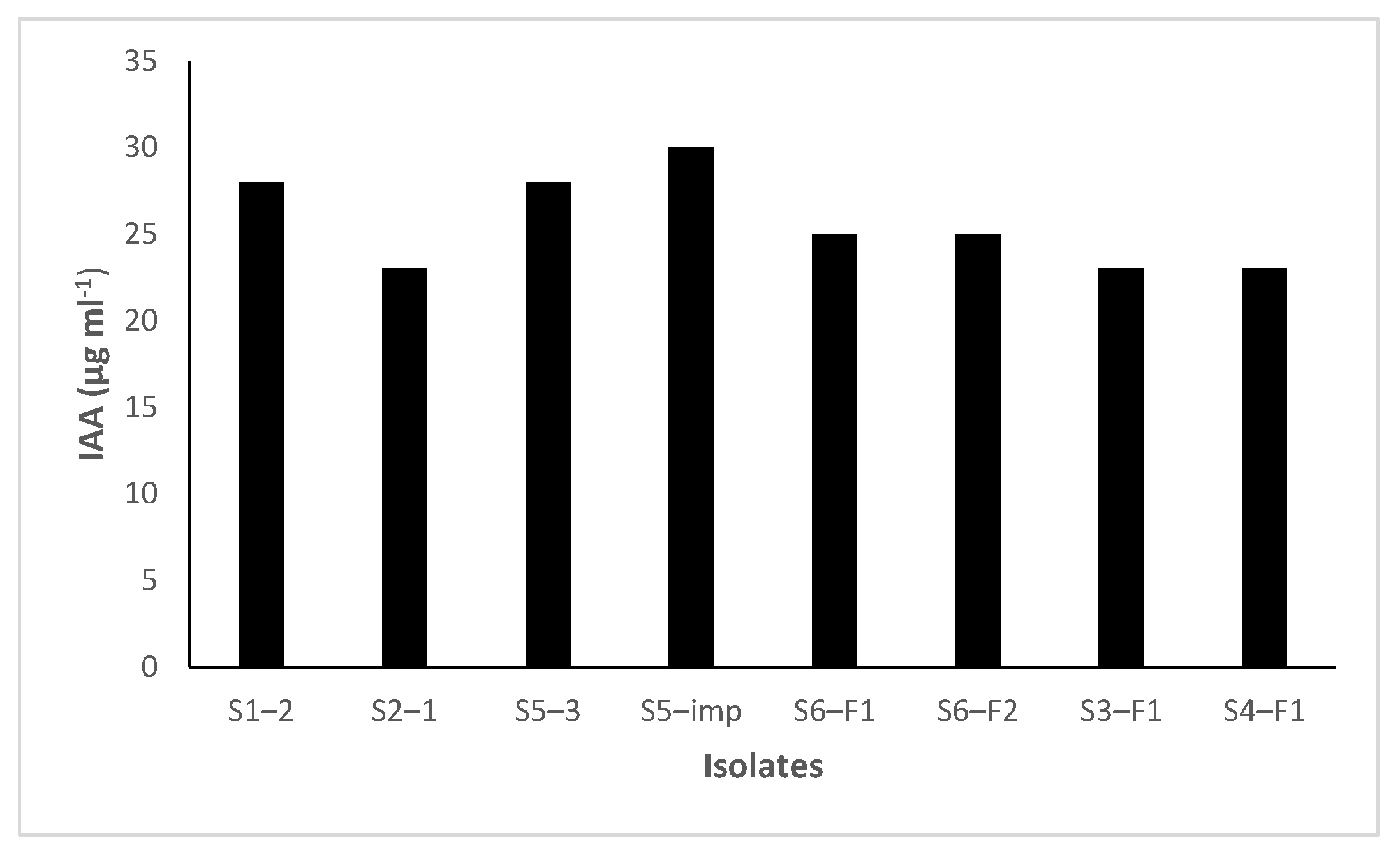
3.3. IAA Production and ACC Deaminase Activity
3.4. Gene Sequencing of Bacterial and Fungal Isolates
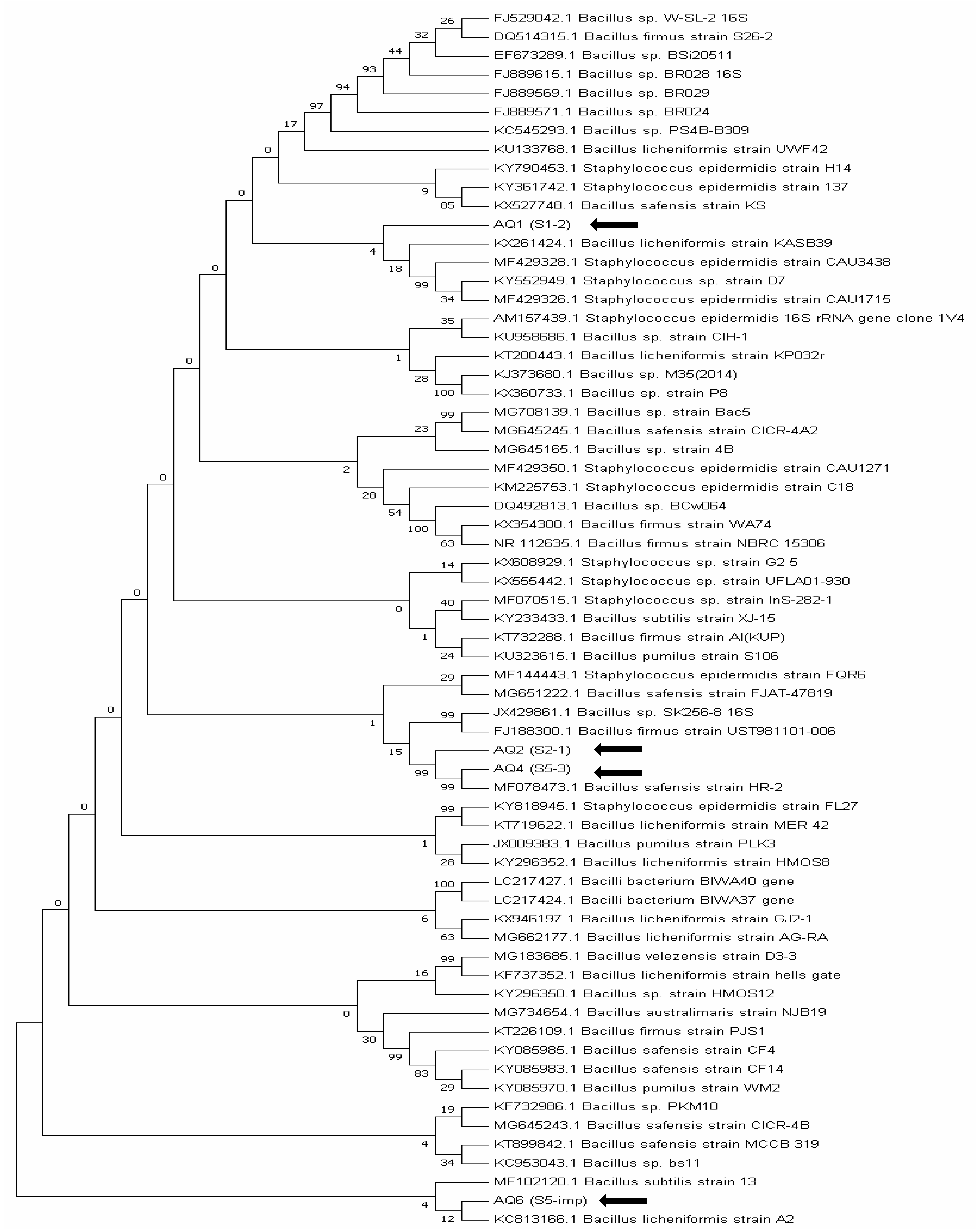
3.4.1. 16S rRNA Gene Sequencing of Bacterial Isolates
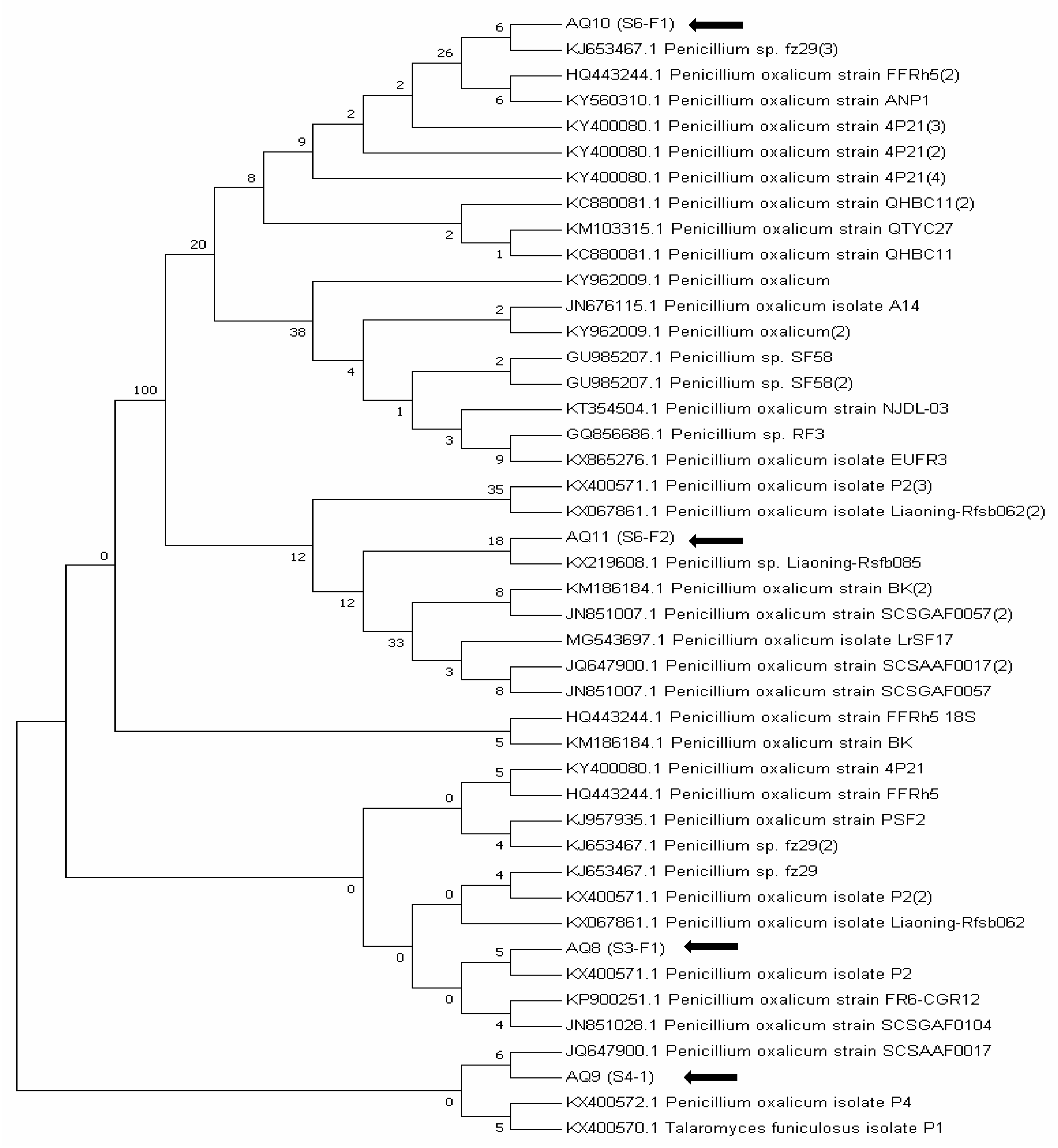
3.4.2. ITS Gene Sequencing of Fungal Isolates
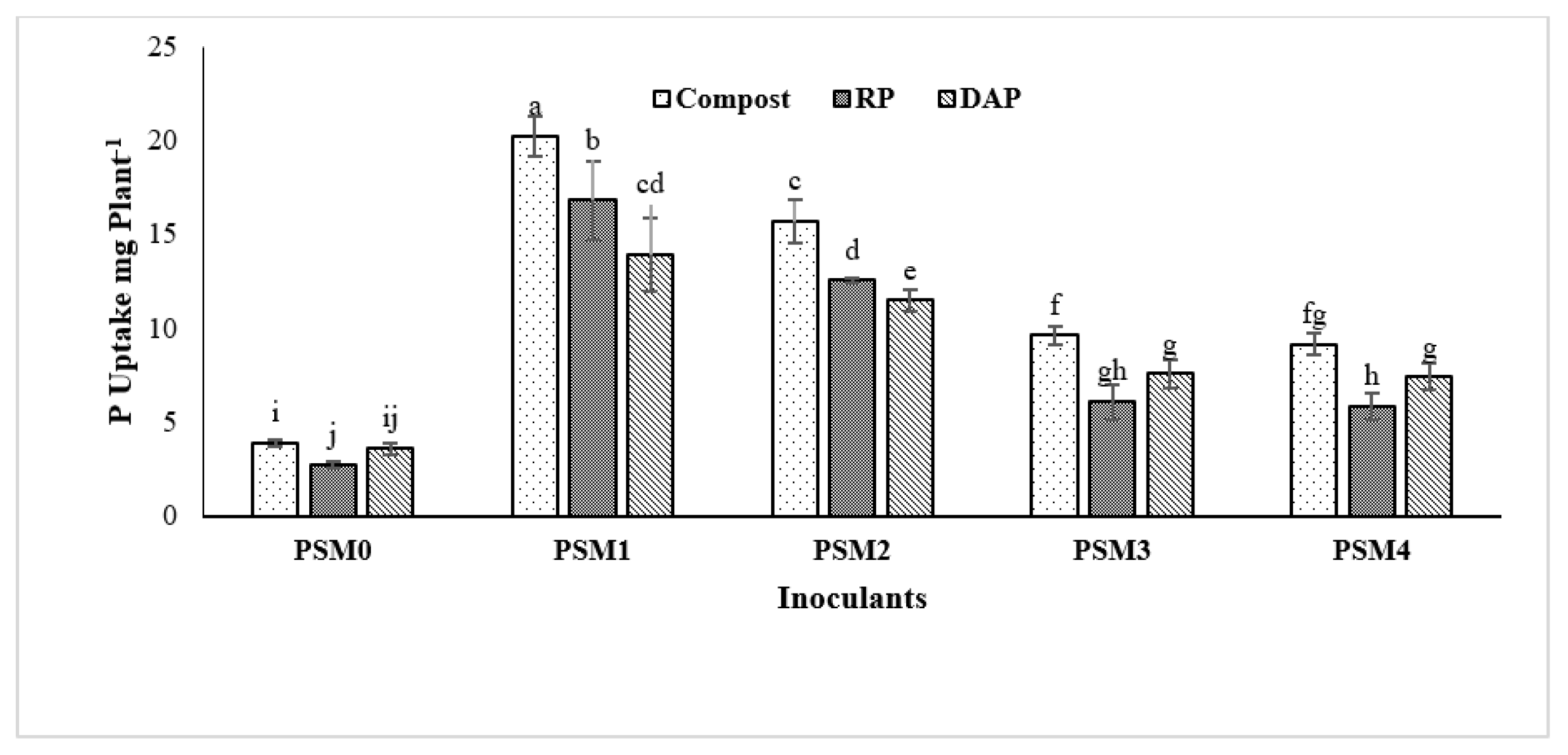
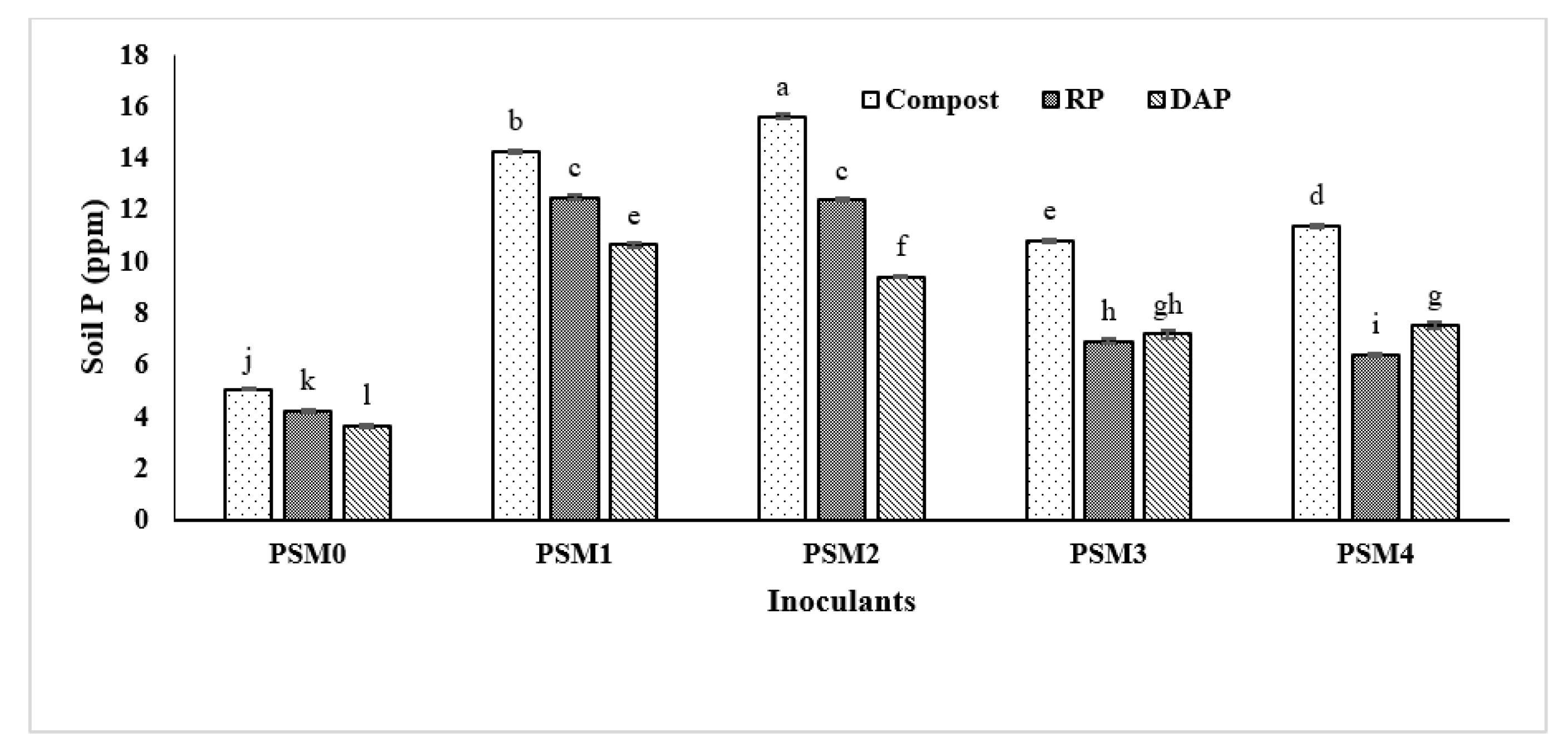
3.5. Effect of Phosphate Solubilizing Microbes on Maize Growth
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, C.; Wei, X.; Sun, H.L.; Wang, Z.Q. Phosphate availability alters lateral root anatomy and root architecture of Fraxinus mandshuricaRupr. Seedlings. J. Integr. Plant Biol. 2005, 7, 292–301. [Google Scholar] [CrossRef]
- Havlin, J.L.; Beaton, J.D.; Tisdale, S.L.; Nelson, W.L. Soil Fertility and Fertilizers: An Introduction to Nutrient Management, 7th ed.; Prentice-Hall: Upper Saddle River, NJ, USA, 2005. [Google Scholar]
- Wei, Y.; Wei, Z.; Cao, Z.; Zhao, Y.; Zhao, X.; Lu, Q.; Wang, X.; Zhang, X. A regulating method for the distribution of phosphorus fractions based on environmental parameters related to the key phosphate-solubilizing bacteria during composting. Biores. Technol. 2016, 211, 610–617. [Google Scholar] [CrossRef]
- Owen, D.; Williams, A.P.; Griffith, G.W.; Withers, P.J. Use of commercial bio-inoculants to increase agricultural production through improved phosphrous acquisition. Appl. Soil Ecol. 2015, 86, 41–54. [Google Scholar] [CrossRef]
- Mustafa, A.; Naveed, M.; Saeed, Q.; Ashraf, M.N.; Hussain, A.; Abbas, T.; Kamran, M.; Minggang, X. Application Potentials of Plant Growth Promoting Rhizobacteria and Fungi as an Alternative to Conventional Weed Control Methods. In Sustainable Crop Production; IntechOpen: London, UK, 2019. [Google Scholar]
- Khan, M.S.; Zaidi, A.; Ahmad, E. Mechanism of Phosphate Solubilization and Physiological Functions of Phosphate-Solubilizing Microorganisms. In Phosphate Solubilizing Microorganisms; Springer: Cham, Switzerland, 2014; pp. 31–62. [Google Scholar]
- Onyia, C.E.; Anyawu, C.U.; Ikegbunam, M.N. Ability of fungi, isolated from nsukka peppers and garden-egg plant rhizospheres, to solubilize phosphate and tolerate cadmium. Advanc. Microbiol. 2015, 5, 500. [Google Scholar] [CrossRef]
- Zhu, J.; Li, M.; Whelan, M. Phosphorus activators contribute to legacy phosphorus availability in agricultural soils: A review. Sci. Total Environ. 2018, 15, 522–537. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A. Role of plant growth promoting rhizobacteria and Ag-nano particle in the bioremediation of heavy metals and maize growth under municipal wastewater irrigation. Int. J. Phytorem. 2016, 18, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Roopa, B.; Maya, C.; Makar, H.K. Effect of different PGPR strains along with Rhizobium on nodulation and chickpea productivity. Asian J. Expert Biol. Sci. 2012, 3, 424–426. [Google Scholar]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. Springer Plus 2013, 1, 587. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A. Exopolysaccharide producing rhizobacteria and their impact on growth and drought tolerance of wheat grown under rainfed conditions. PLoS ONE 2019, 14, e0222302. [Google Scholar] [CrossRef] [PubMed]
- Pikovskaya, R.I. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya 1948, 17, 362–370. [Google Scholar]
- Murphy, J.A.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Priyadharsini, P.; Muthukumar, T. The root endophytic fungus Curvularia geniculata from Parthenium hysterophorus roots improves plant growth through phosphate solubilization and phytohormone production. Fungal Ecol. 2017, 27, 69–77. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 7, 1870–1874. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Abdi, H.; Williams, L.J. Principal component analysis. Wiley interdisciplinary reviews. Comput. Stat. 2010, 4, 433–459. [Google Scholar] [CrossRef]
- Paul, D. Research on heavy metal pollution of river Ganga: A review. Ann. Agric. Sci. 2017, 2, 278–286. [Google Scholar] [CrossRef]
- Pande, A.; Pandey, P.; Mehra, S.; Singh, M.; Kaushik, S. Phenotypic and genotypic characterization of phosphate solubilizing bacteria and their efficiency on the growth of maize. J. Genet. Eng. Biotechnol. 2017, 2, 379–391. [Google Scholar] [CrossRef]
- Yin, Z.; Shi, F.; Jiang, H.; Roberts, D.P.; Chen, S.; Fan, B. Phosphate solubilization and promotion of maize growth by Penicillium oxalicum P4 and Aspergillus niger P85 in a calcareous soil. Can. J. Microbiol. 2015, 12, 913–923. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Zandi, P. Effects of exogenously applied plant growth regulators in combination with PGPR on the physiology and root growth of chickpea(Cicer arietinum)and their role in drought tolerance. J. Plant Interact. 2018, 1, 239–247. [Google Scholar] [CrossRef]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 1–15. [Google Scholar] [CrossRef]
- Khamwan, S.; Boonlue, S.; Jogloy, S.; Mongkolthanaruk, W. Characterization of endophytic bacteria and their response to plant growth promotion in Helianthus tuberosus L. Biocatal. Agric. Biotechnol. 2018, 13, 153–159. [Google Scholar] [CrossRef]
- Naveed, M.; Mustafa, A.; Majeed, S.; Naseem, Z.; Saeed, Q.; Khan, A.; Nawaz, A.; Baig, K.S.; Chen, J.T. Enhancing cadmium tolerance and pea plant health through Enterobacter sp. MN17 inoculation together with biochar and gravel sand. Plants 2020, 9, 530. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Curá, J.A. Role of Beneficial Microorganisms and Salicylic Acid in Improving Rainfed Agriculture and Future Food Safety. Microorganism 2020, 8, 1018. [Google Scholar] [CrossRef] [PubMed]
- Ditta, A.; Muhammad, J.; Imtiaz, M.; Mehmood, S.; Qian, Z.; Tu, S. Application of rock phosphate enriched composts increases nodulation, growth and yield of chickpea. Int. J. Recycl. Org. Waste Agric. 2018, 7, 33–40. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, K.P. Enriching vermicompost by nitrogen fixing and phosphate solubilizing bacteria. Bioresour. Technol. 2001, 76, 173–175. [Google Scholar] [CrossRef]
- Mustafa, A.; Naveed, M.; Abbas, T.; Saeed, Q.; Hussain, A.; Ashraf, M.N.; Minggang, X. Growth response of wheat and associated weeds to plant antagonistic rhizobacteria and fungi. Ital. J. Agron. 2019, 14, 191–198. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, S.; Sood, S.; Prasad, R.; Dubey, Y.P. Bioorganic nutrient source effect on growth, biomass, and quality of natural sweetener plant stevia and soil fertility in the Western Himalayas. Commun. Soil Sci. Plant Anal. 2015, 9, 1170–1186. [Google Scholar] [CrossRef]
| Soil Characteristic | Unit | Value |
|---|---|---|
| Soil Textural Class | Silty clay loam | |
| pH | 7.81 | |
| EC | (dSm−1) | 1.21 |
| Organic matter | (%) | 0.56 |
| HCO3 | (%) | 5.79 |
| P(Extractable) | (ppm) | 5.7 |
| K(extractable) | (ppm) | 77 |
| Isolate | Category | Zone Formation | Solubilization Index | IAA Production | ACC Activity | Phosphorus from TCP (ppm) | Phosphorus from Rock Phosphate (ppm) | TCP Media pH 7.0 |
|---|---|---|---|---|---|---|---|---|
| S2–1 | Bacteria | ++ | 1.57 | ++ | ++ | 176.09 | 88.67 | 5.25 |
| S5–4 | Bacteria | ++ | 0.36 | ++ | ++ | 33.67 | - | 3.44 |
| S7–1 | Bacteria | ++ | 0.61 | ++ | ++ | 60.82 | - | 4.4 |
| S1–2 | Bacteria | ++ | 1.86 | ++ | ++ | 273.74 | 156.52 | 4.44 |
| S5–3 | Bacteria | ++ | 1.66 | ++ | ++ | 193.45 | 67.91 | 5.57 |
| S2–2 | Bacteria | ++ | 0.71 | ++ | ++ | 77.41 | - | 5.29 |
| S5–imp | Bacteria | ++ | 1.94 | ++ | ++ | 247.70 | 111.45 | 5.07 |
| S3–F1 | Fungi | ++ | 3 | ++ | - | 387.52 | 693.16 | 2.8 |
| S6–F1 | Fungi | ++ | 4.33 | ++ | - | 394.76 | 542.70 | 2.79 |
| S6–F2 | Fungi | ++ | 4 | ++ | - | 271.78 | 144.84 | 3.93 |
| S4–F1 | Fungi | ++ | 3.5 | ++ | - | 297.10 | 434.19 | 3.36 |
| Microbial Isolate Code | Sequence Length (bp) | Sequence Identity % | Nearest Homologue Sequence | Strain Identified | |
|---|---|---|---|---|---|
| Bacterial Isolate | S1–2 | 1437 | 94 | KY552949 | Staphylococcus sp. |
| S2–1 | 1527 | 99 | FJ188300 | Bacillus firmus | |
| S5–3 | 1546 | 99 | MF078473 | Bacillus safenis | |
| S5–imp | 1215 | 94 | KC813166 | Bacillus licheniformis | |
| Fungal Isolate | S6–F1 | 554 | 99 | KJ653467 | Penicillium sp. |
| S6–F2 | 559 | 99 | KX219608 | Penicillium sp. | |
| S3–F1 | 590 | 99 | KX400571 | Penicillium oxalicum | |
| S4–F1 | 557 | 99 | JQ647900 | Penicillium oxalicum |
| Root Length (cm) | Root Fresh Weight (g) | Root Dry Weight (g) | Dry Biomass (g) | |
|---|---|---|---|---|
| Compost | 34 ± 0.45 i | 9.8 ± 0.23 def | 1.75 ± 0.23 cde | 3.35 ± 0.18 hi |
| Rock Phosphate (RP) | 29 ± 0.43 i | 6.8 ± 0.35 h | 1.28 ± 0.25 e | 2.43 ± 0.17 i |
| DAP | 32 ± 0.32 i | 7.2 ± 0.32 gh | 1.45 ± 0.09 de | 3.19 ± 0.03 hi |
| Penicillium sp. × compost | 73 ± 0.45 a | 19.6 ± 0.30 a | 4.76 ± 0.17 a | 8.90 ± 0.22 a |
| Penicillium sp. × RP | 60 ± 0.52 b | 11 ± 0.04 cde | 2.92 ± 0.09 bc | 6.13 ± 0.39 bcd |
| Penicillium sp. × DAP | 58 ± 1.13 bc | 12 ± 0.39 bcd | 2.83 ± 0.23 bc | 7.36 ± 0.49 bc |
| Penicillium oxalicum × compost | 69 ± 0.45 a | 17.89 ± 0.07 a | 3.56 ± 0.11 ab | 7.61 ± 0.28 ab |
| Penicillium oxalicum × RP | 56 ± 0.68 bcd | 10.4 ± 0.61 def | 2.62 ± 0.12 bcd | 5.1 ± 0.28 cde |
| Penicillium oxalicum × DAP | 59.66 ± 0.68 b | 11.7 ± 1.30 bcd | 2.4 ± 0.11 bcde | 6.24 ± 0.22 cde |
| Staphylococcus sp. × compost | 54 ± 0.68 cde | 14.3 ± 0.19 b | 2.81 ± 0.26 cde | 6.23 ± 0.06 bcd |
| Staphylococcus sp. × RP | 42.6 ± 0.68 gh | 9.2 ± 0.28 efg | 1.71 ± 0.17 cde | 3.90 ± 0.21 gh |
| Staphylococcus sp. × DAP | 50.6 ± 1.57 ef | 8.1 ± 0.10 fgh | 2.14 ± 0.05 cde | 4.67 ± 0.16 fg |
| Bacillus licheniformis × compost | 53 ± 0.89 de | 13.5 ± 0.30 bc | 2.20 ± 0.12 cde | 5.44 ± 0.13 def |
| Bacillus licheniformis × RP | 40.3 ± 1.13 h | 7.9 ±0.49 efg | 1.58 ± 0.09 cde | 3.1 ± 0.20 ghi |
| Bacillus licheniformis × DAP | 46.3 ± 0.68 fg | 8.5 ± 0.22 fgh | 1.85 ± 0.42 cde | 4.78 ± 0.31 fg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qarni, A.; Billah, M.; Hussain, K.; Shah, S.H.; Ahmed, W.; Alam, S.; Sheikh, A.A.; Jafri, L.; Munir, A.; Malik, K.M.; et al. Isolation and Characterization of Phosphate Solubilizing Microbes from Rock Phosphate Mines and Their Potential Effect for Sustainable Agriculture. Sustainability 2021, 13, 2151. https://doi.org/10.3390/su13042151
Qarni A, Billah M, Hussain K, Shah SH, Ahmed W, Alam S, Sheikh AA, Jafri L, Munir A, Malik KM, et al. Isolation and Characterization of Phosphate Solubilizing Microbes from Rock Phosphate Mines and Their Potential Effect for Sustainable Agriculture. Sustainability. 2021; 13(4):2151. https://doi.org/10.3390/su13042151
Chicago/Turabian StyleQarni, Awais, Motsim Billah, Khadim Hussain, Sabir Hussain Shah, Waqas Ahmed, Sadia Alam, Aftab Ahmad Sheikh, Laila Jafri, Asia Munir, Kouser Majeed Malik, and et al. 2021. "Isolation and Characterization of Phosphate Solubilizing Microbes from Rock Phosphate Mines and Their Potential Effect for Sustainable Agriculture" Sustainability 13, no. 4: 2151. https://doi.org/10.3390/su13042151
APA StyleQarni, A., Billah, M., Hussain, K., Shah, S. H., Ahmed, W., Alam, S., Sheikh, A. A., Jafri, L., Munir, A., Malik, K. M., & Khan, N. (2021). Isolation and Characterization of Phosphate Solubilizing Microbes from Rock Phosphate Mines and Their Potential Effect for Sustainable Agriculture. Sustainability, 13(4), 2151. https://doi.org/10.3390/su13042151







