Investigation of Dye Removal Capability of Blast Furnace Slag in Wastewater Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Batch Experiment
Adsorbent Dose
Contact Time
Adsorbate Dose
2.2.2. Kinetic and Isotherm Modelling
2.2.3. Fourier Transform Infrared (FTIR) Spectroscopy
2.2.4. Scanning Electron Microscopy (SEM)
2.2.5. Energy Dispersive Spectroscopy (EDS)
2.2.6. X-ray Diffraction (XRD)
3. Results
3.1. Batch Experiments
3.1.1. Adsorbent Dose
3.1.2. Contact Time
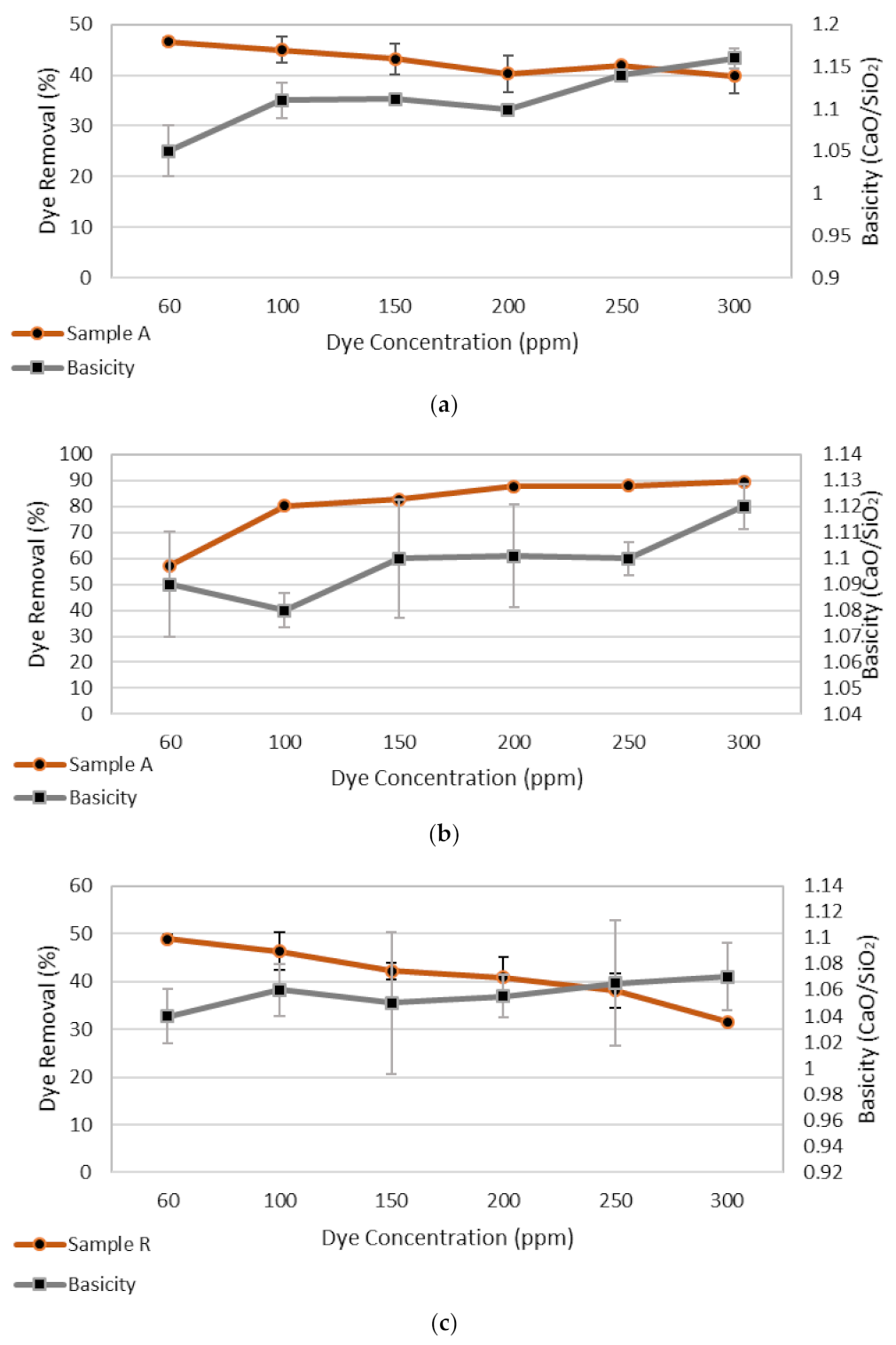
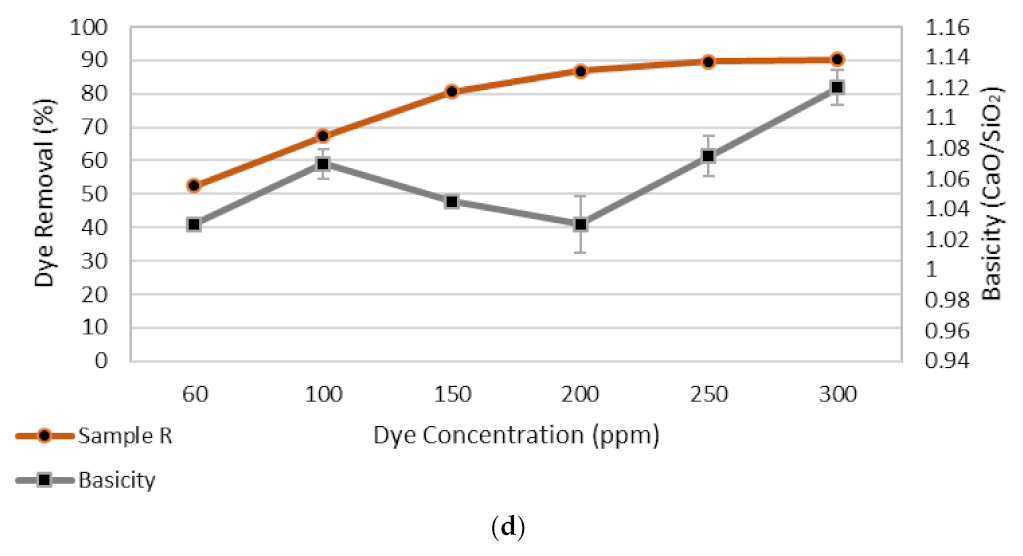
3.1.3. Adsorbate Dose
3.1.4. Kinetic and Isotherm Modelling
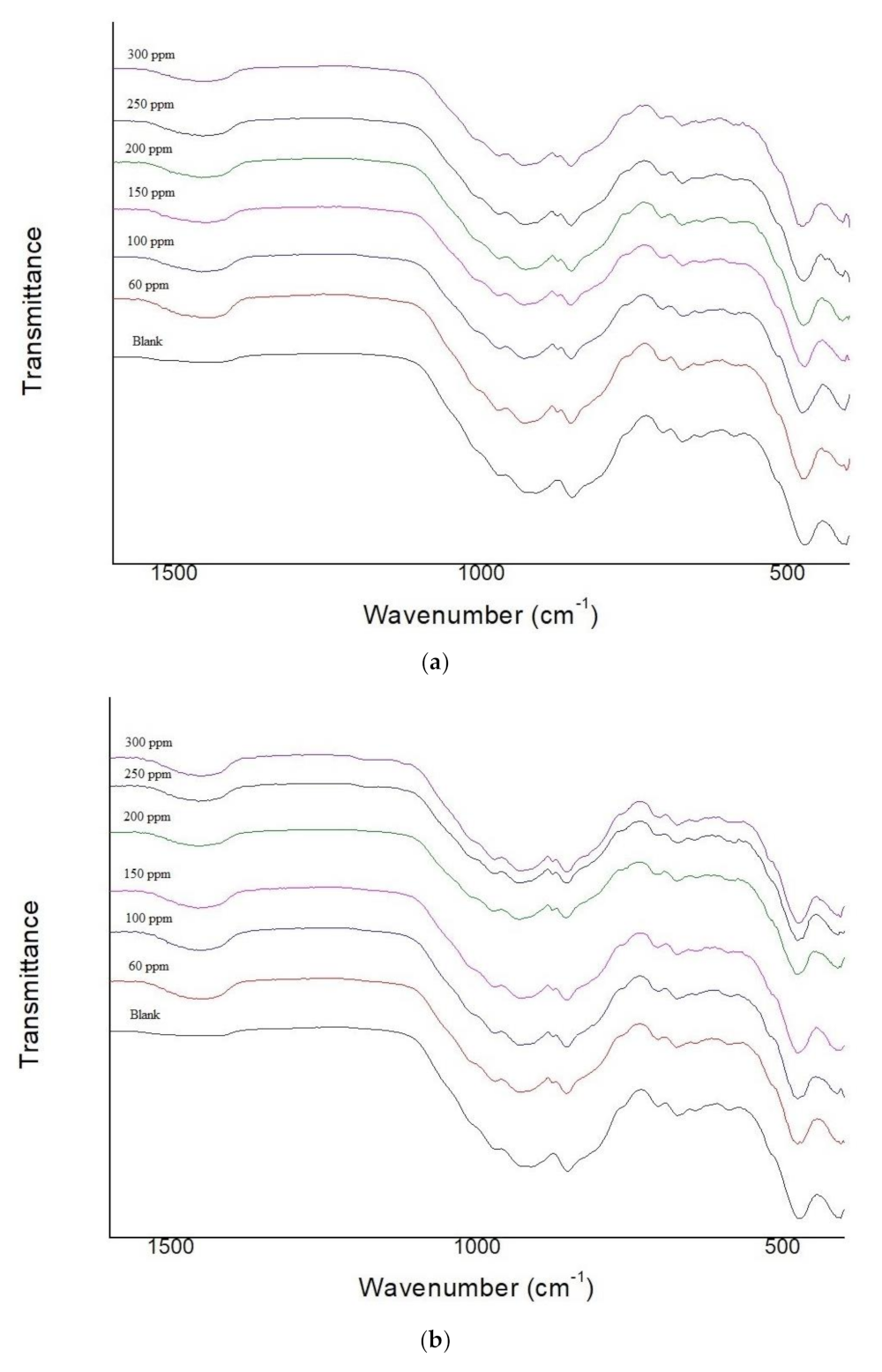
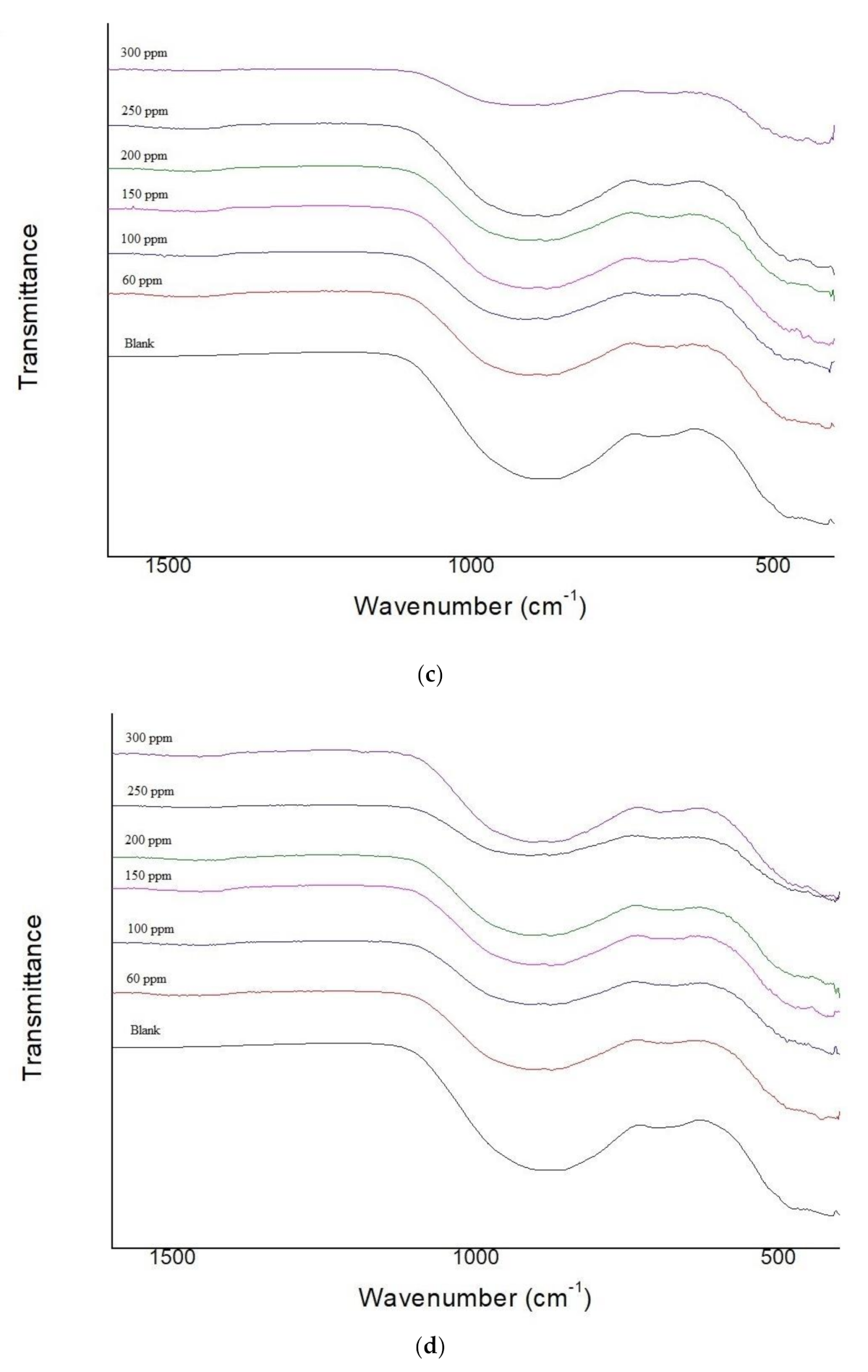
3.2. Fourier Transform Infrared (FTIR) Spectroscopy
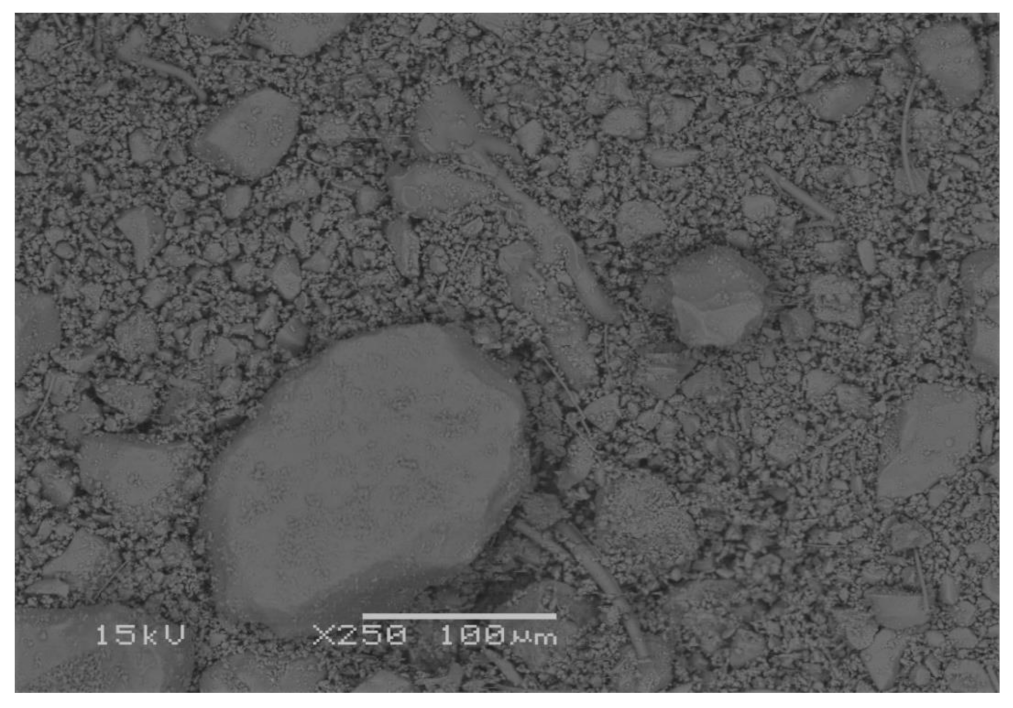
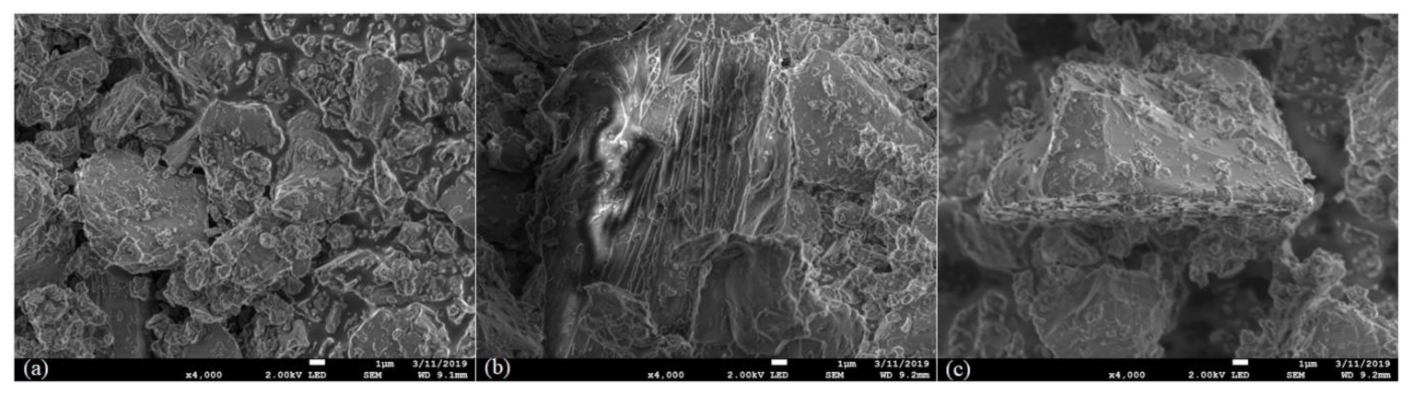
3.3. Scanning Electron Microscopy (SEM)
3.4. Energy Dispersive Spectroscopy (EDS)
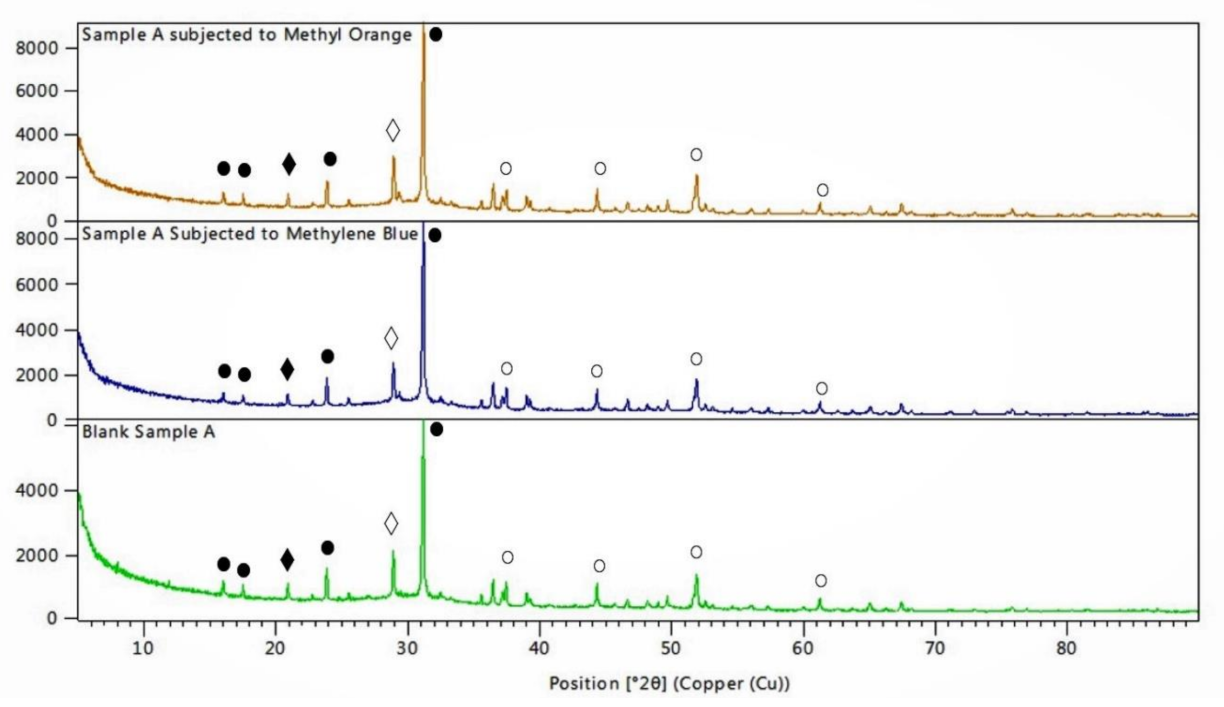
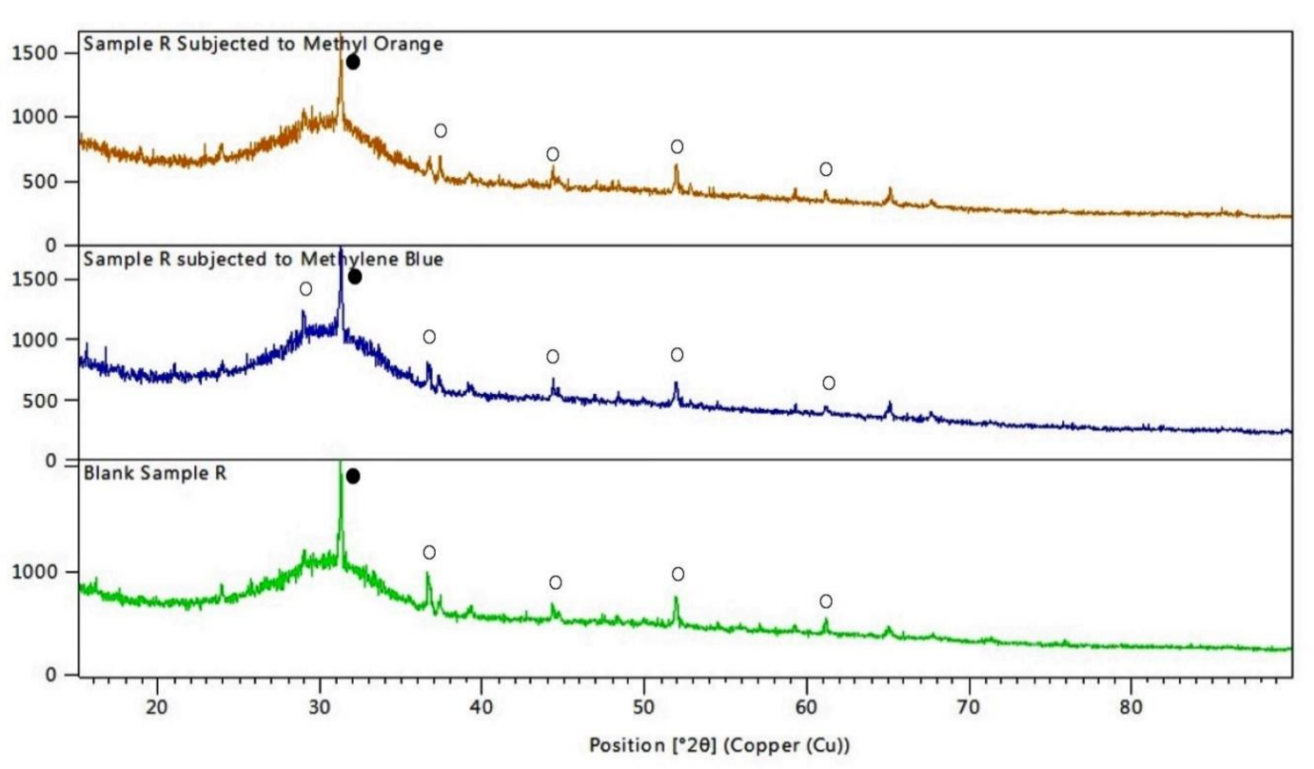
3.5. X-ray Diffraction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jain, A.; Gupta, V.; Bhatnagar, A. Utilisation of industrial waste products as adsorbents for the removal of dyes. J. Hazard. Mater. 2003, 101, 31–42. [Google Scholar] [CrossRef]
- Francis, A. Non-isothermal crystallisation kinetics of a blast furnace slag glass. J. Am. Ceram. Soc. 2005, 88, 1859–1863. [Google Scholar] [CrossRef]
- Taha, G.; Mosaed, T. Blast Furnace Slag of a Ferrosilicon Firm in Aswan Governorate, Upper Egypt, as an Adsorbent for the Removal of Merocyanine Dye from Its Aqueous Solution. Chem. Biodivers. 2010, 7, 878–886. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, G.; An, C.; Li, W.; Chen, L.; Zhang, Z. Transport of Reactive X-3B Dye at the Interface between Cationic Surfactant-Modified Water Quenched Blast Furnace Slag and Aqueous Solution. Can. J. Chem. Eng. 2018, 96, 1240–1249. [Google Scholar] [CrossRef]
- Gao, H.; Song, Z.; Zhang, W.; Yand, X.; Wang, X.; Wang, D. Synthesis of highly effective absorbents with waste quenching blast furnace slag to remove Methyl Orange from aqueous solution. J. Environ. Sci. 2017, S3, 68–77. [Google Scholar] [CrossRef]
- Shi, J.; Kuwahara, Y.; An, T.; Yamashita, H. The fabrication of TiO2 supported on slag-made calcium silicate as low-cost photocatalyst with high adsorption ability for the degradation of dye pollutants in water. Catal. Today 2017, 281, 21–28. [Google Scholar] [CrossRef]
- Yasipourtehrani, S.; Strezov, V.; Bliznyukov, S.; Evans, T. Investigation of Thermal Properties of Blast Furnace Slag to Improve Process Energy Efficiency. J. Clean. Prod. 2017, 149, 137–145. [Google Scholar] [CrossRef]
- Rivera, A.R.; Sanjuán, M.Á.; Martin, D.A. Granulated Blast-Furnace Slag and Coal Fly Ash Ternary Portland Cements Optimization. Sustainability 2020, 12, 5783. [Google Scholar] [CrossRef]
- Rios, J.D.; Vahi, A.; Leiva, C.; Martinez-De La Concha, A.; Cifuentez, H. Analysis of the Utilization of Air-Cooled Blast Furnace Slag as Industrial Waste Aggregates in Self-Compacting Concrete. Sustainability 2019, 11, 1702. [Google Scholar] [CrossRef]
- Yasipourtehrani, S.; Strezov, V.; Evans, T.J. Investigation of Phosphate Removal Capability of Blast Furnace Slag in Wastewater Treatment. Sci. Rep. 2019, 9, 7498. [Google Scholar] [CrossRef]
- Ozturk, Z.; Gultekin, E. Preparation of ceramic wall tiling derived from blast furnace slag. Ceram. Int. 2015, 41, 12020–12026. [Google Scholar] [CrossRef]
- Yagub, M.; Sen, T.; Afroze, S.; Ang, H. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Luo, X.; Liang, C.; Hu, Y. Comparison of Different Enhanced Coagulation Methods for Azo Dye Removal from astewater. Sustainability 2019, 11, 4760. [Google Scholar] [CrossRef]
- Shaban, M.; Abukhadra, M.; Hamd, A. Recycling of glass in synthesis of MCM 48 mesoporous silica as catalyst support for Ni2O3 photocatalyst for Congo red dye removal. Clean Technol. Environ. Policy 2017, 20, 13–28. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Jain, A. A comparative adsorption study with different industrial wastes as adsorbents for the removal of cationic dyes from water. J. Colloid Interface Sci. 2005, 281, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Dhmees, A.; Khaleel, N.; Mahmoud, S. Synthesis of silica nanoparticles from blast furnace slag as cost effective adsorbent for efficient azo-dye removal. Egypt. J. Pet. 2018, 27, 1113–1121. [Google Scholar] [CrossRef]
- Ramakrishna, K.; Viraraghavan, T. Use of Slag for Dye removal. J. Waste Manag. 1997, 17, 483–488. [Google Scholar] [CrossRef]
- Gupta, V.; Ali, I.; Mohan, D. Equilibrium uptake and sorption dynamics for the removal of a basic dye (basic red) using low-cost adsorbents. J. Colloid Interface Sci. 2003, 265, 257–264. [Google Scholar] [CrossRef]
- Yan, L.; Qin, L.; Yu, H.; Li, S.; Shan, R.; Dy, B. Adsorption of acid dyes from aqueous solution by CTMAB modified bentonite: Kinetic and isotherm modeling. J. Mol. Liq. 2015, 211, 1074–1081. [Google Scholar] [CrossRef]
- Mazouz, R.; Filali, N.; Hattab, Z.; Guerfi, K. Valorization of granulated slag of Arcelor-Mittal (Algeria) in cationic dye adsorption from aqueous solution: Column studies. J. Water Reuse Desalin. 2016, 6, 2004–2013. [Google Scholar] [CrossRef]
- Nassar, N.; Marei, N.; Vitale, G.; Arar, L. Adsorptive Removal of Dyes from Synthetic and Real Textile Wastewater Using Magnetic Iron Oxide Nanoparticles: Thermodynamic and Mechanistic Insights. Can. J. Chem. Eng. 2015, 93, 1965–1974. [Google Scholar] [CrossRef]
- Ferrero, F. Dye removal from aqueous solution using coal fly ash for continuous flow adsorption. Clean Technol. Environ. Policy 2015, 17, 1907–1915. [Google Scholar] [CrossRef]
- Shang, S.M. Process Control In Printing Of Textiles. In Process Control In Textile Manufacturing; Majumdar, A., Das, R., Alagirusamy, V., Kothari, K., Eds.; Woodhead Publishing Limited in Association with the Textile Institute: Oxford, MI, USA, 2013; pp. 339–362. [Google Scholar]
- Bhatnagar, A.; Minocha, A.K.; Jeun, B.-H.; Song, H.; Seo, Y.-C. Removal of Anionic Dyes from Water using Citrus limonum (Lemon) Peel: Equilibrium Studies and Kinetic Modeling. Sep. Sci. Technol. 2009, 44, 316–334. [Google Scholar] [CrossRef]
- Wagner, L. Failure Analysis of Integrated Circuits; Springer: New York, NY, USA, 1999; pp. 205–206. [Google Scholar]
- Johansson, L.; Gustafsson, J. Phosphate Removal Using Blast Furnace Slags and Opoka-Mechanism. Water Res. 2000, 34, 259–265. [Google Scholar] [CrossRef]
- Memon, S.; Loa, T.; Barbhuiya, S.; Xu, W. Development of form-stable composite phase change material byincorporation of dodecyl alcohol into ground granulated blast furnace. Energ. Build. 2013, 62, 360–367. [Google Scholar] [CrossRef]
- Fleet, M.; Liu, X.; King, P. Accommodation of the carbonate ion in apatite: An FTIR and X-ray structure study of crystals synthesised at 2–4 GPa. Am. Mineral. 2004, 89, 1422–1432. [Google Scholar] [CrossRef]
- Kuo, Y.; Wang, J.; Wang, C.; Tsai, C. Effect of water quenching and SiO2 addition during vitrification of fly ash Part 1: On the crystalline characteristics of slag. J. Hazard. Mater. 2008, 152, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Gonzalez, R.; Paz, H.; Madrid, A.; Casillas, G.; Hernandez, M.; Perez, M. Catalytic activity of palladium nanocubes/multiwalled carbon nanotubes structures for methyl orange dye removal. Catal. Today 2017, 282, 168–173. [Google Scholar] [CrossRef]
- Genc, A.; Oguz, A. Sorption of acid dyes from aqueous solution by using non-ground ash and slag. Desalination 2010, 264, 78–83. [Google Scholar] [CrossRef]



| Slag (%) | Fe | SiO2 | CaO | MgO | Al2O3 | MnO | TiO2 | Basicity (CaO/SiO2 Ratio) |
|---|---|---|---|---|---|---|---|---|
| Sample A | 0.43 | 35.93 | 40.41 | 8.38 | 13.54 | 0.26 | 0.5 | 1.12 |
| Sample R | 0.39 | 30.78 | 33.83 | 13.34 | 19.01 | 0.84 | 0.97 | 1.09 |
| Adsorbent Dose | |||||
|---|---|---|---|---|---|
| Removal rate of MB (%) | 20 g/L | 40 g/L | 60 g/L | 80 g/L | 100 g/L |
| Sample A | 30.8 | 31.7 | 40.2 | 46.5 | 39.2 |
| Sample R | 29.8 | 32.8 | 39.3 | 48.3 | 41.9 |
| Removal rate of MO (%) | |||||
| Sample A | 23.6 | 43.4 | 49.7 | 57.3 | 56.0 |
| Sample R | 27.9 | 40.7 | 46.6 | 52.9 | 51.4 |
| Methylene Blue | Sample A (%) | Sample R (%) | Methyl Orange | Sample A (%) | Sample R (%) |
|---|---|---|---|---|---|
| 10 min | 35.6 | 45.4 | 10 min | 17.7 | 21.0 |
| 30 min | 32.2 | 44.4 | 30 min | 47.6 | 51.6 |
| 1 h | 47.0 | 48.2 | 1 h | 57.0 | 52.6 |
| 2 h | 44.4 | 42.7 | 2 h | 55.6 | 51.0 |
| Methylene Blue | Methyl Orange | |||||||
|---|---|---|---|---|---|---|---|---|
| Dye Concentration (mg/L) | 100 | 150 | 200 | 250 | 100 | 150 | 200 | 250 |
| pH Before Adsorption | 8.8 | 8.9 | 8.9 | 9 | 3.3 | 3.3 | 3.1 | 3.0 |
| pH After Adsorption | 10.3 | 10.2 | 10 | 10.1 | 5.4 | 5.3 | 5.5 | 5.9 |
| Methylene Blue | Methyl Orange | |||
|---|---|---|---|---|
| Sample A | Sample R | Sample A | Sample R | |
| qe (mg/g) | 0.354 | 0.361 | 0.496 | 0.422 |
| ks (g/mg min) | 0.449 | 0.480 | 0.113 | 0.263 |
| R2 | 0.985 | 0.998 | 0.969 | 0.981 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasipourtehrani, S.; Strezov, V.; Kan, T.; Evans, T. Investigation of Dye Removal Capability of Blast Furnace Slag in Wastewater Treatment. Sustainability 2021, 13, 1970. https://doi.org/10.3390/su13041970
Yasipourtehrani S, Strezov V, Kan T, Evans T. Investigation of Dye Removal Capability of Blast Furnace Slag in Wastewater Treatment. Sustainability. 2021; 13(4):1970. https://doi.org/10.3390/su13041970
Chicago/Turabian StyleYasipourtehrani, Sara, Vladimir Strezov, Tao Kan, and Tim Evans. 2021. "Investigation of Dye Removal Capability of Blast Furnace Slag in Wastewater Treatment" Sustainability 13, no. 4: 1970. https://doi.org/10.3390/su13041970
APA StyleYasipourtehrani, S., Strezov, V., Kan, T., & Evans, T. (2021). Investigation of Dye Removal Capability of Blast Furnace Slag in Wastewater Treatment. Sustainability, 13(4), 1970. https://doi.org/10.3390/su13041970








