1. Introduction
As there is an intrinsic link between the condition of these mangroves and climate change, information about their status can serve as a barometer to measure and quantify some of its effects. As defined by the IPCC [
1], climate change is a persistent, permanent alteration to climate properties, and they affect all aspects of life on this planet. Human health, infrastructures, and safety can all be influenced by climate change, not just the ecosystems, coastal systems, fire occurrence patterns, and overall availability of potable water and food [
2]. Furthermore, climate change can and has resulted in significant transformations in the status of the Earth’s oceans, landmasses, and ice sheets, which may well have substantial long-term side effects on related systems. Levels of carbon dioxide in the atmosphere, a key greenhouse gas that contributes to climate change, increased from 280 to 411 ppmv in the 169 years from 1850 to 2019 [
3]. A considerable proportion of all the greenhouse gases from anthropogenic sources is represented by deforestation and the production and subsequent use of fossil fuels. Pendleton et al. [
4] estimated in their research that this accounted for around 8–20% of total global emissions.
Soil is an important factor in the provision of ecosystem services [
5]. Soils play an intrinsic role in the global carbon cycle, sequestering carbon and regulating greenhouse gases [
6]. In 1980, Jenny [
7] noted the strong relationship between climate and soil quality. Therefore, climate changes resulting from increased concentrations of greenhouse gases may have negative effects on soil structure and its stored organic carbon content, in addition to obstructing water, carbon, and many other important element cycles, causing adverse effects on plant productivity [
8]. The relationship between soil and the different characteristics of mangrove forests in the tropics was studied by Hossain and Nuruddin in 2016, where they indicated that soil properties (such as physico-chemical properties) have an impact on the vegetational structure of mangrove forests [
9].
The term used to specifically refer to any carbon dioxide that coastal ocean ecosystems generate or capture from the atmosphere is ‘blue carbon’ [
10,
11,
12]. Mangroves represent a sizeable blue carbon system in coastal oceans, and they play a counter-intuitively large role in the attenuation of greenhouse gases, given their small relative area to tropical forests [
12,
13,
14,
15]. Across the globe, the blue carbon stored only by mangroves accounts for 11.7 Pg C, a tremendous quantity for their size. Uniquely positioned, Twilley et al. [
15] proposed that it is a combination of their potential nutrient exchange with coastal water, and their straddling of the terrestrial-ocean interface, that results in their high degree of contribution to coastal ocean carbon biogeochemistry.
Other environmental factors positively influenced by mangroves include the augmentation of the marine food web in oligotrophic water in its proximity [
16], habitat and nutrition provisions for many aquatic and bird species, coral reef protection, and a number of various physical attributes [
17,
18,
19]. By acting as a nutrient filter, mangroves fulfil crucial roles from other perspectives aside from blue carbon, such as ecological and socioeconomic, as humans can benefit from them in many ways [
20]. In addition to the boon of the enhanced marine food web and the various ecological factors, Almahasheer et al. [
21] observed in their research that the mangroves functioned as wind, wave, and even hurricane barriers for the local population, and their root systems helped to stave off erosion through sediment stabilisation. As stated previously, proportional to their size, mangroves perform a significant role in the carbon cycle. Despite covering only around 137,600 km
2 of ground split between 118 countries [
22], mangroves’ carbon input into ocean systems makes up 11% of the total terrestrial carbon input [
23]. It is for these reasons, Sasmito et al. [
24] asserted that, of the whole globe, mangroves are one of the most productive ecosystems currently known. Predominantly comprised of trees and shrubs, this forested wetland can develop into broad swathes, and favours the muddy and carbonate coastal areas of tropical and subtropical regions [
25,
26].
Given their inherent importance in managing carbon levels and their other environmental contributions, these systems should be observed, studied, and preserved wherever possible; however, mangrove ecosystems are highly threatened [
27] and in a state of decline, with a rate worse than the rainforests and coral reefs [
28,
29]. Forest harvesting and other human actions have resulted in a significant deterioration in mangrove ecosystem numbers, with an estimated 35% of the total global mangrove area destroyed over the past two decades alone [
28,
29]. Despite harvesting nearly all live biomass, Sasmito et al. [
24], in its study on mangroves of West Papua Province, Indonesia, asserted that soil carbon stocks were left relatively undisturbed, while aquaculture conversion withdrew 85% of its live biomass carbon stock and 60% of its soil carbon stock.
The mangrove forests are remarkably resilient; Edwards and Head [
30] insisted that they could not possibly thrive in proximity to the Red Sea, given the local environmental conditions. However, in the past 40 years, and possibly because of rejuvenation initiatives, the proliferation rate of mangroves for that area has increased by 12%, such that they now occupy 135 km
2 of the Red Sea coast [
31]. In the eastern and western coastal areas of the Arabian Peninsula, mangroves also thrive [
32]. This all has given the mangrove forest a reputation for its capacity to thrive in usually harsh and inhospitable circumstances; many living things struggle in environments with high concentrations of salt, sparse rainfall, dehydrated land, and a complete lack of riverine systems, making the mangrove forests the subject of much attention [
33]. A pioneer species,
Avicennia marina (
Figure S1) stores carbon in both biomass and sediments [
27], and paves the way for full mangrove forests to develop in otherwise challenging areas. In many areas of Saudi Arabia, the conditions are inhibitive for these mangrove forests, however, along the Red Sea coast’s intertidal zones, some isolated stands manage to survive, grow, and thrive [
32]. Over 48 km
2 of this coastline is now inhabited by
A. marina (Almahasheer, personal communication), and it is particularly more prolific in southern or coastal regions as opposed to inland or northern [
34].
Though there was not a substantial amount for arid and semiarid environments, an analysis of the available research revealed a focus on reliably and accurately estimating soil carbon and aboveground biomass [
10,
21,
35,
36,
37]. Consideration by region allowed some academics to produce estimations for the average values for tropical wet, tropical dry, and subtropical mangroves’ AGB growth rates; at 9.9, 3.3, and 18.1 Mg of dry matter ha
−1 year
−1 [
38,
39,
40]. However, some authors looking into more specific areas, such as Wang et al. [
41], elected to quantify total mangrove AGB in their study instead of its growth rate; giving a value in the northeast of China’s Hainan Island of 312,806.3 Mg. Parvaresh [
42] also looked at total instead of rate, however, like many other authors, they accounted for area when they performed their own study into the AGB of
A. marina trees in Iran, focusing on those in the arid climate of Sirik. They found the total AGB to be 17.2 Mg ha
−1, a value that is comparatively low when considering other similar research. In the Mangawhai Harbour in New Zealand, Tran et al. [
43] estimated the ABG of the same species at between 0.3–0.9 Mg ha
−1, significantly less than in other similarly temperate zones, both within the same country and Australia. In the same study, they additionally recorded the BGB, which was interestingly in line with that of the larger tree locations, despite less AGB, at a value between 1.2–1.5 Mg ha
−1. Although the majority of authors in this field centred their work on AGB, Tamooh [
44] in Kenya, in the Gazy Bay, investigated multiple species’ BGB instead, estimating a value of 43 Mg ha
−1 for
A. marina, while Mackey [
45] reported 109–126 Mg ha
−1 in Australia for the same species a few years prior.
While there are many papers in other locations worldwide on the status of mangroves, their carbon contents, and estimations of their biomasses, there are not as many specifically in the Arabian region. Two of those that are, include one study in Egypt and one in Saudi Arabia. Mashaly et al. [
27] performed their research in the former, estimating total biomass carbon content (in Mg C ha
−1) and an average value for single-tree biomass (in kg) for various types of mangroves in South Sinai. Respectively, their reported figures for those were as follows: 109.3 and 92.3 for intertidal mangroves, 41.9 and 16.6 for shoreline mangroves, 70.3 and 40.5 for salt plain mangroves, and 29.2 and 21.2 for transplanted mangroves. Within Saudi Arabia, Abohassan et al. [
46] investigated two locations, Shuaiba and Yanbu, along the Red Sea coastline. They estimated both the AGB and BGB for these regions, discovering that the total AGB in Shuaiba was 18.6 Mg ha
−1, a substantial 7.8 Mg ha
−1 higher than that of Yanbu. They also observed that in addition to having more AGB, Shuaiba also possessed a much greater quantity of aerial and fine roots than Yanbu, with 23.7 and 96.4 Mg ha
−1, respectively, for Shuaiba and 10.1 and 39.1 Mg ha
−1 for Yanbu.
At the time of writing, despite some existing evaluations into the biomass carbon of the mangrove forests for some discrete areas along the Saudi Arabian Red Sea coast, no studies were found to have representatively accounted for the whole of the coastline. From the city of Duba in the north to Jazan in the south, the coast of the Red Sea stretches across around 1134 km of Saudi Arabia, and this study aims to fill the research gap by assessing the AGB and BGB of A. marina mangrove trees along its length. The salinity gradient and nutrient availability in the various locations throughout the coast were likely to affect biomass carbon, and this paper aims to evaluate that influence.
Thus, the objectives of the present study were to (1) evaluate the environmental determinants of the ability of mangroves to capture carbon along the Saudi Red Sea coast; (2) formulate regression equations to anticipate mangrove biomass; and (3) assess the overall tree carbon in mangrove stands of natural occurrence. Some soil management strategies (such as organic cultivation) have a great impact on improving and raising the soil’s organic carbon content, which helps mitigate climate changes resulting from increased concentrations of greenhouse gases [
47]. Therefore, the findings of this study are likely to have a broad range of derivative applications for contexts across the globe; possibly assisting in the discovery of new carbon emission reduction and management techniques, such as through the mitigation of mangrove forest or other high-value ecosystem deterioration.
3. Results
The sediment and sea water sample results illustrate the environmental conditions at each location, and can be seen in
Table 1 and
Table 2. EC and TDS values of the sediment samples remained relatively consistent; however, the TP and TN values were vastly dissimilar, lowest in the northern location and highest in the central one (
Table 1).
Sea water samples were in accordance with sediment samples for TP and TN, also showing the northern location with the least, and the central with the most (
Table 2). However, in contrast to the sediment samples, the EC and TDS values demonstrated a dramatic concentration gradient, this time greatest in the north and smallest in the south.
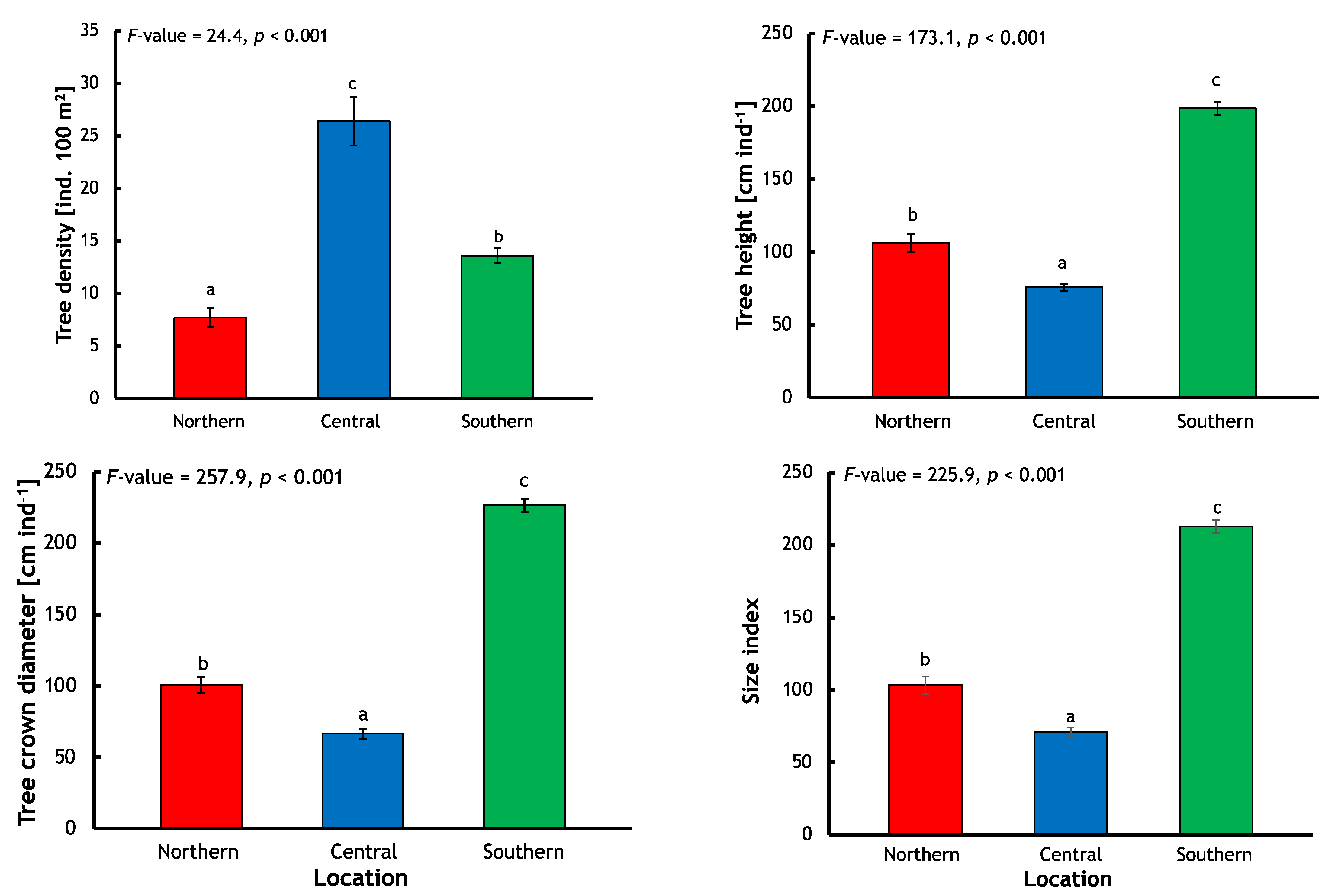
Avicennia marina population characteristics in each location served to elucidate the particular circumstance and condition of the species within those different settings (
Figure 2). It was found that while the southern location mangroves had the greatest values for size index (212.6), tree height (198.6 cm ind.
−1), and tree crown diameter (226.5 cm ind.
−1), they only had a medium tree density (13.6 ind. 100 m
2). Contrastingly, despite the central location having the smallest size index (71.7), tree height (75.6 cm ind.
−1), and tree crown diameter (66.5 cm ind.
−1) properties, it possessed the greatest tree density (26.4 ind. 100 m
2) (
Figure 2).
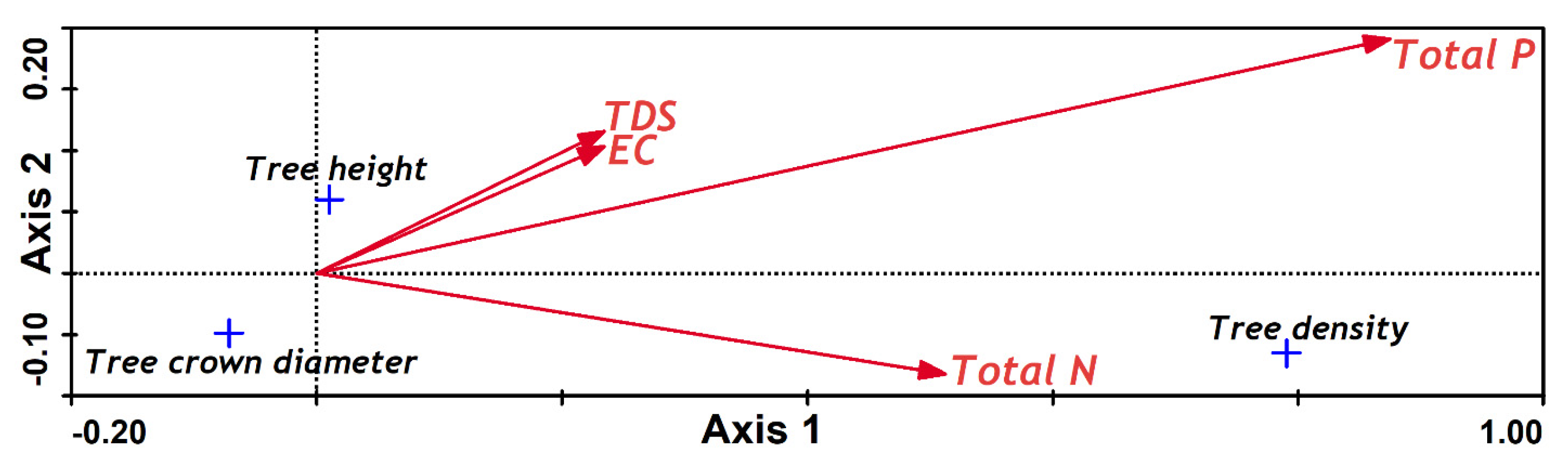
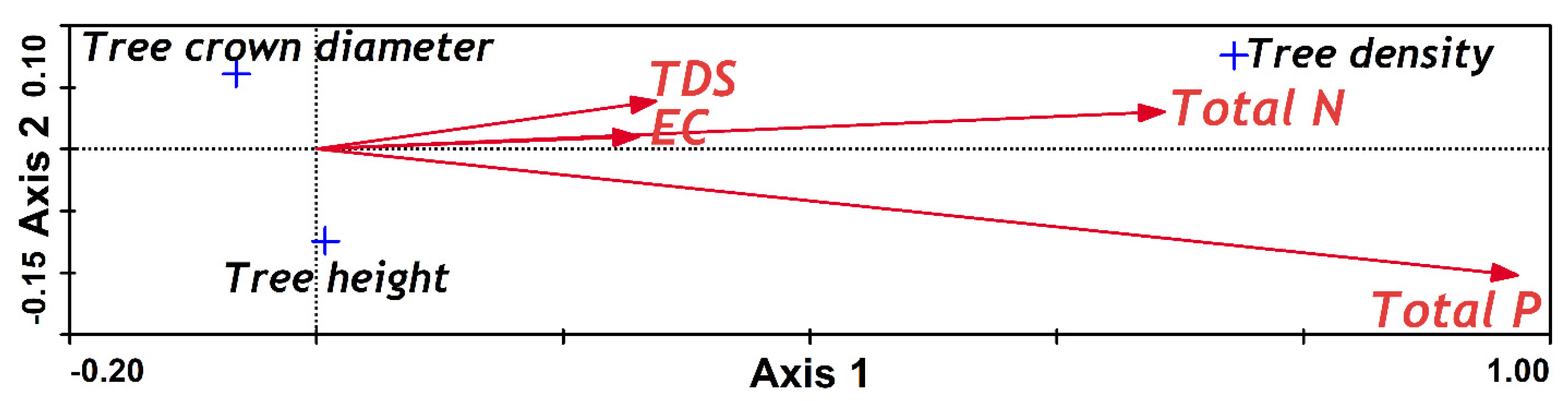
Two CCA were conducted, both between the first two population axes, with one against sediment properties and the other against sea water properties (
Figure 3 and
Figure 4); TP and TN were found to positively affect the separation of the population characteristics in both analyses. However, for the sediment properties CCA, the second axis was negatively correlated with TN (
Table 3), whereas for water properties, it was negatively correlated with TP (
Table 4).
The final regression equation employed in the prediction of both AGB and BGB components was most accurate for all seven sites in all three locations when it was plotted linearly, in the form of
, where
is the biomass,
is the parameter, and both
and
are constants. As briefly touched on in the statistical analysis section of this paper, the Student’s
t-test determined a lack of significant difference between actual sample measurements of biomasses and their estimated quantities using the regression equations. Furthermore, the location biomass for both total biomass and each particular biomass component was evaluated, taking advantage of generated prediction models (
Table 5).
As can be seen in
Table 6, there were significant variations in both the calculated biomass levels and proportions of AGB to BGB between locations. In terms of total biomass (Mg DM ha
−1), the mangrove in the south possessed the greatest at 1188.2, the central mangrove was almost half that at 660.9, and the north location was only 197.9.
Proportionally, the percentages of total biomass that were above-ground for each location provided further insights; the location in the north had 58.4%, the centre had 62%, and the south had around 75%. Only a quarter of the southern mangrove’s biomass was below-ground. As can be observed in
Table 7, the total biomass carbon content in Mg C ha
−1 for the selected three locations is, in descending order: southern, 412.5; central, 294.6; and northern, with the least, 87.6.
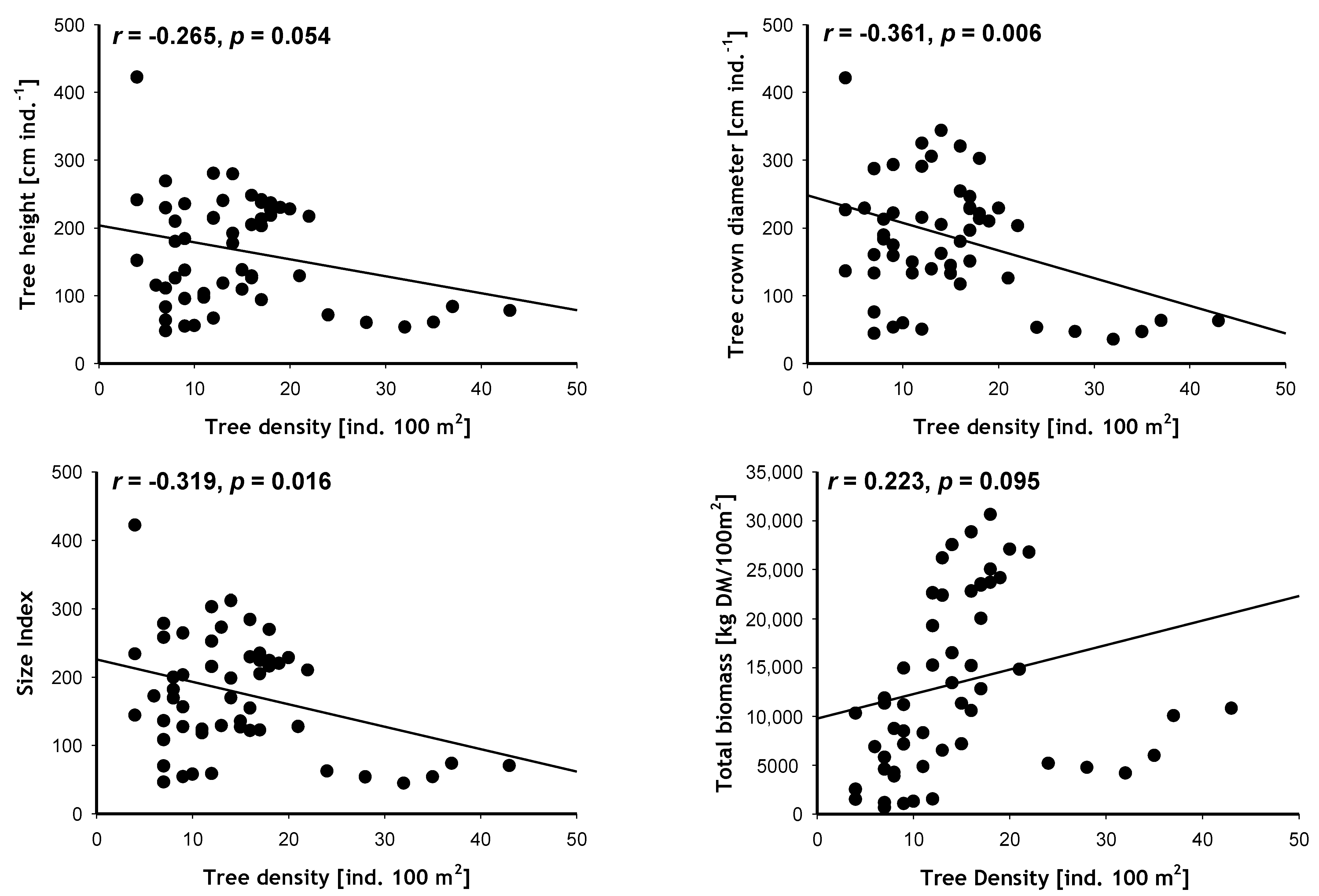
Results also indicated a negative correlation between tree size characteristics and mangrove density, i.e., the larger the size index, height, or crown diameter of each tree, the less dense the forest is likely to be (
Figure 5).
Interestingly, it is the density factor and not the individual size factor that is positively correlated with total biomass; counter-intuitively, it is the shorter but denser central mangroves that possess a higher total biomass, rather than the taller but sparser northern ones.
4. Discussion
In accordance with existing research [
14,
65,
66], these results illustrated the critical dependence of natural carbon stocks and plant establishment in
A. marina forests on multiple environmental factors. They also served to demonstrate how, even when within the same or very similar habitats, these factors can change dramatically, causing observable alterations to the ecosystems. It can be inferred from the results that the increase in TN and TP had a negative effect on the tree density characteristic of the mangroves, and that greater values for EC and TDS contributed positively to tree height. Both the sediment analyses and the water analyses indicated the mangroves in the central location held the greatest magnitude for TP and TN values, whereas the northern location was shown to have held the smallest. Despite similar findings from both sediment and sea water analyses in those areas, the sediment analyses suggest that the central location possesses the highest EC and TDS values, whereas the water analyses evince a decreasing gradient for those values progressing from north to south.
In terms of the productivity of the mangroves, both the nutrient abundance and salinity have been shown to have an influence. Given the elevated salinity levels in both the northern and central locations, it has been suggested that
A. marina growing there would experience physiological stress, as they would have to focus a greater proportion of their energy on osmotic regulation, as opposed to growth [
10,
67,
68]. Arshad et al. [
59] asserted this link as causative in their research, stating the salinity levels have direct and negative implications for stem and leaf development. In addition to the salinity level impact at the central site, a higher degree of pollution was observed there, likely from a combination of fertiliser run-off, residential wastewater, industrial point sources, and other human activities [
21,
69], contributing to the ongoing eutrophication of the water. Aside from causing eutrophication, a further contributor of physiological stress, the pollution itself also negatively affects the survivability of mangroves, as Vaiphasa et al. [
70] found in their observation, wherein effluents from shrimp ponds were in the vicinity. Arshad et al. [
59] further argued that the improper disposal of sewage into the water was causing mangrove pneumatophores to die off. This directly impedes plant growth, as the respiration rate of the root system cannot keep up with nutrient uptake with its pollution-reduced aeration surface area [
71]. In all, existing studies by Saifullah [
49], Alongi [
72], and Triantafyllou et al. [
73], seem to align with the results from this research, suggesting reliability.
The results align with previous research, suggesting that the relatively small size and fragmentary distribution of mangroves in the central location has many contributing factors, including alterations in land use, low level of precipitation, a stiffer substrate, significantly high concentrations of nitrogen and phosphorous in sea water and substrates, and pollution types and levels [
21,
32,
49,
72,
74]. However, it still cannot be stated with certainty from the new results in this paper whether or not intraspecific competition may be an additional reason. Shaltout and Ayyad [
75] proposed that, given the growth of a plant more tightly encompassed by others is less in comparison with a plant that has more space [
76], the indirect correlation in the central location between small size and high density could be partly explained by intraspecific competition. In comparison to the central location, the data from the southern location could be argued to indicate intraspecific competition is a factor, as its high tree size but relatively low density suggests the size was augmented by a decrease in that competition. Nonetheless, this study presents data that also seems to indicate that the density change from southern to central locations is triggered by the differing levels of TN and TP in the sea water and sediments, not intraspecific competition. For this to be known for certain, more empirical studies would be necessary to specifically investigate how intraspecific competition affects population characteristics and dynamics of Saudi Red Sea coast mangroves. Existing research has also presented similar overall results [
18,
58,
75,
77,
78,
79,
80]. Appearing to signify a negative correlation between tree density and tree size factors (height and crown diameter), and a positive correlation between overall biomass and tree density, the results from this research continue to be in accordance with earlier studies (e.g., Fromard et al. [
81]; Xiao [
82]; Slik [
83]; Eid et al. [
84]; Fajardo [
85]) and predicted outcomes. As previously stated, the results infer that the total biomass per unit area of each location varies as a result of the size and density of the trees.
At 1188.2 Mg DM ha
−1 in the south, total biomass was found to incrementally decline northwards, to 660.9 Mg DM ha
−1 in the centre, and down to 197.9 Mg DM ha
−1 in the north. It is highly probable that this gradient is caused by the southernmost zone’s relatively high precipitation, nutrient abundance, higher quantity of wadis, tropical climate, and slightly reduced salinity [
32,
49], as was observed in the measured environmental properties. The average AGB for this dataset was 469.4 Mg DM ha
−1 (
Table 8), which was significantly higher than the global mangrove AGB average Hu et al. [
86] proposed of 115.2 Mg DM ha
−1, and also higher than the averages of mangroves in many countries (e.g., United Arab Emirates, Australia, Japan, and Egypt). Sitoe et al. [
87] asserted that the average tree height causes this significant variance in the biomasses of the same species; however, Abohassan et al. [
46] purport that it is the environmental conditions instead that have greater influence. Each location’s relative proportions of AGB to BGB of the total biomass also diverged conspicuously; the southern location’s AGB was 74.3% of its total, the central location’s AGB was 62.1% of its total, and the northern location’s AGB was 58.4%. These values are akin to results found for the same or similar mangroves within analogous environments; measurements of the Sinai Peninsula’s salt plain (78% AGB) and southern coastline (75.3% AGB) are accordant with the southern location’s AGB [
88]. For the northern or central locations, it is the transplanted mangroves in Egypt’s Nabq and Ras Mohammed Protected Area (53.1% AGB) [
88], Bangladesh’s Oligohaline zone of the Sundarbans (64.8% AGB) [
89], and Mozambique’s Sofala Bay (52% AGB) [
87] that show similar values. Biomass overall was purported to differ considerably over climate gradients by Simard et al. [
90], and a significant number of authors concur that geomorphological settings also have a substantial impact on biomass [
25,
26,
66]. Although the proportion of AGB was still greater than BGB in the central and northern locations, they were both significantly closer to being evenly split than the southern zone; i.e., the BGB-to-AGB ratios were 0.7 in the north, 0.6 in the centre, and 0.4 in the south. It is possible that
A. marina, prioritising establishment of a supportive and stable root network at early stages to compensate for oxygen-deprived loose sediments, grows more AGB as it matures and augments its biomass [
27]. The southern location, possessing the greatest overall biomass, also possessed the largest biomass carbon content, at 412.5 Mg C ha
−1. Values of 294.6 Mg C ha
−1 and 87.6 Mg C ha
−1 were calculated for the central and northern locations, respectively. Results shared by Mashaly et al. [
27] investigating
A. marina show a value of 109.3 Mg C ha
−1 in southern Sinai for intertidal mangrove forests. As can be seen in
Table 8, the average total biomass carbon content was 264.9 Mg C ha
−1 for this study, comparative averages from other studies suggest this is fairly high: Schile et al. [
10] reported 7.3–147.5 Mg C ha
−1 from the UAE, Afefe et al. [
91] had 33.8 Mg C ha
−1 for Egypt’s Gebel Elba Protected Area, and Sitoe et al. [
87] found an average in Mozambique’s Sofala Bay of 264.9 Mg C ha
−1. Furthermore, a higher AGB carbon value than average was also determined in this study (180.3 Mg C ha
−1), when compared with the mean AGB carbon values of 40.2 and 125 Mg C ha
−1 for Australian [
92] and New Zealand [
93] mangroves, respectively. Contrastingly, and despite a higher than average value for AGB carbon, the BGB carbon values calculated for the three locations averaged at 84.6 Mg C ha
−1, much lower than the largest reported value worldwide (263 Mg C ha
−1), and still significantly lower than an average obtained for a study in Brazil (104.4 Mg C ha
−1) by Santos et al. [
94]. These discrepancies could be explained by the hydrogeomorphic setting directing the mangrove’s blue carbon stock dynamics, in parallel to long-term transformations of land-use having a significant impact on carbon gains and losses [
24].
Despite having such a crucial role in counteracting climate change and sequestering carbon [
14,
108,
109], mangrove forests are one of the worst-suffering ecosystems, deteriorating at an alarming rate [
108,
110,
111,
112,
113]. According to Almahasheer et al. [
31], a 12% augmentation of the area of mangroves has been provoked by governmental initiatives along the Red Sea coastline in the past 40 years. Even with this level of input, the degree of risk to mangroves in the area continues to climb, with existing factors’ effects being exacerbated by new ones, such as excessive tree felling and grazing, the development of resorts or the oil industry [
114], invasion of territory by shrimp farmers [
53], and pollution from sewage and oil [
80]. In 2017, to tackle this issue and be held accountable and responsible for protecting the mangrove habitats, the Council of Ministers of Saudi Arabia created the Standing Committee for the Protection of the Environment of Coastal Areas. While this committee is a landmark first step in the road to preserving Red Sea coast mangroves, more work will be necessary to reap their other benefits, and improve upon not only the mangroves’ quality, but also their capacity to sequester carbon.
 ).
).