Evaluation of Symbiotic Association between Various Rhizobia, Capable of Producing Plant-Growth-Promoting Biomolecules, and Mung Bean for Sustainable Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Preservation of Rhizobial Isolates
2.2. Experimental Setup and Growth Attributes
2.3. Physiological and Photosynthetic Attributes
2.4. Extraction of Enzymes, Determination of Total Soluble Protein (TSP) Content and Malondialdehyde (MDA) Content
2.5. Biomolecular Characterization and Identification of Selected Rhizobial Isolates
2.6. Statistical Analyses
3. Results
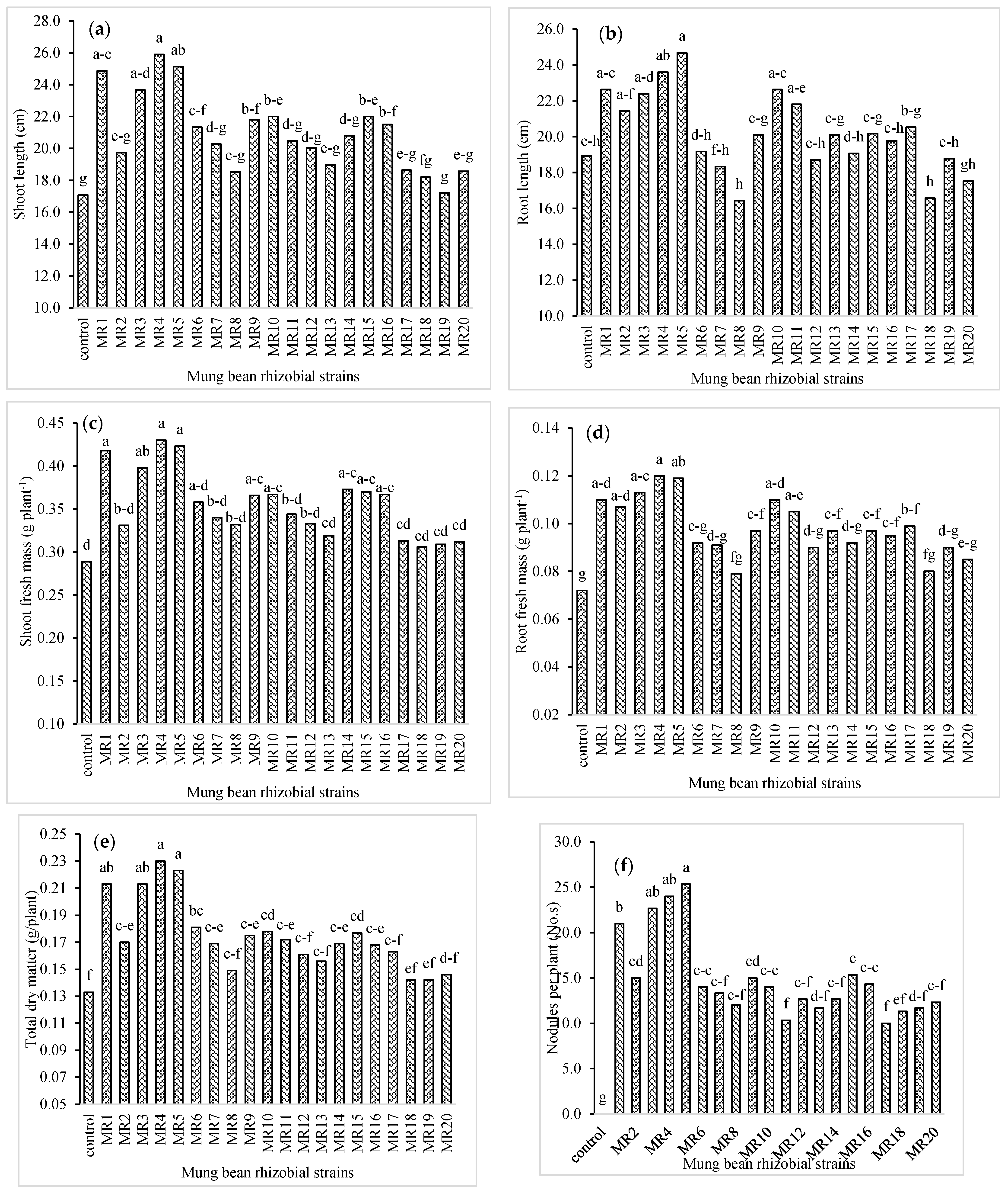
3.1. Plant and Biomass Growth, and Nodulation of Mung Bean
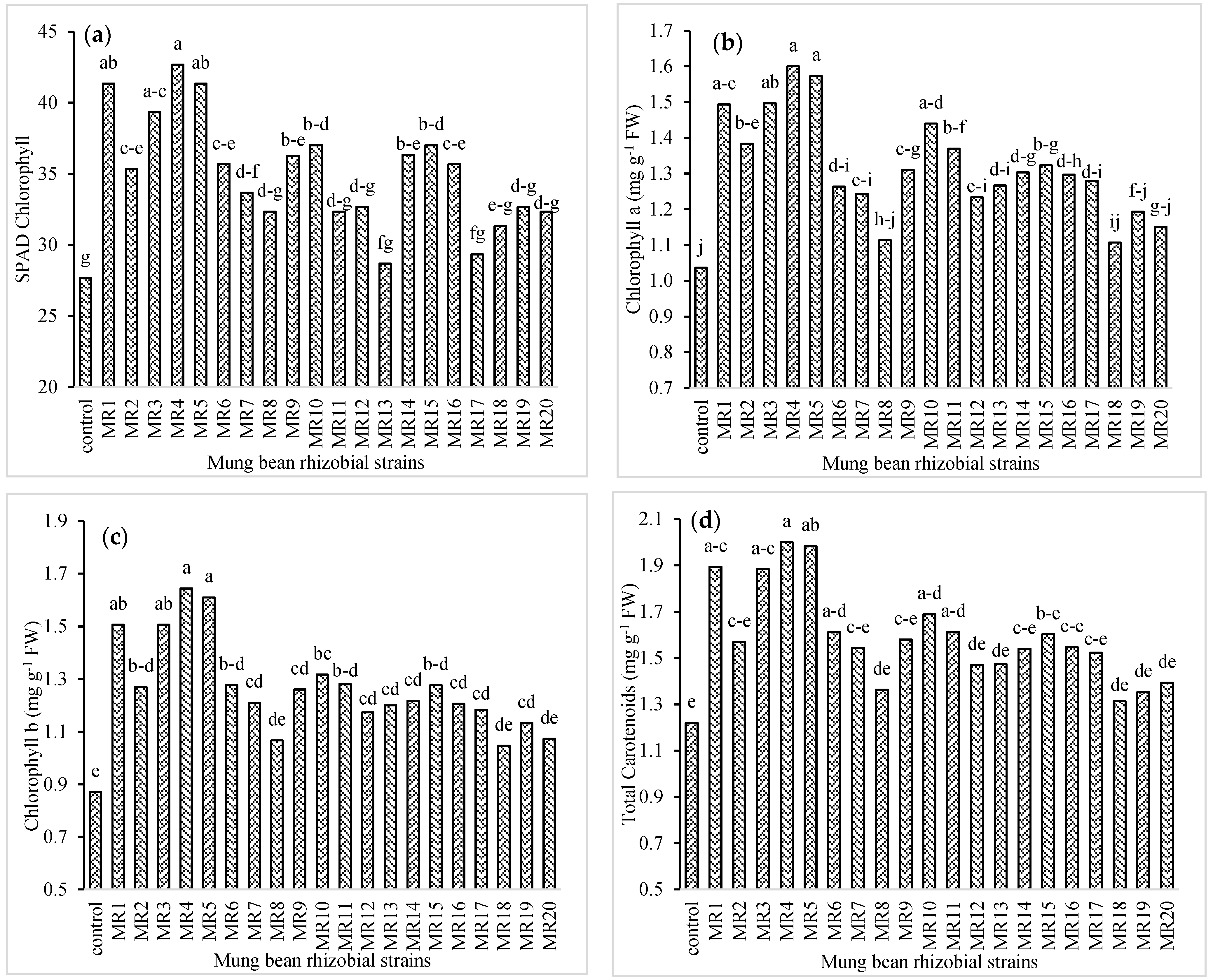
3.2. Physiological Attributes of Mung Bean
3.3. Photosynthetic Attributes of Mung Bean
3.4. Biochemical Properties of Mung Bean
3.5. Selection of Rhizobial Isolates of Mung Bean
3.6. Identification and Biochemical Characterization of the Selected Strains
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Laranjo, M.; Alexandre, A.; Oliveira, S. Legume growth-promoting rhizobia: An overview on the Mesorhizobium genus. Microbiol. Res. 2014, 169, 2–17. [Google Scholar] [CrossRef]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef]
- Oldroyd, G.E. Speak, friend, and enter: Signalling systems that promote beneficial symbiotic associations in plants. Nat. Rev. Microbiol. 2013, 11, 252–263. [Google Scholar] [CrossRef]
- Jensen, E.S.; Peoples, M.B.; Boddey, R.M.; Gresshoff, P.M.; Hauggaard-Nielsen, H.; Alves, B.J.; Morrison, M.J. Legumes for mitigation of climate change and the provision of feedstock for biofuels and biorefineries. A review. Agron. Sustain. Dev. 2012, 32, 329–364. [Google Scholar] [CrossRef]
- Lupwayi, N.; Clayton, G.; Hanson, K.; Rice, W.; Biederbeck, V. Endophytic rhizobia in barley, wheat and canola roots. Can. J. Plant Sci. 2004, 84, 37–45. [Google Scholar] [CrossRef]
- Perret, X.; Staehelin, C.; Broughton, W.J. Molecular basis of symbiotic promiscuity. Microbiol. Mol. Biol. Rev. 2000, 64, 180–201. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, U.; Ibrahim, M.; Abbas, F.; Yamin, M.; Jabeen, F.; Shahzadi, A.; Farooque, A.A.; Imtiaz, M.; Ditta, A.; Ali, S. Phytoextraction of Lead Using a Hedge Plant [Alternanthera bettzickiana (Regel) G. Nicholson]: Physiological and Biochemical Alterations through Bioresource Management. Sustainability 2021, 13, 5074. [Google Scholar] [CrossRef]
- Stephens, J.; Rask, H. Inoculant production and formulation. Field Crop. Res. 2000, 65, 249–258. [Google Scholar] [CrossRef]
- Chernin, L.; Glick, B.R. The use of ACC deaminase to increase the tolerance of plants to various phytopathogens. In Bacteria in Agrobiology: Stress Management; Springer: Berlin, Germany, 2012; pp. 279–299. [Google Scholar]
- Berg, G. Plant–microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 2009, 84, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Bal, H.B.; Das, S.; Dangar, T.K.; Adhya, T.K. ACC deaminase and IAA producing growth promoting bacteria from the rhizosphere soil of tropical rice plants. J. Basic Microbiol. 2013, 53, 972–984. [Google Scholar] [CrossRef]
- Dakora, F.D.; Matiru, V.; Kanu, A.S. Rhizosphere ecology of lumichrome and riboflavin, two bacterial signal molecules eliciting developmental changes in plants. Front. Plant Sci. 2015, 6, 700. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Müller, K.M.; Charles, T.C.; Vesely, S.; Glick, B.R. 1-aminocyclopropane-1-carboxylate (ACC) deaminase genes in rhizobia from southern Saskatchewan. Microb. Ecol. 2009, 57, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Shokri, D.; Emtiazi, G. Indole-3-acetic acid (IAA) production in symbiotic and non-symbiotic nitrogen-fixing bacteria and its optimization by Taguchi design. Curr. Microbiol. 2010, 61, 217–225. [Google Scholar] [CrossRef]
- Avis, T.J.; Gravel, V.; Antoun, H.; Tweddell, R.J. Multifaceted beneficial effects of rhizosphere microorganisms on plant health and productivity. Soil Biol. Biochem. 2008, 40, 1733–1740. [Google Scholar] [CrossRef]
- Thakur, A.; Panwar, J. Effect of rhizobium-VAM interactions on growth and yield in mungbean (Vigna radiata (L) Wilczek) under field conditions. Indian J. Plant Physiol. 1995, 38, 62–65. [Google Scholar]
- PARC. National Coordinated Program for Sugar and Food Legume Crops. 2021. Available online: http://www.parc.gov.pk/index.php/en/national-coordinated-research-system/108-national-coordinated-research-systems/324-national-coordinated-program-for-sugar-and-food-legume-crops (accessed on 2 July 2021).
- Abd-Alla, M.H.; Morsy, F.M.; El-Enany, A.-W.E.; Ohyama, T. Isolation and characterization of a heavy-metal-resistant isolate of Rhizobium leguminosarum bv. viciae potentially applicable for biosorption of Cd2+ and Co2+. Int. Biodeterior. Biodegrad. 2012, 67, 48–55. [Google Scholar] [CrossRef]
- Ben-Asher, J.; Tsuyuki, I.; Bravdo, B.-A.; Sagih, M. Irrigation of grapevines with saline water: I. Leaf area index, stomatal conductance, transpiration and photosynthesis. Agric. Water Manag. 2006, 83, 13–21. [Google Scholar] [CrossRef]
- Hussain, F.; Bronson, K.; Yadvinder, S.; Singh, B.; Peng, S. Use of chlorophyll meter sufficiency indices for nitrogen management of irrigated rice in Asia. Agron. J. 2000, 92, 875–879. [Google Scholar]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef]
- Aery, N. Manual of Environmental Analysis; Ane Books Pvt Ltd.: New Delhi, India, 2010. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Cakmak, I.; Horst, W.J. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol. Plant. 1991, 83, 463–468. [Google Scholar] [CrossRef]
- Sarwar, M.; Arshad, M.; Martens, D.A.; Frankenberger, W. Tryptophan-dependent biosynthesis of auxins in soil. Plant Soil 1992, 147, 207–215. [Google Scholar] [CrossRef]
- Borrow, A.; Brian, P.; Chester, V.; Curtis, P.; Hemming, H.; Henehan, C. Gibberellic acid, a metabolic product of the fungus Gibberella fujikuroi: Some observations on its production and isolation. J. Sci. Food Agric. 1955, 6, 340–348. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Fasim, F.; Ahmed, N.; Parsons, R.; Gadd, G.M. Solubilization of zinc salts by a bacterium isolated from the air environment of a tannery. FEMS Microbiol. Lett. 2002, 213, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Nautiyal, C.S. An efficient method for qualitative screening of phosphate-solubilizing bacteria. Curr. Microbiol. 2001, 43, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, A.; Sharma, S.K.; Sharma, M.P.; Yadav, N.; Joshi, O.P. Inoculation of zinc solubilizing Bacillus aryabhattai strains for improved growth, mobilization and biofortification of zinc in soybean and wheat cultivated in Vertisols of central India. Appl. Soil Ecol. 2014, 73, 87–96. [Google Scholar] [CrossRef]
- Schwyn, B.; Neilands, J. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Ashraf, M.; Hasnain, S.; Berge, O.; Mahmood, T. Inoculating wheat seedlings with exopolysaccharide-producing bacteria restricts sodium uptake and stimulates plant growth under salt stress. Biol. Fertil. Soils 2004, 40, 157–162. [Google Scholar] [CrossRef]
- Chernin, L.S.; Winson, M.K.; Thompson, J.M.; Haran, S.; Bycroft, B.W.; Chet, I. Chitinolytic activity in Chromobacterium violaceum: Substrate analysis and regulation by quorum sensing. J. Bacteriol. 1998, 180, 4435–4441. [Google Scholar] [CrossRef]
- MacFaddin, J.F. Biochemical Tests for Identification of Medical Bacteria; Lippincott Williams: Philadelphia, PA, USA, 2000. [Google Scholar]
- Nutman, P. A Manual for the Practical Study of Root-Nodule Bacteria; Wiley-Blackwell: Hoboken, NJ, USA, 1970. [Google Scholar]
- Steel, R.; Torrie, J.; Dicky, D. Principles and Procedures of Statistics. Multiple Comparisons; McGraw Hill Book Co.: New York, NY, USA, 1997. [Google Scholar]
- Huang, H.; Erickson, R. Effect of seed treatment with Rhizobium leguminosarum on Pythium damping-off, seedling height, root nodulation, root biomass, shoot biomass, and seed yield of pea and lentil. J. Phytopathol. 2007, 155, 31–37. [Google Scholar] [CrossRef]
- Etesami, H.; Alikhani, H.A.; Akbari, A.A. Evaluation of plant growth hormones production (IAA) ability by Iranian soils rhizobial strains and effects of superior strains application on wheat growth indexes. World Appl. Sci. J. 2009, 6, 1576–1584. [Google Scholar]
- Dey, R.; Pal, K.; Bhatt, D.; Chauhan, S. Growth promotion and yield enhancement of peanut (Arachis hypogaea L.) by application of plant growth-promoting rhizobacteria. Microbiol. Res. 2004, 159, 371–394. [Google Scholar] [CrossRef]
- Jadhav, R.; Thaker, N.; Desai, A. Involvement of the siderophore of cowpea Rhizobium in the iron nutrition of the peanut. World J. Microbiol. Biotechnol. 1994, 10, 360–361. [Google Scholar] [CrossRef]
- Van Rhijn, P.; Fujishige, N.A.; Lim, P.O.; Hirsch, A.M. Sugar-binding activity of pea lectin enhances heterologous infection of transgenic alfalfa plants by Rhizobium leguminosarum biovar viciae. Plant Physiol. 2001, 126, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Afzal, A.; Bano, A. Rhizobium and phosphate solubilizing bacteria improve the yield and phosphorus uptake in wheat (Triticum aestivum). Int. J. Agric. Biol. 2008, 10, 85–88. [Google Scholar]
- Grover, M.; Ali, S.Z.; Sandhya, V.; Rasul, A.; Venkateswarlu, B. Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World J. Microbiol. Biotechnol. 2011, 27, 1231–1240. [Google Scholar] [CrossRef]
- Saïdi, S.; Chebil, S.; Gtari, M.; Mhamdi, R. Characterization of root-nodule bacteria isolated from Vicia faba and selection of plant growth promoting isolates. World J. Microbiol. Biotechnol. 2013, 29, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Choure, K.; Dubey, R.C.; Maheshwari, D.K. Rhizosphere competent Mesorhizobiumloti MP6 induces root hair curling, inhibits Sclerotinia sclerotiorum and enhances growth of Indian mustard (Brassica campestris). Braz. J. Microbiol. 2007, 38, 124–130. [Google Scholar] [CrossRef]
- Elkoca, E.; Turan, M.; Donmez, M.F. Effects of single, dual and triple inoculations with Bacillus subtilis, Bacillus megaterium and Rhizobium leguminosarum bv. Phaseoli on nodulation, nutrient uptake, yield and yield parameters of common bean (Phaseolus vulgaris l. cv.‘elkoca-05’). J. Plant Nutr. 2010, 33, 2104–2119. [Google Scholar] [CrossRef]
- Hussain, M.B.; Mahmood, S.; Ahmed, N.; Nawaz, H. Rhizobial inoculation for improving growth physiology, nutrition and yield of maize under drought stress conditions. Pak. J. Bot. 2018, 50, 1681–1689. [Google Scholar]
- Ahmad, M.; Zahir, Z.A.; Khalid, M.; Nazli, F.; Arshad, M. Efficacy of Rhizobium and Pseudomonas strains to improve physiology, ionic balance and quality of mung bean under salt-affected conditions on farmer’s fields. Plant Physiol. Biochem. 2013, 63, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Zahir, Z.A.; Asghar, H.N.; Asghar, M. Inducing salt tolerance in mung bean through coinoculation with rhizobia and plant-growth-promoting rhizobacteria containing 1-aminocyclopropane-1-carboxylate deaminase. Can. J. Microbiol. 2011, 57, 578–589. [Google Scholar] [CrossRef]
- Bambara, S.K.; Ndakidemi, P.A. Effects of Rhizobium inoculation, lime and molybdenum on photosynthesis and chlorophyll content of Phaeseolus vulgaris L. Afr. J. Microbiol. Res. 2009, 3, 791–798. [Google Scholar]
- Saghafi, D.; Ghorbanpour, M.; Lajayer, B.A. Efficiency of Rhizobium strains as plant growth promoting rhizobacteria on morpho-physiological properties of Brassica napus L. under salinity stress. J. Soil Sci. Plant Nutr. 2018, 18, 253–268. [Google Scholar] [CrossRef][Green Version]
- Jiménez-Gómez, A.; Flores-Félix, J.D.; García-Fraile, P.; Mateos, P.F.; Menéndez, E.; Velázquez, E. Probiotic activities of Rhizobium laguerreae on growth and quality of spinach. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Tairo, E.V.; Ndakidemi, P.A. Bradyrhizobium japonicum inoculation and phosphorus supplementation on growth and chlorophyll accumulation in soybean (Glycine max L.). Am. J. Plant Sci. 2013, 4, 40398. [Google Scholar] [CrossRef][Green Version]
- Nyoki, D.; Ndakidemi, P.A. Effects of phosphorus and Bradyrhizobium japonicum on growth and chlorophyll content of cowpea (Vigna unguiculata (L) Walp). J. Exp. Agric. Int. 2014, 1120–1136. [Google Scholar] [CrossRef]
- Routray, S.; Khanna, V. Characterization of rhizobacteria for multiple plant growth promoting traits from mung bean rhizosphere. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 2264–2269. [Google Scholar] [CrossRef]
- Kaur, G.; Khanna, V. Evaluation of thermotolerant rhizobacteria for multiple plant growth promoting traits from pigeonpea rhizosphere. J. Appl. Nat. Sci. 2017, 9, 920–923. [Google Scholar] [CrossRef]
- Hafeez, F.Y.; Abaid-Ullah, M.; Hassan, M.N. Plant growth-promoting rhizobacteria as zinc mobilizers: A promising approach for cereals biofortification. In Bacteria in Agrobiology: Crop Productivity; Springer: Berlin, Germany, 2013; pp. 217–235. [Google Scholar]
- Chen, Y.; Rekha, P.; Arun, A.; Shen, F.; Lai, W.-A.; Young, C.C. Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl. Soil Ecol. 2006, 34, 33–41. [Google Scholar] [CrossRef]
- Shahab, S.; Ahmed, N.; Khan, N.S. Indole acetic acid production and enhanced plant growth promotion by indigenous PSBs. Afr. J. Agric. Res. 2009, 4, 1312–1316. [Google Scholar]


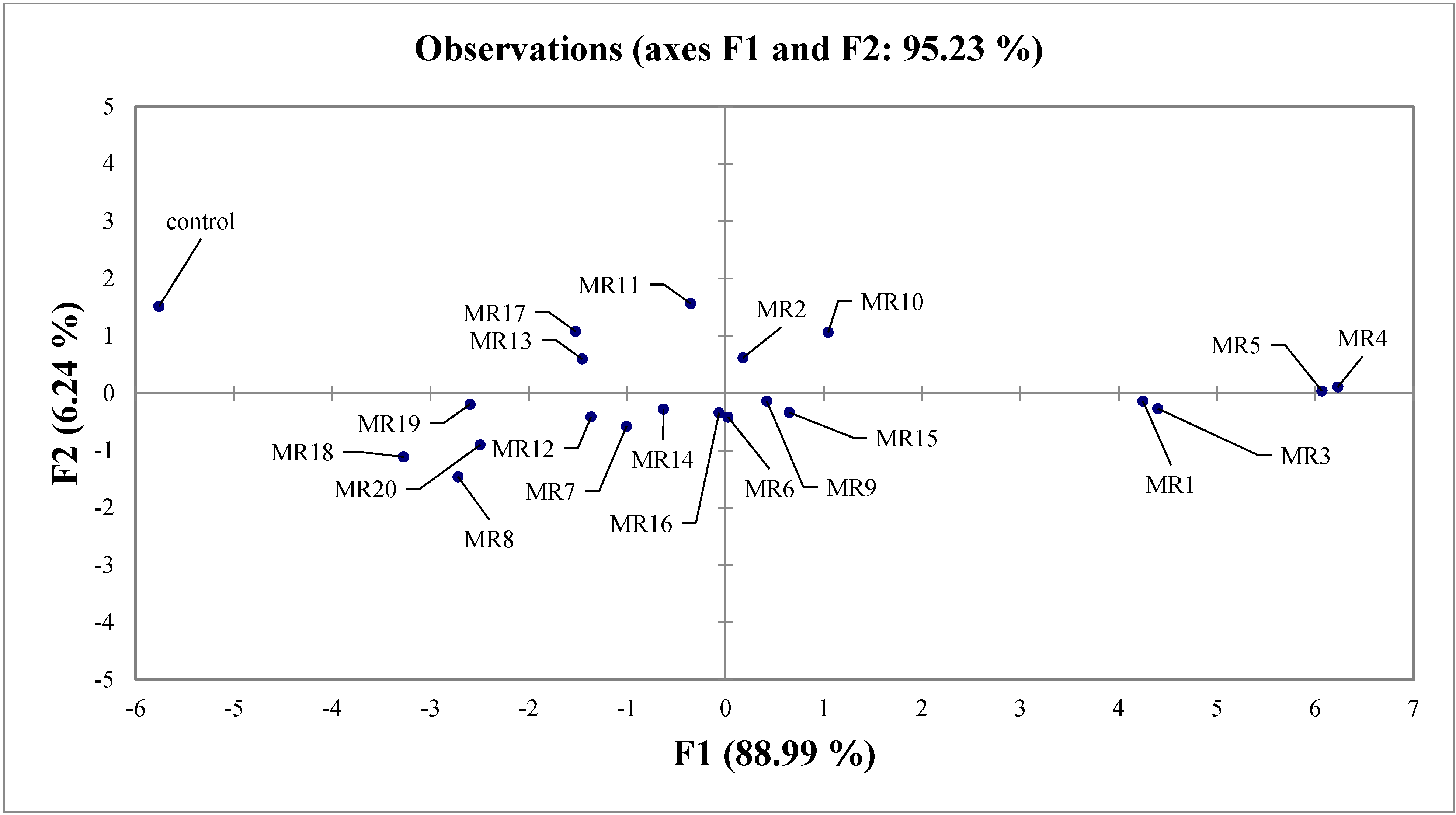


| Observation | F1 | F2 | F3 | F4 | F5 |
|---|---|---|---|---|---|
| Control | −5.763 | 1.515 | 1.093 | 0.292 | −0.090 |
| MR1 | 4.246 | −0.137 | 0.656 | −0.152 | 0.088 |
| MR2 | 0.179 | 0.618 | −0.989 | −0.210 | 0.296 |
| MR3 | 4.397 | −0.269 | −0.301 | 0.319 | 0.130 |
| MR4 | 6.228 | 0.109 | 0.110 | 0.545 | −0.238 |
| MR5 | 6.067 | 0.039 | −0.287 | −0.133 | −0.012 |
| MR6 | 0.025 | −0.417 | 0.614 | 0.324 | 0.212 |
| MR7 | −1.006 | −0.579 | 0.315 | 0.090 | 0.400 |
| MR8 | −2.720 | −1.460 | 0.187 | −0.055 | 0.070 |
| MR9 | 0.421 | −0.138 | 0.228 | 0.003 | −0.321 |
| MR10 | 1.045 | 1.064 | 0.029 | −0.754 | 0.093 |
| MR11 | −0.355 | 1.563 | 0.082 | 0.012 | 0.034 |
| MR12 | −1.368 | −0.417 | 0.047 | −0.022 | 0.106 |
| MR13 | −1.459 | 0.597 | −0.764 | 0.197 | −0.282 |
| MR14 | −0.630 | −0.281 | 0.792 | −0.194 | −0.131 |
| MR15 | 0.650 | −0.336 | 0.338 | −0.220 | −0.080 |
| MR16 | −0.066 | −0.342 | 0.330 | −0.386 | −0.234 |
| MR17 | −1.524 | 1.079 | −0.518 | 0.292 | 0.234 |
| MR18 | −3.275 | −1.111 | −0.294 | 0.109 | 0.005 |
| MR19 | −2.598 | −0.192 | −1.134 | −0.058 | −0.255 |
| MR20 | −2.496 | −0.904 | −0.536 | 0.002 | −0.027 |
| Codes | MR1 | MR3 | MR4 | MR5 |
|---|---|---|---|---|
| Strains | Rhizobium phaseoli | Rhizobium phaseoli | Rhizobium phaseoli | Rhizobium phaseoli |
| Cell shape | Rod-shaped | Rod-shaped | Rod-shaped | Rod-shaped |
| Colony color | Milky white | Milky white | Milky white | Milky white |
| Colony appearance | Shiny | Shiny | Shiny | Shiny |
| Colony shape | Round | Round | Round | Round |
| Gram’s test | Negative | Negative | Negative | Negative |
| Oxidase test | Positive | Positive | Positive | Positive |
| Catalase test | Positive | Positive | Positive | Positive |
| Exoplysaccahride activity | Positive | Positive | Positive | Positive |
| Auxin biosynthesis | Highly positive | Highly positive | Highly positive | Highly positive |
| Chitinase activity | Positive | Positive | Positive | Positive |
| Phosphate solubilization | Positive | Positive | Positive | Positive |
| Siderophore production | Positive | Negative | Positive | Positive |
| Organic acid production | Negative | Negative | Positive | Positive |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmood, A.; Shahzad, T.; Hussain, S.; Ali, Q.; Ali, H.M.; Yasin, S.; Ibrahim, M.; Salem, M.Z.M.; Khalid, M. Evaluation of Symbiotic Association between Various Rhizobia, Capable of Producing Plant-Growth-Promoting Biomolecules, and Mung Bean for Sustainable Production. Sustainability 2021, 13, 13832. https://doi.org/10.3390/su132413832
Mahmood A, Shahzad T, Hussain S, Ali Q, Ali HM, Yasin S, Ibrahim M, Salem MZM, Khalid M. Evaluation of Symbiotic Association between Various Rhizobia, Capable of Producing Plant-Growth-Promoting Biomolecules, and Mung Bean for Sustainable Production. Sustainability. 2021; 13(24):13832. https://doi.org/10.3390/su132413832
Chicago/Turabian StyleMahmood, Abid, Tanvir Shahzad, Sabir Hussain, Qasim Ali, Hayssam M. Ali, Sanaullah Yasin, Muhammad Ibrahim, Mohamed Z. M. Salem, and Muhammad Khalid. 2021. "Evaluation of Symbiotic Association between Various Rhizobia, Capable of Producing Plant-Growth-Promoting Biomolecules, and Mung Bean for Sustainable Production" Sustainability 13, no. 24: 13832. https://doi.org/10.3390/su132413832
APA StyleMahmood, A., Shahzad, T., Hussain, S., Ali, Q., Ali, H. M., Yasin, S., Ibrahim, M., Salem, M. Z. M., & Khalid, M. (2021). Evaluation of Symbiotic Association between Various Rhizobia, Capable of Producing Plant-Growth-Promoting Biomolecules, and Mung Bean for Sustainable Production. Sustainability, 13(24), 13832. https://doi.org/10.3390/su132413832








