Diminishing Heavy Metal Hazards of Contaminated Soil via Biochar Supplementation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Samples
2.2. Biochar Preparation
2.3. Pot Experiment
2.4. US EPA Extraction
2.5. Sequential Extraction Procedure (SEP)
2.6. Quality Control
2.7. Statistical Analysis
3. Results
3.1. Biochar Characterization
3.2. Soil Properties
3.3. Heavy Metal Mobility and Redistribution
3.3.1. TCLP-Extractable Metals
3.3.2. Acid-Soluble Fraction
3.3.3. Oxide-Bound Fraction
3.3.4. Organic Matter Bound Fraction
3.3.5. Residual Metals
4. Discussion
4.1. Biochar Effect
4.2. Pyrolysis Effect
4.3. Metal Mobility and Redistribution
4.4. Heavy Metal Immobilization Mechanisms
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murtaza, G.; Ditta, A.; Ullah, N.; Usman, M.; Ahmed, Z. Biochar for the management of nutrient impoverished and metal contaminated soils: Preparation, applications, and prospects. J. Soil Sci. Plant Nutr. 2021, 21, 2191–2213. [Google Scholar] [CrossRef]
- Saleh, T.A.; Gupta, V.K. Column with CNT/magnesium oxide composite for lead (II) removal from water. Environ. Sci. Pollut. Res. 2012, 19, 1224–1228. [Google Scholar] [CrossRef]
- Awad, M.; Moustafa-Farag, M.; Wei, L.; Huang, Q.; Liu, Z. Effect of garden waste biochar on the bioavailability of heavy metals and growth of Brassica juncea (L.) in a multi-contaminated soil. Arab. J. Geosci. 2020, 13, 439. [Google Scholar] [CrossRef]
- Murtaza, G.; Ahmed, Z.; Usman, M.; Tariq, W.; Ullah, Z.; Shareef, M.; Iqbal, H.; Waqas, M.; Tariq, A.; Wu, Y.; et al. Biochar induced modifications in soil properties and its impacts on crop growth and production. J. Plant Nutr. 2021, 44, 1677–1691. [Google Scholar] [CrossRef]
- Khan, K.Y.; Ali, B.; Cui, X.; Feng, Y.; Yang, X.; Stoffella, P.J. Impact of different feedstocks derived biochar amendment with cadmium low uptake affinity cultivar of pak choi (Brassica rapa ssb. chinensis L.) on phytoavoidation of Cd to reduce potential dietary toxicity. Ecotoxicol. Environ. Saf. 2017, 141, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Yang, X.; Gielen, G.; Bolan, N.; Ok, Y.S.; Niazi, N.K.; Xu, S.; Yuan, G.; Chen, X.; Zhang, X.; et al. Effect of bamboo and rice straw biochars on the mobility and redistribution of heavy metals (Cd, Cu, Pb and Zn) in contaminated soil. J. Environ. Manag. 2017, 186, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Awad, M.Y.M.; El-Desoky, M.; Abdel-Mawly, S.E.; Ghallab, A. Mobility of Heavy Metals in Some Contaminated Egyptian Soils Treated with Certain Organic Materials. Ph.D. Thesis, Assiut University, Assiut, Egypt, 2007. [Google Scholar]
- LI, J.; XU, Y. Immobilization of Cd in paddy soil using moisture management and amendment. Environ. Sci. Pollut. Res. 2015, 22, 5580–5586. [Google Scholar] [CrossRef] [PubMed]
- Irshad, S.; Xie, Z.; Mehmood, S.; Nawaz, A.; Ditta, A.; Mahmood, Q. Insights into conventional and recent technologies for arsenic bioremediation: A systematic review. Environ. Sci. Pollut. Res. 2021, 28, 18870–18892. [Google Scholar] [CrossRef]
- Younis, U.; Rahi, A.A.; Danish, S.; Younis, U.; Ali, M.A.; Ahmed, N.; Datta, R.; Fahad, S.; Holatko, J.; Hammerschmiedt, T.; et al. Fourier Transform Infrared Spectroscopy vibrational bands study of Spinacia oleracea and Trigonella corniculata under biochar amendment in naturally contaminated soil. PLoS ONE 2021, 16, e0253390. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Rahman, M.H.u.; Haider, G.; Amir, R.; Ikram, R.M.; Ahmad, S.; Schofield, H.K.; Riaz, B.; Iqbal, R.; Fahad, S.; et al. Chemical and Biological Enhancement Effects of Biochar on Wheat Growth and Yield under Arid Field Conditions. Sustainability 2021, 13, 5890. [Google Scholar] [CrossRef]
- Sarfraz, R.; Hussain, A.; Sabir, A.; Fekih, I.B.; Ditta, A.; Xing, S. Role of Biochar and plant growth-promoting rhizobacteria to enhance soil carbon sequestration—A review. Environ. Monit. Assess. 2019, 191, 251. [Google Scholar] [CrossRef]
- Rahman, S.U.; Xuebin, Q.; Riaz, L.; Yasin, G.; Shah, A.N.; Shahzad, U.; Jahan, M.S.; Ditta, A.; Bashir, M.A.; Rehim, A.; et al. The interactive effect of pH variation and cadmium stress on wheat (Triticum aestivum L.) growth, physiological and bio-chemical parameters. PLoS ONE 2021, 16, e0253798. [Google Scholar] [CrossRef] [PubMed]
- Lima, I.M.; Boykin, D.L.; Klasson, K.T.; Uchimiya, M. Influence of post-treatment strategies on the properties of activated chars from broiler manure. Chemosphere 2014, 95, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Uchimiya, M.; Lima, I.M.; Klasson, T.; Wartelle, L.H. Contaminant immobilization and nutrient release by biochar soil amendment: Roles of natural organic matter. Chemosphere 2010, 80, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Shi, Y.; Qiu, Y.; Che, L.; Xue, C. Performance and mechanisms of emerging animal-derived biochars for immobilization of heavy metals. Sci. Total Environ. 2019, 646, 1281–1289. [Google Scholar] [CrossRef]
- Ozcan, N.; Altundag, H. Speciation of heavy metals in street dust samples from sakarya I. Organized industrial district using the BCR sequential extraction procedure by ICP-OES. Bull. Chem. Soc. Ethiop. 2013, 27, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Awad, M.Y.M.; El-Desoky, M.A.; Ghallab, A.; Abdel-Mawly, S.E. Changes in Soil Zn and Mn Forms of Some Contaminated Egyptian Soils Treated with Organic Materials. Assiut J. Agric. Sci. 2017, 48, 269–285. [Google Scholar]
- Zang, F.; Wang, S.; Nan, Z.; Ma, J.; Wang, Y.; Chen, Y.; Zhang, Q.; Li, Y. Influence of pH on the release and chemical fractionation of heavy metals in sediment from a suburban drainage stream in an arid mine-based oasis. J. Soils Sediments 2017, 17, 2524–2536. [Google Scholar] [CrossRef]
- Shiowatana, J.; McLaren, R.G.; Chanmekha, N.; Samphao, A. Fractionation of arsenic in soil by a continuous-flow sequential extraction method. J. Environ. Qual. 2001, 30, 1940–1949. [Google Scholar] [CrossRef] [Green Version]
- Ullah, I.; Ditta, A.; Imtiaz, M.; Mehmood, S.; Rizwan, M.; Rizwan, M.S.; Jan, A.U.; Ahmad, I. Assessment of health and ecological risks of heavy metal contamination: A case study of agricultural soils in Thall, Dir-Kohistan. Environ. Monit. Assess. 2020, 192, 786. [Google Scholar] [CrossRef]
- Buekers, J. Fixation of Cadmium, Copper, Nickel and Zinc in Soil: Kinetics, Mechanisms and Its Effect on Metal Bioavailability. Ph.D. Thesis, Katholieke Universiteit Lueven, Leuven, Belgium,, 2007. Dissertationes De Agricultura, Doctoraatsprooefschrift nr.. [Google Scholar]
- Levy, D.B.; Barbarick, K.A.; Siemer, E.G.; Sommers, L.E. Distribution and partitioning of trace metals in contaminated soils near Leadville, Colorado. J. Environ. Qual. 1992, 21, 185–195. [Google Scholar] [CrossRef]
- Saracoglu, S.; Soylak, M.; Elci, L. Extractable trace metals content of dust from vehicle air filters as determined by sequential extraction and flame atomic absorption spectrometry. J. AOAC Int. 2009, 92, 1196–1202. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Martín, M.J.; García-Delgado, M.; Lorenzo, L.F.; Rodríguez-Cruz, M.S.; Arienzo, M. Heavy metals in sewage sludge amended soils determined by sequential extractions as a function of incubation time of soils. Geoderma 2007, 142, 262–273. [Google Scholar] [CrossRef]
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils. Soil Sci. 1954, 78, 154. [Google Scholar] [CrossRef]
- Nelson, D.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Ed.; John Wiley & Sons: New York, NY, USA, 1982; pp. 539–579. [Google Scholar]
- Burt, R. Soil Survey Laboratory Methods Manual. In Soil Survey Investigations Report No. 42, Version 4.0; Natural Resources Conservation Service, US Department of Agriculture: Washington, DC, USA, 2004. [Google Scholar]
- USEPA. Method 1311 TCLP-Toxicity Characteristic Leaching Procedure. In Test Methods for Evaluating Solid Waste, 3rd ed.; Environmental Protection Agency: Washington, DC, USA, 1992. [Google Scholar]
- Žemberyová, M.; Barteková, J.; Hagarová, I. The utilization of modified BCR three-step sequential extraction procedure for the fractionation of Cd, Cr, Cu, Ni, Pb and Zn in soil reference materials of different origins. Talanta 2006, 70, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Awad, M.Y.M. Effect of Some Organic Compounds on Soil Properties and Plant Growth. Master’s Thesis, Menoufia University, Al Minufya, Egypt, 2001. [Google Scholar]
- Rekaby, S.A.; Awad, M.Y.M.; Hegab, S.A.; Eissa, M.A. Effect of some organic amendments on barley plants under saline condition. J. Plant Nutr. 2020, 43, 1840–1851. [Google Scholar] [CrossRef]
- Rekaby, S.A.; Awad, M.; Majrashi, A.; Ali, E.F.; Eissa, M.A. Corn Cob-Derived Biochar Improves the Growth of Saline-Irrigated Quinoa in Different Orders of Egyptian Soils. Horticulturae 2021, 7, 221. [Google Scholar] [CrossRef]
- Naveed, M.; Tanvir, B.; Xiukang, W.; Brtnicky, M.; Ditta, A.; Kucerik, J.; Subhani, Z.; Nazir, M.Z.; Radziemska, M.; Saeed, Q.; et al. Co-composted biochar enhances growth, physiological and phytostabilization efficiency of Brassica napus and reduces associated health risks under Cr stress. Front. Plant Sci. 2021. not published. [Google Scholar] [CrossRef]
- Awad, M.; Liu, Z.; Skalicky, M.; Dessoky, E.S.; Brestic, M.; Mbarki, S.; Rastogi, A.; EL Sabagh, A. Fractionation of Heavy Metals in Multi-Contaminated Soil Treated with Biochar Using the Sequential Extraction Procedure. Biomolecules 2021, 11, 448. [Google Scholar] [CrossRef]
- Awad, M.; El-Desoky, M.A.; Ghallab, A.; Kubes, J.; Abdel-Mawly, S.E.; Danish, S.; Ratnasekera, D.; Sohidul Islam, M.; Skalicky, M.; Brestic, M.; et al. Ornamental Plant Efficiency for Heavy Metals Phytoextraction from Contaminated Soils Amended with Organic Materials. Molecules 2021, 26, 3360. [Google Scholar] [CrossRef]
- Uchimiya, M.; Chang, S.; Klasson, K.T. Screening biochars for heavy metal retention in soil: Role of oxygen functional groups. J. Hazard. Mater. 2011, 190, 432–441. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, W.; Yang, Y.; Huang, X.; Wang, S.; Qiu, R. Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar. Water Res. 2012, 46, 854–862. [Google Scholar] [CrossRef]
- Ali, A.M.; Hegab, S.A.; Abd El Gawad, A.M.; Awad, M. Integrated Effect of Filter Mud Cake Combined with Chemical and Biofertilizers to Enhance Potato Growth and Its Yield. J. Soil Sci. Plant Nutr. 2021, 4, 1–10. [Google Scholar] [CrossRef]
- Beesley, L.; Marmiroli, M. The immobilization and retention of soluble arsenic, cadmium and zinc by biochar. Environ. Pollut. 2011, 159, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Houben, D.; Evrard, L.; Sonnet, P. Mobility, bioavailability and pH-dependent leaching of cadmium, zinc and lead in a contaminated soil amended with biochar. Chemosphere 2013, 92, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Solaiman, Z.M.; Meney, K.; Murphy, D.V.; Rengel, Z. Biochars immobilize soil cadmium, but do not improve growth of emergent wetland species Juncus subsecundus in cadmium-contaminated soil. J. Soils Sediments 2013, 13, 140–151. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; McGrouther, K.; Huang, H.; Lu, K.; Guo, X.; He, L.; Lin, X.; Che, L.; Ye, Z.; et al. Effect of biochar on the extractability of heavy metals (Cd, Cu, Pb, and Zn) and enzyme activity in soil. Environ. Sci. Pollut. Res. 2016, 23, 974–984. [Google Scholar] [CrossRef]
- Mehmood, S.; Ahmed, W.; Rizwan, M.; Imtiaz, M.; Elnahal, A.S.M.A.; Ditta, A.; Irshad, S.; Ikram, M.; Li, W. Comparative efficacy of raw and HNO3-modified biochar derived from rice straw on vanadium transformation and its uptake by rice (Oryza sativa L.): Insights from photosynthesis, antioxidative response, and gene-expression profile. Environ. Pollut. 2021, 289, 117916. [Google Scholar] [CrossRef]
- Yuan, J.H.; Xu, R.K. The amelioration effects of low temperature biochar generated from nine crop residues on an acidic Ultisol. Soil Use Manag. 2011, 27, 110–115. [Google Scholar] [CrossRef]
- Uchimiya, M.; Bannon, D.I.; Wartelle, L.H.; Lima, I.M.; Klasson, K.T. Lead retention by broiler litter biochars in small arms range soil: Impact of pyrolysis temperature. J. Agric. Food Chem. 2012, 60, 5035–5044. [Google Scholar] [CrossRef]
- Ijaz, M.; Rizwan, M.S.; Sarfraz, M.; Ul-Allah, S.; Sher, A.; Sattar, A.; Ali, L.; Ditta, A.; Yousaf, B. Biochar reduced cadmium uptake and enhanced wheat productivity in alkaline contaminated soil. Int. J. Agric. Biol. 2020, 24, 1633–1640. [Google Scholar]
- Cao, C.Y.; Yu, B.; Wang, M.; Zhao, Y.Y.; Wan, X.; Zhao, S. Immobilization of cadmium in simulated contaminated soils using thermal-activated serpentine. Soil Sci. Plant Nutr. 2020, 66, 499–505. [Google Scholar] [CrossRef]
- Sabir, A.; Naveed, M.; Bashir, M.A.; Hussain, A.; Mustafa, A.; Zahir, Z.A.; Kamran, M.; Ditta, A.; Núñez-Delgado, A.; Saeed, Q.; et al. Cadmium mediated phytotoxic impacts in Brassica napus: Managing growth, physiological and oxidative disturbances through combined use of biochar and Enterobacter sp. MN17. J. Environ. Manag. 2020, 265, 110522. [Google Scholar] [CrossRef]
- Feng, X.H.; Zhai, L.M.; Tan, W.F.; Liu, F.; He, J.Z. Adsorption and redox reactions of heavy metals on synthesized Mn oxide minerals. Environ. Pollut. 2007, 147, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, S.; Rizwan, M.; Bashir, S.; Ditta, A.; Aziz, O.; Yong, L.Z.; Dai, Z.; Akmal, M.; Ahmed, W.; Adeel, M.; et al. Comparative Effects of Biochar, Slag and Ferrous–Mn Ore on Lead and Cadmium Immobilization in Soil. Bull. Environ. Contamin. Toxicol. 2018, 100, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Lucchini, P.; Quilliam, R.S.; DeLuca, T.H.; Vamerali, T.; Jones, D.L. Increased bioavailability of metals in two contrasting agricultural soils treated with waste wood-derived biochar and ash. Environ. Sci. Pollut. Res. 2014, 21, 3230–3240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, O.; Sun, K.; Liu, X.; Zheng, W.; Zhaoil, Y. Sorption of simazine to corn straw biochars prepared at different pyrolytic temperatures. Environ. Pollut. 2011, 159, 2594–2601. [Google Scholar] [CrossRef] [PubMed]
- Beesley, L.; Moreno-Jimenez, E.; Gomez-Eyles, J.L. Effects of biochar and greenwaste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environ. Pollut. 2010, 158, 2282–2287. [Google Scholar] [CrossRef]
- Jalali, M.; Moharami, S. Effects of the addition of phosphorus on the redistribution of cadmium, copper, lead, nickel, and zinc among soil fractions in contaminated calcareous soil. Soil Sediment Contamin. 2009, 19, 88–102. [Google Scholar] [CrossRef]
- Mehmood, S.; Imtiaz, M.; Bashir, S.; Rizwan, M.; Irshad, S.; Yuvaraja, G.; Ikram, M.; Aziz, O.; Ditta, A.; Rehman, S.U.; et al. Leaching behavior of Pb and Cd and transformation of their speciation in co-contaminated soil receiving different passivators. Environ. Eng. Sci. 2019, 36, 749–759. [Google Scholar] [CrossRef]
- Mehmood, S.; Saeed, D.A.; Rizwan, M.; Khan, M.N.; Aziz, O.; Bashir, S.; Ibrahim, M.; Ditta, A.; Akmal, M.; Mumtaz, M.A.; et al. Impact of different amendments on biochemical responses of sesame (Sesamum indicum L.) plants grown in lead-cadmium contaminated soil. Plant Physiol. Biochem. 2018, 132, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Banat, K.M.; Howari, F.M.; To’Mah, M.M. Chemical fractionation and heavy metal distribution in agricultural soils, north of Jordan Valley. Soil Sediment Contamin. 2007, 16, 89–107. [Google Scholar] [CrossRef]
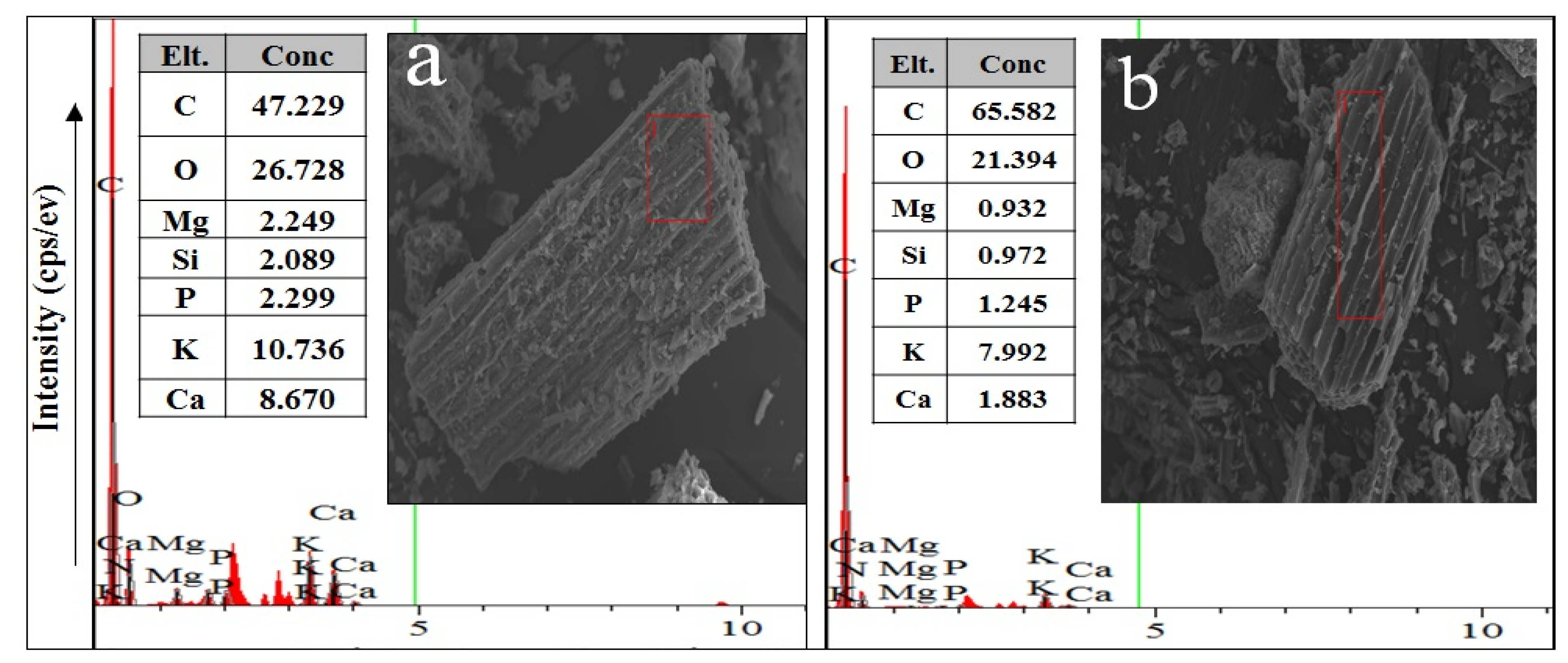
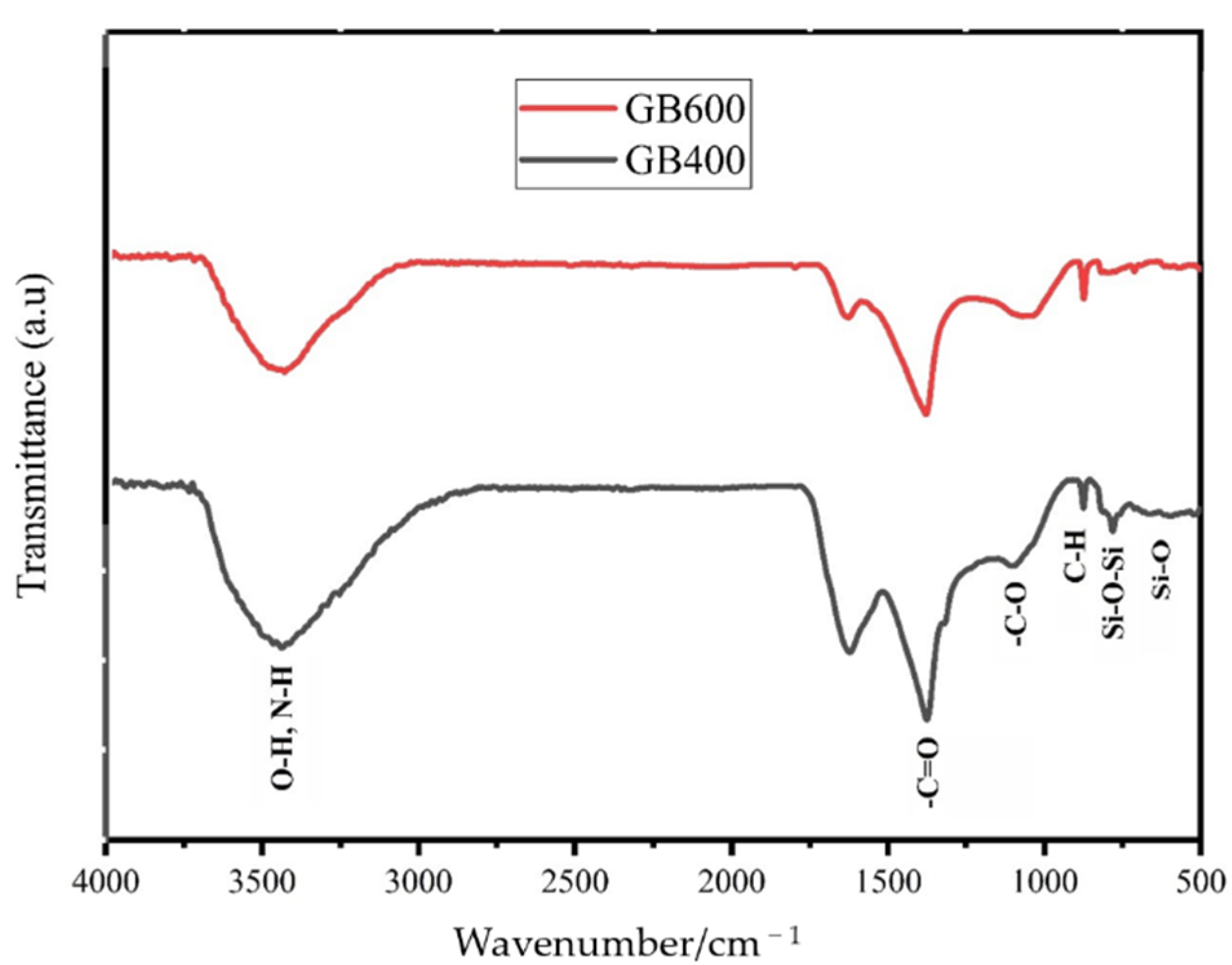

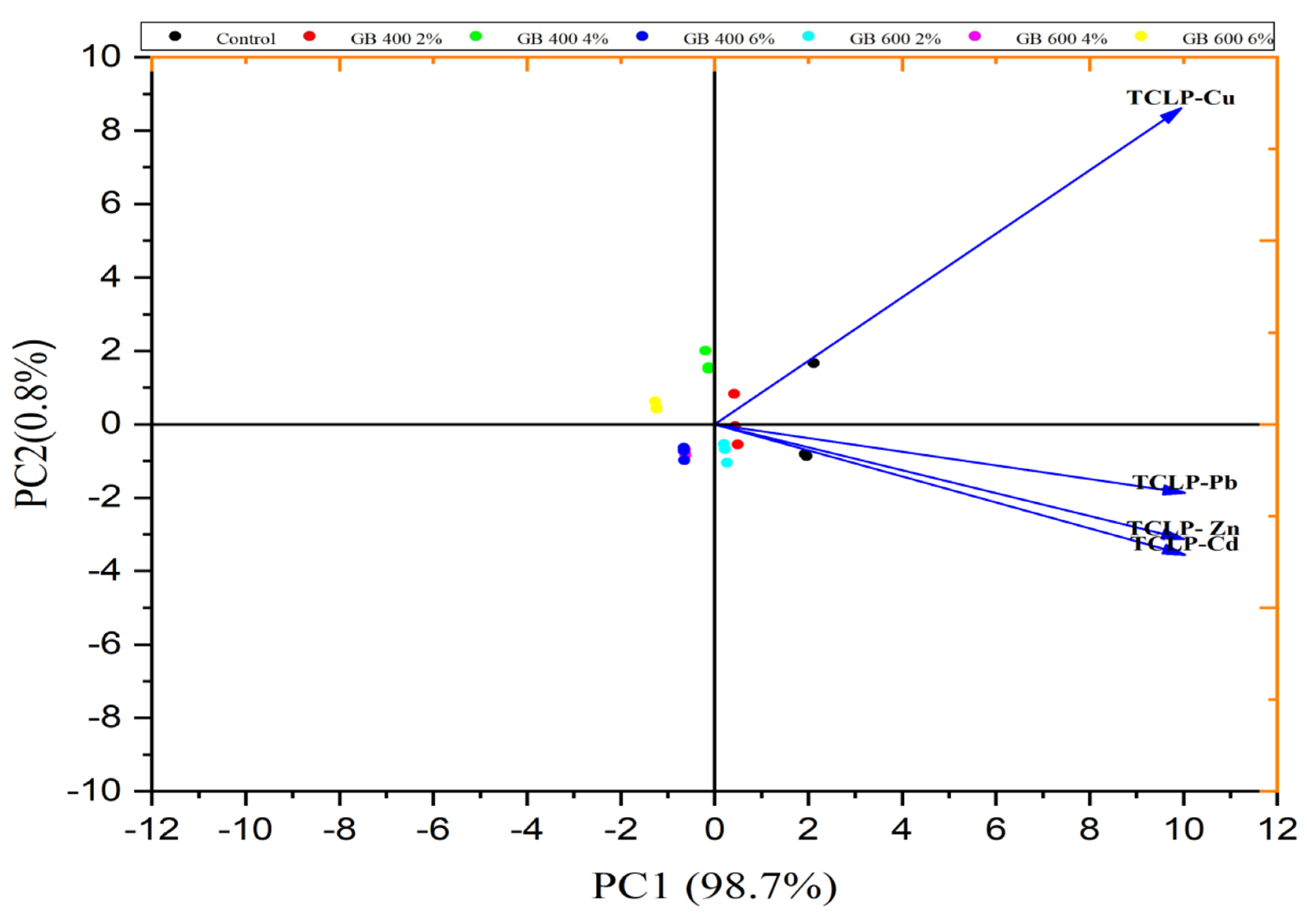
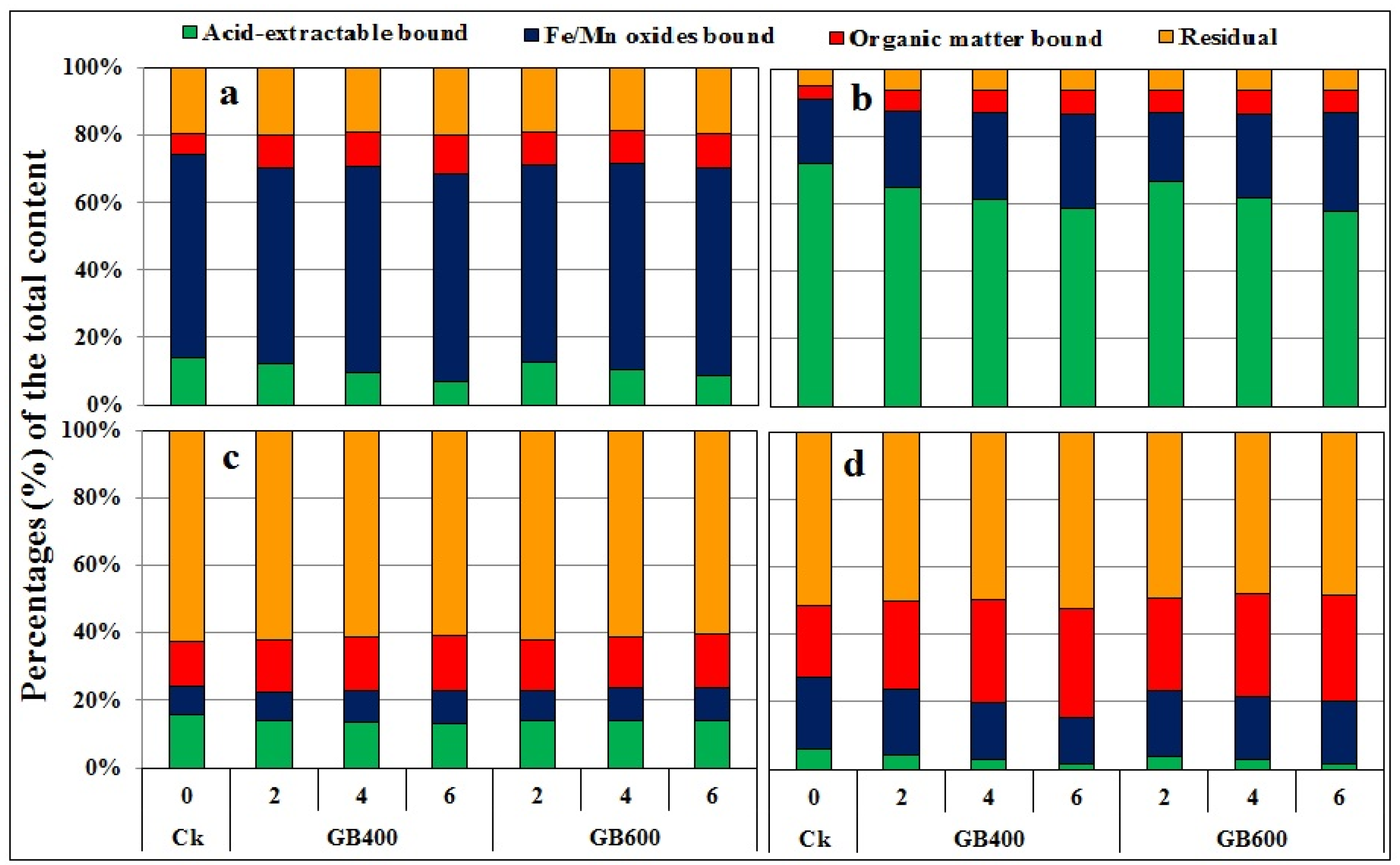
| Property | Unit | Soil | Garden Waste Biochars (GB) | |
|---|---|---|---|---|
| GB400 | GB600 | |||
| Clay | g kg−1 | 258 | - | - |
| Silt | g kg−1 | 319 | - | - |
| Sand | g kg−1 | 423 | - | - |
| Texture | Loam | - | - | |
| Organic matter | g kg−1 | 23.45 | 867 | 862 |
| EC (1:5) | dS m‒1 | 0.92 | 5.50 | 7.00 |
| pH (1:2.5) | - | 5.54 | 11.00 | 11.50 |
| Total Cd | mg kg−1 | 2.44 | ND | ND |
| Total Pb | mg kg−1 | 980 | 7.00 | 11.25 |
| Total Zn | mg kg−1 | 1019 | 82.00 | 107.00 |
| Total Cu | mg kg−1 | 34 | 18.50 | 26.90 |
| Biochar Treatment | Biochar Rate (%) | OM (g kg−1) | pH (1:2.5) | EC (1:10) |
|---|---|---|---|---|
| CK | 0 | 23.7 ± 0.09 g | 5.39 ± 0.02 h | 0.45 ± 0.05 f |
| GB400 | 2 | 33.8 ± 0.31 f | 6.16 ± 0.10 d | 0.33 ± 0.10 g |
| 4 | 49.8 ± 0.12 c,d | 7.04 ± 0.25 b | 0.60 ± 0.01 c,d | |
| 6 | 55.2 ± 0.48 a,b | 7.27 ± 0.30 a | 0.62 ± 0.04 b,c | |
| GB600 | 2 | 33.8 ± 0.39 f | 6.15 ± 0.12 d | 0.54 ± 0.07 e |
| 4 | 43.6 ± 0.40 e | 7.02 ± 0.01 b | 0.56 ± 0.02 d,e | |
| 6 | 48.0 ± 0.22 d | 7.26 ± 0.29 a | 0.64 ± 0.03 a,b,c |
| Biochar Treatment | Biochar Rate (%) | Pb | Cd | Zn | Cu |
|---|---|---|---|---|---|
| CK | 0 | 137.79 ± 1 a | 1.83 ± 0.14 a | 168.01 ± 3 a | 2.01 ± 0.13 a |
| GB400 | 2 | 119.84 ± 2 c | 1.54 ±0.15 b | 150.70 ± 3 b | 1.28 ± 0.01 b |
| 4 | 95.01 ± 1 e | 1.44 ± 0.10 c | 146.82 ± 13 b | 0.91 ± 0.05 c | |
| 6 | 66.77 ± 3 g | 1.38 ± 0.08 c | 141.68 ± 1 c | 0.52 ± 0.02 d | |
| GB600 | 2 | 125.16 ± 3 b | 1.59± 0.07 b | 153.24 ± 2 b | 1.28 ± 0.01 b |
| 4 | 103.93 ± 5 d | 1.44 ± 0.10 c | 152.55 ± 3 b | 0.90 ± 0.01 c | |
| 6 | 85.69 ± 5 f | 1.42 ± 0.09 c | 151.56 ± 1 b | 0.52 ± 0.02 d |
| Biochar Treatment | Biochar Rate (%) | Pb | Cd | Zn | Cu |
|---|---|---|---|---|---|
| CK | 0 | 602.33 ± 10 a,b | 0.494 ± 0.013 f | 85.83 ± 4 c | 6.94 ± 0.71 a |
| GB400 | 2 | 561.02 ± 6 d,c | 0.537 ± 0.006 e | 93.66 ± 1 b | 6.25 ± 0.01 b |
| 4 | 614.15 ± 6 a | 0.602 ± 0.007 c | 99.50 ± 1 a | 5.48 ±0.01 c | |
| 6 | 593.48 ± 13 a,b | 0.669 ± 0.010 b | 103.42 ± 3 a | 4.34 ± 0.01 d | |
| GB600 | 2 | 582.65 ± 1 b | 0.486 ± 0.013 f | 94.22 ± 3 b | 6.63 ±0.02 a,b |
| 4 | 615.87 ± 18 a | 0.569 ± 0.078 d | 101.70 ± 3 a | 6.38 ± 0.44 b | |
| 6 | 592.60 ± 10 a,b | 0.706 ± 0.003 a | 102.83 ± 10 a | 6.39 ± 0.42 b |
| Biochar Treatment | Biochar Rate (%) | Pb | Cd | Zn | Cu |
|---|---|---|---|---|---|
| CK | 0 | 62.03 ± 1 e | 0.104 ± 0.011 c | 141.82 ± 1 a | 7.34 ± 0.52 e |
| GB400 | 2 | 96.30 ± 1 d | 0.145 ± 0.014 b | 168.09 ± 10 a | 8.15 ± 0.05 d |
| 4 | 104.91 ± 2 b | 0.156 ± 0.005 a | 173.15 ± 5 a | 9.81 ± 0.45 b | |
| 6 | 109.24 ± 1 a | 0.167 ± 0.015 a | 175.46 ± 1 a | 10.32 ± 0.88 a,b | |
| GB600 | 2 | 96.64 ± 2 d | 0.161 ± 0.007 a | 160.48 ± 3 a | 9.30 ± 0.05 c |
| 4 | 99.75 ±1 c | 0.163 ± 0.015 a | 166.14 ± 12 a | 10.19 ± 0.89 a,b | |
| 6 | 99.96 ± 3 c | 0.167 ± 0.004 a | 169.02 ± 9 b | 10.70 ± 0.44 a |
| Biochar Treatment | Biochar Rate (%) | Pb | Cd | Zn | Cu |
|---|---|---|---|---|---|
| CK | 0 | 196.61 ± 2 a | 0.131 ± 0.010 b | 664.67 ± 45 a | 17.01 ± 0.31 a |
| GB400 | 2 | 193.89 ± 8 a | 0.148 ± 0.002 a | 676.47 ± 16 a | 15.93 ± 1.25 a |
| 4 | 193.55 ± 10 a | 0.150 ± 0.001 a | 665.08 ± 17 a | 16.05 ± 0.93 a | |
| 6 | 193.89 ± 26 a | 0.148 ± 0.007 a | 657.42 ± 25 a | 16.81 ± 1.46 a | |
| GB600 | 2 | 193.06 ± 4 a | 0.153 ± 0.012 a | 673.63 ± 6 a | 16.75± 0.22 a |
| 4 | 187.09 ± 5 a | 0.151 ± 0.006 a | 659.60 ± 5 a | 16.11 ± 0.39 a | |
| 6 | 188.25 ± 11 a | 0.154 ± 0.001 a | 646.27 ± 5 a | 16.51 ± 1.38 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awad, M.; El-Sayed, M.M.; Li, X.; Liu, Z.; Mustafa, S.K.; Ditta, A.; Hessini, K. Diminishing Heavy Metal Hazards of Contaminated Soil via Biochar Supplementation. Sustainability 2021, 13, 12742. https://doi.org/10.3390/su132212742
Awad M, El-Sayed MM, Li X, Liu Z, Mustafa SK, Ditta A, Hessini K. Diminishing Heavy Metal Hazards of Contaminated Soil via Biochar Supplementation. Sustainability. 2021; 13(22):12742. https://doi.org/10.3390/su132212742
Chicago/Turabian StyleAwad, Mahrous, Mahmuod M. El-Sayed, Xiang Li, Zhongzhen Liu, Syed Khalid Mustafa, Allah Ditta, and Kamel Hessini. 2021. "Diminishing Heavy Metal Hazards of Contaminated Soil via Biochar Supplementation" Sustainability 13, no. 22: 12742. https://doi.org/10.3390/su132212742
APA StyleAwad, M., El-Sayed, M. M., Li, X., Liu, Z., Mustafa, S. K., Ditta, A., & Hessini, K. (2021). Diminishing Heavy Metal Hazards of Contaminated Soil via Biochar Supplementation. Sustainability, 13(22), 12742. https://doi.org/10.3390/su132212742









