Effect of Leaf Area Size on the Main Composition in Grape Must of Three Varieties of Vitis vinifera L. in an Organic Vineyard
Abstract
:1. Introduction
2. Materials and Methods
2.1. Location of the Experiment
2.2. Weather Conditions
2.3. Grape Varieties and Treatments of Experiment
2.4. Measurement of Leaf Area and Sampling
2.5. Titratable Acidity (TA) and pH
2.6. Total Soluble Solids (TSS)
2.7. Tartaric and Malic Acid (TtA, MA)
2.8. Statistical Analysis
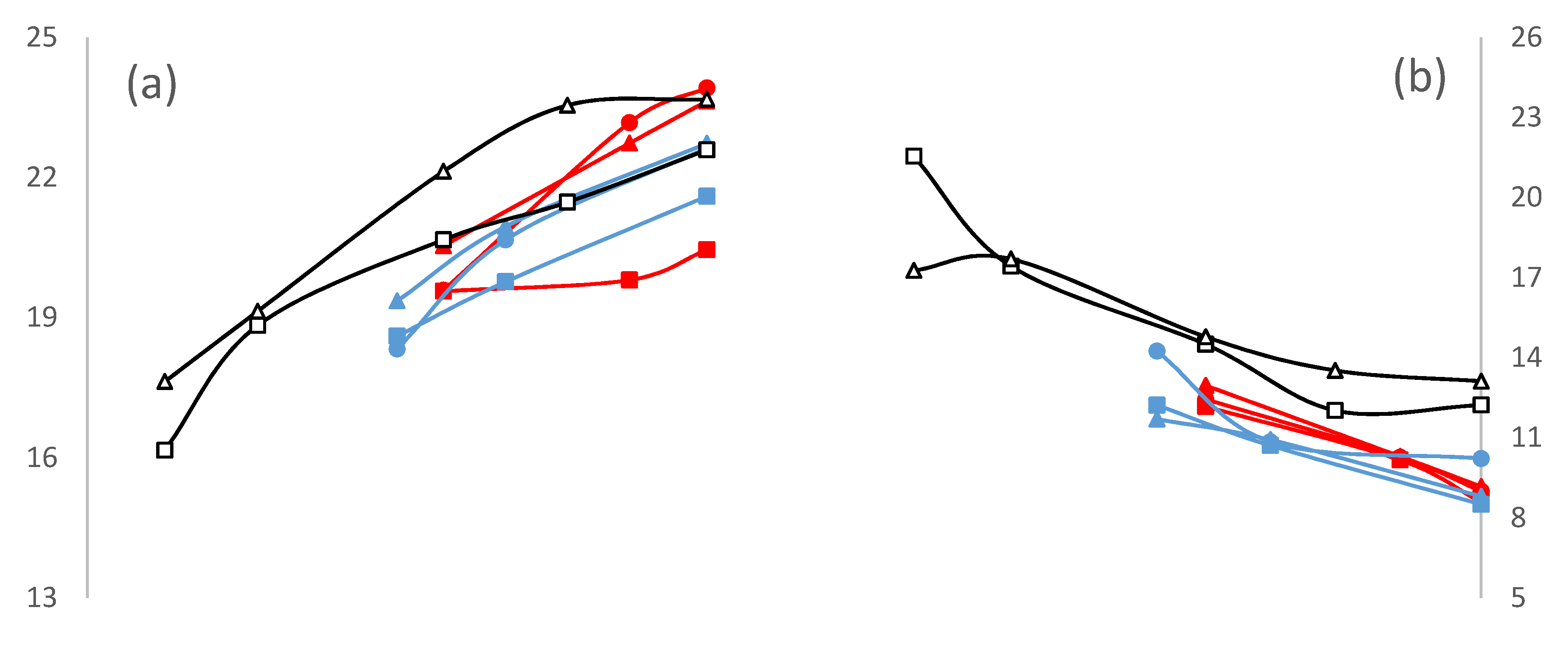
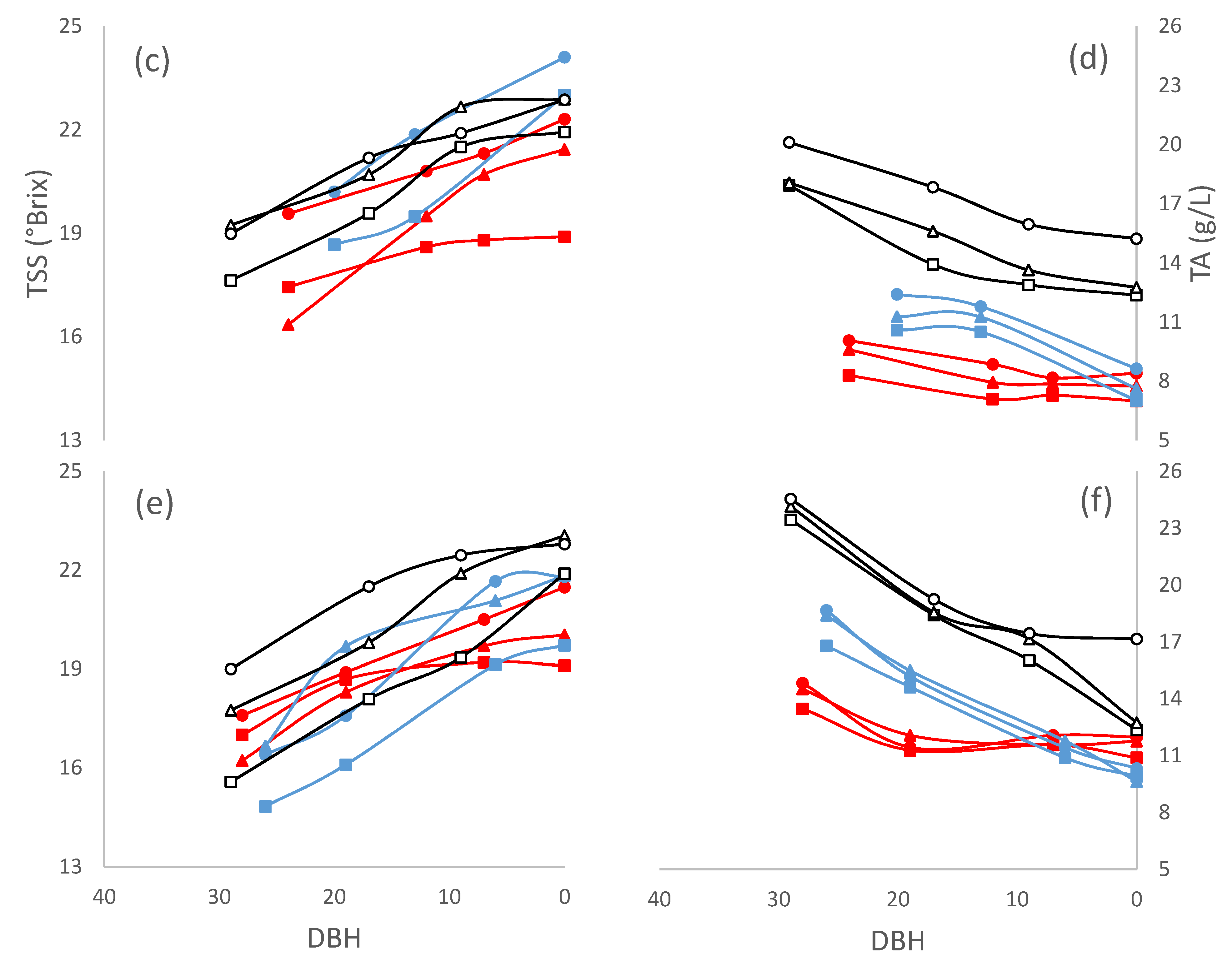
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sivčev, B.V.; Sivčev, I.L.; Rankovic-Vasic, Z.Z. Natural process and use of natural matters in organic viticulture. J. Agric. Sci. 2010, 55, 195–215. [Google Scholar]
- Bayramoglu, Z.; Gundogmus, E. Cost efficiency on organic farming: A comparison between organic and conventional raisin-producing households in turkey. Span. J. Agric. Res. 2008, 1, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Willer, H. Organic Viticulture in Europe: Development and Current Statistics. In Proceedings of the 16th IFOAM Organic Congress, Modena, Italy, 16–20 June 2008. [Google Scholar]
- Guesmi, B.; Serra, T.; Kallas, Z. The productive efficiency of organic farming: The case of grape sector in Catalonia. Span. J. Agric. Res. 2012, 10, 552–566. [Google Scholar] [CrossRef] [Green Version]
- Rojas-méndez, J.I.; Le nestour, M.; Rod, M. Understanding attitude and behavior of canadian consumers toward organic wine. J. Food Prod. Market 2015, 21, 375–396. [Google Scholar] [CrossRef]
- Brandt, K.; Mølgaard, J.P. Organic agriculture: Does it enhance or reduce the nutritional value of plant foods? J. Sci. Food Agric. 2001, 81, 924–931. [Google Scholar] [CrossRef]
- Moreno-Labanda, J.F.; Mallavia, R.; Pérez-Fons, L.; Lizama, V.; Saura, D.; Micol, V. Determination of piceid and resveratrol in Spanish wines deriving from monastrell (Vitis vinifera L.) grape variety. J. Agric. Food Chem. 2004, 52, 5396–5403. [Google Scholar] [CrossRef]
- Ruffner, H.P.; Brem, S.; Rast, D.M. Pathway of photosynthetic malate formation in Vitis vinifera. Plant. Physiol. 1983, 73, 582. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J. The Oxford Companion to Wine, 3rd ed.; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Saito, K.; Kasai, Z. Accumulation of tartaric acid in the ripening process of grapes. Plant. Cell Physiol. 1968, 9, 529–537. [Google Scholar]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. The grape and its maturation. In Handbook of Enology: The Microbiology of Wine and Vinifications, 2nd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2006; Volume 1. [Google Scholar] [CrossRef]
- Ruffner, H.P. Metabolism of tartaric and malic acids in Vitis: A review—Part A. Vitis 1982, 21, 247–259. [Google Scholar]
- Esteban, M.A.; Villanueva, M.J.; Lissarrugae, J.R. Effect of irrigation on changes in berry composition of tempranillo during maturation. sugars, organic acids, and mineral elements. Am. J. Enol. Vitic. 1999, 50, 418–434. [Google Scholar]
- Ninio, R.; Lewinsohn, E.; Mizrahi, Y.; Sitrit, Y. Changes in sugars, acids, and volatiles during ripening of koubo [Cereus peruvianus (L.) Miller] fruits. J. Agric. Food Chem. 2003, 51, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.C.; Sang, M.; Fan, M.; Wu, B.; Wang, L.; Duan, W.; Li, S. Changes of polyphenols, sugars, and organic acid in 5 vitis genotypes during berry ripening. J. Food Sci. 2011, 76, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Ollat, N.; Gaudillere, J.P. The effect of limiting leaf area during stage i of berry growth on development and composition of berries of Vitis vinifera L. cv. Cabernet Sauvignon. Am. J. Enol. Vitic. 1998, 49, 251–258. [Google Scholar]
- Poni, S.; Giachino, E. Growth, photosynthesis and cropping of potted grapevines (Vitis vinifera L. cv. Cabernet Sauvignon) in relation to shoot trimming. Aust. J. Grape. Wine R. 2000, 6, 216–226. [Google Scholar] [CrossRef]
- Petrie, P.R.; Trought, M.C.T.; Howell, G.S.; Buchan, G.D. The effect of leaf removal and canopy height on whole-vine gas exchange and fruit development of Vitis vinifera L. ‘Sauvignon Blanc’. Funct. Plant. Biol. 2003, 30, 711–717. [Google Scholar] [CrossRef]
- Hüvely, A.; Pető, J.; Pölös, E.; Cserni, I. Changes in nutrient content of grape leaves according to weather changes. Lucr. Stiint. Ser. I 2014, 16, 109–114. [Google Scholar]
- Lobit, P.; Genard, M.; Soing, P.; Habib, R. Modelling malic acid accumulation in fruits: Relationships with organic acids, potassium, and temperature. J. Exp. Bot. 2006, 57, 1471–1483. [Google Scholar] [CrossRef] [Green Version]
- Conde, C.; Silva, P.; Fontes, N.; Dias, A.C.P.; Tavares, R.M.; Sousa, J.M.; Agasse, A.; Delrot, S.; Gerós, H. Biochemical changes throughout grape berry developmentand fruit and wine quality. Food 2007, 1, 1–22. [Google Scholar]
- Intrieri, C.; Filippetti, I. Matturazione accelerata delle uve ed eccessivo grado alcolico dei vini: Cosa può fare la ricerca se cambia il clima? Rivista Frutticoltura Ortofloricoltura 2009, 71, 60–62. [Google Scholar]
- De Toda, F.M. Forcing vine regrowth: A new technique to delay grape ripening until a cooler period: Sourced from the research article “Preliminary results on forcing vine regrowth to delay ripening to a cooler period” (Vitis, 2019). Original language of the article: English. IVES Tech. Rev. Vine Wine 2020. [Google Scholar] [CrossRef]
- Reynolds, A.G.; Vanden Heuvel, J.E. Influence of grapevine training systems on vine growth and fruit composition: A review. Am. J. Enol. Vitic. 2009, 60, 251–268. [Google Scholar]
- Gao, Z.; Tong, Y.; Zheng, C.; Zhai, H.; Yao, Y.; Du, Y. Dark inhibits leaf size by controlling carbohydrate and auxin catabolism in grape. Sci. Hort. 2021, 288, 110377. [Google Scholar] [CrossRef]
- Petrie, P.R.; Trought, M.C.T.; Howell, G.S. Influence of leaf ageing, leaf area and crop load on photosynthesis, stomatal conductance and senescence of grapevine (Vitis vinifera L. cv. Pinot Noir) leaves. Vitis 2000, 39, 31–36. [Google Scholar]
- Sweetman, C.; Deluc, L.G.; Cramer, G.R.; Ford, C.M.; Soole, K.L. Regulation of malate metabolism in grape berry and other developing fruits. Phytochemistry 2009, 70, 1329–1344. [Google Scholar] [CrossRef]
- Jones, G.V.; Moriondo, M.; Bois, B.; Hall, A.; Duff, A. Analysis of the Spatial Climate Structure in Viticulture Regions Worldwide. In Proceedings of the 32nd World Congress of the Vine and Wine and 7th General Assembly of the International Organisation of Vine and Wine, Zagreb, Croatia, 28 June–3 July 2009. [Google Scholar]
- Huglin, P. Nouveau Mode d’Evaluation des Possibilites Heliothermiques d’un Milieu Viticole. C. R. Acad. Agric. Franc. 1978, 64, 1117–1126. [Google Scholar]
- Winkler, A.J.; Cook, A.; Kliewere, W.M.; Lider, L.A. General Viticulture, 4th ed.; University of California Press: Berkeley, CA, USA, 1974; p. 740. [Google Scholar]
- OIV. Compendium of International Methods of Wine and Must Analysis; OIV: Paris, France, 2016; ISBN 979-10-91799-46-1. [Google Scholar]
- Geller, J.P.; Kurtural, S.K. Mechanical canopy and crop-load management of ‘pinot gris’ in a warm climate. Am. J. Enol. Vitic. 2013, 64, 65–73. [Google Scholar] [CrossRef]
- Martínez De Toda, F.; Sancha, J.C.; Balda, P. Reducing the sugar and pH of the grape (Vitis vinifera L. cvs. ‘Grenache’ and ‘Tempranillo’) through a single shoot trimming. S. Afr. J. Enol. Vitic. 2013, 34, 246–251. [Google Scholar]
- Sabbatini, P.; Howell, G.S. Effects of early defoliation on yield, fruit composition, and harvest season cluster rot complex of grapevines. Hortscience 2010, 45, 1804–1808. [Google Scholar] [CrossRef]
- Naor, A.; Gal, Y.; Bravdo, B. Shoot and cluster thinning influence vegetative growth, fruit yield, and wine quality of “Sauvignon Blanc” grapevines. J. Am. Soc. Hortic. Sci. 2002, 127, 628–634. [Google Scholar] [CrossRef] [Green Version]
- Schiefer, H.C.; Thin, G. Vorteile einer hoeheren Laubwand? Das Deutsche Weinmagazin 2014, 12, 18–20. [Google Scholar]
- Candolfi-Vasconcelos, M.C.; Koblet, W. Yield, fruit quality, bud fertility and starch reserves of the wood as a function of leaf removal in Vitis vinifera—Evidence of compensation and stress recovering. Vitis 1990, 29, 199–221. [Google Scholar]
- Kozina, B.; Karoglan, M.; Herjavec, S.; Jeromel, A.; Orlic, S. Influence of basal leaf removal on the chemical composition of ‘Sauvignon Blanc’ and Riesling wines. J. Food Agric. Environ. 2008, 1, 28–33. [Google Scholar]
- Gutiérrez-Gamboa, G.; Díaz-Galvéz, I.; Verdugo-Vásquez, N.; Moreno-Simunovic, Y. Leaf-to-Fruit Ratios in Vitis vinifera L. cv. “Sauvignon Blanc”, “Carmenère”, “Cabernet Sauvignon”, and “Syrah” Growing in Maule Valley (Chile): Influence on Yield and Fruit Composition. Agriculture 2019, 9, 8. [Google Scholar] [CrossRef] [Green Version]
- Rösti, J.; Schumann, M.; Cleroux, M.; Lorenzini, F.; Zufferey, V.; Rienth, M. Effect of drying on tartaric acid and malic acid in Shiraz and Merlot berries. Aust. J. Grape Wine Res. 2018, 24, 421–429. [Google Scholar] [CrossRef]
- Rienth, M.; Torregrosa, L.; Gautier, S.; Ardisson, M.; Brillouet, J.-M.; Romieu, C. Temperature desynchronizes sugar and organic acid metabolism in ripening grapevine fruits and remodels their transcriptome. BMC Plant. Biol. 2016, 16, 164. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, C.; Friant, P.; Choné, X.; Tregoat, O.; Koundouras, S.; Dubourdieu, D. Influence of climate, soil, and cultivar on terroir. Am. J. Enol. Viticult. 2004, 55, 207–217. [Google Scholar]
- Rühl, E.H. Effect of rootstocks and K+ supply on ph and acidity of grape juice. Acta Hortic. 2000, 512, 31–38. [Google Scholar] [CrossRef]

| April | May | June | July | August | September | October | Average Value (April—October) | |
|---|---|---|---|---|---|---|---|---|
| 2017 | 8.3 | 15.2 | 20.2 | 20.4 | 20.9 | 13.3 | 10.2 | 15.5 |
| 2018 | 14.4 | 17.6 | 19.2 | 21 | 22.6 | 15.9 | 11.4 | 17.4 |
| 2019 | 10.9 | 11.9 | 22 | 20.1 | 20.7 | 14.7 | 10.4 | 15.8 |
| long-term average | 9.3 | 14.4 | 17.2 | 19.3 | 18.8 | 14.1 | 9 | 14.6 |
| Leaf Area (m2/vine) | ||||
|---|---|---|---|---|
| Variant of Leaf Area Treatment | Season | ‘Pinot Gris’ | ‘Sauvignon Blanc’ | ‘Rhine Riesling’ |
| A | 3.03 c | 2.67 d | 4.25 c | |
| B | 2017 | 1.97 b | 1.54 b | 2.61 b |
| C | 1.02 a | 0.99 a | 1.17 a | |
| A | 3.38 c | 2.90 d | 4.82 d | |
| B | 2018 | 1.95 b | 1.71 b,c | 2.99 b |
| C | 1.14 a | 1.12 a | 1.40 a | |
| A | x | 3.29 e | 4.63 c,d | |
| B | 2019 | 2.20 b | 1.95 c | 2.72 b |
| C | 1.17 a | 1.14 a | 1.39 a | |
| Variety | Season | Variant of Leaf Area Treatment | TtA/MA | TtA (g/L) | MA (g/L) | pH |
|---|---|---|---|---|---|---|
| ‘Pinot Gris’ | 2017 | A | 2.41 a | 8.14 b | 3.38 c | 3.11 a |
| B | 2.38 a | 8.05 b | 3.38 c | 3.11 a | ||
| C | 2.33 a | 7.37 a | 3.16 c | 3.09 a | ||
| 2018 | A | 2.92 b | 9.18 c | 3.14 c | 3.37 b | |
| B | 3.57 c | 8.52 b | 2.39 b | 3.42 c | ||
| C | 4.12 d | 8.07 b | 1.96 a | 3.39 b,c | ||
| 2019 | A B | × 2.45 a | × 10.50 d | × 4.90 d | × 3.62 d | |
| C | 2.14 a | 11.27 e | 4.60 d | 3.65 d | ||
| ‘Sauvignon Blanc’ | 2017 | A | 2.89 b | 8.81 c,d | 3.04 d | 3.03 a |
| B | 3.75 c,d | 7.14 a | 1.90 b | 3.01 a | ||
| C | 4.90 e | 7.11 a | 1.45 a | 3.10 a | ||
| 2018 | A | 3.18 b,c | 8.08 b | 2.54 c | 3.49 b | |
| B | 4.00 d | 7.82 b | 1.97 b | 3.56 c | ||
| C | 4.42 d,e | 8.28 b,c | 1.87 b | 3.36 c | ||
| 2019 | A | 1.52 a | 9.90 f | 6.50 e | 3.67 c | |
| B | 2.69 a,b | 9.18 e | 3.41 d | 3.69 c | ||
| C | 2.62 a,b | 9.25 d | 3.52 d | 3.69 c | ||
| ‘Rhine Riesling’ | 2017 | A | 3.84 e | 11.35 e | 2.96 b | 2.78 a |
| B | 3.29 d | 9.71 d | 2.95 b | 2.87 b | ||
| C | 4.10 e | 9.05 b,c | 2.21 a | 2.89 b | ||
| 2018 | A | 2.69 c | 8.45 a | 3.14 b | 3.18 c | |
| B | 3.02 c,d | 8.75 a,b | 2.89 b | 3.19 c | ||
| C | 3.18 d | 9.24 c | 2.91 b | 3.14 c | ||
| 2019 | A | 1.28 a | 10.92 e | 8.54 d | 3.67 d | |
| B | 1.85 b | 10.08 d | 5.46 c | 3.68 d | ||
| C | 1.68 a,b | 9.30 c | 5.55 c | 3.66 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horák, M.; Balík, J.; Bieniasz, M. Effect of Leaf Area Size on the Main Composition in Grape Must of Three Varieties of Vitis vinifera L. in an Organic Vineyard. Sustainability 2021, 13, 13298. https://doi.org/10.3390/su132313298
Horák M, Balík J, Bieniasz M. Effect of Leaf Area Size on the Main Composition in Grape Must of Three Varieties of Vitis vinifera L. in an Organic Vineyard. Sustainability. 2021; 13(23):13298. https://doi.org/10.3390/su132313298
Chicago/Turabian StyleHorák, Miroslav, Josef Balík, and Monika Bieniasz. 2021. "Effect of Leaf Area Size on the Main Composition in Grape Must of Three Varieties of Vitis vinifera L. in an Organic Vineyard" Sustainability 13, no. 23: 13298. https://doi.org/10.3390/su132313298
APA StyleHorák, M., Balík, J., & Bieniasz, M. (2021). Effect of Leaf Area Size on the Main Composition in Grape Must of Three Varieties of Vitis vinifera L. in an Organic Vineyard. Sustainability, 13(23), 13298. https://doi.org/10.3390/su132313298






