Changes in Volatile Organic Compounds from Salt-Tolerant Trichoderma and the Biochemical Response and Growth Performance in Saline-Stressed Groundnut
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of Salt-Tolerant Trichoderma
2.2. Identification of Volatile Organic Compounds from Salt-Tolerant Trichoderma
2.3. Trichoderma Conidia and Salinity Treatments for Seeds
2.4. Chlorophyll Content in Groundnut Leaves
2.5. Determination of Indole-3-Acetic Acid (IAA) Synthase in Groundnut
2.6. Determination of Total Phenolic and Flavonoid Contents
2.7. Determination of Minerals
2.8. Statistical Analysis
3. Results
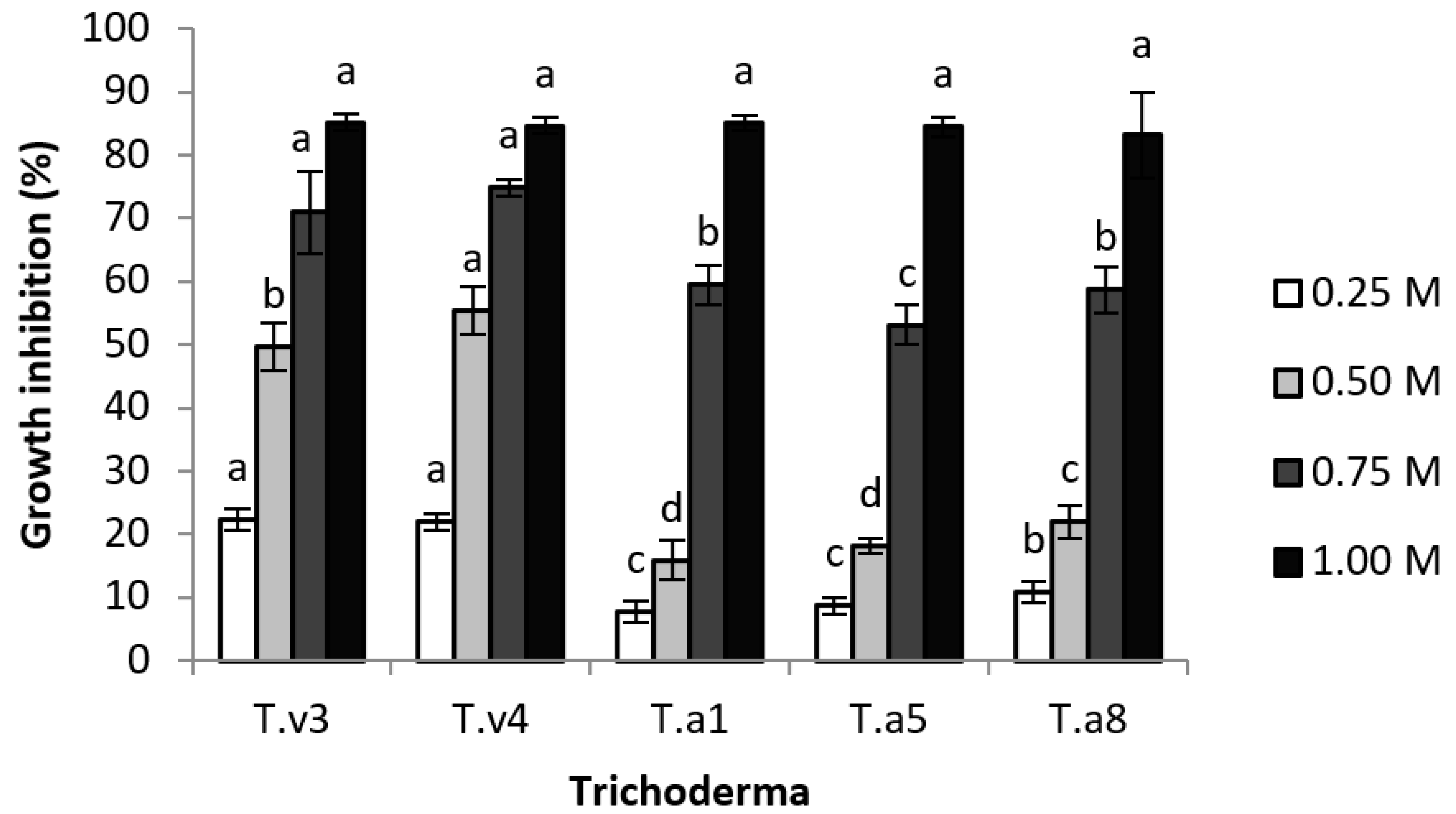
3.1. Growth Inhibition of Trichoderma in PDA Containing Salt
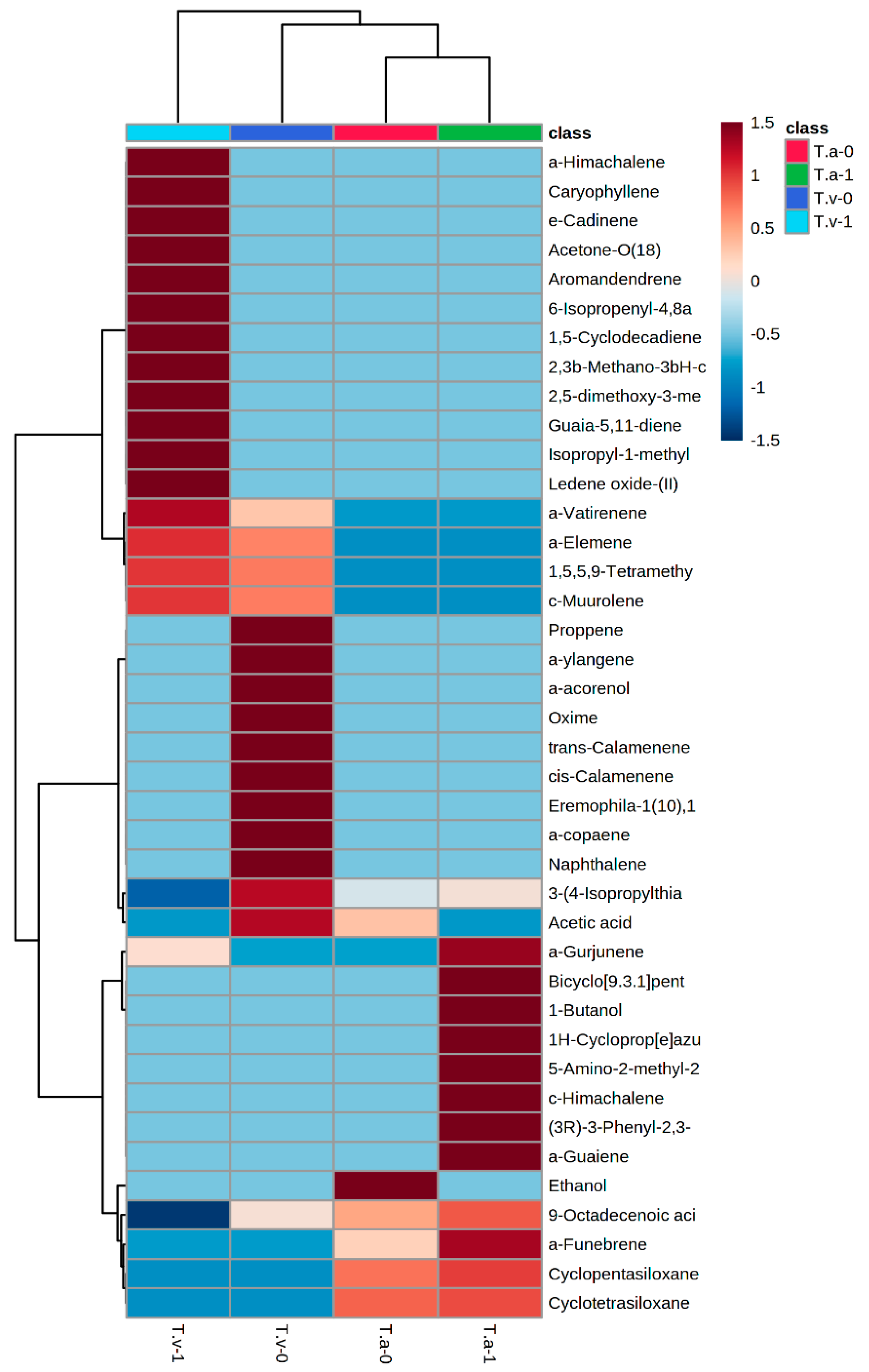
3.2. Identification of Volatile Organic Compounds from Salt-Tolerant Trichoderma
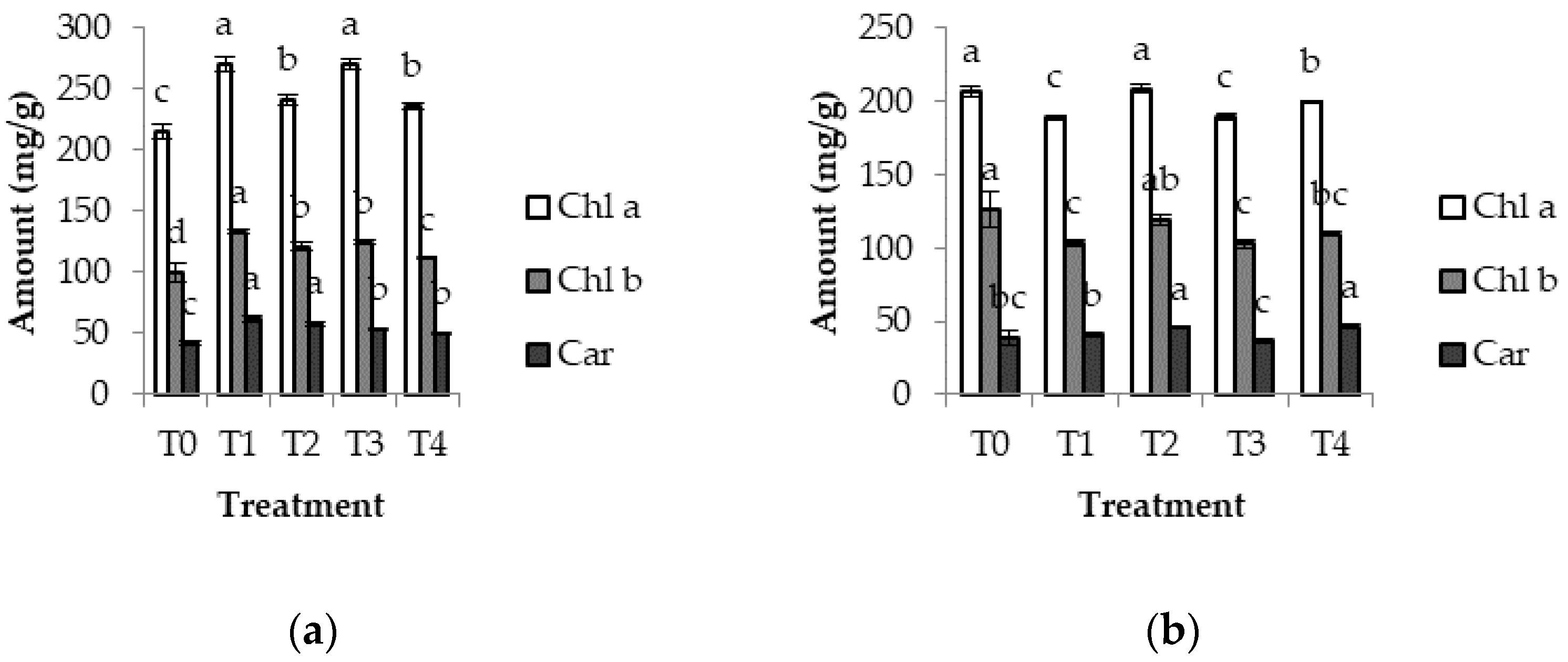
3.3. Chlorophyll Content
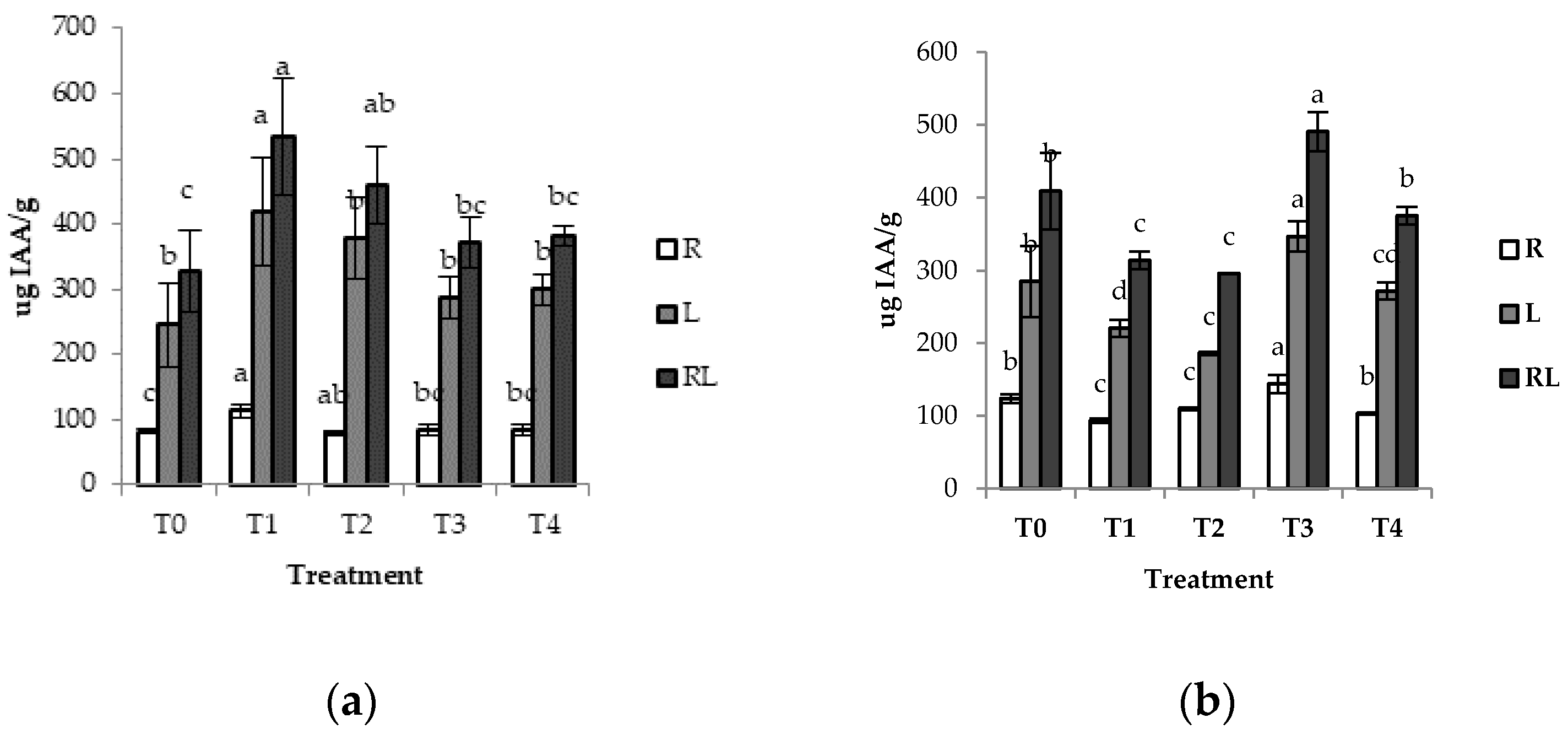
3.4. Determination of Indole-3-Acetic Acid (IAA) Synthase
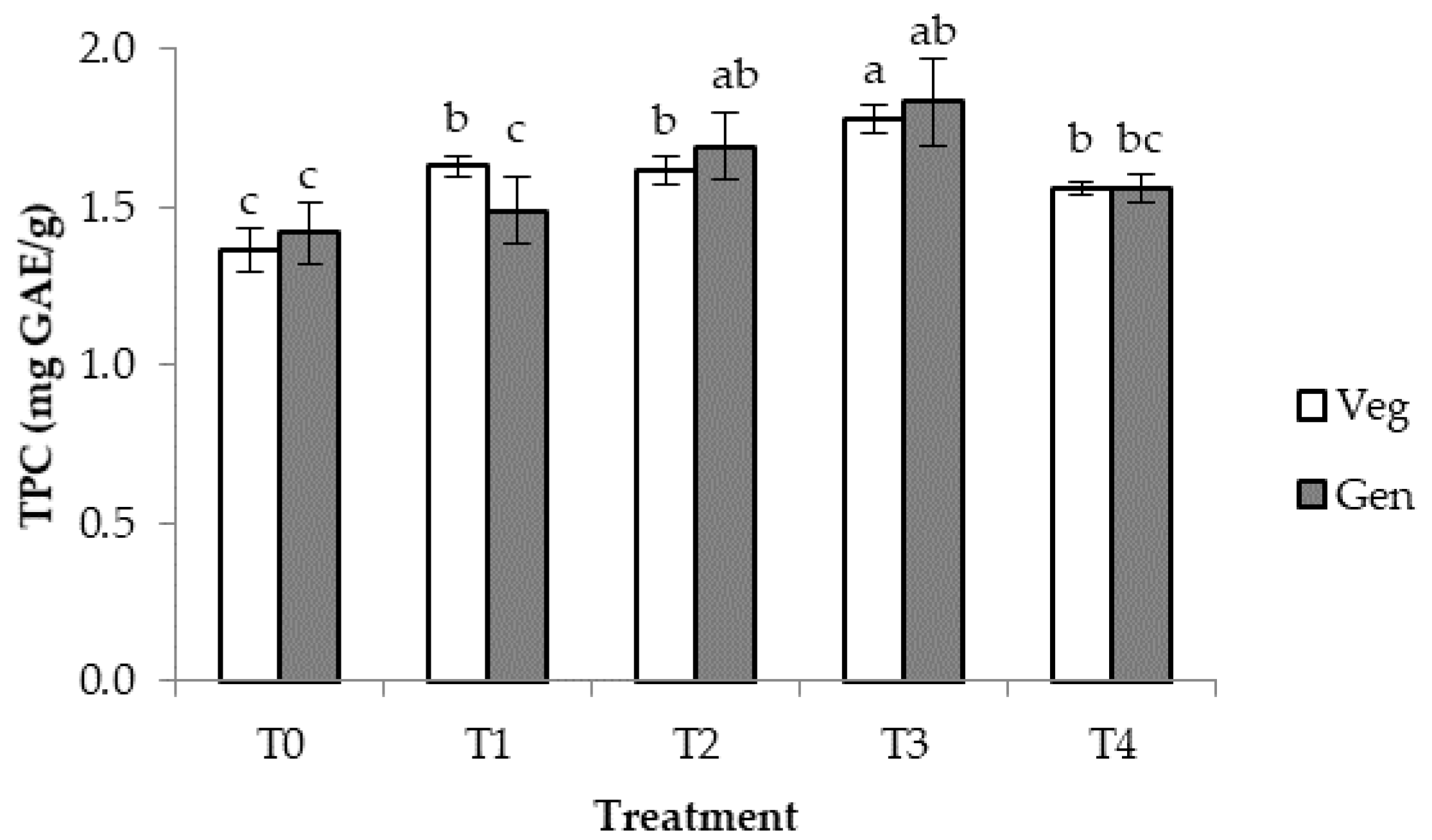
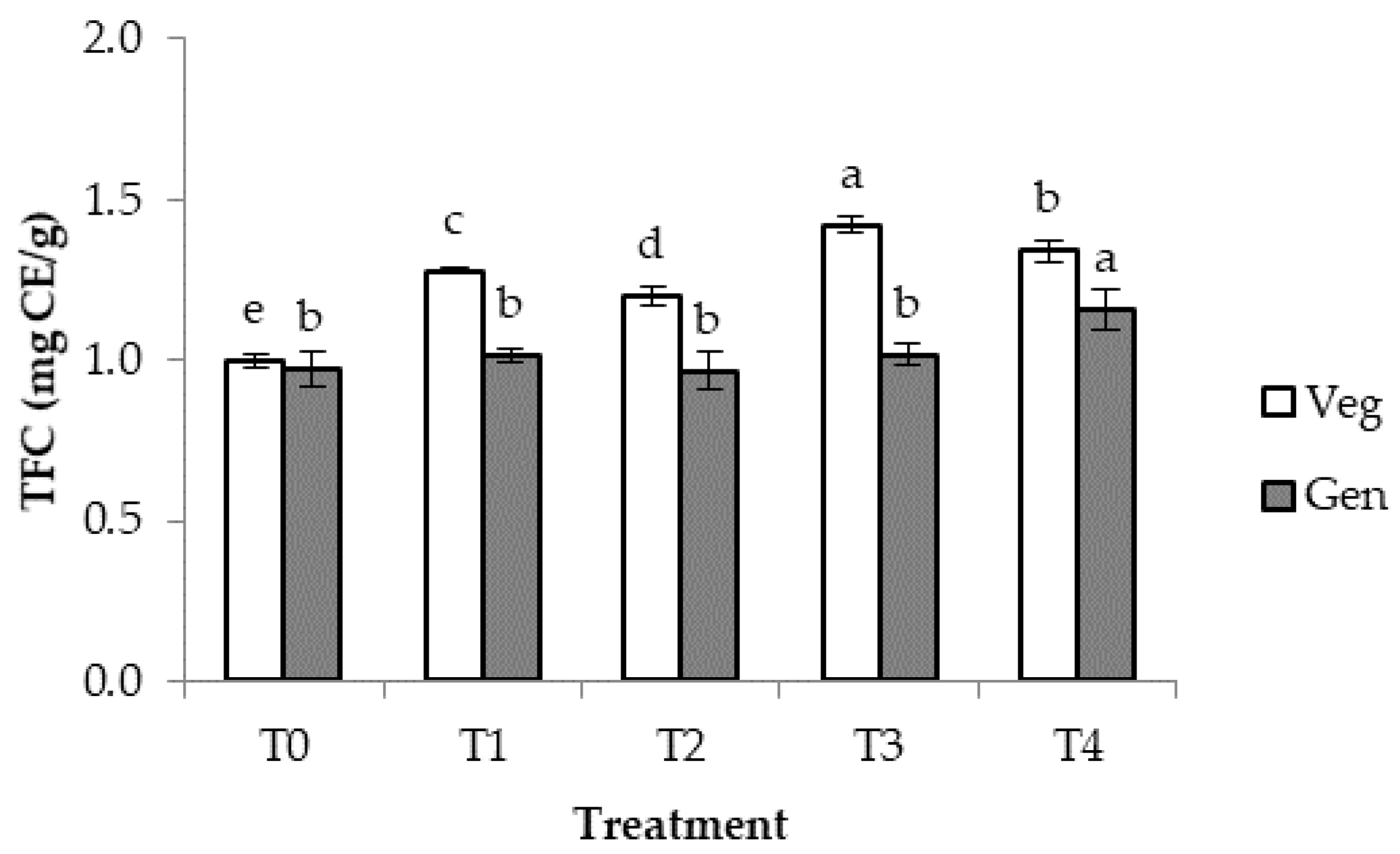
3.5. Total Phenolic and Flavonoid Contents at Vegetative and Generative Growth Stages
3.6. Changes in Growth Parameters
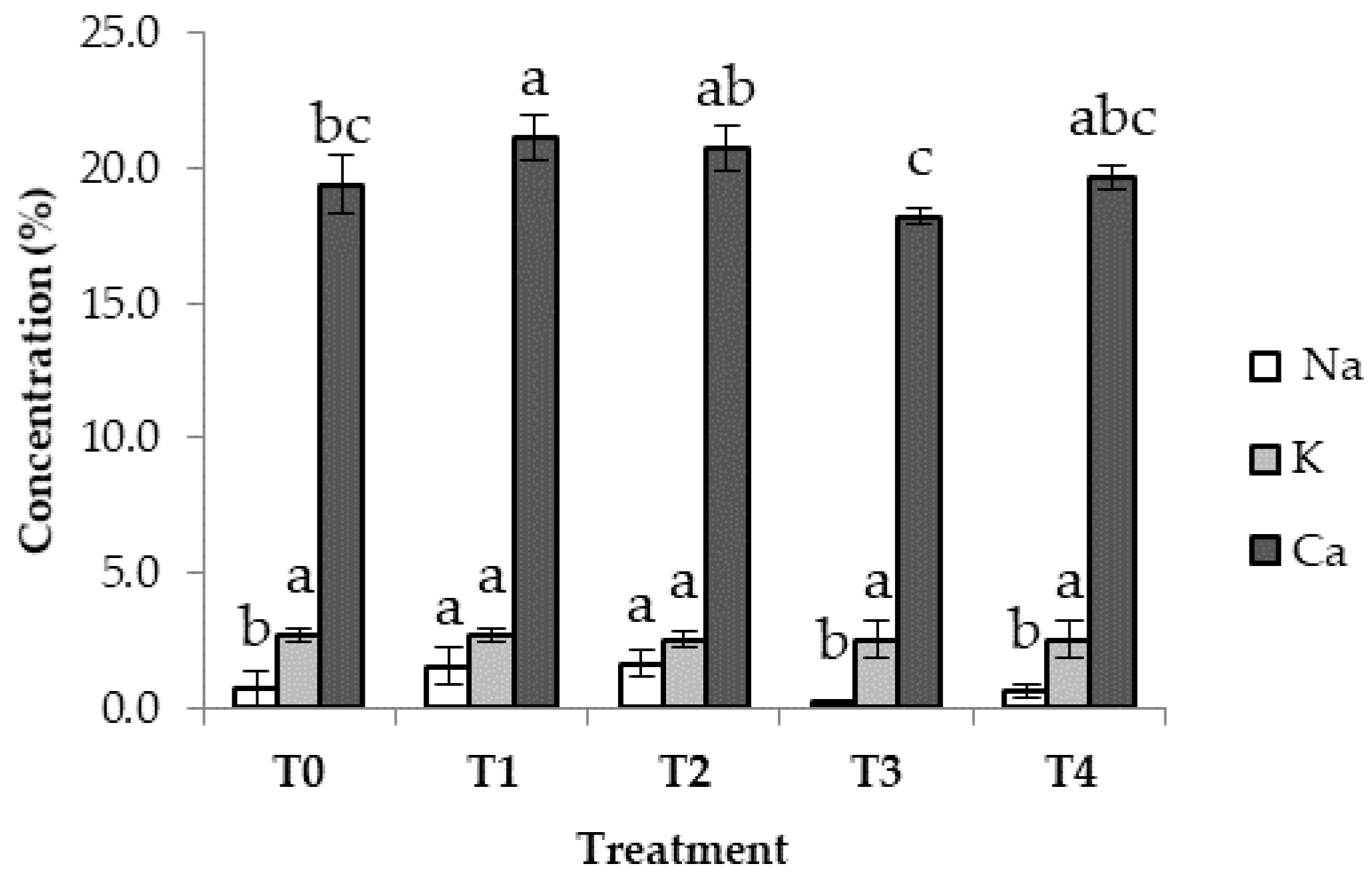
3.7. Minerals in Groundnut Plants
4. Discussion
4.1. Growth Inhibition of Trichoderma
4.2. Volatile Organic Compounds of Salt-Tolerant Trichoderma
4.3. Chlorophylls in Groundnut
4.4. Indole-3-Acetic Acid (IAA) Synthase
4.5. Total Phenolic and Flavonoid Contents in Groundnut
4.6. Changes in Growth Parameters and Minerals in Groundnut
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Safdar, H.; Amin, A.; Shafiq, Y.; Ali, A.; Yasin, R.; Shoukat, A.; Hussan, M.U.; Sarwar, M.I. A review: Impact of salinity on plant growth. Nat. Sci. 2019, 17, 34–40. [Google Scholar]
- Otlewska, A.; Migliore, M.; Dybka-Stępień, K.; Manfredini, A.; Struszczyk-Świta, K.; Napoli, R.; Białkowska, A.; Canfora, L.; Pinzari, F. When salt meddles between plant, soil, and microorganisms. Front. Plant Sci. 2020, 11, 1429. [Google Scholar] [CrossRef]
- Etesami, H.; Maheshwari, D.K. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Verma, H.; Kumar, D.; Kumar, V.; Kumari, M.; Singh, S.K.; Sharma, V.K.; Droby, S.; Santoyo, G.; White, J.F.; Kumar, A. The potential application of endophytes in management of stress from drought and salinity in crop plants. Microorganisms 2021, 9, 1729. [Google Scholar] [CrossRef] [PubMed]
- Jithesh, M.; Prashanth, S.; Sivaprakash, K.; Parida, A.K. Antioxidative response mechanisms in halophytes: Their role in stress defence. J. Genet. 2006, 85, 237. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Alfaro-Cuevas, R.; López-Bucio, J. Trichoderma spp. improve growth of Arabidopsis seedlings under salt stress through enhanced root development, osmolite production, and Na+ elimination through root exudates. Mol. Plant-Microbe Interact. 2014, 27, 503–514. [Google Scholar] [CrossRef] [Green Version]
- Katori, T.; Ikeda, A.; Iuchi, S.; Kobayashi, M.; Shinozaki, K.; Maehashi, K.; Sakata, Y.; Tanaka, S.; Taji, T. Dissecting the genetic control of natural variation in salt tolerance of Arabidopsis thaliana accessions. J. Exp. Bot. 2010, 61, 1125–1138. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Tak, Y.; Potkule, J.; Choyal, P.; Tomar, M.; Meena, N.L.; Kaur, C. Phenolics as Plant Protective Companion Against Abiotic Stress. In Plant Phenolics in Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2020; pp. 277–308. [Google Scholar]
- Rahnama, A.; James, R.A.; Poustini, K.; Munns, R. Stomatal conductance as a screen for osmotic stress tolerance in durum wheat growing in saline soil. Funct. Plant Biol. 2010, 37, 255–263. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef]
- Rawat, L.; Bisht, T.; Upadhayay, R.; Kukreti, A. Selection of salinity tolerant Trichoderma isolates and evaluating their performance in alleviating salinity stress in rice (Oryzae sativa L.). Int. J. Natl. Acad. Agric. Sci. 2016, 34, 1869–1875. [Google Scholar]
- Zhang, S.; Song, J.; Wang, H.; Feng, G. Effect of salinity on seed germination, ion content and photosynthesis of cotyledons in halophytes or xerophyte growing in Central Asia. J. Plant Ecol. 2010, 3, 259–267. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Singh, A.; Singh, S.; Singh, H.B. Biological management of Sclerotinia sclerotiorum in pea using plant growth promoting microbial consortium. J. Basic Microbiol. 2015, 55, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Jain, A.; Sarma, B.K.; Upadhyay, R.S.; Singh, H.B. Beneficial compatible microbes enhance antioxidants in chickpea edible parts through synergistic interactions. LWT—Food Sci. Technol. 2014, 56, 390–397. [Google Scholar] [CrossRef]
- Yadav, R.S.; Singh, V.; Pal, S.; Meena, S.K.; Meena, V.S.; Sarma, B.K.; Singh, H.B.; Rakshit, A. Seed bio-priming of baby corn emerged as a viable strategy for reducing mineral fertilizer use and increasing productivity. Sci. Hortic. 2018, 241, 93–99. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Cortés-Penagos, C.; López-Bucio, J. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol. 2009, 149, 1579–1592. [Google Scholar] [CrossRef] [Green Version]
- Gravel, V.; Antoun, H.; Tweddell, R.J. Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida or Trichoderma atroviride: Possible role of indole acetic acid (IAA). Soil Biol. Biochem. 2007, 39, 1968–1977. [Google Scholar] [CrossRef]
- Rawat, L.; Singh, Y.; Shukla, N.; Kumar, J. Seed biopriming with salinity tolerant isolates of Trichoderma harzmnum alleviates salt stress in rice: Growth, physiological and biochemical characteristics. J. Plant Pathol. 2012, 94, 353–365. [Google Scholar]
- Lee, S.; Yap, M.; Behringer, G.; Hung, R.; Bennett, J.W. Volatile organic compounds emitted by Trichoderma species mediate plant growth. Fungal Biol. Biotechnol. 2016, 3, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Poosapati, S.; Ravulapalli, P.D.; Tippirishetty, N.; Vishwanathaswamy, D.K.; Chunduri, S. Selection of high temperature and salinity tolerant Trichoderma isolates with antagonistic activity against Sclerotium rolfsii. SpringerPlus 2014, 3, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, V.; Keswani, C.; Ray, S.; Upadhyay, R.S.; Singh, D.P.; Prabha, R.; Sarma, B.K.; Singh, H.B. Isolation and screening of high salinity tolerant Trichoderma spp. with plant growth property and antagonistic activity against various soilborne phytopathogens. Arch. Phytopathol. Plant Prot. 2019, 52, 667–680. [Google Scholar] [CrossRef]
- Kumar, K.; Manigundan, K.; Amaresan, N. Influence of salt tolerant Trichoderma spp. on growth of maize (Zea mays) under different salinity conditions. J. Basic Microbiol. 2017, 57, 141–150. [Google Scholar] [CrossRef]
- Martínez, R.S.; Chavera, G.S.; Mamani, W.H.; Molina, M.R. Native strains of Trichoderma from northern Chile: Adaptive tolerance in boric saline soils. Interciencia 2015, 40, 263–269. [Google Scholar]
- Ikram, M.; Ali, N.; Jan, G.; Iqbal, A.; Hamayun, M.; Jan, F.G.; Hussain, A.; Lee, I.-J. Trichoderma reesei improved the nutrition status of wheat crop under salt stress. J. Plant Interact. 2019, 14, 590–602. [Google Scholar] [CrossRef] [Green Version]
- Kashyap, P.L.; Solanki, M.K.; Kushwaha, P.; Kumar, S.; Srivastava, A.K. Biocontrol potential of salt-tolerant Trichoderma and Hypocrea isolates for the management of tomato root rot under saline environment. J. Soil Sci. Plant Nutr. 2020, 20, 160–176. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Y.; Liu, C.; Chen, F.; Ge, H.; Tian, F.; Yang, T.; Ma, K.; Zhang, Y. Trichoderma harzianum mitigates salt stress in cucumber via multiple responses. Ecotoxicol. Environ. Saf. 2019, 170, 436–445. [Google Scholar] [CrossRef]
- Abdullah, T.; Yusnawan, E. Influence of Trichoderma as a seed treatment on the growth and yield of groundnut under saline environment. J. Degrad. Min. Lands Manag. 2020, 8, 2401–2409. [Google Scholar] [CrossRef]
- Inayati, A.; Sulistyowati, L.; Aini, L.Q.; Yusnawan, E. Trichoderma virens-Tv4 enhances growth promoter and plant defense-related enzymes of mungbean (Vigna radiata) against soil-borne pathogen Rhizoctonia solani. Biodiversitas J. Biol. Divers. 2020, 21, 2410–2419. [Google Scholar] [CrossRef]
- Yusnawan, E.; Inayati, A.; Baliadii, Y. Isolation of antagonistic fungi from rhizospheres and its biocontrol activity against different isolates of soil borne fungal pathogens infected legumes. Biodiversitas J. Biol. Divers. 2019, 20, 2048–2054. [Google Scholar] [CrossRef] [Green Version]
- Inayati, A.; Sulistyowati, L.; Aini, L.Q.; Yusnawan, E. Antifungal Activity of Volatile Organic Compounds from Trichoderma virens. In Proceedings of the AIP Conference Proceedings, Malang, Indonesia, 13–14 March 2019; p. 080012. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4. [Google Scholar] [CrossRef]
- Yusnawan, E.; Inayati, A. Antifungal activity of crude extracts of Ageratum conyzoides, Cyperus rotundus, and Amaranthus spinosus against rust disease. AGRIVITA J. Agric. Sci. 2018, 40, 12. [Google Scholar] [CrossRef]
- Karpiuk, U.V.; Al Azzam, K.M.; Abudayeh, Z.H.M.; Kislichenko, V.; Naddaf, A.; Cholak, I.; Yemelianova, O. Qualitative and quantitative content determination of macro-minor elements in Bryonia alba L. roots using flame atomic absorption spectroscopy technique. Adv. Pharm. Bull. 2016, 6, 285. [Google Scholar] [CrossRef]
- Ben Alaya, A.; Rabhi, F.; Hessini, K.; Djébali, N. Pyrenophora teres growth and severity of net blotch on barley under salt stress. Eur. J. Plant Pathol. 2021, 161, 709–722. [Google Scholar] [CrossRef]
- Kadhim, M.J.; Mohammed, G.J.; Hameed, I.H. In vitro antibacterial, antifungal and phytochemical analysis of methanolic extract of fruit Cassia fistula. Orient. J. Chem. 2016, 32, 1329. [Google Scholar] [CrossRef] [Green Version]
- Palmer-Young, E.C.; Veit, D.; Gershenzon, J.; Schuman, M.C. The sesquiterpenes (E)-ß-farnesene and (E)-α-bergamotene quench ozone but fail to protect the wild tobacco Nicotiana attenuata from ozone, UVB, and drought stresses. PLoS ONE 2015, 10, e0127296. [Google Scholar]
- Rinkel, J.; Dickschat, J.S. Mechanistic investigations on multiproduct β-himachalene synthase from Cryptosporangium arvum. Beilstein J. Org. Chem. 2019, 15, 1008–1019. [Google Scholar] [CrossRef]
- Yin, J.; Li, X.; Huang, F.F.; Lu, M.H.; Yang, J.; Zhu, L.Y. Chemical composition, antioxidant and anticancer activity of the essential oil from Myric rubra leaves. IOP Conf. Ser. Earth Environ. Sci. 2019, 346, 012085. [Google Scholar] [CrossRef]
- Del Rosario Cappellari, L.; Banchio, E. Microbial volatile organic compounds produced by Bacillus amyloliquefaciens GB03 ameliorate the effects of salt stress in Mentha piperita principally through acetoin emission. J. Plant Growth Regul. 2020, 39, 764–775. [Google Scholar] [CrossRef]
- Nieto-Jacobo, M.F.; Steyaert, J.M.; Salazar-Badillo, F.B.; Nguyen, D.V.; Rostás, M.; Braithwaite, M.; De Souza, J.T.; Jimenez-Bremont, J.F.; Ohkura, M.; Stewart, A. Environmental growth conditions of Trichoderma spp. affects indole acetic acid derivatives, volatile organic compounds, and plant growth promotion. Front. Plant Sci. 2017, 8, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, C.V. Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves. Sci. Hortic. 2004, 103, 93–99. [Google Scholar] [CrossRef]
- Al Hassan, M.; Fuertes, M.M.; Sánchez, F.J.R.; Vicente, O.; Boscaiu, M. Effects of salt and water stress on plant growth and on accumulation of osmolytes and antioxidant compounds in cherry tomato. Not. Bot. Horti Agrobot. Cluj-Napoca 2015, 43, 1–11. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Kumar, K.; Thakur, P.; Rathore, U.S.; Kumar, S.; Mishra, R.; Amaresan, N.; Pandey, S.; Mishra, M. Plant beneficial effects of Trichoderma spp. suppressing Fusarium wilt and enhancing growth in Tomato. Vegetos 2021, 3, 1–8. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.-Q. Effects of phosphate solubilization and phytohormone production of Trichoderma asperellum Q1 on promoting cucumber growth under salt stress. J. Integr. Agric. 2015, 14, 1588–1597. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeng, T.L.; Shih, Y.J.; Wu, M.T.; Sung, J.M. Comparisons of flavonoids and anti-oxidative activities in seed coat, embryonic axis and cotyledon of black soybeans. Food Chem. 2010, 123, 1112–1116. [Google Scholar] [CrossRef]
- Chen, S.; Wu, F.; Li, Y.; Qian, Y.; Pan, X.; Li, F.; Wang, Y.; Wu, Z.; Fu, C.; Lin, H.; et al. NtMYB4 and NtCHS1 are critical factors in the regulation of flavonoid biosynthesis and are involved in salinity responsiveness. Front. Plant Sci. 2019, 10, 178. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Smith, D.L. Flavonoids in agriculture: Chemistry and roles in, biotic and abiotic stress responses, and microbial associations. Agronomy 2020, 10, 1209. [Google Scholar] [CrossRef]
- Rasool, S.; Ahmad, A.; Siddiqi, T.; Ahmad, P. Changes in growth, lipid peroxidation and some key antioxidant enzymes in chickpea genotypes under salt stress. Acta Physiol. Plant. 2013, 35, 1039–1050. [Google Scholar] [CrossRef]
- Fu, J.; Xiao, Y.; Wang, Y.-F.; Liu, Z.-H.; Yang, K. Saline–alkaline stress in growing maize seedlings is alleviated by Trichoderma asperellum through regulation of the soil environment. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Munns, R.; Passioura, J.B.; Colmer, T.D.; Byrt, C.S. Osmotic adjustment and energy limitations to plant growth in saline soil. New Phytol. 2020, 225, 1091–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Schillaci, M.; Walker, R.; Smith, P.M.; Watt, M.; Roessner, U. Alleviation of salinity stress in plants by endophytic plant-fungal symbiosis: Current knowledge, perspectives and future directions. Plant Soil 2021, 461, 219–244. [Google Scholar] [CrossRef]
- Huang, X.; Chen, L.; Ran, W.; Shen, Q.; Yang, X. Trichoderma harzianum strain SQR-T37 and its bio-organic fertilizer could control Rhizoctonia solani damping-off disease in cucumber seedlings mainly by the mycoparasitism. Appl. Microbiol. Biotechnol. 2011, 91, 741–755. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Abd_Allah, E.; Alqarawi, A.; Al Huqail, A.A.; Egamberdieva, D. Alleviation of abiotic salt stress in Ochradenus baccatus (Del.) by Trichoderma hamatum (Bonord.) Bainier. J. Plant Interact. 2014, 9, 857–868. [Google Scholar] [CrossRef]
- Zhang, S.; Gan, Y.; Xu, B. Application of plant-growth-promoting fungi Trichoderma longibrachiatum T6 enhances tolerance of wheat to salt stress through improvement of antioxidative defense system and gene expression. Front. Plant Sci. 2016, 7, 1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Treatment | Shoot Height (cm) | Root Length (cm) | Shoot Dry Weight (g) | Root Dry Weight (g) | Biomass Dry Weight (g) |
|---|---|---|---|---|---|
| T0 | 6.86 b | 5.16 a | 0.59 a,b | 0.034 a | 0.62 a,b |
| T1 | 9.02 a | 3.88 b | 0.52 b,c | 0.023 b,c | 0.55 b,c |
| T2 | 9.12 a | 4.52 a,b | 0.45 c | 0.020 c | 0.49 c |
| T3 | 8.81a | 5.06 a | 0.64 a | 0.030 a,b | 0.69 a |
| T4 | 8.87 a | 4.93 a | 0.56 b,c | 0.029 a,b | 0.55 b,c |
| Treatment | Shoot Height (cm) | Root Length (cm) | Shoot Dry Weight (g) | Root Dry Weight (g) | Biomass Dry Weight (g) |
|---|---|---|---|---|---|
| T0 | 10.80 a | 6.30 a | 3.12 b,c | 0.15 a,b | 3.27 b,c |
| T1 | 10.48 a | 6.80 a | 2.36 c,d | 0.11 b | 2.38 c,d |
| T2 | 10.64 a | 7.90 a | 2.16 d | 0.13 a,b | 1.89 d |
| T3 | 11.70 a | 3.90 b | 4.53 a | 0.18 a | 4.71 a |
| T4 | 11.80 a | 5.60 a,b | 3.81 a,b | 0.13 a,b | 3.94 a,b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yusnawan, E.; Taufiq, A.; Wijanarko, A.; Susilowati, D.N.; Praptana, R.H.; Chandra-Hioe, M.V.; Supriyo, A.; Inayati, A. Changes in Volatile Organic Compounds from Salt-Tolerant Trichoderma and the Biochemical Response and Growth Performance in Saline-Stressed Groundnut. Sustainability 2021, 13, 13226. https://doi.org/10.3390/su132313226
Yusnawan E, Taufiq A, Wijanarko A, Susilowati DN, Praptana RH, Chandra-Hioe MV, Supriyo A, Inayati A. Changes in Volatile Organic Compounds from Salt-Tolerant Trichoderma and the Biochemical Response and Growth Performance in Saline-Stressed Groundnut. Sustainability. 2021; 13(23):13226. https://doi.org/10.3390/su132313226
Chicago/Turabian StyleYusnawan, Eriyanto, Abdullah Taufiq, Andy Wijanarko, Dwi Ningsih Susilowati, Raden Heru Praptana, Maria V. Chandra-Hioe, Agus Supriyo, and Alfi Inayati. 2021. "Changes in Volatile Organic Compounds from Salt-Tolerant Trichoderma and the Biochemical Response and Growth Performance in Saline-Stressed Groundnut" Sustainability 13, no. 23: 13226. https://doi.org/10.3390/su132313226
APA StyleYusnawan, E., Taufiq, A., Wijanarko, A., Susilowati, D. N., Praptana, R. H., Chandra-Hioe, M. V., Supriyo, A., & Inayati, A. (2021). Changes in Volatile Organic Compounds from Salt-Tolerant Trichoderma and the Biochemical Response and Growth Performance in Saline-Stressed Groundnut. Sustainability, 13(23), 13226. https://doi.org/10.3390/su132313226






