Exploring the Microbial Dynamics of Organic Matter Degradation and Humification during Co-Composting of Cow Manure and Bedding Material Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Composting Process and Sampling
2.2. Physicochemical Analysis
2.3. DNA Extraction and High-Throughput Sequencing
2.4. DNA Extraction and High-Throughput Sequencing
3. Results and Discussion
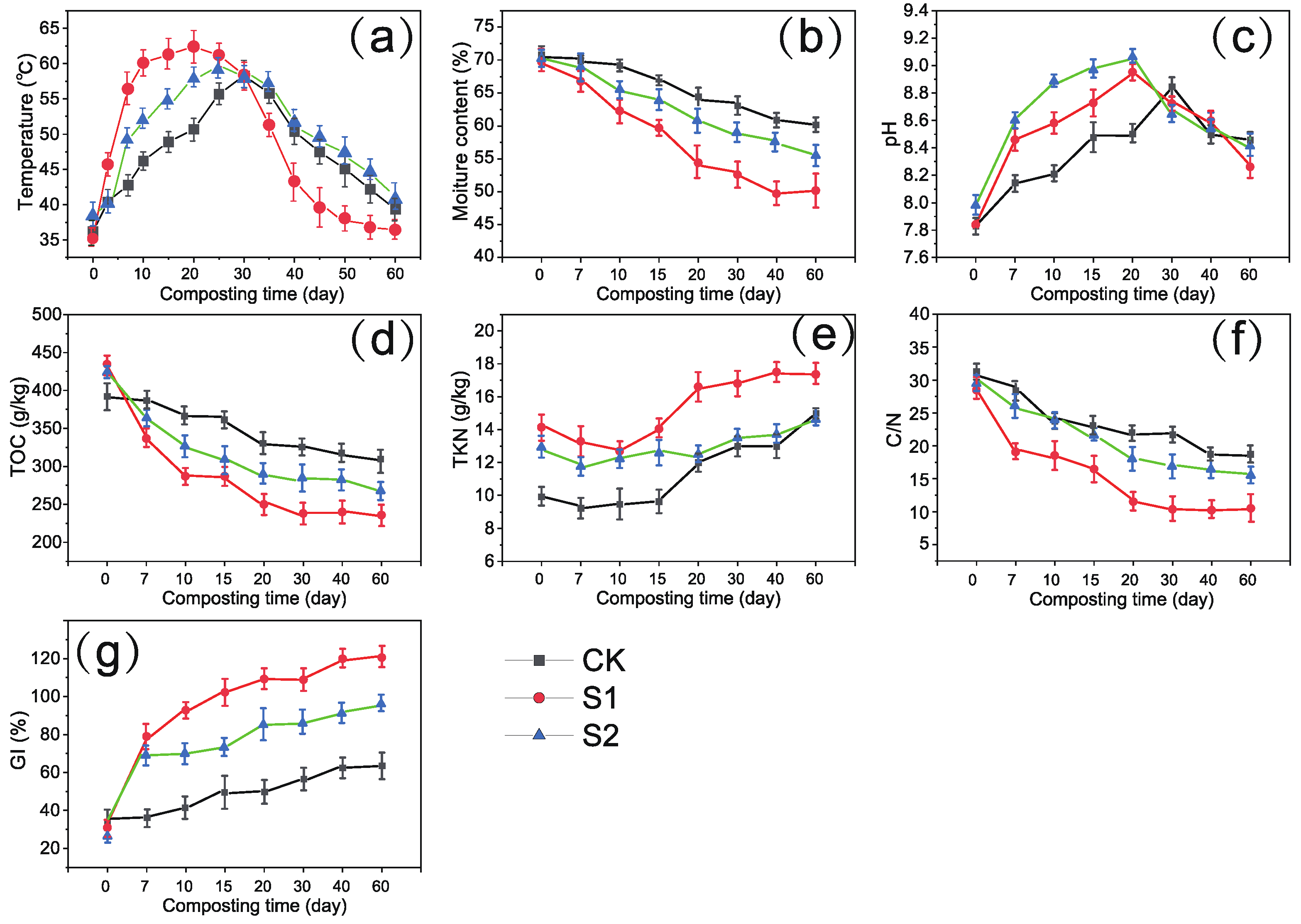
3.1. Changes in Physicochemical Characteristics during Composting
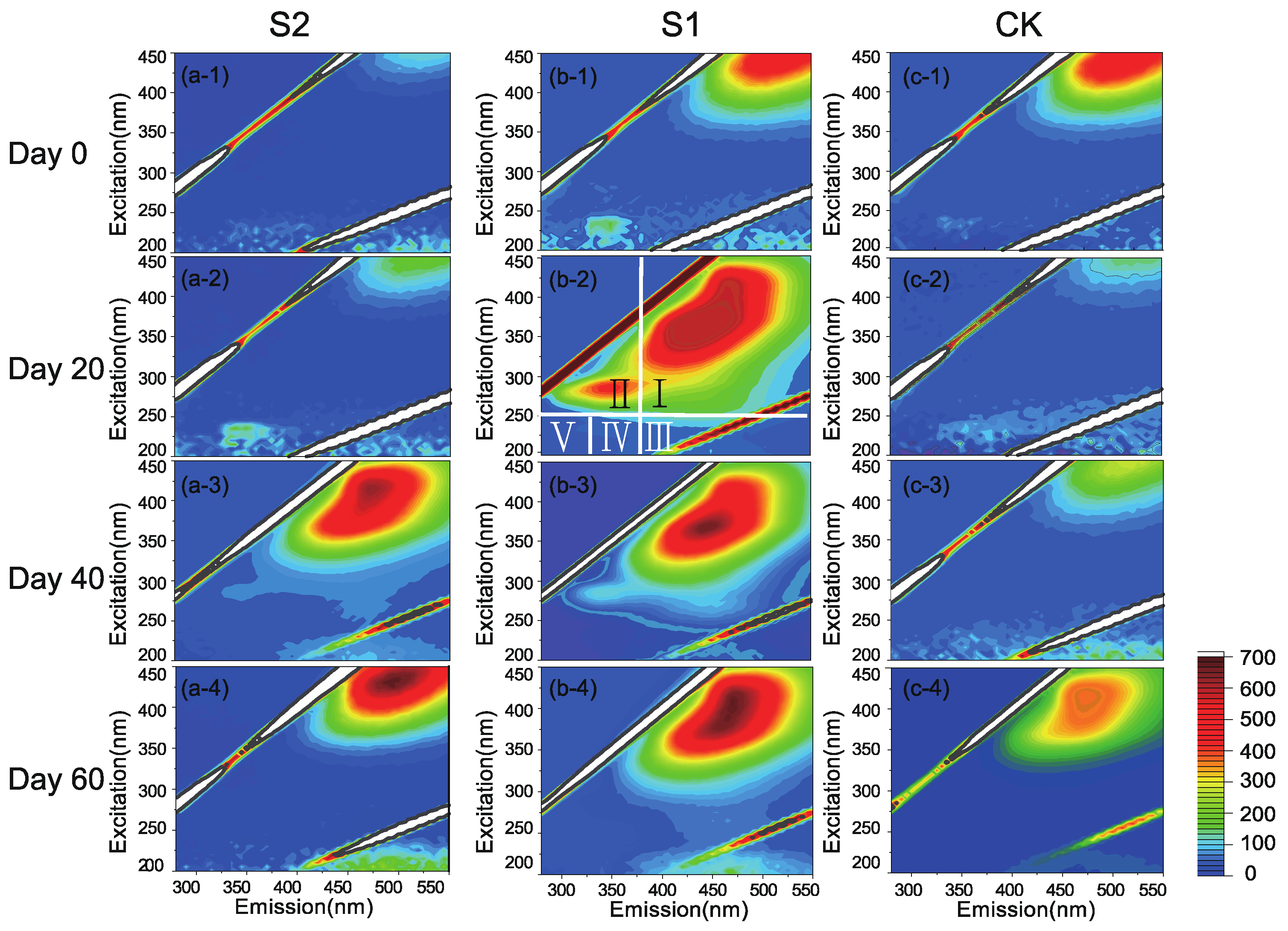
3.2. Degradation of OM and Formation of HS
3.3. Similarity and Diversity of Microbial Communities
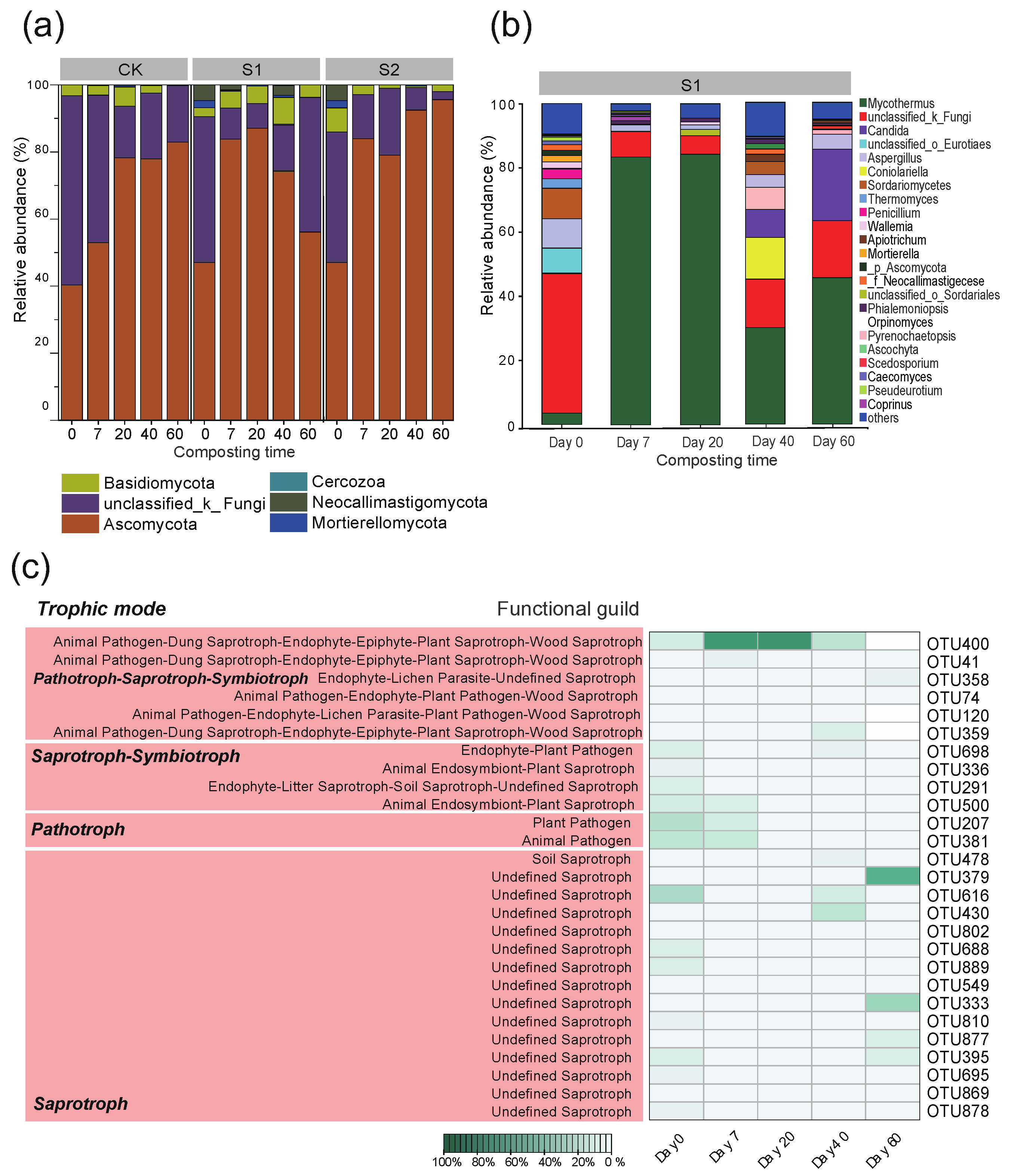
3.4. Bacterial Community Succession and Predicted Bacterial Functions
3.5. Fungal Community Succession and Predicted Fungal Functions
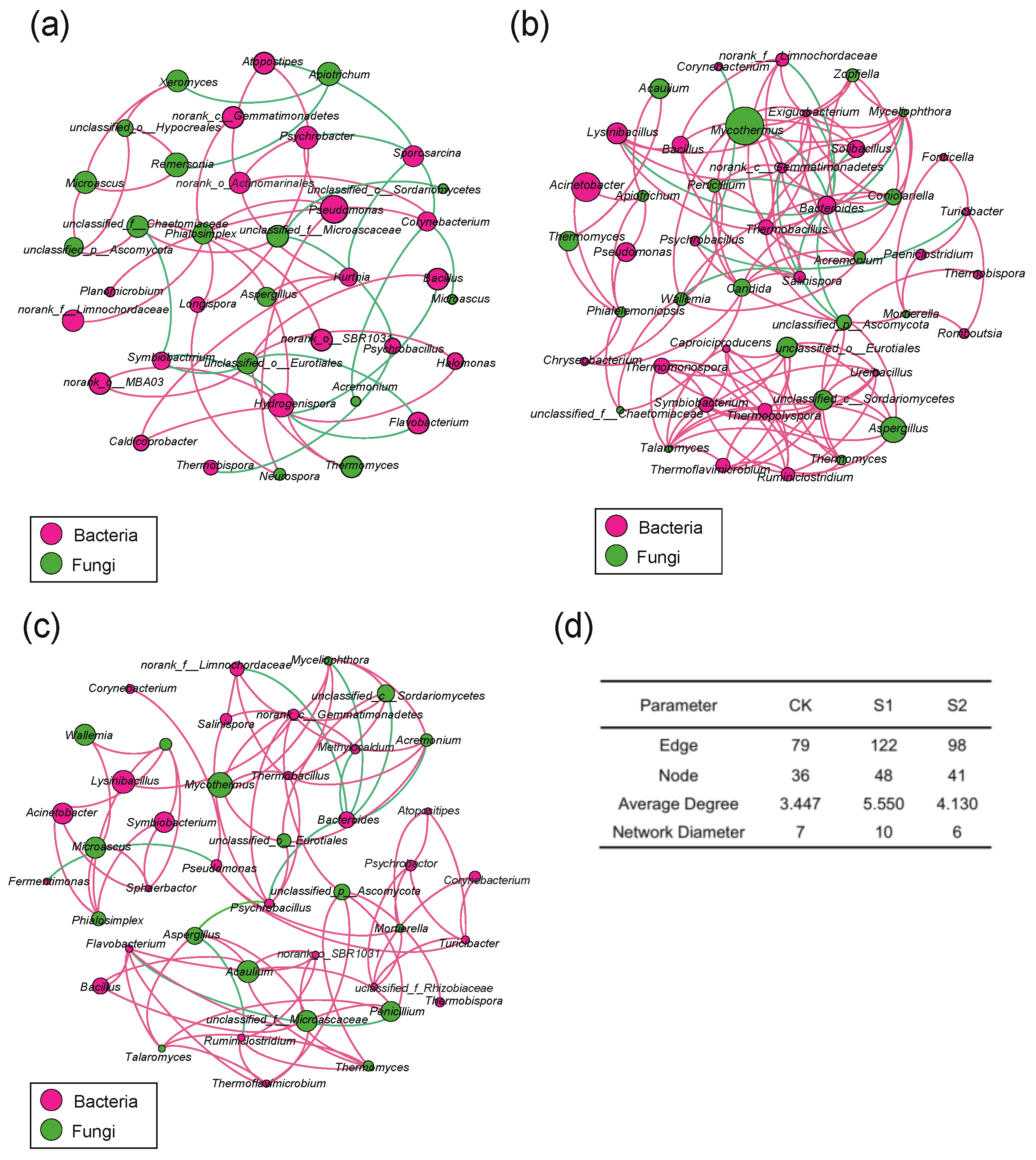
3.6. Co-Occurrence Network Analysis for Correlations in Microbial Community
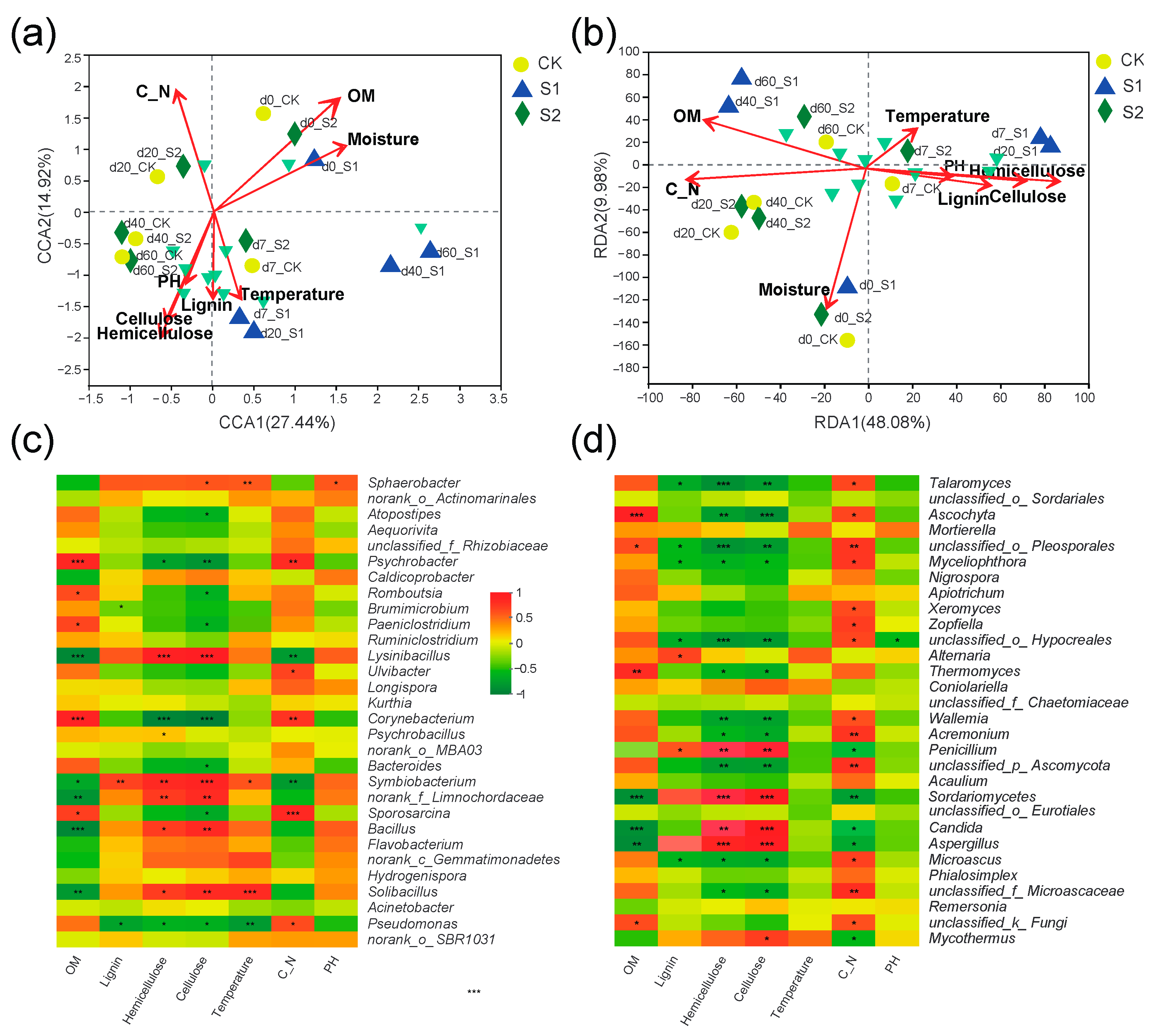
3.7. Relationships of the Bacterial/Fungal Community with Physicochemical Characteristics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Report on the Global Tobacco Epidemic, 2017: Monitoring Tobacco Use and Prevention Policies; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Wang, K.; Chu, C.; Li, X.; Wang, W.; Ren, N. Succession of Bacterial Community Function in Cow Manure Composing. Bioresour. Technol. 2018, 267, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Safferman, S.I.; Wallace, J.M. Cow Manure: Waste Or Resource? IEEE Potentials 2015, 34, 25–29. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, J.; Li, J.; Xin, Y.; Hao, Z.; Chen, C.; Li, H.; Wang, B.; Ding, M.; Li, W.; et al. Insights into Bacterial Diversity in Compost: Core Microbiome and Prevalence of Potential Pathogenic Bacteria. Sci. Total Environ. 2020, 718, 137304. [Google Scholar] [CrossRef]
- Husfeldt, A.W.; Endres, M.I.; Salfer, J.A.; Janni, K.A. Management and Characteristics of Recycled Manure Solids Used for Bedding in Midwest Freestall Dairy Herds. J. Dairy Sci. 2012, 95, 2195–2203. [Google Scholar] [CrossRef] [PubMed]
- Fournel, S.; Godbout, S.; Ruel, P.; Fortin, A.; Duquette-Lozeau, K.; Létourneau, V.; Généreux, M.; Lemieux, J.; Potvin, D.; Côté, C.; et al. Production of Recycled Manure Solids for Use as Bedding in Canadian Dairy Farms: II. Composting Methods. J. Dairy Sci. 2019, 102, 1847–1865. [Google Scholar] [CrossRef]
- Leso, L.; Barbari, M.; Lopes, M.A.; Damasceno, F.A.; Galama, P.; Taraba, J.L.; Kuipers, A. Invited Review: Compost-Bedded Pack Barns for Dairy Cows. J. Dairy Sci. 2020, 103, 1072–1099. [Google Scholar] [CrossRef]
- Atencio, J.L.; Fernandez, J.A.; Gernat, A.G.; Murillo, J.G. Effect of Pine Wood Shavings, Rice Hulls and River Bed Sand on Broiler Productivity When Used as a Litter Sources. Int. J. Poult. Sci. 2010, 9, 240–243. [Google Scholar] [CrossRef]
- Wolfe, T.; Vasseur, E.; Devries, T.J.; Bergeron, R. Effects of Alternative Deep Bedding Options on Dairy Cow Preference, Lying Behavior, Cleanliness, and Teat End Contamination. J. Dairy Sci. 2018, 101, 530–536. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Chen, H.; Wang, Q.; Liu, T.; Duan, Y.; Awasthi, S.K.; Ren, X.; Tu, Z.; Li, J.; Zhao, J.; et al. Succession of Bacteria Diversity in the Poultry Manure Composted Mixed with Clay: Studies upon Its Dynamics and Associations with Physicochemical and Gaseous Parameters. Bioresour. Technol. 2018, 267, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Li, G.; Jiang, T.; Schuchardt, F.; Chen, T.; Zhao, Y.; Shen, Y. Effect Of Aeration Rate, C/N Ratio And Moisture Content On The Stability And Maturity Of Compost. Bioresour. Technol. 2012, 112, 171–178. [Google Scholar] [CrossRef]
- He, X.S.; Xi, B.D.; Wei, Z.M.; Jiang, Y.H.; Geng, C.M.; Yang, Y.; Yuan, Y.; Liu, H.L. Physicochemical and Spectroscopic Characteristics of Dissolved Organic Matter Extracted from Municipal Solid Waste (MSW) and Their Influence on the Landfill Biological Stability. Bioresour. Technol. 2011, 102, 2322–2327. [Google Scholar] [CrossRef]
- Esmaeili, A.; Khoram, M.R.; Gholami, M.; Eslami, H. Pistachio Waste Management Using Combined Composting-Vermicomposting Technique: Physico-Chemical Changes and Worm Growth Analysis. J. Clean Prod. 2020, 242, 118523. [Google Scholar] [CrossRef]
- Guo, A.; Zhao, Z.; Zhang, P.; Yang, Q.; Li, Y.; Wang, G. Linkage between Soil Nutrient and Microbial Characteristic in an Opencast Mine, China. Sci. Total Environ. 2019, 671, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.H.; Kim, J.K.; Ravindran, B.; Lee, D.J.; Wong, J.W.; Selvam, A.; Karthikeyan, O.P.; Kwag, J.H. Evaluation of Pilot-Scale in-Vessel Composting for Hanwoo Manure Management. Bioresour. Technol. 2017, 245, 201–206. [Google Scholar] [CrossRef]
- Ren, L.; Schuchardt, F.; Shen, Y.; Li, G.; Li, C. Impact of Struvite Crystallization on Nitrogen Losses during Composting of Pig Manure and Cornstalk. Waste Manag. 2010, 30, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Kebibeche, H.; Khelil, O.; Kacem, M.; Kaid Harche, M. Addition of Wood Sawdust during the Co-Composting of Sewage Sludge and Wheat Straw Influences Seeds Germination. Ecotoxicol. Environ. Saf. 2019, 168, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Awasthi, S.K.; Liu, T.; Chen, H.; Zhang, Z.; Wang, Q.; Ren, X.; Tu, Z.; Awasthi, M.K.; Taherzadeh, M.J. Dynamics of Fungal Diversity and Interactions with Environmental Elements in Response to Wheat Straw Biochar Amended Poultry Manure Composting. Bioresour. Technol. 2019, 274, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Yang, W.; Men, M.; Bello, A.; Xu, X.; Xu, B.; Deng, L.; Jiang, X.; Sheng, S.; Wu, X.; et al. Microbial Community Succession and Response to Environmental Variables During Cow Manure and Corn Straw Composting. Front. Microbiol. 2019, 10, 529. [Google Scholar] [CrossRef]
- Lopez-Gonzalez, J.A.; Suarez-Estrella, F.; Vargas-Garcia, M.C.; Lopez, M.J.; Jurado, M.M.; Moreno, J. Dynamics Of Bacterial Microbiota during Lignocellulosic Waste Composting: Studies upon Its Structure, Functionality and Biodiversity. Bioresour. Technol. 2015, 175, 406–416. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Aziz, H.A. Evaluation of Thermochemical Pretreatment and Continuous Thermophilic Condition in Rice Straw Composting Process Enhancement. Bioresour. Technol. 2013, 133, 240–247. [Google Scholar] [CrossRef]
- Taha, M.; Foda, M.; Shahsavari, E.; Aburto-Medina, A.; Adetutu, E.; Ball, A. Commercial Feasibility of Lignocellulose Biodegradation: Possibilities and Challenges. Curr. Opin. Biotechnol. 2016, 38, 190–38197. [Google Scholar] [CrossRef]
- Jain, M.S.; Daga, M.; Kalamdhad, A.S. Physical Parameters Evaluation during Production of Soil Conditioner from Aquatic Waste: Hydrilla Verticillata (L.F.) Royle. Environ. Technol. Innov. 2018, 11, 64–73. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X. Addition of Fish Pond Sediment and Rock Phosphate Enhances the Composting of Green Waste. Bioresour. Technol. 2017, 233, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Holman, D.B.; Hao, X.; Topp, E.; Yang, H.E.; Alexander, T.W. Effect of Co-Composting Cattle Manure with Construction and Demolition Waste on the Archaeal, Bacterial, and Fungal Microbiota, and on Antimicrobial Resistance Determinants. PLoS ONE 2016, 11, E0157539. [Google Scholar]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. Funguild: An Open Annotation Tool for Parsing Fungal Community Datasets by Ecological Guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Du, G.; Feng, W.; Cai, H.; Ma, Z.; Liu, X.; Yuan, C.; Shi, J.; Zhang, B. Exogenous Enzyme Amendment Accelerates Maturity and Changes Microflora Succession in Horse and Wildlife Animal Manure Co-Composting. Environ. Sci. Pollut. Res. 2021, 28, 21610–21620. [Google Scholar] [CrossRef]
- Wu, D.; Wei, Z.; Zhao, Y.; Zhao, X.; Mohamed, T.A.; Zhu, L.; Wu, J.; Meng, Q.; Yao, C.; Zhao, R. Improved Lignocellulose Degradation Efficiency Based on Fenton Pretreatment during Rice Straw Composting. Bioresour. Technol. 2019, 294, 122132. [Google Scholar] [CrossRef]
- Zhou, H.; Zhao, Y.; Yang, H.; Zhu, L.; Cai, B.; Luo, S.; Cao, J.; Wei, Z. Transformation of Organic Nitrogen Fractions with Different Molecular Weights during Different Organic Wastes Composting. Bioresour. Technol. 2018, 262, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Torres, M.; Oviedo-Ocana, E.R.; Dominguez, I.; Komilis, D.; Sanchez, A. A Systematic Review on the Composting of Green Waste: Feedstock Quality and Optimization Strategies. Waste Manag. 2018, 77, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Luo, W.; Li, Y.; Wang, G.; Li, G. Performance of Co-Composting Sewage Sludge and Organic Fraction of Municipal Solid Waste at Different Proportions. Bioresour. Technol. 2018, 250, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Gou, C.; Wang, Y.; Zhang, X.; Lou, Y.; Gao, Y. Inoculation with a Psychrotrophic-Thermophilic Complex Microbial Agent Accelerates Onset and Promotes Maturity of Dairy Manure-Rice Straw Composting under Cold Climate Conditions. Bioresour. Technol. 2018, 243, 339–346. [Google Scholar] [CrossRef]
- Nakasaki, K.; Araya, S.; Mimoto, H. Inoculation of Pichia Kudriavzevii RB1 Degrades the Organic Acids Present in Raw Compost Material and Accelerates Composting. Bioresour. Technol. 2013, 144, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Wei, Y.; Zhang, Z.; Zhang, A.K.; Li, Y.; Li, J. Effects of Different C/N Ratios on the Maturity and Microbial Quantity of Composting with Sesame Meal and Rice Straw Biochar. Biochar 2021, 3, 557–564. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Pandey, A.K.; Khan, J.; Bundela, P.S.; Wong, J.W.; Selvam, A. Evaluation of Thermophilic Fungal Consortium for Organic Municipal Solid Waste Composting. Bioresour. Technol. 2014, 168, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.-G.; Zeng, G.-M.; Yuan, X.-Z.; Dai, F.; Liu, J.; Wu, X.-H. The Stimulatory Effects of Surfactants on Composting of Waste Rich in Cellulose. World J. Microbiol. Biotechnol. 2006, 22, 1121–1127. [Google Scholar] [CrossRef]
- Lv, B.; Xing, M.; Yang, J.; Qi, W.; Lu, Y. Chemical and Spectroscopic Characterization of Water Extractable Organic Matter during Vermicomposting of Cattle Dung. Bioresour. Technol. 2013, 132, 320–326. [Google Scholar] [CrossRef]
- Rajput, A.A.; Zeshan, C. Visvanathan. Effect of Thermal Pretreatment on Chemical Composition, Physical Structure and Biogas Production Kinetics of Wheat Straw. J. Environ.Manag. 2018, 221, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Paradelo, R.; Moldes, A.B.; Barral, M.T. Evolution of Organic Matter during the Mesophilic Composting of Lignocellulosic Winery Wastes. J. Environ. Manag. 2013, 116, 18–26. [Google Scholar] [CrossRef]
- Glasser, W.G. About Making Lignin Great Again-Some Lessons from the Past. Front. Chem. 2019, 7, 565. [Google Scholar] [CrossRef]
- Li, J.; Xing, W.; Bao, H.; Wang, J.; Tong, X.; Zhang, H.; Luo, W.; Wu, F. Impact of Pine Leaf Biochar Amendment on Bacterial Dynamics and Correlation of Environmental Factors during Pig Manure Composting. Bioresour. Technol. 2019, 293, 122031. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Duan, Y.; Awasthi, S.K.; Liu, T.; Zhang, Z.; Kim, S.-H.; Pandey, A. Effect of Biochar on Emission, Maturity and Bacterial Dynamics during Sheep Manure Compositing. Renew. Energy 2020, 152, 421–429. [Google Scholar] [CrossRef]
- Li, B.; Zhang, X.; Guo, F.; Wu, W.; Zhang, T. Characterization of Tetracycline Resistant Bacterial Community in Saline Activated Sludge Using Batch Stress Incubation With High-Throughput Sequencing Analysis. Water Res. 2013, 47, 4207–4216. [Google Scholar] [CrossRef] [PubMed]
- Palisoc, M.; Guy, J.K.; Peacock, J.P.; Trinh, D.C.; Dodsworth, J.A.; Hedlund, B.P. Novel Thermophilic Cellulolytic Isolates Belonging to the Phylum Chloroflexi; Undergraduate Research, University Of Nevada: Las Vegas, NV, USA, 2011. [Google Scholar]
- Franke-Whittle, I.H.; Knapp, B.A.; Fuchs, J.; Kaufmann, R.; Insam, H. Application of COMPOCHIP Microarray to Investigate the Bacterial Communities of Different Composts. Microb. Ecol. 2009, 57, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Al Atrouni, A.; Joly-Guillou, M.-L.; Hamze, M.; Kempf, M. Reservoirs of Non-Baumannii Acinetobacter Species. Front. Microbiol. 2016, 7, 49. [Google Scholar] [CrossRef]
- Goldberg, J.B.; Hancock, R.E.; Parales, R.E.; Loper, J.; Cornelis, P. Pseudomonas 2007. J. Bacteriol. 2008, 190, 2649–2662. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xiang, W.; Gan, B.; Peng, W.; Jia, D.; Xie, L.; Huang, Z.; Jian, G. Screening and Identification of a Cellulose-Decomposing Strain Lysinibacillus Fusiformis. J. Environ. Eng. 2013, 7, 5041–5046. [Google Scholar]
- Chantarasiri, A.; Boontanom, P.; Nuiplot, N.O. Isolation and Characterization of Lysinibacillus Sphaericus BR2308 from Coastal Wetland in Thailand for the Biodegradation of Lignin. Aquac. Aquar. Conserv. Legis. 2017, 10, 200–209. [Google Scholar]
- Ganguly, R.K.; Chakraborty, S.K. Assessment of Microbial Roles in the Bioconversion of Paper Mill Sludge through Vermicomposting. J. Environ. Health Sci. 2018, 16, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; L, D.; Shah, G.M.; Chen, W.; Wang, W.; Xu, Y.; Huang, H. Hyperthermophilic Pretreatment Composting Significantly Accelerates Humic Substances Formation by Regulating Precursors Production and Microbial Communities. Waste Manag. 2019, 92, 89–96. [Google Scholar] [CrossRef]
- Poomai, N.; Siripornadulsil, W.; Siripornadulsil, S. Cellulase Enzyme Production from Agricultural Waste by Acinetobacter Sp. KKU44. Adv. Mater. Res. 2014, 931–932, 1106–1110. [Google Scholar] [CrossRef]
- Dexter, S.V.; Boopathy, R. Biodegradation of Phenol by Acinetobacter Tandoii Isolated from the Gut of the Termite. Environ. Sci. Pollut. Res. 2019, 26, 34067–34072. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhao, Y.; Qi, H.; Zhao, X.; Yang, T.; Du, Y.; Zhang, H.; Wei, Z. Identifying the Key Factors that Affect the Formation of Humic Substance during Different Materials Composting. Bioresour. Technol. 2017, 244, 1193–1196. [Google Scholar] [CrossRef]
- Zhang, J.; Zeng, G.; Chen, Y.; Yu, M.; Yu, Z.; Hui, L.; Yu, Y.; Huang, H. Effects of Physico-Chemical Parameters on the Bacterial and Fungal Communities during Agricultural Waste Composting. Bioresour. Technol. 2011, 102, 2950–2956. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Wang, X.; Zhen, L.; Gu, J.; Zhang, K.; Wang, Q.; Ma, J.; Peng, H.; Lei, L.; Zhao, W. Effects of Inoculating with Lignocellulose-Degrading Consortium on Cellulose-Degrading Genes and Fungal Community during Co-Composting of Spent Mushroom Substrate With Swine Manure. Bioresour. Technol. 2019, 291, 121876. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Lu, Y.; Xu, J.; Li, M.; Shan, G.; Li, Q. Exploring The Characteristics of Dissolved Organic Matter and Succession of Bacterial Community during Composting. Bioresour. Technol. 2019, 292, 121942. [Google Scholar] [CrossRef] [PubMed]
- Zavrel, M.; Hoot, S.J.; White, T.C. Comparison of Sterol Import under Aerobic and Anaerobic Conditions in Three Fungal Species, Candida Albicans, Candida Glabrata, and Saccharomyces Cerevisiae. Eukaryot Cell. 2013, 12, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Kertesz, M.A.; Thai, M. Compost Bacteria and Fungi that Influence Growth and Development of Agaricus Bisporus and Other Commercial Mushrooms. Appl. Microbiol. Biotechnol. 2018, 102, 1639–1650. [Google Scholar] [CrossRef]
- Ma, R.; Bai, Y.; Huang, H.; Luo, H.; Chen, S.; Fan, Y.; Cai, L.; Yao, B. Utility of Thermostable Xylanases of Mycothermus Thermophilus in Generating Prebiotic Xylooligosaccharides. J. Agric. Food Chem. 2017, 65, 1139–1145. [Google Scholar] [CrossRef]
- Wang, X.; Kong, Z.; Wang, Y.; Wang, M.; Liu, D.; Shen, Q. Insights into the Functionality of Fungal Community during the Large Scale Aerobic Co-Composting Process of Swine Manure and Rice Straw. J. Environ. Manag. 2020, 270, 110958. [Google Scholar] [CrossRef]
- Cannon, P.F.; Kirk, P.M. Fungal Families of the World; Cabi: Egham, UK, 2007. [Google Scholar]
- Liu, C.; Liu, J.; Li, J.; He, H.; Peng, S.; Li, C.; Chen, Y. Removal of H2S by Co-Immobilized Bacteria and Fungi Biocatalysts in a Bio-Trickling Filter. Process. Saf. Environ. Prot. 2013, 91, 145–152. [Google Scholar] [CrossRef]
- Meng, L.; Li, W.; Zhang, S.; Wu, C.; Lv, L. Feasibility of Co-Composting of Sewage Sludge, Spent Mushroom Substrate and Wheat Straw. Bioresour. Technol. 2017, 226, 39–45. [Google Scholar] [CrossRef] [PubMed]

| Materials | Moisture (%) | pH | OM (%) | TOC (%) | TKN (%) | C/N |
|---|---|---|---|---|---|---|
| WS | 8.7 ± 1.7 | 6.63 ± 0.05 | 71.1 ± 2.3 | 41.1 ± 1.1 | 0.17 ± 0.01 | 24.2 ± 0.5 |
| CM | 61.6 ± 1.9 | 7.92 ± 0.07 | 87.2 ± 2.1 | 39.9 ± 1.5 | 1.37 ± 0.02 | 29.1 ± 0.3 |
| BM | 54.4 ± 1.6 | 8.84 ± 0.06 | 79.3 ± 2.4 | 49.3 ± 1.8 | 2.21 ± 0.01 | 22.3 ± 0.2 |
| Items | Times (Day) | SEM | Significance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 20 | 40 | 60 | TR | TI | TR × TI | ||
| Temperature (°C) | |||||||||
| CK | 35.1 ± 0.1Ad | 42.4 ± 0.8Cb | 54.1 ± 0.1Ca | 50.7 ± 0.5Aa | 43.7 ± 0.5Ac | 0.00 | |||
| S1 | 35.3 ± 0.5Ae | 55.2 ± 0.6Ab | 64.3 ± 0.2Aa | 42.9 ± 0.8Bc | 37.2 ± 0.1Bd | *** | *** | *** | |
| S2 | 35.1 ± 0.6Ad | 49.0 ± 0.4Bb | 57.3 ± 0.1Ba | 39.3 ± 0.8Ab | 39.8 ± 0.1Ac | ||||
| pH | |||||||||
| CK | 7.81 ± 0.01Ae | 8.14 ± 0.01Cd | 8.48 ± 0.01Ca | 8.38 ± 0.01Cc | 8.44 ± 0.03Ab | 0.008 | *** | *** | *** |
| S1 | 7.84 ± 0.02Ae | 8.45 ± 0.01Bc | 8.90 ± 0.01Ba | 8.63 ± 0.01Ab | 8.21 ± 0.01Bd | ||||
| S2 | 7.83 ± 0.01e | 8.57 ± 0.01Ab | 9.08 ± 0.01Aa | 8.51 ± 0.01Bc | 8.35 ± 0.01Bd | ||||
| TOC (g·kg−1) | |||||||||
| CK | 393.6 ± 7.5Ca | 394.3 ± 13.1Aa | 345.0 ± 12.2Ab | 340.1 ± 2.0Ab | 341.3 ± 1.4Ab | 0.00 | *** | *** | 0.002 |
| S1 | 430 ± 7.5Aa | 343.6 ± 6.6Aab | 295.7 ± 17.0Ab | 250.7 ± 17.7Cb | 250.6 ± 5.5Cb | ||||
| S2 | 421.0 ± 6.5Ba | 330.7 ± 6.1Ab | 311.3 ± 12.2Ab | 298.7 ± 6.8Bb | 291.3 ± 8.0Bb | ||||
| TN (g·kg−1) | |||||||||
| CK | 10.0 ± 0.21Cd | 9.2 ± 0.1Ce | 11.7 ± 0.6Bc | 12.8 ± 0.1Cb | 14.1 ± 0.2Ba | 0.018 | *** | *** | *** |
| S1 | 14.1 ± 0.1Ac | 13.6 ± 0.2Ac | 16.1 ± 0.5Ab | 17.1 ± 0.2Aa | 17.1 ± 0.3Aa | ||||
| S2 | 13.5 ± 0.3Bc | 11.7 ± 0.2Bd | 12.5 ± 0.2Bc | 13.8 ± 0.1Bb | 14.5 ± 0.3Ba | ||||
| C/N | |||||||||
| CK | 30.6 ± 0.3Aa | 29.4 ± 0.1Ab | 24.4 ± 0.3Ac | 21.5 ± 0.2Ad | 21.5 ± 0.4Ad | 0.192 | *** | *** | *** |
| S1 | 30.8 ± 0.4Aa | 19.9 ± 0.6Cb | 18.5 ± 0.3Cc | 14.7 ± 0.3Cc | 14.4 ± 0.8Cc | ||||
| S2 | 30.9 ± 0.4Aa | 25.4 ± 4.0Bb | 24.8 ± 5.1Bc | 21.3 ± 3.9Bd | 17.3 ± 4.4Bd | ||||
| GI | |||||||||
| CK | 32.3 ± 1.1Ac | 38.3 ± 0.4Cbc | 48.3 ± 0.5Bab | 54.5 ± 2.7Ca | 57.4 ± 5.5Ca | 0.023 | *** | *** | *** |
| S1 | 33.9 ± 1.1Ad | 77.8 ± 3.9Ac | 89.2 ± 1.1Ab | 119.0 ± 6.4Aa | 123.1 ± 9.7Aa | ||||
| S2 | 33.5 ± 1.2Ae | 67.5 ± 1.1Bd | 83.3 ± 0.9Ac | 88.1 ± 1.3Bb | 91.8 ± 1.5Ba | ||||
| Items | Times (Day) | SEM | Significance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 20 | 40 | 60 | TR | TI | TR × TI | ||
| OM (organic matter, %) | |||||||||
| CK | 83.3 ± 17.6Aa | 82.1 ± 6.0Aa | 78.2 ± 4.6Ab | 77.4 ± 10.5Ab | 76.6 ± 3.0Ac | 0.00 | 0.403 | *** | 0.315 |
| S1 | 84.7 ± 17.4Aa | 82.3 ± 10.7Ab | 77.5 ± 15.5Ac | 76.9 ± 16.1Bcd | 75.2 ± 10.5Bd | ||||
| S2 | 80.7 ± 6.9Ab | 82.1 ± 3.5Aa | 78.3 ± 8.7Ac | 77.2 ± 10.3ABc | 75.7 ± 5.2ABd | ||||
| Cellulose (%) | |||||||||
| CK | 0 | 12.1 ± 1.2Bbc | 15.6 ± 4.1Aa | 13.3 ± 1.4Ab | 11.6 ± 1.0Bc | 0.056 | *** | *** | 0.002 |
| S1 | 0 | 14.3 ± 1.0Ab | 16.7 ± 1.5Aa | 14.3 ± 1.0Ab | 14.3 ± 6.0Ab | ||||
| S2 | 0 | 11.8 ± 0.6Bc | 15.4 ± 1.0AaB | 13.8 ± 1.3Aab | 12.5 ± 0.5Bbc | ||||
| Hemicellulose (%) | |||||||||
| CK | 0 | 18.4 ± 0.5Ac | 38.2 ± 1.2Ba | 40.4 ± 1.2Ba | 32.1.1 ± 1.1Bb | 0.068 | *** | *** | *** |
| S1 | 0 | 20.3 ± 1.0Ab | 45.7 ± 2.5Aa | 46.6 ± 2.3Aa | 42.8 ± 3.0Aa | ||||
| S2 | 0 | 18.3 ± 1.2Ac | 36.3 ± 0.9Bb | 42.2 ± 1.3Ba | 34.2 ± 1.1Bb | ||||
| Lignin (%) | |||||||||
| CK | 0 | 4.5 ± 0.01Cc | 11.9 ± 0.18Bb | 16.1 ± 0.8Ba | 12.5 ± 0.7Bb | 0.024 | *** | *** | 0.002 |
| S1 | 0 | 6.4 ± 0.1Ac | 14.5 ± 0.6Ab | 20.2 ± 1.6Aa | 16.4 ± 0.7Ab | ||||
| S2 | 0 | 5.4 ± 0.8Bc | 13.3 ± 0.6ABb | 17.8 ± 1.3ABa | 13.4 ± 0.9Bb | ||||
| Treatment | Time (Day) | Coverage | Chao 1 Bacteria/FungI | Ace Bacteria/FungI | Shannon Bacteria/FungI | Simpson Bacteria/FungI |
|---|---|---|---|---|---|---|
| Richness | Diversity | |||||
| CK | 0 | 1.0 | 773.72/226.29 | 987.01/219.31 | 4.11/3.43 | 0.16/0.28 |
| 7 | 1.0 | 684.02/223.12 | 718.87/221.05 | 3.26/3.01 | 0.12/0.23 | |
| 20 | 1.0 | 294.23/104.09 | 363.55/106.90 | 2.47/2.84 | 0.10/0.25 | |
| 40 | 1.0 | 676.01/126.01 | 456.99/122.36 | 3.88/1.35 | 0.11/0.18 | |
| 60 | 1.0 | 799.19/131.27 | 480.58/126.16 | 4.82/2.43 | 0.12/0.20 | |
| S1 | 0 | 1.0 | 985.68/303.29 | 992.26/223.35 | 4.19/4.21 | 0.15/0.25 |
| 7 | 1.0 | 747.16/223.27 | 656.44/216.22 | 3.15/2.13 | 0.09/0.23 | |
| 20 | 1.0 | 656.23/184.37 | 589.49/131.97 | 2.13/2.04 | 0.08/0.21 | |
| 40 | 1.0 | 686.57/171.75 | 695.05/170.35 | 3.95/2.38 | 0.25/0.25 | |
| 60 | 1.0 | 652.07/135.38 | 659.59/236.58 | 3.88/2.33 | 0.27/0.26 | |
| S2 | 0 | 1.0 | 949.29/313.05 | 1097.23/283.97 | 2.17/4.11 | 0.23/0.31 |
| 7 | 1.0 | 750.12/203.14 | 719.71/258.62 | 3.12/3.69 | 0.25/0.25 | |
| 20 | 1.0 | 315.55/103.64 | 411.23/112.69 | 2.98/3.28 | 0.11/0.28 | |
| 40 | 1.0 | 580.02/128.05 | 670.02/128.36 | 2.84/3.85 | 0.10/0.18 | |
| 60 | 1.0 | 723.21/201.75 | 776.25/179.62 | 3.24/3.76 | 0.17/0.21 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, H.; Ji, M.; Xie, Y.; Shi, J.; Liu, L.; Zhang, B.; Sun, J. Exploring the Microbial Dynamics of Organic Matter Degradation and Humification during Co-Composting of Cow Manure and Bedding Material Waste. Sustainability 2021, 13, 13035. https://doi.org/10.3390/su132313035
Duan H, Ji M, Xie Y, Shi J, Liu L, Zhang B, Sun J. Exploring the Microbial Dynamics of Organic Matter Degradation and Humification during Co-Composting of Cow Manure and Bedding Material Waste. Sustainability. 2021; 13(23):13035. https://doi.org/10.3390/su132313035
Chicago/Turabian StyleDuan, Haiyan, Minghua Ji, Yukang Xie, Jiping Shi, Li Liu, Baoguo Zhang, and Junsong Sun. 2021. "Exploring the Microbial Dynamics of Organic Matter Degradation and Humification during Co-Composting of Cow Manure and Bedding Material Waste" Sustainability 13, no. 23: 13035. https://doi.org/10.3390/su132313035
APA StyleDuan, H., Ji, M., Xie, Y., Shi, J., Liu, L., Zhang, B., & Sun, J. (2021). Exploring the Microbial Dynamics of Organic Matter Degradation and Humification during Co-Composting of Cow Manure and Bedding Material Waste. Sustainability, 13(23), 13035. https://doi.org/10.3390/su132313035







