Cr(VI) Adsorption from Aqueous Solution by UiO-66 Modified Corncob
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of UiO-66 Modified Corncob
2.3. Characterization
2.4. Adsorption Experiments
2.5. Data Analysis
3. Results and Discussion
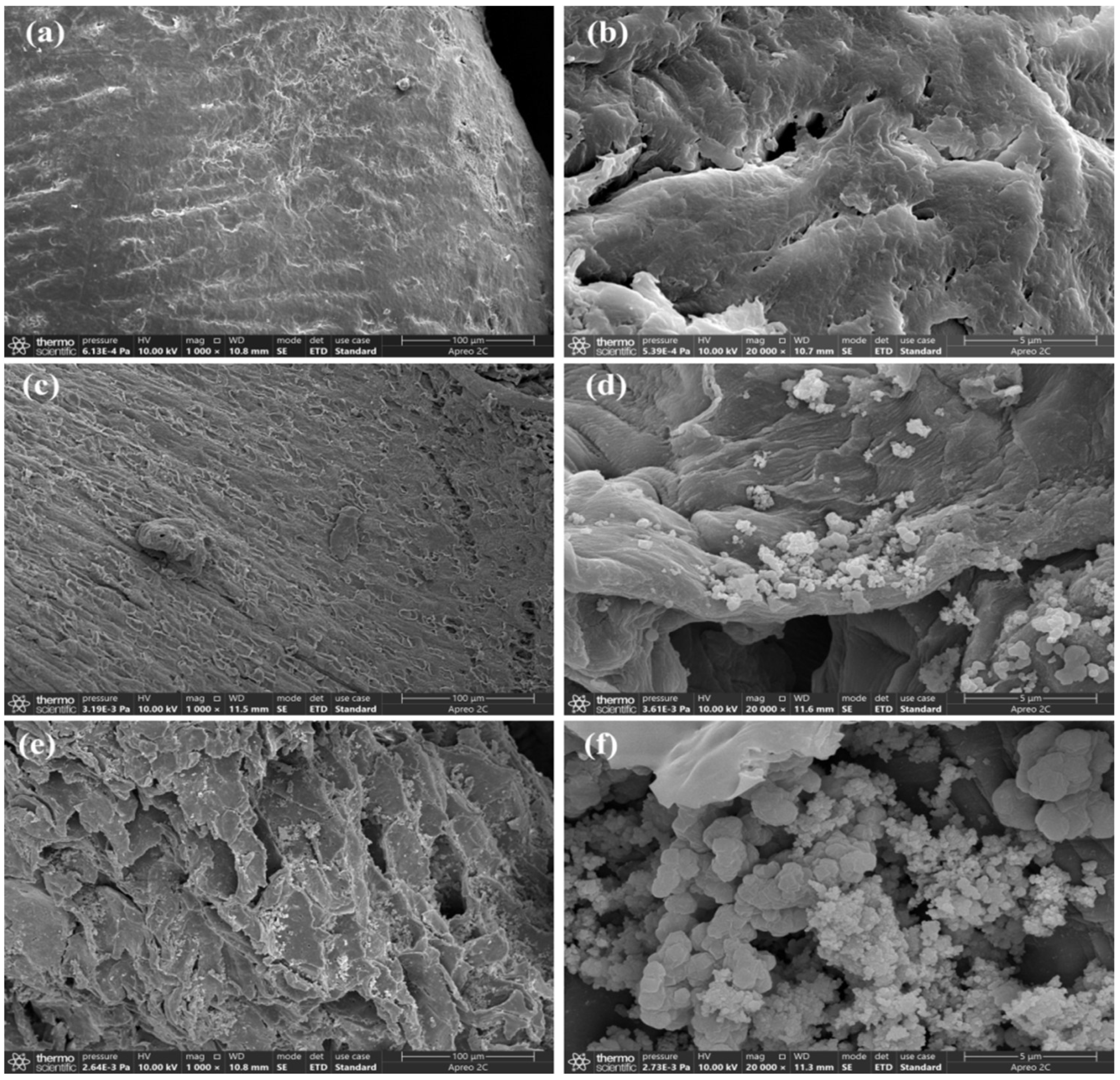
3.1. Characterization of UiO-66 Modified Corncob
3.2. Adsorption Kinetics
3.3. Cr(VI) Adsorption Equilibrium
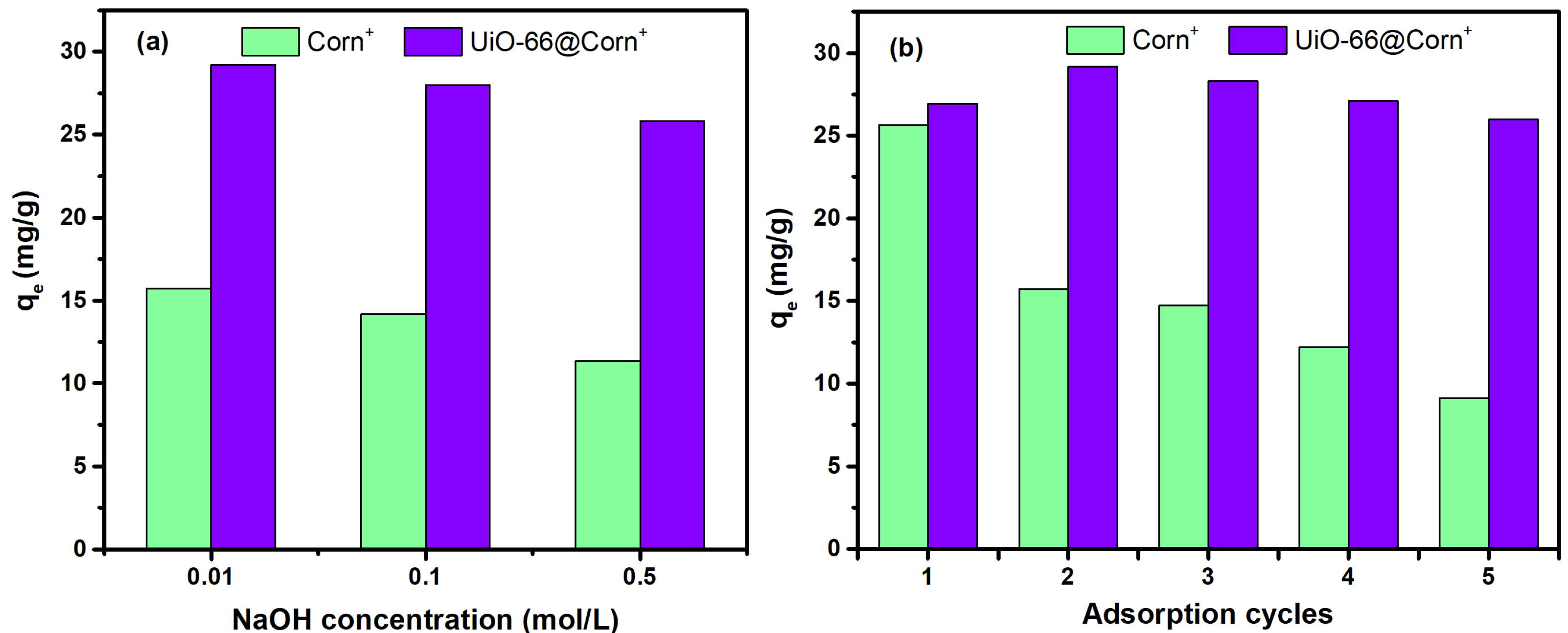
3.4. Effects of Solution Chemistry
3.5. Regeneration Studies
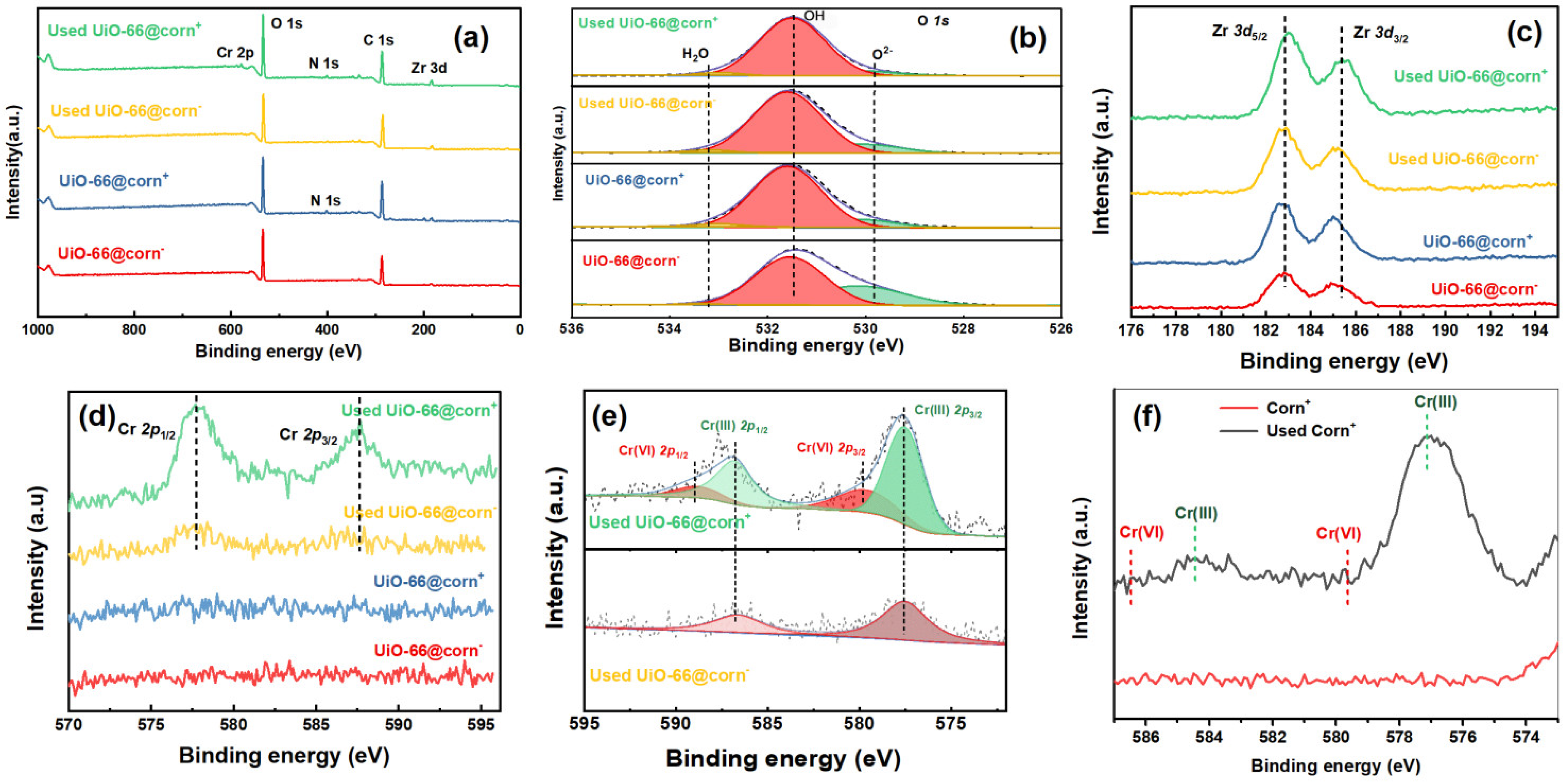
3.6. Adsorption Mechanism of Cr(VI)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ihsanullah; Abbas, A.; Al-Amer, A.M.; Laoui, T.; Al-Marri, M.J.; Nasser, M.S.; Khraisheh, M.; Atieh, M. Heavy metal removal from aqueous solution by advanced carbon nanotubes: Critical review of adsorption applications. Sep. Purif. Technol. 2016, 157, 141–161. [CrossRef]
- Rego, R.M.; Sriram, G.; Ajeya, K.V.; Jung, H.-Y.; Kurkuri, M.D.; Kigga, M. Cerium based UiO-66 MOF as a multipollutant adsorbent for universal water purification. J. Hazard. Mater. 2021, 416, 125941. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Feng, Y.; Tian, R.; Chen, J.; Wang, A.; Yao, J. Defect Rich UiO-66 with Enhanced Adsorption and Photosensitized Reduction of Cr(VI) under Visible Light. Ind. Eng. Chem. Res. 2019, 58, 21562–21568. [Google Scholar] [CrossRef]
- Thach, U.D.; Prelot, B.; Pellet-Rostaing, S.; Zajac, J.; Hesemann, P. Surface Properties and Chemical Constitution as Crucial Parameters for the Sorption Properties of Ionosilicas: The Case of Chromate Adsorption. ACS Appl. Nano Mater. 2018, 1, 2076–2087. [Google Scholar] [CrossRef]
- Burakov, A.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Du, M.; Zhu, H.; Bao, S.; Yang, T.; Zou, M. Structure regulation of silica nanotubes and their adsorption behaviors for heavy metal ions: pH effect, kinetics, isotherms and mechanism. J. Hazard. Mater. 2015, 286, 533–544. [Google Scholar] [CrossRef]
- Fiyadh, S.S.; AlSaadi, M.A.; Jaafar, W.Z.; AlOmar, M.; Fayaed, S.S.; Mohd, N.S.; Hin, L.S.; El-Shafie, A. Review on heavy metal adsorption processes by carbon nanotubes. J. Clean. Prod. 2019, 230, 783–793. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Lee, J.K.; Cho, C.-W.; Jhung, S.H. Remarkably efficient adsorbent for the removal of bisphenol A from water: Bio-MOF-1-derived porous carbon. Chem. Eng. J. 2018, 343, 225–234. [Google Scholar] [CrossRef]
- Xu, G.-R.; An, Z.-H.; Xu, K.; Liu, Q.; Das, R.; Zhao, H.-L. Metal organic framework (MOF)-based micro/nanoscaled materials for heavy metal ions removal: The cutting-edge study on designs, synthesis, and applications. Coord. Chem. Rev. 2020, 427, 213554. [Google Scholar] [CrossRef]
- Wang, C.; Luan, J.; Wu, C. Metal-organic frameworks for aquatic arsenic removal. Water Res. 2019, 158, 370–382. [Google Scholar] [CrossRef]
- Li, Y.; Fang, Y.; Cao, Z.; Li, N.; Chen, D.; Xu, Q.; Lu, J. Construction of g-C3N4/PDI@MOF heterojunctions for the highly efficient visible light-driven degradation of pharmaceutical and phenolic micropollutants. Appl. Catal. B Environ. 2019, 250, 150–162. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, X.; Chen, L. An ultrasensitive electrochemical bisphenol A sensor based on hierarchical Ce-metal-organic framework modified with cetyltrimethylammonium bromide. Sens. Actuators B Chem. 2018, 261, 425–433. [Google Scholar] [CrossRef]
- Ahmad, K.; Shah, H.-U.-R.; Ashfaq, M.; Nawaz, H. Removal of decidedly lethal metal arsenic from water using metal organic frameworks: A critical review. Rev. Inorg. Chem. 2021. accepted. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Santiago-Portillo, A.; Asiri, A.M.; Garcia, H. Engineering UiO-66 Metal Organic Framework for Heterogeneous Catalysis. ChemCatChem 2019, 11, 899–923. [Google Scholar] [CrossRef]
- Audu, C.O.; Nguyen, H.G.T.; Chang, C.-Y.; Katz, M.J.; Mao, L.; Farha, O.K.; Hupp, J.T.; Nguyen, S.T. The dual capture of AsV and AsIII by UiO-66 and analogues. Chem. Sci. 2016, 7, 6492–6498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.-L.; Feng, X.-Q.; Han, R.-P.; Zang, S.-Q.; Yang, G. Cr(VI) removal via anion exchange on a silver-triazolate MOF. J. Hazard. Mater. 2017, 321, 622–628. [Google Scholar] [CrossRef]
- Sun, X.; Yao, S.; Yu, C.; Li, G.; Liu, C.; Huo, Q.; Liu, Y. An ultrastable Zr-MOF for fast capture and highly luminescence detection of Cr2O72− simultaneously in an aqueous phase. J. Mater. Chem. A 2018, 6, 6363–6369. [Google Scholar] [CrossRef]
- Duan, C.; Meng, X.; Liu, C.; Lu, W.; Liu, J.; Dai, L.; Wang, W.; Zhao, W.; Xiong, C.; Ni, Y. Carbohydrates-rich corncobs supported metal-organic frameworks as versatile biosorbents for dye removal and microbial inactivation. Carbohydr. Polym. 2019, 222, 115042. [Google Scholar] [CrossRef]
- Yan, B.; Yan, J.; Li, Y.; Qin, Y.; Yang, L. Spatial distribution of biogas potential, utilization ratio and development potential of biogas from agricultural waste in China. J. Clean. Prod. 2021, 292, 126077. [Google Scholar] [CrossRef]
- Qiu, H.; Ye, M.; Zeng, Q.; Li, W.; Fortner, J.; Liu, L.; Yang, L. Fabrication of agricultural waste supported UiO-66 nanoparticles with high utilization in phosphate removal from water. Chem. Eng. J. 2019, 360, 621–630. [Google Scholar] [CrossRef]
- Ahmad, K.; Shah, H.-U.-R.; Nasim, H.A.; Ayub, A.; Ashfaq, M.; Rauf, A.; Shah, S.S.A.; Ahmad, M.M.; Nawaz, H.; Hussain, E. Synthesis and characterization of water stable polymeric metallo organic composite (PMOC) for the removal of arsenic and lead from brackish water. Toxin Rev. 2021, 40. [Google Scholar] [CrossRef]
- Ahmad, K.; Nazir, M.A.; Qureshi, A.K.; Hussain, E.; Najam, T.; Javed, M.S.; Shah, S.S.A.; Tufail, M.K.; Hussain, S.; Khan, N.A.; et al. Engineering of Zirconium based metal-organic frameworks (Zr-MOFs) as efficient adsorbents. Mater. Sci. Eng. B 2020, 262, 114766. [Google Scholar] [CrossRef]
- Ahmad, K.; Shah, H.-U.-R.; Ashfaq, M.; Shah, S.S.A.; Hussain, E.; Naseem, H.A.; Parveen, S.; Ayub, A. Effect of metal atom in zeolitic imidazolate frameworks (ZIF-8 & 67) for removal of Pb2+ & Hg2+ from water. Food Chem. Toxicol. 2021, 149, 112008. [Google Scholar] [CrossRef]
- Shah, H.U.R.; Ahmad, K.; Naseem, H.A.; Parveen, S.; Ashfaq, M.; Rauf, A.; Aziz, T. Water stable graphene oxide metal-organic frameworks composite (ZIF-67@GO) for efficient removal of malachite green from water. Food Chem. Toxicol. 2021, 154, 112312. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Shah, H.-U.-R.; Parveen, S.; Aziz, T.; Naseem, H.A.; Ashfaq, M.; Rauf, A. Metal Organic Framework (KIUB-MOF-1) as Efficient Adsorbent for Cationic and Anionic Dyes from Brackish Water. J. Mol. Struct. 2021, 1242, 130898. [Google Scholar] [CrossRef]
- Ayub, A.; Irfan, A.; Raza, Z.A.; Abbas, M.; Muhammad, A.; Ahmad, K.; Munwar, A. Development of poly(1-vinylimidazole)-chitosan composite sorbent under microwave irradiation for enhanced uptake of Cd(II) ions from aqueous media. Polym. Bull. 2021. [Google Scholar] [CrossRef]
- Katz, M.J.; Brown, Z.J.; Colón, Y.J.; Siu, P.W.; Scheidt, K.A.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K. A facile synthesis of UiO-66, UiO-67 and their derivatives. Chem. Commun. 2013, 49, 9449–9451. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Hameed, B. Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chem. Eng. J. 2009, 153, 1–8. [Google Scholar] [CrossRef]
- Guo, X.; Liu, A.; Lu, J.; Niu, X.; Jiang, M.; Ma, Y.; Liu, X.; Li, M. Adsorption Mechanism of Hexavalent Chromium on Biochar: Kinetic, Thermodynamic, and Characterization Studies. ACS Omega 2020, 5, 27323–27331. [Google Scholar] [CrossRef]
- Liu, S.S.; Chen, Y.Z.; De Zhang, L.; Hua, G.M.; Xu, W.; Li, N.; Zhang, Y. Enhanced removal of trace Cr(VI) ions from aqueous solution by titanium oxide–Ag composite adsorbents. J. Hazard. Mater. 2011, 190, 723–728. [Google Scholar] [CrossRef]
- Deng, Y.-Y.; Xiao, X.-F.; Wang, D.; Han, B.; Gao, Y.; Xue, J.-L. Adsorption of Cr(VI) from Aqueous Solution by Ethylenediaminetetraacetic Acid-Chitosan-Modified Metal-Organic Framework. J. Nanosci. Nanotechnol. 2020, 20, 1660–1669. [Google Scholar] [CrossRef]
- Periyasamy, S.; Viswanathan, N. Hydrothermal synthesis of hydrocalumite assisted biopolymeric hybrid composites for efficient Cr(vi) removal from water. New J. Chem. 2018, 42, 3371–3382. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, J.; Li, Y.; Zhuang, Q.; Gu, J. Simultaneous Degradation and Removal of CrVI from Aqueous Solution with Zr-Based Metal-Organic Frameworks Bearing Inherent Reductive Sites. Chem. Eur. J. 2017, 23, 15415–15423. [Google Scholar] [CrossRef]
- Mukri, B.; Krushnamurty, K.; Chowdhury, A.; Suryakala, D.; Subrahmanyam, C. Alkali-treated Carbonized Rice Husk for the Removal of Aqueous Cr(VI). BioResources 2016, 11, 9175–9189. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.Q.; Cheng, B.; You, W.; Yu, J.G.; Ho, W.K. 3D hierarchical graphene oxide-NiFe LDH composite with enhanced adsorption affinity to Congo red, methyl orange and Cr(VI) ions. J. Hazard. Mater. 2019, 369, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Bao, D.; Wang, H.; Liao, W.; Li, H.-Q. Multi-Metal Modified Layered Double Hydroxides for Cr(VI) and Fluoride Ion Adsorption in Single and Competitive Systems: Experimental and Mechanism Studies. Environ. Eng. Sci. 2020, 37, 623–636. [Google Scholar] [CrossRef]
- Jin, X.; Wang, H.J.; Jin, X.; Wang, H.; Chen, L.N.; Wang, W.Y.; Lin, T.; Zhu, Z.T. Preparation of keratin/PET nanofiber membrane and its high adsorption performance of Cr(VI). Sci. Total Environ. 2020, 710, 135546. [Google Scholar] [CrossRef]
- Hoang, L.P.; Nguyen, T.M.P.; Van, H.T.; Hoang, T.K.D.; Vu, X.H.; Nguyen, T.V.; Ca, N.X. Cr(VI) Removal from Aqueous Solution Using a Magnetite Snail Shell. Water Air Soil Pollut. 2020, 231, 28. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, J.; Cai, J.; Zhang, L.; Cui, Y.; Yang, Y.; Chen, B.; Qian, G. A porous Zr-cluster-based cationic metal–organic framework for highly efficient Cr2O72− removal from water. Chem. Commun. 2015, 51, 14732–14734. [Google Scholar] [CrossRef]
- Du, Y.; Wang, L.; Wang, J.; Zheng, G.; Wu, J.; Dai, H. Flower-, wire-, and sheet-like MnO2-deposited diatomites: Highly efficient absorbents for the removal of Cr(VI). J. Environ. Sci. 2015, 29, 71–81. [Google Scholar] [CrossRef]
- Liu, S.; Chen, M.; Cao, X.; Li, G.; Zhang, D.; Li, M.; Meng, N.; Yin, J.; Yan, B. Chromium (VI) removal from water using cetylpyridinium chloride (CPC)-modified montmorillonite. Sep. Purif. Technol. 2020, 241, 116732. [Google Scholar] [CrossRef]
- Yang, J.; Huang, B.; Lin, M. Adsorption of Hexavalent Chromium from Aqueous Solution by a Chitosan/Bentonite Composite: Isotherm, Kinetics, and Thermodynamics Studies. J. Chem. Eng. Data 2020, 65, 2751–2763. [Google Scholar] [CrossRef]
- Wang, L.; Xie, Y.; Yang, J.; Zhu, X.; Hu, Q.; Li, X.; Liu, Z. Insight into mechanisms of fluoride removal from contaminated groundwater using lanthanum-modified bone waste. RSC Adv. 2017, 7, 54291–54305. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, N.; Chen, D.; Ma, D.; Liu, G.; Zou, X.; Chen, Y.; Shu, R.; Song, Q.; Lv, W. Facile synthesis of acid-modified UiO-66 to enhance the removal of Cr(VI) from aqueous solutions. Sci. Total Environ. 2019, 682, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Embaby, M.S.; Elwany, S.D.; Setyaningsih, W.; Saber, M.R. The adsorptive properties of UiO-66 towards organic dyes: A record adsorption capacity for the anionic dye Alizarin Red S. Chin. J. Chem. Eng. 2018, 26, 731–739. [Google Scholar] [CrossRef]
- Jiang, Y.; Cai, W.; Tu, W.; Zhu, M. Facile Cross-Link Method To Synthesize Magnetic Fe3O4@SiO2–Chitosan with High Adsorption Capacity toward Hexavalent Chromium. J. Chem. Eng. Data 2018, 64, 226–233. [Google Scholar] [CrossRef]
- Wang, C.; Xiong, C.; He, Y.; Yang, C.; Li, X.; Zheng, J.; Wang, S. Facile preparation of magnetic Zr-MOF for adsorption of Pb(II) and Cr(VI) from water: Adsorption characteristics and mechanisms. Chem. Eng. J. 2021, 415, 128923. [Google Scholar] [CrossRef]






| Adsorbents | qe,exp (mg/g) | Pseudo-First-Order Model | Pseudo-Second-Order Model | Intra-Particle Diffusion Model | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| K1 (min−1) | qe,calculated (mg/g) | R2 | K2 | qe,calculated (mg/g) | R2 | kpi (mg/(g·min0.5) | c | R2 | ||
| (g/(mg·min)) | ||||||||||
| UiO-66@Corn+ | 30.621 | 0.003 | 8.802 | 0.813 | 0.002 | 31.056 | 1.000 | 0.540 | 15.473 | 0.550 |
| UiO-66@Corn− | 12.166 | 0.002 | 4.180 | 0.665 | 0.003 | 12.110 | 0.999 | 0.231 | 5.275 | 0.657 |
| Adsorbents | Langmuir Model | Freundlich Model | Langmuir–Freundlich Model | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| KL (L/mg) | qm (mg/g) | R2 | 1/n | KF (L/g) | R2 | Qg (mg/g) | KLF (L/mg) | n | R2 | |
| Corn+ | 0.053 | 60.232 | 0.993 | 0.452 | 6.944 | 0.950 | 54.971 | 0.066 | 1.175 | 0.994 |
| UiO-66 | 0.031 | 109.88 | 0.990 | 0.484 | 9.334 | 0.961 | 102.00 | 0.037 | 1.115 | 0.989 |
| UiO-66@Corn+ | 0.032 | 90.038 | 0.993 | 0.565 | 5.810 | 0.975 | 84.940 | 0.037 | 1.059 | 0.992 |
| UiO-66@Corn− | 0.066 | 22.383 | 0.990 | 0.470 | 2.787 | 0.939 | 19.539 | 0.090 | 1.272 | 0.994 |
| Adsorbents | qm | Reference | Adsorbents | qm | Reference |
|---|---|---|---|---|---|
| Ce-UiO-66 | 30.00 | [2] | TiO2-Ag | 25.70 | [31] |
| EDTA-chitosan/Cu-BTC | 46.51 | [32] | CaAl-CO3 LDH | 20.24 | [33] |
| UiO-66 | 36.40 | [34] | Rice husk | 25.2 | [35] |
| UiO-66-(OH)2 | 59.20 | [34] | GO-NiFe LDH | 53.6 | [36] |
| CoMgAl-LDH | 59.27 | [37] | Wool keratin/PET composite | 75.86 | [38] |
| MSS | 46.08 | [39] | MOF-867 | 53.4 | [40] |
| MnO2-deposited diatomites | 48.2 | [41] | UiO-66@Corn+ | 90.04 | This study |
| Modified montmorillonite | 43.84 | [42] | UiO-66@Corn− | 22.38 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, H.; Wan, Y.; Chen, H.; Xiong, G.; Wang, L.; Xu, Q.; Li, X.; Zhou, Q. Cr(VI) Adsorption from Aqueous Solution by UiO-66 Modified Corncob. Sustainability 2021, 13, 12962. https://doi.org/10.3390/su132312962
Xie H, Wan Y, Chen H, Xiong G, Wang L, Xu Q, Li X, Zhou Q. Cr(VI) Adsorption from Aqueous Solution by UiO-66 Modified Corncob. Sustainability. 2021; 13(23):12962. https://doi.org/10.3390/su132312962
Chicago/Turabian StyleXie, Hongzhong, Yanlei Wan, Hao Chen, Guangcheng Xiong, Lingqing Wang, Qi Xu, Xiang Li, and Qiuhong Zhou. 2021. "Cr(VI) Adsorption from Aqueous Solution by UiO-66 Modified Corncob" Sustainability 13, no. 23: 12962. https://doi.org/10.3390/su132312962
APA StyleXie, H., Wan, Y., Chen, H., Xiong, G., Wang, L., Xu, Q., Li, X., & Zhou, Q. (2021). Cr(VI) Adsorption from Aqueous Solution by UiO-66 Modified Corncob. Sustainability, 13(23), 12962. https://doi.org/10.3390/su132312962






