Growth of Black Soldier Fly Larvae Reared on Organic Side-Streams
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection and Preparation of the Feed Substrates
2.1.1. Side-Streams
2.1.2. Control Substrates
2.1.3. Feed Preparation
2.2. Substrate Analysis
2.2.1. Pretreatment of the Substrates for Chemical Analysis
2.2.2. Determination of the Proximate Analysis
2.2.3. Determination of Mineral Composition and Fiber Profile
2.3. Rearing Experiment
2.3.1. H. illucens Origin and Maintenance
2.3.2. Feed Experiment
2.4. Calculations
2.5. Statistical Analyses
3. Results
3.1. Substrate Analysis
3.1.1. Proximate Analysis
3.1.2. Determination of Minerals and Fiber Profile
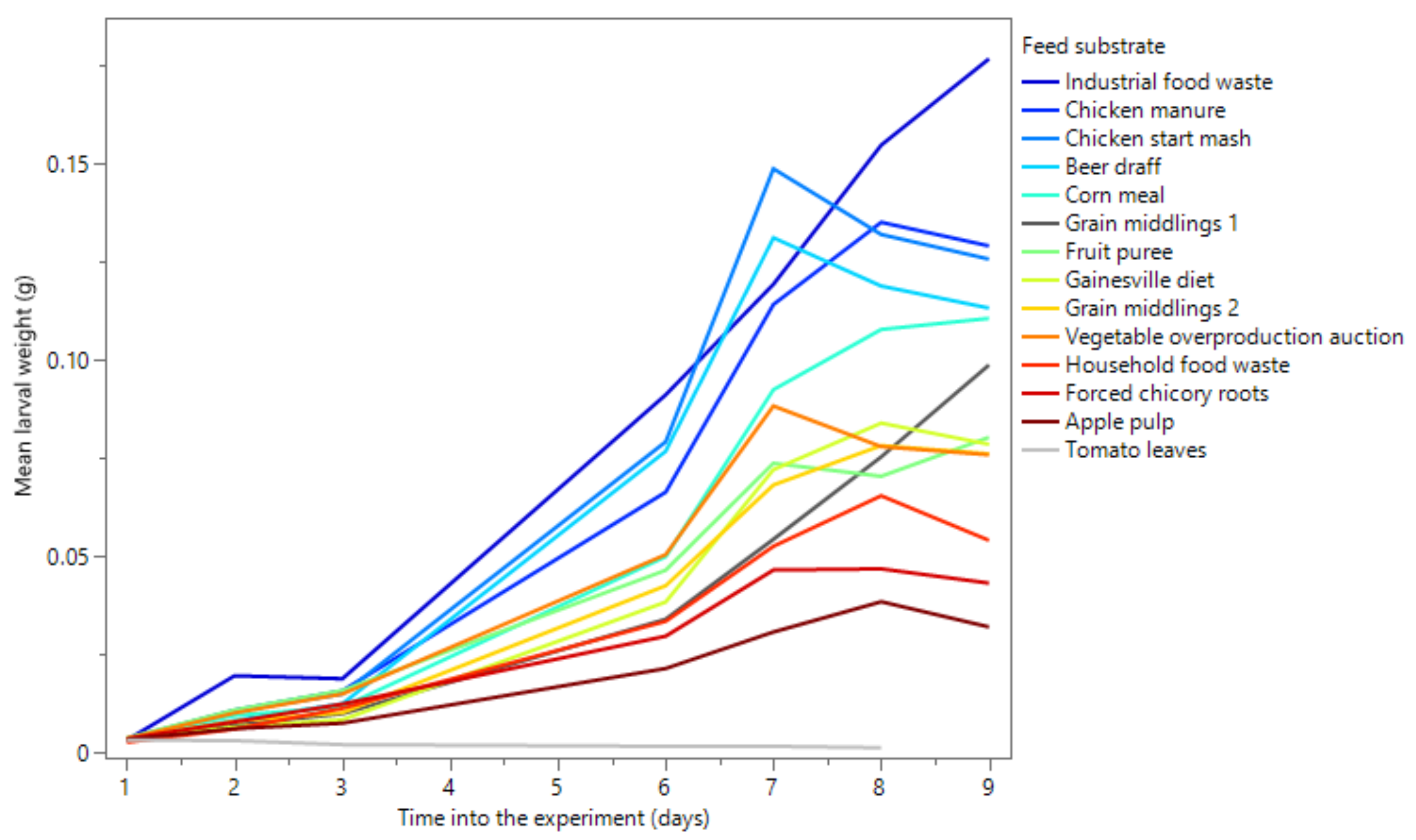
3.2. Rearing Experiment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gardiner, R.; Hajek, P. Municipal waste generation, R&D intensity, and economic growth nexus—A case of EU regions. Waste Manag. 2020, 114, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Fitzherbert, E.B.; Struebig, M.J.; Morel, A.; Danielsen, F.; Brühl, C.A.; Donald, P.F.; Phalan, B. How will oil palm expansion affect biodiversity? Trends Ecol. Evol. 2008, 23, 538–545. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Communication from the Commission to the European Parliament, the European Councip, the European Economic and Social Committee and the Committee of the Regions of the European Green Deal; European Commision: Brussels, Belgium, 2019. [Google Scholar]
- European Union DIRECTIVE 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on waste and repealing certain Directives. Off. J. Eur. Union 2008, 28, 3–30.
- Pimentel, D.; Marklein, A.; Toth, M.A.; Karpoff, M.N.; Paul, G.S.; McCormack, R.; Kyriazis, J.; Krueger, T. Food versus biofuels: Environmental and economic costs. Hum. Ecol. 2009, 37, 1–2. [Google Scholar] [CrossRef]
- Phan, C.; Phi, V.; Walraven, M.; Bézagu, M.; Lefranc, M.; Ray, C. Industrial Symbiosis in Insect Production-A Sustainable Eco-Efficient and Circular Business Model. Sustainability 2020, 12, 333. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; Van Broekhoven, S.; Van Huis, A.; Van Loon, J.J.A. Feed conversion, survival and development, and composition of four insect species on diets composed of food by-products. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Van Huis, A. Potential of Insects as Food and Feed in Assuring Food Security. Annu. Rev. Entomol. 2012, 58, 563–583. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; van Itterbeeck, J.; Heetkamp, M.J.W.; van den Brand, H.; van Loon, J.J.A.; van Huis, A. An Exploration on Greenhouse Gas and Ammonia Production by Insect Species Suitable for Animal or Human Consumption. PLoS ONE 2010, 5, e14445. [Google Scholar] [CrossRef] [Green Version]
- Bjørge, J.D.; Overgaard, J.; Malte, H.; Gianotten, N.; Heckmann, L.H. Role of temperature on growth and metabolic rate in the tenebrionid beetles Alphitobius diaperinus and Tenebrio molitor. J. Insect Physiol. 2018, 107, 89–96. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; De Boer, I.J.M. Environmental Impact of the Production of Mealworms as a Protein Source for Humans-A Life Cycle Assessment. PLoS ONE 2012, 7, 51145. [Google Scholar] [CrossRef] [Green Version]
- Miglietta, P.P.; De Leo, F.; Ruberti, M.; Massari, S.; Pfister, S. Mealworms for Food: A Water Footprint Perspective. Water 2015, 7, 6190–6203. [Google Scholar] [CrossRef]
- Bosch, G.; van Zanten, H.H.E.; Zamprogna, A.; Veenenbos, M.; Meijer, N.P.; van der Fels-Klerx, H.J.; van Loon, J.J.A. Conversion of organic resources by black soldier fly larvae: Legislation, efficiency and environmental impact. J. Clean. Prod. 2019, 222, 355–363. [Google Scholar] [CrossRef]
- Gold, M.; Tomberlin, J.K.; Diener, S.; Zurbrügg, C.; Mathys, A. Decomposition of biowaste macronutrients, microbes, and chemicals in black soldier fly larval treatment: A review. Waste Manag. 2018, 82, 302–318. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Shelomi, M. Review of Black Soldier Fly (Hermetia illucens) as Animal Feed and Human Food. Foods 2017, 6, 91. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.C.; Liang, S.H.; Li, S.Y.; Su, C.H.; Chien, C.C.; Chen, Y.J.; Huong, D.T.M. Direct transesterification of black soldier fly larvae (Hermetia illucens) for biodiesel production. J. Taiwan Inst. Chem. Eng. 2018, 85, 165–169. [Google Scholar] [CrossRef]
- Verheyen, G.; Theunis, M.; Vreysen, S.; Naessens, T.; Noyens, I.; Ooms, T.; Goossens, S.; Pieters, L.; Foubert, K.; Miert, S. V Glycine-acyl Surfactants Prepared from Black Soldier Fly Fat, Coconut Oil and Palm Kernel Oil. Curr. Green Chem. 2020, 7, 239–248. [Google Scholar] [CrossRef]
- Barbi, S.; Messori, M.; Manfredini, T.; Pini, M.; Montorsi, M. Rational design and characterization of bioplastics from Hermetia illucens prepupae proteins. Biopolymers 2018, 110, e23250. [Google Scholar] [CrossRef]
- Surendra, K.C.; Tomberlin, J.K.; van Huis, A.; Cammack, J.A.; Heckmann, L.H.L.; Khanal, S.K. Rethinking organic wastes bioconversion: Evaluating the potential of the black soldier fly (Hermetia illucens (L.)) (Diptera: Stratiomyidae) (BSF). Waste Manag. 2020, 117, 58–80. [Google Scholar] [CrossRef]
- Smetana, S.; Palanisamy, M.; Mathys, A.; Heinz, V. Sustainability of insect use for feed and food: Life Cycle Assessment perspective. J. Clean. Prod. 2016, 137, 741–751. [Google Scholar] [CrossRef]
- Raksasat, R.; Lim, J.W.; Kiatkittipong, W.; Kiatkittipong, K.; Ho, Y.C.; Lam, M.K.; Font-Palma, C.; Mohd Zaid, H.F.; Cheng, C.K. A review of organic waste enrichment for inducing palatability of black soldier fly larvae: Wastes to valuable resources. Environ. Pollut. 2020, 267, 115488. [Google Scholar] [CrossRef]
- Barragán-Fonseca, K.B. Flies are What They Eat—Tailoring Nutrition of BSF (Hermetia illucens) for Larval Biomass Production and Fitness. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2018. [Google Scholar]
- Gligorescu, A.; Toft, S.; Hauggaard-Nielsen, H.; Axelsen, J.A.; Nielsen, S.A. Development, metabolism and nutrient composition of black soldier fly larvae (Hermetia illucens; Diptera: Stratiomyidae) in relation to temperature and diet. J. Insects Food Feed 2018, 4, 123–133. [Google Scholar] [CrossRef]
- Gold, M.; Cassar, C.M.; Zurbrügg, C.; Kreuzer, M.; Boulos, S.; Diener, S.; Mathys, A. Biowaste treatment with black soldier fly larvae: Increasing performance through the formulation of biowastes based on protein and carbohydrates. Waste Manag. 2020, 102, 319–329. [Google Scholar] [CrossRef]
- Laganaro, M.; Bahrndorff, S.; Eriksen, N.T. Growth and metabolic performance of black soldier fly larvae grown on low and high-quality substrates. Waste Manag. 2021, 121, 198–205. [Google Scholar] [CrossRef]
- Danieli, P.P.; Lussiana, C.; Gasco, L.; Amici, A.; Ronchi, B.; The Effects of Diet Formulation on the Yield, Proximate Composition, and Fatty Acid Profile of the Black Soldier Fly (Hermetia illucens L. ) Prepupae Intended for Animal Feed. Animals 2019, 9, 178. [Google Scholar] [CrossRef] [Green Version]
- Pliantiangtam, N.; Chundang, P.; Kovitvadhi, A. Growth performance, waste reduction efficiency and nutritional composition of black soldier fly (Hermetia illucens) larvae and prepupae reared on coconut endosperm and soybean curd residue with or without supplementation. Insects 2021, 12, 682. [Google Scholar] [CrossRef]
- Nguyen, T.T.X.; Tomberlin, J.K.; Vanlaerhoven, S. Ability of Black Soldier Fly (Diptera: Stratiomyidae) Larvae to Recycle Food Waste. Environ. Entomol. 2015, 44, 406–410. [Google Scholar] [CrossRef]
- Meneguz, M.; Schiavone, A.; Gai, F.; Dama, A.; Lussiana, C.; Renna, M.; Gasco, L. Effect of rearing substrate on growth performance, waste reduction efficiency and chemical composition of black soldier fly (Hermetia illucens ) larvae. J. Sci. Food Agric. 2018, 98, 5776–5784. [Google Scholar] [CrossRef]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef]
- Lim, J.W.; Mohd-Noor, S.N.; Wong, C.Y.; Lam, M.K.; Goh, P.S.; Beniers, J.J.A.; Oh, W.D.; Jumbri, K.; Ghani, N.A. Palatability of black soldier fly larvae in valorizing mixed waste coconut endosperm and soybean curd residue into larval lipid and protein sources. J. Environ. Manage. 2019, 231, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Sideris, V.; Georgiadou, M.; Papadoulis, G.; Mountzouris, K.; Tsagkarakis, A. Effect of Processed Beverage By-Product-Based Diets on Biological Parameters, Conversion Efficiency and Body Composition of Hermetia illucens (L) (Diptera: Stratiomyidae). Insects 2021, 12, 475. [Google Scholar] [CrossRef] [PubMed]
- Hogsette, J.A. New Diets for Production of House Flies and Stable Flies (Diptera: Muscidae) in the Laboratory. J. Econ. Entomol. 1992, 85, 2291–2294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISO. Belgian Standard Cereals, Cereals-Based Products and Animal Feeding Stuffs-Determination of Crude Fat and Total Fat Content by the Randall Extraction Method (ISO 11085:2015); ISO: London, UK, 2015. [Google Scholar]
- ISO. NBN EN ISO 5983-1 : 2005 COR 2009 Animal Feeding Stuffs—Determination of Nitrogen Content and Calculation of Crude Protein Content—Part 1: Kjeldahl Method; ISO: London, UK, 2005. [Google Scholar]
- Chen, X.; Zhao, G.; Zhang, Y.; Han, L.; Xiao, W. Nitrogen-to-Protein Conversion Factors for Crop Residues and Animal Manure Common in China. J. Agric. Food Chem. 2017, 65, 9186–9190. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, S.; Kasuga, A.; Aoyagi, Y. Nitrogen-to-protein conversion factors for common vegetables in Japan. J. Food Sci. 2001, 66, 412–415. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- FAO; WHO; UNU. The Relationship Between Food Composition and Available Energy; FAO: Rome, Italy; WHO: Geneva, Switzerland; UNU: Tokyo, Japan, 1981. [Google Scholar]
- Emis. Compendium Voor Monsterneming en Analyses van Afvalstoffen en Bodem (CMA)—Bereiding van Extracten en Analyseoplossingen; Emis: Mol, Belgium, 2021. [Google Scholar]
- Lynch, K.M.; Steffen, E.J.; Arendt, E.K. Brewers’ spent grain: A review with an emphasis on food and health. J. Inst. Brew. 2016, 122, 553–568. [Google Scholar] [CrossRef]
- EAAP European Federation of Animal Science—Leading European Organisation of Animal Science. Available online: https://www.eaap.org/ (accessed on 5 September 2021).
- Cammack, J.A.; Tomberlin, J.K. The impact of diet protein and carbohydrate on select life-history traits of the black soldier fly Hermetia illucens (L.) (Diptera: Stratiomyidae). Insects 2017, 8, 56. [Google Scholar] [CrossRef] [Green Version]
- Lalander, C.; Diener, S.; Zurbrügg, C.; Vinnerås, B. Effects of feedstock on larval development and process efficiency in waste treatment with black soldier fly (Hermetia illucens). J. Clean. Prod. 2019, 208, 211–219. [Google Scholar] [CrossRef]
- Meneguz, M.; Gasco, L.; Tomberlin, J.K. Impact of pH and feeding system on black soldier fly (Hermetia illucens, L; Diptera: Stratiomyidae) larval development. PLoS ONE 2018, 13, 1–15. [Google Scholar] [CrossRef]
- Liland, N.S.; Biancarosa, I.; Araujo, P.; Biemans, D.; Bruckner, C.G.; Waagbø, R.; Torstensen, B.E.; Lock, E.-J. Modulation of nutrient composition of black soldier fly (Hermetia illucens) larvae by feeding seaweed-enriched media. PLoS ONE 2017, 12, e0183188. [Google Scholar] [CrossRef]
- Scala, A.; Cammack, J.A.; Salvia, R.; Scieuzo, C.; Franco, A.; Bufo, S.A.; Tomberlin, J.K.; Falabella, P. Rearing substrate impacts growth and macronutrient composition of Hermetia illucens (L.) (Diptera: Stratiomyidae) larvae produced at an industrial scale. Sci. Rep. 2020, 10, 19448. [Google Scholar] [CrossRef]
- Barbi, S.; Macavei, L.I.; Fuso, A.; Luparelli, A.V.; Caligiani, A.; Ferrari, A.M.; Maistrello, L.; Montorsi, M. Valorization of seasonal agri-food leftovers through insects. Sci. Total Environ. 2020, 709, 136209. [Google Scholar] [CrossRef]
- Jucker, C.; Erba, D.; Leonardi, M.G.; Lupi, D.; Savoldelli, S. Assessment of Vegetable and Fruit Substrates as Potential Rearing Media for Hermetia illucens (Diptera: Stratiomyidae) Larvae. Environ. Entomol. 2017, 46, 1415–1423. [Google Scholar] [CrossRef]
- Murakami, A.E.; Watkins, S.E.; Saleh, E.A.; England, J.A.; Waldroup, P.W. Estimation of the sodium and chloride requirements for the young broiler chick. J. Appl. Poult. Res. 1997, 6, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Perelman, B.; Farnoushi, Y.; Krispin, H.; Rish, D. Salt Intoxication in Chickens Salt Intoxication in Commercial Broilers and Breeders – a Clinical and Pathological Description. Isr. J. Vet. Med. 2016, 71, 53–57. [Google Scholar]
- Fausto-Castro, L.; Rivas-García, P.; Gómez-Nafte, J.A.; Rico-Martínez, R.; Rico-Ramírez, V.; Gomez-Gonzalez, R.; Cuarón-Ibargüengoytia, J.A.; Botello-Álvarez, J.E. Selection of food waste with low moisture and high protein content from Mexican restaurants as a supplement to swine feed. J. Clean. Prod. 2020, 256, 120137. [Google Scholar] [CrossRef]
- Martin, K.M.; Vargas-Jurado, N.; Purdum, S.E. Prediction model for manure zinc excretion in laying hens. Poult. Sci. 2018, 97, 267–270. [Google Scholar] [CrossRef]
- Ur Rehman, K.; Ur Rehman, R.; Somroo, A.A.; Cai, M.; Zheng, L.; Xiao, X.; Ur Rehman, A.; Rehman, A.; Tomberlin, J.K.; Yu, Z.; et al. Enhanced bioconversion of dairy and chicken manure by the interaction of exogenous bacteria and black soldier fly larvae. J. Environ. Manage. 2019, 237, 75–83. [Google Scholar] [CrossRef]
- Ghada, E.A.; Manal, E.A.E.; Amal, E.M.; Hala, E.M. Application Of Tomato Leaves Extract as Pesticide Against Aphis Gosstpii Glover (Hemiptera: Aphididae). Int. J. Adv. Res. 2017, 5, 286–290. [Google Scholar] [CrossRef] [Green Version]
- Williams, J.S.; Cooper, R.M. The oldest fungicide and newest phytoalexin – a reappraisal of the fungitoxicity of elemental sulphur. Plant Pathol. 2004, 53, 263–279. [Google Scholar] [CrossRef]
- Robertson, J.A.; Eastwood, M.A. An examination of factors which may affect the water holding capacity of dietary fibre. Br. J. Nutr. 1981, 45, 83–88. [Google Scholar] [CrossRef] [Green Version]
- Oonincx, D.G.A.B.; van Huis, A.; van Loon, J.J.A. Nutrient utilisation by black soldier flies fed with chicken, pig, or cow manure. J. Insects Food Feed 2015, 1, 131–139. [Google Scholar] [CrossRef]
- Bosch, G.; Oonincx, D.G.A.B.; Jordan, H.R.; Zhang, J.; Van Loon, J.J.A.; Van Huis, A.; Tomberlin, J.K. Standardisation of quantitative resource conversion studies with black soldier fly larvae Abstract. J. Insects Food Feed. 2020, 6, 95–109. [Google Scholar] [CrossRef]
- Parra Paz, A.S.; Carrejo, N.S.; Gómez Rodríguez, C.H. Effects of Larval Density and Feeding Rates on the Bioconversion of Vegetable Waste Using Black Soldier Fly Larvae Hermetia illucens (L.), (Diptera: Stratiomyidae). Waste and Biomass Valorization 2015, 6, 1059–1065. [Google Scholar] [CrossRef]
- Diener, S.; Zurbrugg, C.; Tockner, K. Conversion of organic material by black soldier fly larvae: Establishing optimal feeding rates. Waste Manag. Res. 2009, 27, 603–610. [Google Scholar] [CrossRef]
- de Bono, M.; Tobin, D.M.; Davis, M.W.; Avery, L.; Bargmann, C.I. Social feeding in Caenorhabditis elegans is induced by neurons that detect aversive stimuli. Nature 2002, 419, 899–903. [Google Scholar] [CrossRef]
- Tschirner, M.; Simon, A. Influence of different growing substrates and processing on the nutrient composition of black soldier fly larvae destined for animal feed. J. Insects Food Feed 2015, 1, 249–259. [Google Scholar] [CrossRef]
- Somroo, A.A.; ur Rehman, K.; Zheng, L.; Cai, M.; Xiao, X.; Hu, S.; Mathys, A.; Gold, M.; Yu, Z.; Zhang, J. Influence of Lactobacillus buchneri on soybean curd residue co-conversion by black soldier fly larvae (Hermetia illucens) for food and feedstock production. Waste Manag. 2019, 86, 114–122. [Google Scholar] [CrossRef]
- Smetana, S.; Spykman, R.; Heinz, V. Environmental aspects of insect mass production. J. Insects Food Feed 2021, 7, 553–571. [Google Scholar] [CrossRef]
- Shumo, M.; Khamis, F.M.; Tanga, C.M.; Fiaboe, K.K.M.; Subramanian, S.; Ekesi, S.; Van Huis, A.; Borgemeister, C. Influence of Temperature on Selected Life-History Traits of Black Soldier Fly (Hermetia illucens) Reared on Two Common Urban Organic Waste Streams in Kenya. Animals 2019, 9, 79. [Google Scholar] [CrossRef] [Green Version]


| Substrate | DM 1 | EE 2 | CP 2 | CA 2 | aNDF 2 | NFC 2 | GE 3 |
|---|---|---|---|---|---|---|---|
| Gainesville diet | 90.5 ± 0.0 | 2.6 ± 0.2 | 15.7 ± 0.3 | 6.9 ± 0.3 | 41.7 ± 0.3 | 33.1 ± 1.1 | 219 |
| Chicken start mash | 90.6 ± 0.1 | 4.6 ± 0.4 | 20.4 ± 2.4 | 6.0 ± 1.3 | 20.9 ± 1.2 | 48.2 ± 5.3 | 315 |
| Apple pulp | 25.9 ± 0.1 | 4.6 ± 0.1 | 3.4 ± 0.0 | 1.9 ± 0.1 | 43.3 ± 1.0 | 47.0 ± 1.2 | 242 |
| Beer draff | 29.4 ± 0.5 | 5.5 ± 0.2 | 19.4 ± 0.6 | 4.5 ± 0.1 | 50.6 ± 0.4 | 20.0 ± 1.3 | 207 |
| Industrial Food Waste | 17.8 ± 0.7 | 10.9 ± 0.6 | 18.5 ± 0.1 | 9.2 ± 0.0 | 7.8 ± 0.4 | 53.5 ± 1.1 | 386 |
| Chicken manure | 67.3 ± 3.2 | 3.3 ± 0.1 | 13.1 ± 0.2 | 13.0 ± 0.1 | 41.5 ± 0.6 | 29.1 ± 1.0 | 198 |
| Corn meal | 87.0 ± 0.0 | 8.1 ± 0.1 | 9.5 ± 0.2 | 2.3 ± 0.1 | 32.8 ± 2.0 | 47.3 ± 2.4 | 300 |
| Forced chicory roots | 15.7 ± 0.2 | 0.9 ± 0.0 | 4.6 ± 0.1 | 31.0 ± 0.9 | 14.2 ± 0.5 | 49.3 ± 1.5 | 224 |
| Fruit puree | 6.2 ± 0.1 | 4.5 ± 0.1 | 10.0 ± 0.1 | 12.6 ± 0.2 | 33.3 ± 0.1 | 39.9 ± 0.5 | 239 |
| Grain middlings 1 | 90.2 ± 0.1 | 2.9 ± 0.1 | 14.1 ± 0.1 | 7.9 ± 0.2 | 22.0 ± 0.9 | 53.1 ± 1.3 | 295 |
| Grain middlings 2 | 87.8 ± 0.1 | 1.4 ± 0.0 | 9.6 ± 0.4 | 19.7 ± 0.7 | 45.9 ± 0.6 | 23.4 ± 1.7 | 144 |
| Household food waste | 23.9 ± 0.7 | 14.6 ± 0.2 | 16.7 ± 0.6 | 8.4 ± 0.3 | 15.5 ± 0.1 | 44.8 ± 1.2 | 377 |
| Tomato leaves | 88.4 ± 0.0 | 1.2 ± 0.1 | 12.1 ± 0.3 | 29.0 ± 0.2 | 21.2 ± 2.2 | 36.5 ± 2.8 | 205 |
| Vegetable overproduction auction | 8.1 ± 0.2 | 2.0 ± 0.3 | 10.5 ± 0.4 | 15.3 ± 0.1 | 30.2 ± 1.1 | 42.0 ± 1.9 | 228 |
| Substrate | P | Mg | K | Na | Ca |
|---|---|---|---|---|---|
| Gainesville diet | 1.11 ± 0.02 | 0.33 ± 0.02 | 1.61 ± 0.12 | 0.01 ± 0.00 | 0.60 ± 0.04 |
| Chicken start mash | 0.85 ± 0.09 | 0.25 ± 0.02 | 0.82 ± 0.06 | 0.13 ± 0.02 | 1.14 ± 0.50 |
| Apple pulp | 0.14 ± 0.01 | 0.11 ± 0.00 | 0.61 ± 0.01 | 0.01 ± 0.00 | 0.11 ± 0.00 |
| Beer draff | 0.48 ± 0.04 | 0.16 ± 0.01 | 0.15 ± 0.01 | 0.00 ± 0.00 | 0.18 ± 0.01 |
| Industrial food waste | 0.56 ± 0.03 | 0.16 ± 0.01 | 1.36 ± 0.04 | 1.30 ± 0.03 | 1.14 ± 0.05 |
| Chicken manure | 0.93 ± 0.02 | 0.62 ± 0.01 | 2.76 ± 0.03 | 0.33 ± 0.00 | 1.05 ± 0.02 |
| Corn meal | 0.68 ± 0.02 | 0.22 ± 0.07 | 0.63 ± 0.18 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Forced chicory roots | 0.18 ± 0.01 | 0.20 ± 0.01 | 2.04 ± 0.06 | 0.29 ± 0.01 | 0.98 ± 0.01 |
| Fruit puree | 0.65 ± 0.01 | 0.38 ± 0.02 | 4.41 ± 0.12 | 0.28 ± 0.01 | 0.57 ± 0.02 |
| Grain middlings 1 | 0.59 ± 0.01 | 0.16 ± 0.00 | 0.66 ± 0.01 | 0.08 ± 0.00 | 2.29 ± 0.11 |
| Grain middlings 2 | 0.30 ± 0.01 | 0.13 ± 0.01 | 0.68 ± 0.01 | 0.00 ± 0.00 | 0.68 ± 0.04 |
| Household food waste | 0.50 ± 0.01 | 0.15 ± 0.03 | 1.15 ± 0.09 | 0.55 ± 0.02 | 1.24 ± 0.09 |
| Tomato leaves | 0.57 ± 0.02 | 1.06 ± 0.06 | 3.29 ± 0.16 | 0.05 ± 0.00 | 8.14 ± 0.12 |
| Vegetable overproduction auction | 0.58 ± 0.02 | 0.29 ± 0.01 | 3.09 ± 0.08 | 0.06 ± 0.01 | 1.22 ± 0.01 |
| Substrate | Zn | Cu | Fe |
|---|---|---|---|
| Gainesville diet | 0.005 ± 0.000 | 0.001 ± 0.000 | 0.016 ± 0.001 |
| Chicken start mash | 0.012 ± 0.003 | 0.002 ± 0.000 | 0.016 ± 0.002 |
| Apple pulp | 0.001 ± 0.000 | 0.001 ± 0.000 | 0.003 ± 0.000 |
| Beer draff | 0.006 ± 0.000 | 0.001 ± 0.000 | 0.008 ± 0.001 |
| Industrial food waste | 0.015 ± 0.000 | 0.010 ± 0.001 | 0.091 ± 0.000 |
| Chicken manure | 0.041 ± 0.001 | 0.009 ± 0.000 | 0.073 ± 0.002 |
| Corn meal | 0.004 ± 0.000 | 0.000 ± 0.000 | 0.006 ± 0.000 |
| Forced chicory roots | 0.004 ± 0.000 | 0.002 ± 0.000 | 0.567 ± 0.024 |
| Fruit puree | 0.006 ± 0.000 | 0.001 ± 0.000 | 0.011 ± 0.000 |
| Grain middlings 1 | 0.007 ± 0.000 | 0.002 ± 0.000 | 0.018 ± 0.002 |
| Grain middlings 2 | 0.006 ± 0.000 | 0.001 ± 0.000 | 0.308 ± 0.037 |
| Household food waste | 0.004 ± 0.000 | 0.001 ± 0.000 | 0.048 ± 0.003 |
| Tomato leaves | 0.004 ± 0.000 | 0.001 ± 0.000 | 0.010 ± 0.001 |
| Vegetable overproduction auction | 0.002 ± 0.000 | 0.001 ± 0.000 | 0.060 ± 0.003 |
| Substrate | CF | aNDF | ADF | ADL (Lignin) | Cellulose | Hemicellulose |
|---|---|---|---|---|---|---|
| Gainesville diet | 5.1 ± 0.1 | 41.7 ± 0.3 | 17.9 ± 0.3 | 4.2 ± 0.0 | 13.7 | 23.8 |
| Chicken start mash | 12.0 ± 0.1 | 20.9 ± 1.2 | 6.7 ± 0.3 | 1.1 ± 0.2 | 5.7 | 14.1 |
| Apple pulp | 25.7 ± 0.1 | 43.3 ± 1.0 | 35.7 ± 1.0 | 14.3 ± 0.3 | 21.5 | 7.5 |
| Beer draff | 18.4 ± 0.4 | 50.6 ± 0.4 | 22.9 ± 0.4 | 6.0 ± 0.2 | 16.9 | 27.7 |
| Industrial food waste | 3.2 ± 0.1 | 7.8 ± 0.4 | 6.7 ± 0.1 | 1.3 ± 0.1 | 5.3 | 1.2 |
| Chicken manure | 14.2 ± 0.1 | 41.5 ± 0.6 | 21.4 ± 0.3 | 3.5 ± 0.1 | 17.9 | 20.1 |
| Corn meal | 4.6 ± 0.1 | 32.8 ± 2.0 | 6.6 ± 0.0 | 0.7 ± 0.1 | 5.9 | 26.2 |
| Forced chicory roots | 8.7 ± 0.3 | 14.2 ± 0.5 | 16.9 ± 0.3 | 1.9 ± 0.0 | 15.0 | -2.7 |
| Fruit puree | 17.6 ± 0.1 | 33.3 ± 0.1 | 24.5 ± 0.3 | 3.8 ± 0.0 | 20.7 | 8.8 |
| Grain middlings 1 | 6.6 ± 0.7 | 22.0 ± 0.9 | 6.8 ± 0.1 | 1.1 ± 0.1 | 5.8 | 15.2 |
| Grain middlings 2 | 20.9 ± 0.2 | 45.9 ± 0.6 | 18.7 ± 0.4 | 2.8 ± 0.2 | 15.9 | 27.2 |
| Household food waste | 6.6 ± 0.1 | 15.5 ± 0.1 | 12.6 ± 0.4 | 3.5 ± 0.1 | 9.1 | 2.9 |
| Tomato leaves | 13.7 ± 0.4 | 21.2 ± 2.2 | 18.8 ± 0.1 | 0.8 ± 0.1 | 18.0 | 2.4 |
| Vegetable overproduction auction | 8.6 ± 0.2 | 30.2 ± 1.1 | 29.8 ± 0.7 | 9.9 ± 0.2 | 19.8 | 0.4 |
| Substrate | n | Survival Rate (%) | Maximal Mean Larval Weight (mg) | Time to Reach Maximum Weight (day) |
|---|---|---|---|---|
| Gainesville diet | 6 | 97.2 ± 1.8 a | 83.8 ± 13.8 a,f,g | 8 |
| Chicken start mash | 6 | 96.3 ± 1.9 a | 148.4 ± 21.8 a,b | 7 |
| Apple pulp | 6 | 95.5 ± 3.5 a | 38.3 ± 4.4 h | 8 |
| Beer draff | 6 | 95.2 ± 3.1 a | 130.9 ± 19.6 b,c,d | 7 |
| Industrial food waste | 5 | 88.8 ± 3.2 a | 176.4 ± 15.3 a | 9 |
| Chicken manure | 6 | 97.7 ± 1.4 a | 134.9 ± 11.8 b,c | 8 |
| Corn meal | 6 | 91.8 ± 4.0 a | 110.4 ± 13.2 c,d,e | 9 |
| Forced chicory roots | 3 | 98.0 ± 1.7 a | 46.7 ± 4.9 g,h | 8 |
| Fruit puree | 3 | 99.0 ± 1.0 a | 80.1 ± 8.0 a,f,g | 9 |
| Grain middlings 1 | 6 | 50.0 ± 25.5 b | 98.5 ± 26.3 d,e,f | 9 |
| Grain middlings 2 | 6 | 97.5 ± 2.1 a | 78.0 ± 12.5 a,g | 8 |
| Household food waste | 6 | 83.2 ± 8.0 a | 65.3 ± 13.9 g,h | 8 |
| Tomato leaves | 3 | 0.0 ± 0.0 c | 3.1 ± 0.0 i | 1 |
| Vegetable overproduction auction | 3 | 98.0 ± 0.4 a | 88.1 ± 5.8 d,e,f,g | 7 |
| Substrate | n | Bioconversion Efficiency (%) | Feed Conversion Ratio (FCR) | Waste Reduction (%) |
|---|---|---|---|---|
| Gainesville diet | 6 | 7,72 ± 1.46 d | 3.43 ± 0.74 c,d,e | 45.9 ± 5.7 a,b |
| Chicken start mash | 6 | 17.56 ± 3.37 a,b | 2.08 ± 0.35 d,e | 49.9 ± 9.6 a,b |
| Apple pulp | 6 | 3.59 ± 0.39 a | 8.90 ± 0.80 a | 17.0 ± 7.5 c |
| Beer draff | 6 | 13.91 ± 0.40 b,c | 2.32 ± 0.17 d,e | 46.2 ± 10.4 a,b |
| Industrial food waste | 5 | 20.71 ± 2.71 a | 1.58 ± 0.19 a | 58.9 ± 4.8 a |
| Chicken manure | 6 | 15.43 ± 1.65 b | 2.00 ± 0.33 d,e | 36.4 ± 13.1 b,c |
| Corn meal | 6 | 15.61 ± 2.24 b | 2.49 ± 0.30 d,e | 42.5 ± 7.2 a,b |
| Forced chicory roots | 3 | 4.49 ± 0.10 d,e | 6.20 ± 0.14 a,b,c,d | 30.2 ± 9.3 b,c |
| Fruit puree | 3 | 9.32 ± 0.56 c,d | 3.19 ± 0.32 b,c,d,e | 39.3 ± 5.3 a,b,c |
| Grain middlings 1 | 6 | 3.68 ± 1.59 a | 7.35 ± 4.95 a,b | 36.6 ± 10.5 b,c |
| Grain middlings 2 | 6 | 7.06 ± 1.75 d,e | 3.53 ± 0.77 c,d,e | 35.5 ± 21.6 b,c |
| Household food waste | 6 | 6.47 ± 2.52 d,e | 6.32 ± 2.44 a,b,c | 18.6 ± 4.9 c |
| Tomato leaves | 3 | - | - | - |
| Vegetable overproduction auction | 3 | 7.59 ± 0.69 d,e | 3.43 ± 0.42 b,c,d,e | 53.9 ± 3.4 a,b |
| Term | Estimate | Std Error | t Ratio | Prob > |t| |
|---|---|---|---|---|
| Intercept | 0.00996 | 0.02202 | 0.45 | 0.6527 |
| Substrate protein content (%) | 0.00508 | 0.00085 | 5.97 | <0.0001 |
| Substrate fat content (%) | −0.00393 | 0.00138 | −2.84 | 0.0063 |
| Substrate NFC content (%) | 0.00105 | 0.00046 | 2.27 | 0.0268 |
| (Substrate protein content (%) − 13.1387) × (Substrate fat content (%) − 5.5629) | −0.00117 | 0.00039 | −3.05 | 0.0035 |
| (Substrate fat content (%) − 5.5629) × (Substrate NFC content (%) − 39.0081) | 0.00054 | 0.00015 | 3.68 | 0.0005 |
| (Substrate protein content (%) − 13.1387) × (Substrate fat content (%) − 5.5629) × (Substrate NFC content (%) − 39.0081) | 0.00007 | 0.00004 | 2.03 | 0.0476 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Broeckx, L.; Frooninckx, L.; Slegers, L.; Berrens, S.; Noyens, I.; Goossens, S.; Verheyen, G.; Wuyts, A.; Van Miert, S. Growth of Black Soldier Fly Larvae Reared on Organic Side-Streams. Sustainability 2021, 13, 12953. https://doi.org/10.3390/su132312953
Broeckx L, Frooninckx L, Slegers L, Berrens S, Noyens I, Goossens S, Verheyen G, Wuyts A, Van Miert S. Growth of Black Soldier Fly Larvae Reared on Organic Side-Streams. Sustainability. 2021; 13(23):12953. https://doi.org/10.3390/su132312953
Chicago/Turabian StyleBroeckx, Laurens, Lotte Frooninckx, Laurien Slegers, Siebe Berrens, Isabelle Noyens, Sarah Goossens, Geert Verheyen, Ann Wuyts, and Sabine Van Miert. 2021. "Growth of Black Soldier Fly Larvae Reared on Organic Side-Streams" Sustainability 13, no. 23: 12953. https://doi.org/10.3390/su132312953
APA StyleBroeckx, L., Frooninckx, L., Slegers, L., Berrens, S., Noyens, I., Goossens, S., Verheyen, G., Wuyts, A., & Van Miert, S. (2021). Growth of Black Soldier Fly Larvae Reared on Organic Side-Streams. Sustainability, 13(23), 12953. https://doi.org/10.3390/su132312953










