High Sulfur Content of Mesoporous Activated Carbon Composite Derived from Water Hyacinth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Activated Carbon
2.2. Preparation of Activated Carbon-Sulfur Composite
2.3. Characterization
2.4. Conductivity Measurement
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eftekhari, A.; Kim, D.W. Cathode materials for lithium–sulfur batteries: A practical perspective. J. Mater. Chem. A 2017, 5, 17734–17776. [Google Scholar] [CrossRef]
- Adelhelm, P.; Hartmann, P.; Bender, C.L.; Busche, M.; Eufinger, C.; Janek, J. From lithium to sodium: Cell chemistry of room temperature sodium–air and sodium–sulfur batteries. Beilstein J. Nanotechnol. 2015, 6, 1016–1055. [Google Scholar] [CrossRef]
- Zhang, S.S. Liquid electrolyte lithium/sulfur battery: Fundamental chemistry, problems, and solutions. J. Power Sources 2013, 231, 153–162. [Google Scholar] [CrossRef]
- Swiderska-Mocek, A.; Rudnicka, R. Lithium sulphur battery with activated carbon cloth-sulphur cathode and ionic liquid as electrolyte. J. Power Sources 2015, 273, 162–167. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Liu, J. Biomass-derived porous carbon materials for advanced lithium sulfur batteries. J. Energy Chem. 2019, 34, 171–185. [Google Scholar] [CrossRef] [Green Version]
- Yuan, S.; Guo, Z.; Wang, L.; Hu, S.; Wang, Y.; Xia, Y. Leaf-like graphene-oxide-wrapped sulfur for high-performance lithium-sulfur battery. Adv. Sci. 2015, 2, 1500071. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Luo, Y.; Wu, H.; Jiang, K.; Li, Q.; Fan, S.; Li, J.; Wang, J. Ultrastretchable carbon nanotube composite electrodes for flexible lithium-ion batteries. Nanoscale 2018, 10, 19972–19978. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Guan, R.; Wang, S.; Xiao, M.; Han, D.; Sun, L.; Meng, Y. Polymers for high performance Li-S batteries: Material selection and structure design. Prog. Polym. Sci. 2019, 89, 19–60. [Google Scholar] [CrossRef]
- Gu, X.; Wang, Y.; Lai, C.; Qiu, J.; Li, S.; Hou, Y.; Martens, W.; Mahmood, N.; Zhang, S. Microporous bamboo biochar for lithium−sulfur batteries. Nano Res. 2015, 8, 129–139. [Google Scholar] [CrossRef]
- De, S.; Balu, A.M.; Jan, C.; Waal, V.W.; Luque, R. Biomass-Derived Porous Carbon Materials: Synthesis and Catalytic Applications. ChemCatChem 2015, 7, 1608–1629. [Google Scholar] [CrossRef]
- Yang, K.; Gao, Q.; Tan, Y.; Tian, W.; Zhu, L.; Yang, C. Microporous carbon derived from Apricot shell as cathode material for lithium–sulfur battery. Microporous Mesoporous Mater. 2014, 204, 235–241. [Google Scholar] [CrossRef]
- Yan, Y.; Shi, M.; Wei, Y.; Zhao, C.; Carnie, M.; Yang, R.; Xu, Y. Process optimization for producing hierarchical porous bamboo derived carbon materials with ultrahigh specific surface area for lithium-sulfur batteries. J. Alloys Compd. 2018, 738, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Geng, Z.; Yi, G.; Chen, C.; Xue, M.; Li, B.; Zhang, C. Corncob-derived Porous Carbon as an Interlayer Coating to Improve the Performance of Lithium Sulphur Battery. Int. J. Electrochem. Sci. 2017, 12, 4515–4527. [Google Scholar] [CrossRef]
- Chen, Z.H.; Du, X.L.; He, J.B.; Li, F.; Wang, Y.; Li, Y.L.; Li, B.; Xin, S. Porous coconut shell carbon offering high retention and deep lithiation of sulfur for Lithium–Sulfur batteries. ACS Appl. Mater. Interfaces 2017, 9, 33855–33862. [Google Scholar] [CrossRef] [PubMed]
- Moreno, N.; Caballero, A.; Morales, J.; Rodríguez-Castell, E. Improved performance of electrodes based on carbonized olive stones/S composites by impregnating with mesoporous TiO2 for advanced LieS batteries. J. Power Sources 2016, 313, 21–29. [Google Scholar] [CrossRef]
- Li, F.; Qin, F.; Zhang, K.; Fang, J.; Lai, Y.; Li, J. Hierarchically porous carbon derived from banana peel for lithium sulfur battery with high areal and gravimetric sulfur loading. J. Power Sources 2017, 362, 160–167. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; He, Y.; Liu, N.; Tan, T.; Liang, C. Biomass Derived Nitrogen-Doped Highly Porous Carbon Material with a Hierarchical Porous Structure for High-Performance Lithium/Sulfur Batteries. Materials 2017, 10, 1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, M.; Hu, Q.; Zhang, S.; Tang, H.; Li, L.; Pang, H. Macroporous Activated Carbon Derived from Rapeseed Shell for Lithium–Sulfur Batteries. Appl. Sci. 2017, 7, 1036. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhang, X.; Liu, X.; Zhang, Y.; Zhao, W.; Li, Y.; Qin, C.; Bakenov, Z. High specific surface area bimodal porous carbon derived from biomass reed flowers for high performance lithium-sulfur batteries. J. Colloid Interface Sci. 2020, 569, 22–33. [Google Scholar] [CrossRef]
- Liang, J.; Tang, D.; Huang, L.; Chen, Y.; Ren, W.; Sun, J. High oxygen reduction reaction performance nitrogen-doped biochar cathode: A strategy for comprehensive utilizing nitrogen and carbon in water hyacinth. Bioresour. Technol. 2018, 267, 524–531. [Google Scholar] [CrossRef]
- Sindhu, R.; Binod, P.; Pandey, A.; Madhavan, A.; Jose, A.A.; Narisetty, V.; Edgard, G.; Eulogio, C.; Vincenza, F. Water hyacinth a potential source for value addition: An overview. Bioresour. Technol. 2017, 230, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Ilo, O.P.; Simatele, M.D.; Nkomo, S.L.; Mkhize, N.M.; Prabhu, N.G. The Benefits of Water Hyacinth (Eichhornia crassipes) for Southern Africa: A Review. Sustainability 2020, 12, 9222. [Google Scholar] [CrossRef]
- Rop, K.; Mbui, D.; Njomo, N.; Karuku, G.N.; Michira, I.; Ajayi, R.F. Biodegradable water hyacinth cellulose-graft-poly (ammonium acrylate-co-acrylic acid) polymer hydrogel for potential agricultural application. Heliyon 2019, 5, 01416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, G.; Zhou, B.; Yi, L.; Li, H.; Hu, X.; Li, Y. Green and facile fabrication of hierarchical N-doped porous carbon from water hyacinths for high performance lithium/sodium ion batteries. Sustain. Energy Fuels 2017, 2, 855–861. [Google Scholar] [CrossRef]
- Flygare, J.D.; Riet, A.A.; Mazzeo, B.A.; Wheelera, D.R. Mathematical Model of Four-Line Probe to Determine Conductive Properties of Thin-Film Battery Electrodes. J. Electrochem. Soc. 2015, 162, A2136–A2144. [Google Scholar] [CrossRef]
- Lanterman, B.J.; Riet, A.A.; Gates, N.S.; Flygare, J.D.; Cutler, A.D.; Vogel, J.E.; Wheeler, D.R.; Mazzeoa, B.A. Micro-Four-Line Probe to Measure Electronic Conductivity and Contact Resistance of Thin-Film Battery Electrodes. J. Electrochem. Soc. 2015, 162, A2145–A2151. [Google Scholar] [CrossRef]
- Sotomayor, F.J.; Cychosz, K.A.; Thommes, M. Characterization of Micro/Mesoporous Materials by Physisorption: Concepts and Case Studies. Acc. Mater. Surf. Res. 2018, 3, 34–50. [Google Scholar]
- Zhang, Y.; Zhao, Y.; Konarov, A.; Li, Z.; Chen, P. Effect of mesoporous carbon microtube prepared by carbonizing the poplar catkin on sulfur cathode performance in Li/S batteries. J. Alloys Compd. 2015, 619, 298–302. [Google Scholar] [CrossRef]
- Imammuddin, A.M.; Soeparman, S.; Suprapto, W.; Sonief, A.A. Effect of Carbonization Temperature on Electrical Conductivity of Biocarbon Water Hyacinth Composites. Int. J. Control Autom. 2019, 12, 23–30. [Google Scholar] [CrossRef]
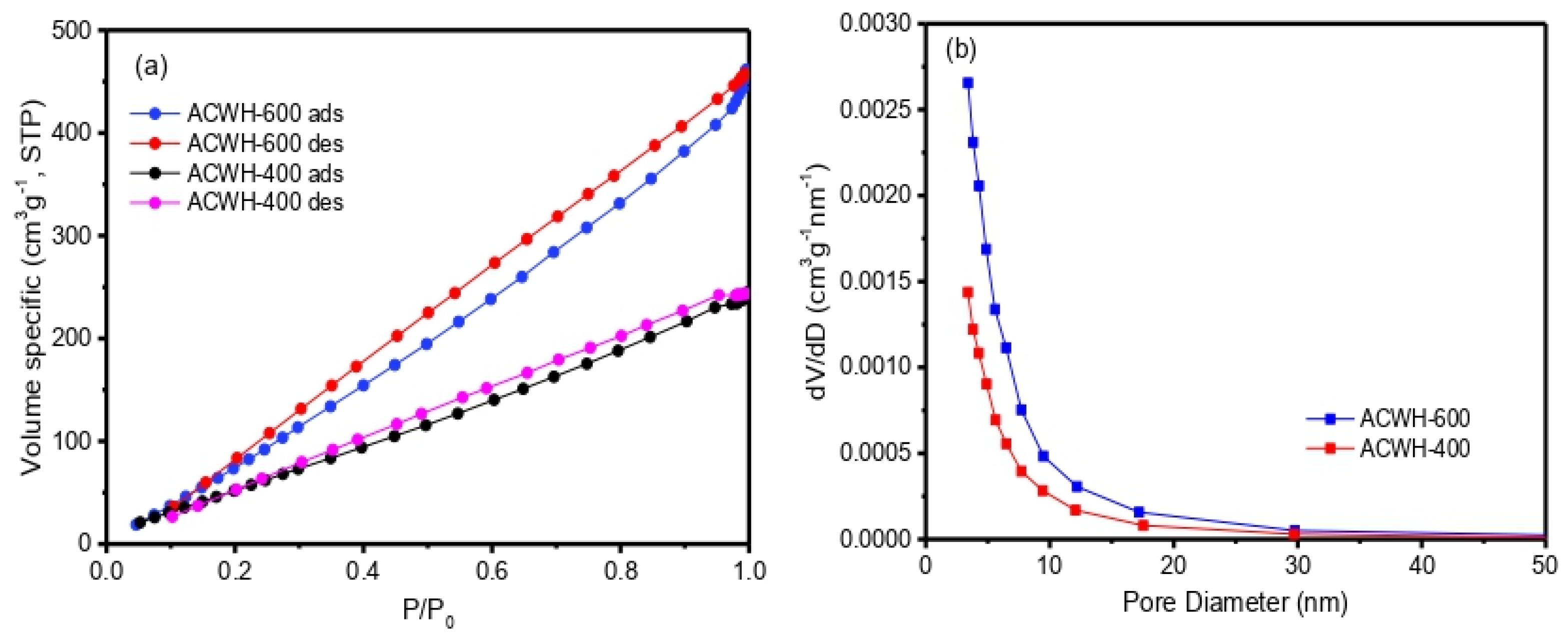
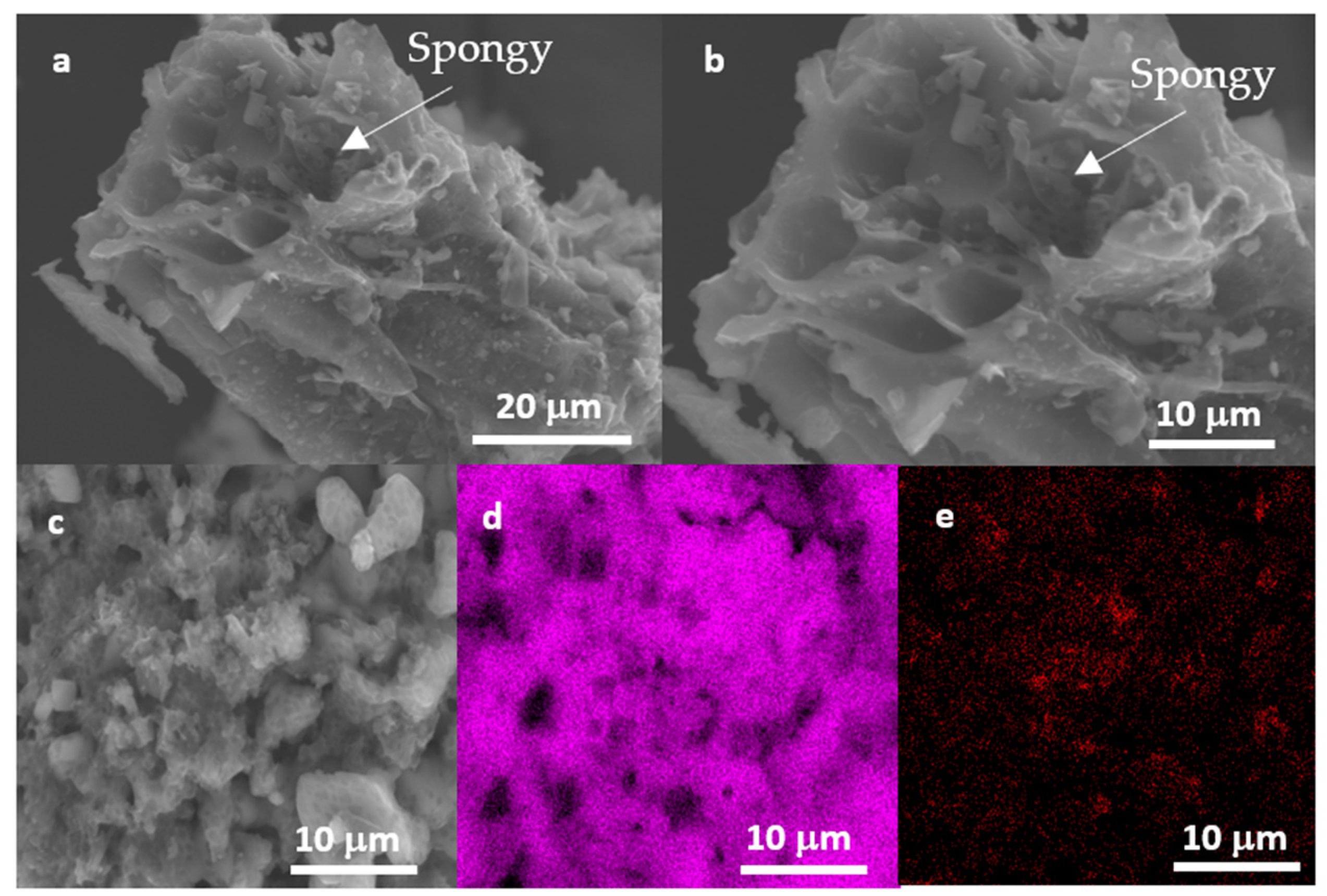

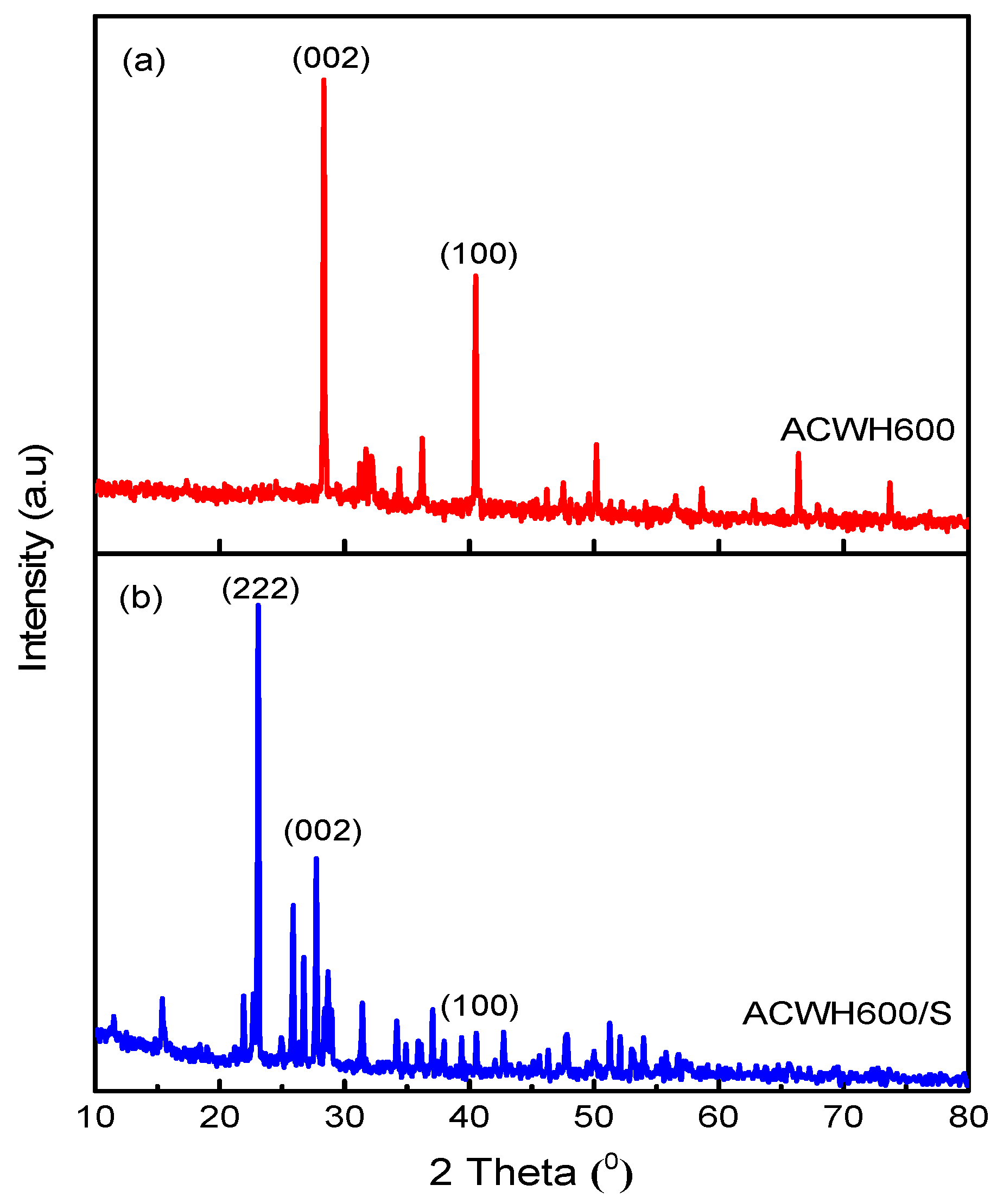
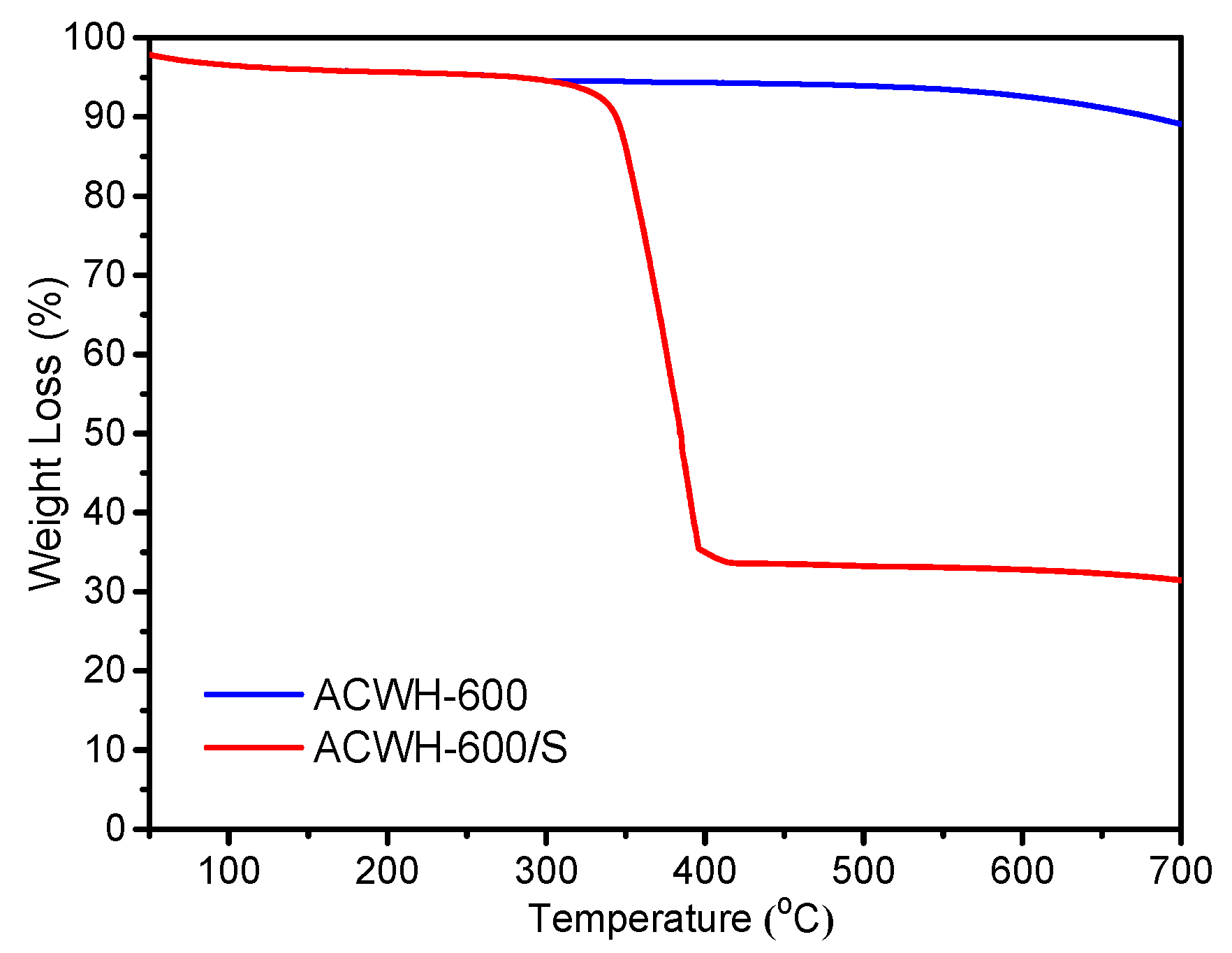
| Sample | SBET (m2 g−1) | VMeso (cm3 g−1) | RMeso (nm) |
|---|---|---|---|
| ACWH-400 | 297.76 | 0.378 | 2.54 |
| ACWH-600 | 642.39 | 0.714 | 2.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurhilal, O.; Winarsih, S.; Hidayat, S.; Sumiarsa, D.; Risdiana, R. High Sulfur Content of Mesoporous Activated Carbon Composite Derived from Water Hyacinth. Sustainability 2021, 13, 12880. https://doi.org/10.3390/su132212880
Nurhilal O, Winarsih S, Hidayat S, Sumiarsa D, Risdiana R. High Sulfur Content of Mesoporous Activated Carbon Composite Derived from Water Hyacinth. Sustainability. 2021; 13(22):12880. https://doi.org/10.3390/su132212880
Chicago/Turabian StyleNurhilal, Otong, Suci Winarsih, Sahrul Hidayat, Dadan Sumiarsa, and Risdiana Risdiana. 2021. "High Sulfur Content of Mesoporous Activated Carbon Composite Derived from Water Hyacinth" Sustainability 13, no. 22: 12880. https://doi.org/10.3390/su132212880
APA StyleNurhilal, O., Winarsih, S., Hidayat, S., Sumiarsa, D., & Risdiana, R. (2021). High Sulfur Content of Mesoporous Activated Carbon Composite Derived from Water Hyacinth. Sustainability, 13(22), 12880. https://doi.org/10.3390/su132212880






