Combining Genetic Gain and Diversity in Plant Breeding: Heritability of Root Selection in Wheat Populations
Abstract
:1. Introduction
- Is selection for seminal root length in hydroponics heritable?
- How strong is the genetic gain achieved in one breeding cycle on a population level?
2. Materials and Methods
2.1. Plant Material
2.2. Hydroponic System
2.3. Hydroponic Root Length Selection, Seed Propagation, and Evaluation
2.4. Competition Experiment
2.5. Data Handling and Statistical Anlysis
3. Results
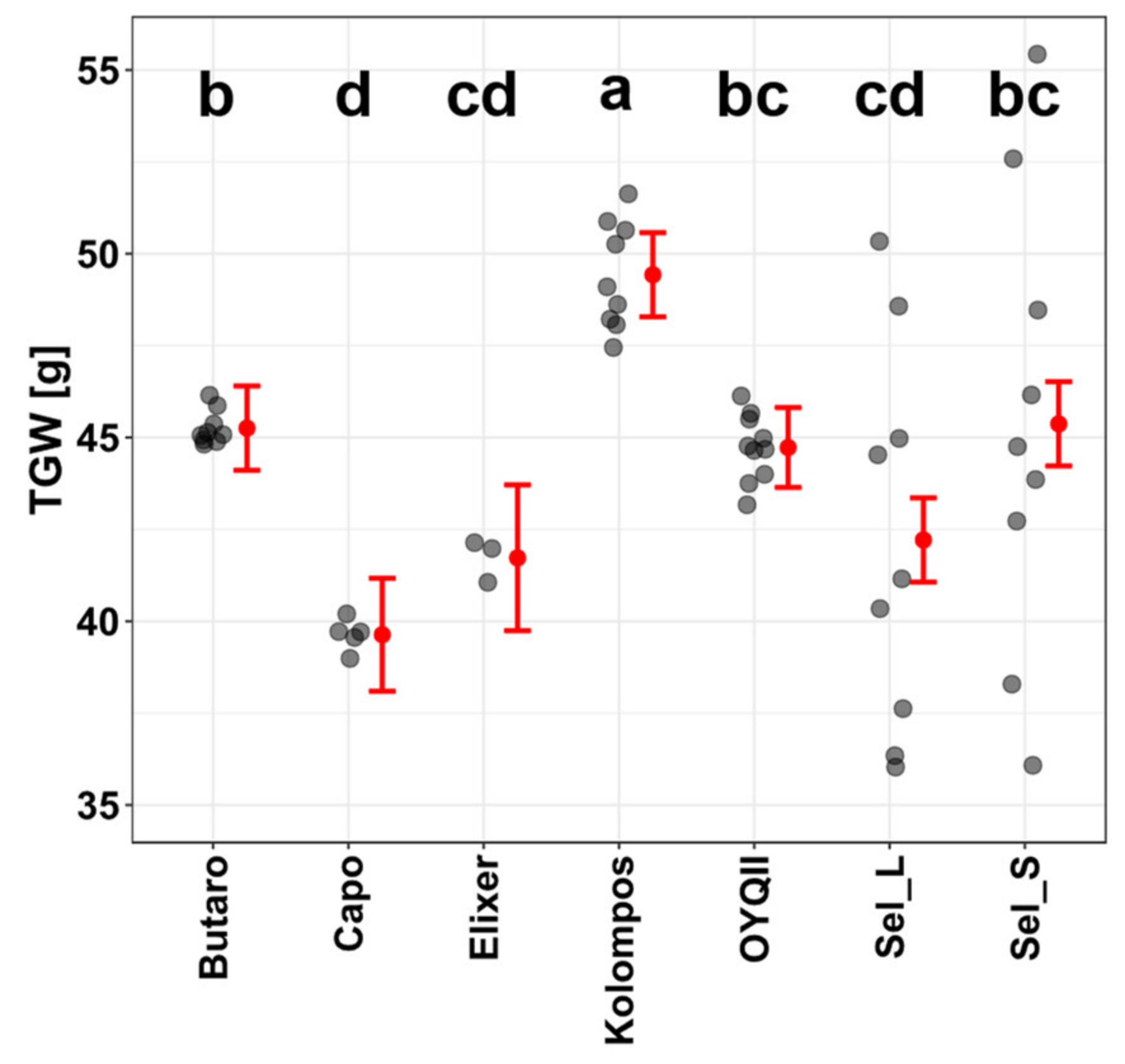
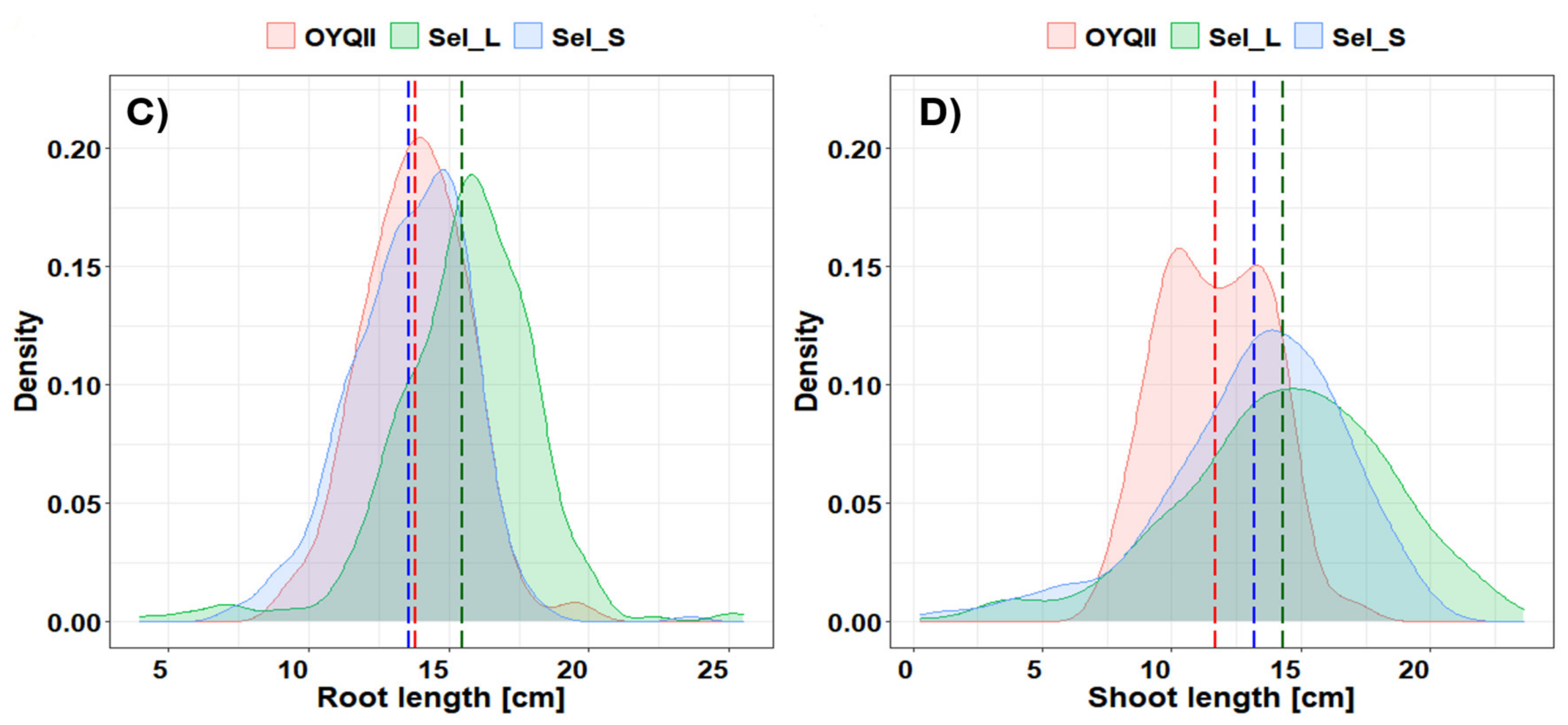
3.1. Hydroponic Selection for Root Phenotype and Soil Test
3.2. Competition Experiment with Mustard
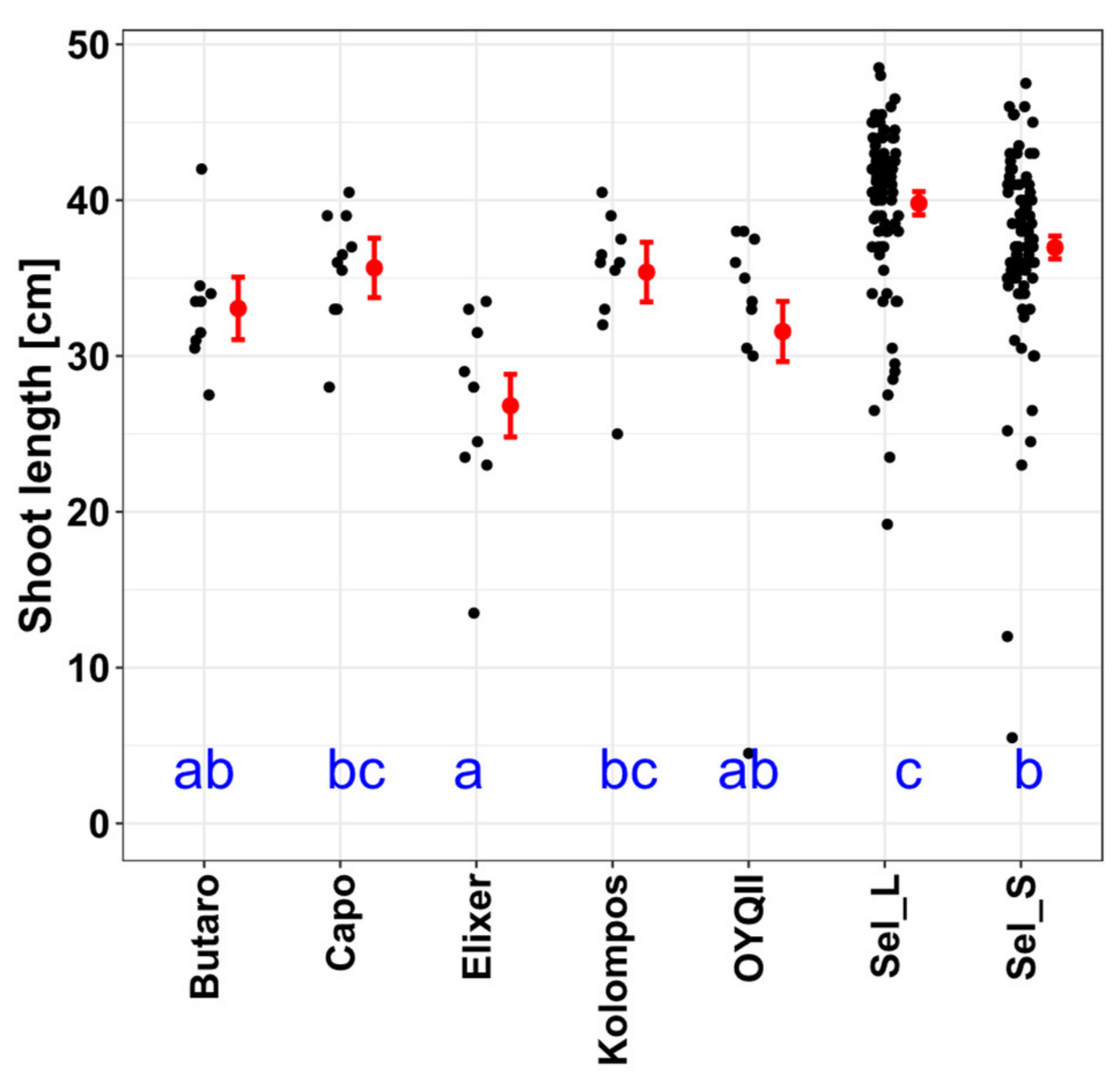
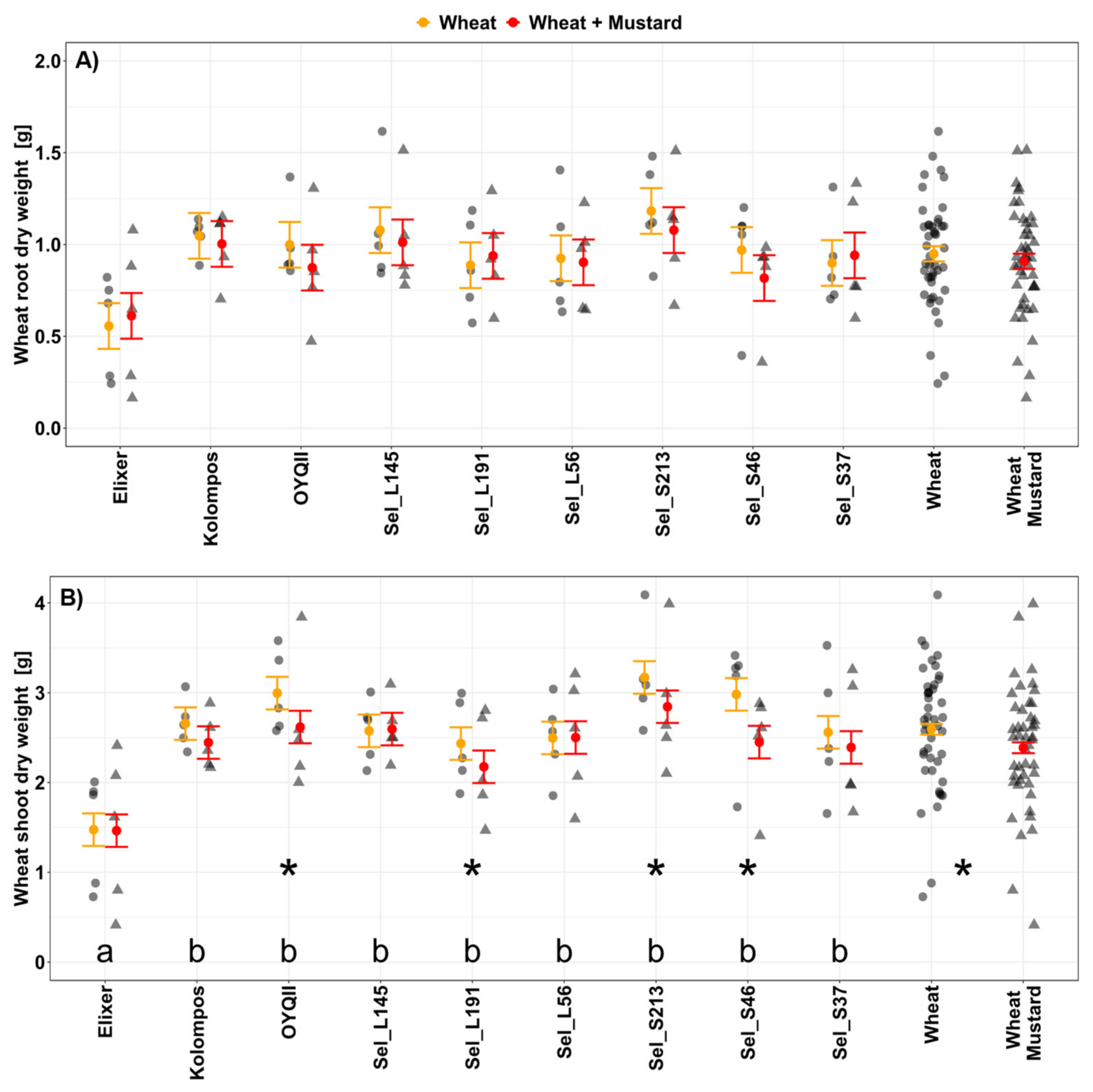
3.2.1. Soil Cover, Plant Length, Root and Shoot Biomass
3.2.2. Wheat Leaf Traits
3.2.3. Competitive Response of Wheat
4. Discussion
4.1. Selection Effects on Root Vigor
4.2. Competetive Response of Wheat and Competetive Effects on Mustard
4.3. Outlook for Future Breeding
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Van Bueren, E.L.; Jones, S.; Tamm, L.; Murphy, K.; Myers, J.; Leifert, C.; Messmer, M. The need to breed crop varieties suitable for organic farming, using wheat, tomato and broccoli as examples: A review. NJAS Wagening. J. Life Sci. 2011, 58, 193–205. [Google Scholar] [CrossRef]
- Winter, E.; Grovermann, C.; Aurbacher, J.; Orsini, S.; Schäfer, F.; Lazzaro, M.; Solfanelli, F.; Messmer, M.M. Sow What You Sell: Strategies for Integrating Organic Breeding and Seed Production into Value Chain Partnerships. Agroecol. Sustain. Food Syst. 2021, 45, 1500–1527. [Google Scholar] [CrossRef]
- Hallmann, J.; von Tiedemann, A. Phytomedizin; Eugen Ulmer KG: Stuttgart, Germany, 2019. [Google Scholar]
- Kahiluoto, H.; Kaseva, J.; Balek, J.; Olesen, J.E.; Ruiz-Ramos, M.; Gobin, A.; Kersebaum, K.C.; Takáč, J.; Ruget, F.; Ferrise, R.; et al. Decline in climate resilience of European wheat. Proc. Natl. Acad. Sci. USA 2019, 116, 123–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Döring, T.F.; Knapp, S.; Kovacs, G.; Murphy, K.; Wolfe, M.S. Evolutionary Plant Breeding in Cereals—Into a New Era. Sustainability 2011, 3, 1944–1971. [Google Scholar] [CrossRef] [Green Version]
- Finckh, M.R. Integration of breeding and technology into diversification strategies for disease control in modern agriculture. Eur. J. Plant Pathol. 2008, 121, 399–409. [Google Scholar] [CrossRef]
- Suneson, C.A. An Evolutionary Plant Breeding Method. Agron. J. 1956, 48, 188–191. [Google Scholar] [CrossRef]
- Brumlop, S.; Pfeiffer, T.; Finckh, M.R. Evolutionary Effects on Morphology and Agronomic Performance of Three Winter Wheat Composite Cross Populations Maintained for Six Years under Organic and Conventional Conditions. Org. Farming 2017, 3, 34–50. [Google Scholar] [CrossRef] [Green Version]
- Weedon, O.D.; Finckh, M.R. Heterogeneous Winter Wheat Populations Differ in Yield Stability Depending on their Genetic Background and Management System. Sustainability 2019, 11, 6172. [Google Scholar] [CrossRef] [Green Version]
- Weedon, O.D.; Finckh, M.R. Response of Wheat Composite Cross Populations to Disease and Climate Variation over 13 Generations. Front. Agric. Sci. Eng. 2021, 8, 400–415. [Google Scholar] [CrossRef]
- Brumlop, S.; Weedon, O.; Link, W.; Finckh, M.R. Effective Population Size (Ne) of Organically and Conventionally Grown Composite Cross Winter Wheat Populations Depending on Generation. Eur. J. Agron. 2019, 109, 125922. [Google Scholar] [CrossRef]
- Spieß, H.; Vollenweider, C. Populationssorten: Strategie. Bioland 2016, Biolandbau im Klimawandel, 20–21. [Google Scholar]
- OSS Winterweizen “EQuality”. Available online: https://www.opensourceseeds.org/saatgut/winterweizen-equality (accessed on 15 February 2021).
- Mundt, C.C. Use of Multiline Cultivars and Cultivar Mixtures for Disease Management. Annu. Rev. Phytopathol. 2002, 40, 381–410. [Google Scholar] [CrossRef] [Green Version]
- An, D.; Su, J.; Liu, Q.; Zhu, Y.; Tong, Y.; Li, J.; Jing, R.; Li, B.; Li, Z. Mapping QTLs for Nitrogen Uptake in Relation to the Early Growth of Wheat (Triticum Aestivum L.). Plant Soil 2006, 284, 73–84. [Google Scholar] [CrossRef]
- Wang, Y.; Thorup-Kristensen, K.; Jensen, L.S.; Magid, J. Vigorous Root Growth Is a Better Indicator of Early Nutrient Uptake than Root Hair Traits in Spring Wheat Grown under Low Fertility. Front. Plant Sci. 2016, 7, 865. [Google Scholar] [CrossRef] [Green Version]
- Hassani, M.A.; Durán, P.; Hacquard, S. Microbial interactions within the plant holobiont. Microbiome 2018, 6, 58. [Google Scholar] [CrossRef]
- Reinhold-Hurek, B.; Bünger, W.; Burbano, C.S.; Sabale, M.; Hurek, T. Roots Shaping Their Microbiome: Global Hotspots for Microbial Activity. Annu. Rev. Phytopathol. 2015, 53, 403–424. [Google Scholar] [CrossRef]
- Wille, L.; Messmer, M.M.; Studer, B.; Hohmann, P. Insights to Plant–Microbe Interactions Provide Opportunities to Improve Resistance Breeding against Root Diseases in Grain Legumes. Plant Cell Environ. 2019, 42, 20–40. [Google Scholar] [CrossRef] [Green Version]
- Bertholdsson, N.-O. Use of Multivariate Statistics to Separate Allelopathic and Competitive Factors Influencing Weed Suppression Ability in Winter Wheat. Weed Res. 2011, 51, 273–283. [Google Scholar] [CrossRef]
- Larsson, S. A Simple, Rapid and Non-Destructive Screening Method Useful for Drought Resistance Breeding in Oats (Avena Sativa L.). Pflanzenzüchtung 1982, 89, 206–221. [Google Scholar]
- Bertholdsson, N.; Weedon, O.; Brumlop, S.; Finckh, M. Evolutionary Changes of Weed Competitive Traits in Winter Wheat Composite Cross Populations in Organic and Conventional Farming Systems. Eur. J. Agron. 2016, 79, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Vijaya Bhaskar, A.V.; Baresel, J.P.; Weedon, O.; Finckh, M.R. Effects of ten years organic and conventional farming on early seedling traits of evolving winter wheat composite cross populations. Sci. Rep. 2019, 9, 9053. [Google Scholar] [CrossRef] [PubMed]
- Vijaya Bhaskar, A.V.; Weedon, O.D.; Finckh, M.R. Exploring the Differences between Organic and Conventional Breeding in Early Vigour Traits of Winter Wheat. Eur. J. Agron. 2019, 105, 86–95. [Google Scholar]
- Rutkoski, J.E. Chapter Four—A practical guide to genetic gain. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 157, pp. 217–249. [Google Scholar]
- Döring, T.F.; Annicchiarico, P.; Clarke, S.; Haigh, Z.; Jones, H.E.; Pearce, H.; Snape, J.; Zhan, J.; Wolfe, M.S. Comparative Analysis of Performance and Stability among Composite Cross Populations, Variety Mixtures and Pure Lines of Winter Wheat in Organic and Conventional Cropping Systems. Field Crop. Res. 2015, 183, 235–245. [Google Scholar] [CrossRef]
- Timaeus, J.; Weedon, O.; Finckh, M.R. Wheat-Pea Species Mixtures as Resource Efficient and High-Performance Food Cropping Systems: Evaluation of Contrasting Wheat Genotypes. In Proceedings of the Intercropping for Sustainability: Research Developments and Their Application, Reading, UK, 18–20 January 2021. [Google Scholar]
- Chanda, S.V.; Singh, Y.D. Estimation of Leaf Area in Wheat Using Linear Measurements. Plant Breed Seed Sci 2002, 46, 75–79. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; Version 4.1.1; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.R-project.org (accessed on 17 November 2021).
- Wickham, H.; François, R.; Henry, L.; Müller, K. RStudio Dplyr: A Grammar of Data Manipulation. Version 1.0.7. 2020. Available online: https://cran.r-project.org/web/packages/dplyr/index.html (accessed on 17 November 2021).
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D. RStudio Ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics. Version 3.3.2. 2020. Available online: https://CRAN.R-project.org/package=ggplot2 (accessed on 17 November 2021).
- Kassambara, A. Ggpubr: “ggplot2” Based Publication Ready Plots. Version 0.4.0. 2020. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 17 November 2021).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S.; Singmann, H.; Dai, B.; Scheipl, F.; Grothendieck, G.; Green, P.; Fox, J.; et al. Lme4: Linear Mixed-Effects Models Using “Eigen” and S4. Version 1.1-27.1. 2020. Available online: https://CRAN.R-project.org/package=lme4 (accessed on 17 November 2021).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; Heisterkamp, S.; Johannes, R. R-Core Nlme: Linear and Nonlinear Mixed Effects Models. Version 3.1-153. 2020. Available online: https://CRAN.R-project.org/package=nlme (accessed on 17 November 2021).
- Lenth, R.V.; Buerkner, P.; Herve, M.; Love, J.; Riebl, H.; Singmann, H. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. Version 1.5.3. 2020. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 17 November 2021).
- Wang, P.; Stieglitz, T.; Zhou, D.W.; Cahill, J.F., Jr. Are competitive effect and response two sides of the same coin, or fundamentally different? Funct. Ecol. 2010, 24, 196–207. [Google Scholar] [CrossRef]
- Schloerke, B.; Cook, D.; Larmarange, J.; Briatte, F.; Marbach, M.; Thoen, E.; Elberg, A.; Toomet, O.; Crowley, J.; Hofmann, H.; et al. GGally: Extension to “Ggplot2”. 2020. Version 2.1.2. Available online: https://CRAN.R-project.org/package=GGally, (accessed on 17 November 2021).
- Becker, H. Pflanzenzüchtung; Ulmer UTB: Stuttgart, Germany, 2019. [Google Scholar]
- Zhang, L.; Richards, R.A.; Condon, A.G.; Liu, D.C.; Rebetzke, G.J. Recurrent selection for wider seedling leaves increases early biomass and leaf area in wheat (Triticum aestivum L.). J. Exp. Bot. 2014, 66, 1215–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heřmanská, A.; Středa, T.; Chloupek, O. Improved wheat grain yield by a new method of root selection. Agron. Sustain. Dev. 2014, 35, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Svačina, P.; Středa, T.; Chloupek, O. Uncommon selection by root system size increases barley yield. Agron. Sustain. Dev. 2013, 34, 545–551. [Google Scholar] [CrossRef]
- Shavrukov, Y.; Kurishbayev, A.; Jatayev, S.; Shvidchenko, V.; Zotova, L.; Koekemoer, F.; de Groot, S.; Soole, K.; Langridge, P. Early Flowering as a Drought Escape Mechanism in Plants: How Can It Aid Wheat Production? Front. Plant Sci. 2017, 8, 1950. [Google Scholar] [CrossRef]
- Mwendwa, J.M.; Brown, W.B.; Weidenhamer, J.D.; Weston, P.A.; Quinn, J.C.; Wu, H.; Weston, L.A. Evaluation of Commercial Wheat Cultivars for Canopy Architecture, Early Vigour, Weed Suppression, and Yield. Agronomy 2020, 10, 983. [Google Scholar] [CrossRef]
- Liu, H.; Fiorani, F.; Jäck, O.; Colombi, T.; Nagel, K.; Weih, M. Shoot and Root Traits Underlying Genotypic Variation in Early Vigor and Nutrient Accumulation in Spring Wheat Grown in High-Latitude Light Conditions. Plants 2021, 10, 174. [Google Scholar] [CrossRef]
- Atkinson, J.A.; Wingen, L.U.; Griffiths, M.; Pound, M.P.; Gaju, O.; Foulkes, M.J.; Le Gouis, J.; Griffiths, S.; Bennett, M.J.; King, J.; et al. Phenotyping pipeline reveals major seedling root growth QTL in hexaploid wheat. J. Exp. Bot. 2015, 66, 2283–2292. [Google Scholar] [CrossRef] [Green Version]
- Olsen, J.; Kristensen, L.; Weiner, J. Influence of sowing density and spatial pattern of spring wheat (Triticum aestivum) on the suppression of different weed species. Weed Biol. Manag. 2006, 6, 165–173. [Google Scholar] [CrossRef]
- Behdarvand, P.; Chinchanikar, G.S.; Dhumal, K.N. Influences of Different Nitrogen Levels on Competition between Spring Wheat (Triticum aestivum L.) and Wild Mustard (Sinapis arvensis L.). J. Agric. Sci. 2012, 4, p134. [Google Scholar] [CrossRef]
- Abdolahi, F.; MohammaddoustChamanabad, H.R.; Asghari, A. The Competitive Response Investigation of Eighteen Wheat (Triticum Aestivum L.) Cultivars with Wild Mustard (Sinapis Arvensis L.). J. Plant Prot. 2018, 31, 581–591. [Google Scholar]
- Li, H.; Jiang, D.; Wollenweber, B.; Dai, T.; Cao, W. Effects of shading on morphology, physiology and grain yield of winter wheat. Eur. J. Agron. 2010, 33, 267–275. [Google Scholar] [CrossRef]
- Franklin, K.A. Shade Avoidance. New Phytol. 2008, 179, 930–944. [Google Scholar] [CrossRef]
- Wille, W.; Pipper, C.B.; Rosenqvist, E.; Andersen, S.B.; Weiner, J. Reducing shade avoidance responses in a cereal crop. AoB Plants 2017, 9, plx039. [Google Scholar] [CrossRef] [Green Version]
- Meilhac, J.; Deschamps, L.; Maire, V.; Flajoulot, S.; Litrico, I. Both Selection and Plasticity Drive Niche Differentiation in Experimental Grasslands. Nat. Plants 2020, 6, 28–33. [Google Scholar] [CrossRef]
- Brooker, R.W.; George, T.S.; Homulle, Z.; Karley, A.J.; Newton, A.C.; Pakeman, R.J.; Schöb, C. Facilitation and Biodiversity Ecosystem Function (BEF) Relationships in Crop Production Systems and Their Role in Sustainable Farming. J. Ecol. 2021, 109, 2054–2067. [Google Scholar] [CrossRef]
- Rebetzke, G.J.; Richards, R.A. Genetic improvement of early vigour in wheat. Aust. J. Agric. Res. 1999, 50, 291–302. [Google Scholar] [CrossRef]
- Středa, T.; Haberle, J.; Klimešová, J.; Klimek-Kopyra, A.; Středová, H.; Bodner, G.; Chloupek, O. Field phenotyping of plant roots by electrical capacitance—A standardized methodological protocol for application in plant breeding: A Review. Int. Agrophysics 2020, 34, 173–184. [Google Scholar] [CrossRef]
- Prey, L.; Von Bloh, M.; Schmidhalter, U. Evaluating RGB Imaging and Multispectral Active and Hyperspectral Passive Sensing for Assessing Early Plant Vigor in Winter Wheat. Sensors 2018, 18, 2931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wuest, S.E.; Peter, R.; Niklaus, P.A. Ecological and Evolutionary Approaches to Improving Crop Variety Mixtures. Nat. Ecol. Evol. 2021, 5, 1068–1077. [Google Scholar] [CrossRef]

| Hydroponics Experiment | Soil Experiment | ||
|---|---|---|---|
| Progeny | rel. Increase SRL (%) | rel. Increase SL (%) | rel. Increase SL (%) |
| Sel_L102 | 10.7 | 31.5 * | 38.3 * |
| Sel_L145 | 18.7 * | 13.5 | 30.1 * |
| Sel_L146 | 17.7 * | 23.2 | 26.1 |
| Sel_L191 | 17 * | 27.3 * | 34.5 * |
| Sel_L198 | 7.8 | 31.9 * | 30.8 * |
| Sel_L214 | 5.4 | −1.3 | −3.9 |
| Sel_L56 | 18.9 * | 31.3 * | 30.1 |
| Sel_L83 | 10.8 | 19.6 | 27.3 |
| Sel_L85 | 4.2 | 22.9 | 23.9 |
| Sel_S120 | 8.2 | 25.5 | 10.2 |
| Sel_S149 | −5.8 | 16 | 15.1 |
| Sel_S187 | 5.8 | 8.9 | 17 |
| Sel_S213 | −5.6 | 9.2 | 21.6 |
| Sel_S33 | −3.1 | 2.2 | 4.4 |
| Sel_S37 | −5.3 | 10.9 | 22.9 |
| Sel_S46 | −20.5 * | 27 * | 35.2 * |
| Sel_S57 | 1.3 | 4 | 6.5 |
| Sel_S58 | 10.8 | 7.9 | 25 |
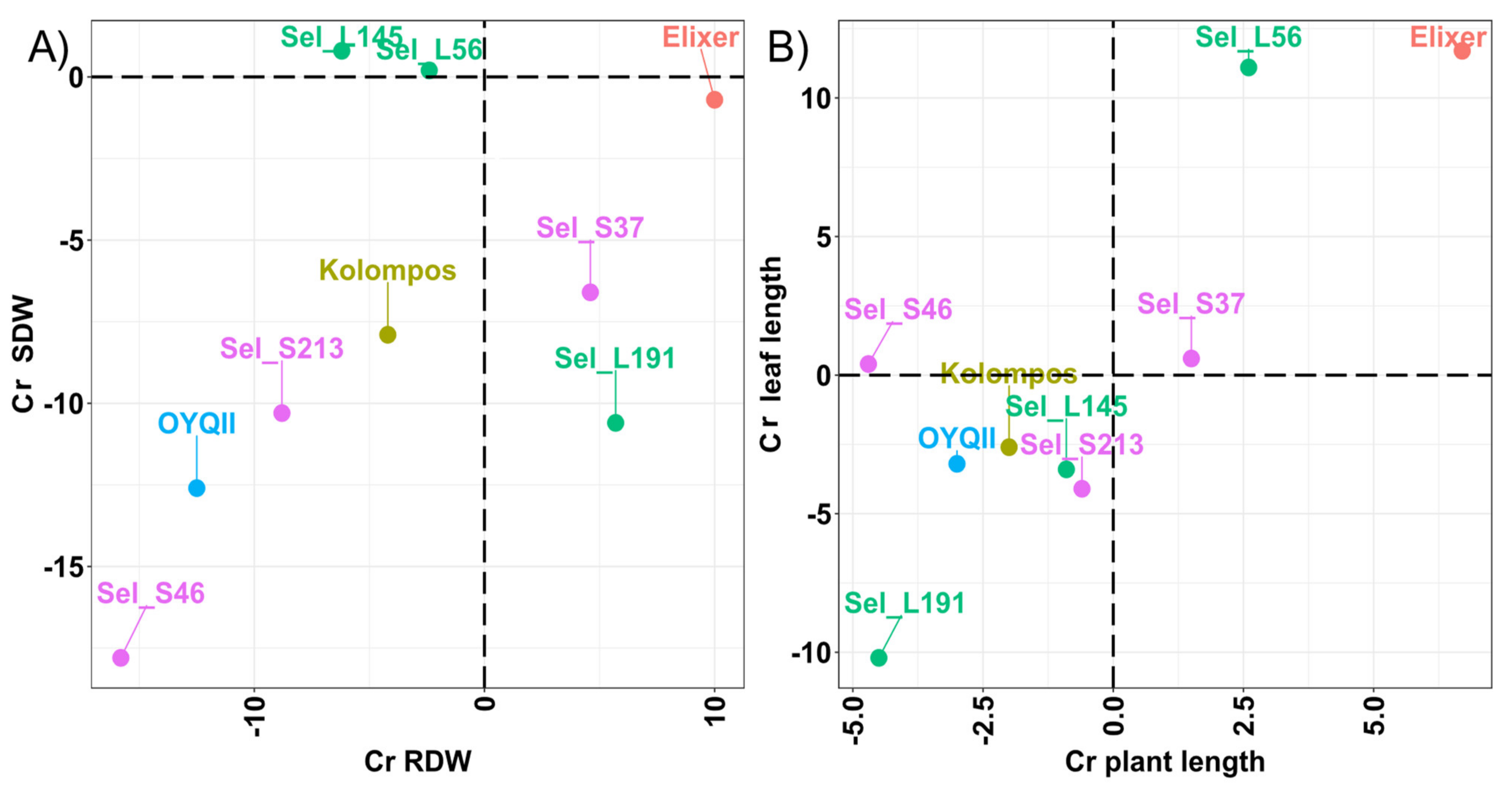
| Genotype | Cover | Plant Length | Leaf Length | RDW | SDW |
|---|---|---|---|---|---|
| Elixer | −44.4 * | 6,7 * | 11.7 * | 10.0 | −0.7 |
| Kolompos | −33.3 * | −2.0 | −2.6 | −4.2 | −7.9 |
| OYQII | −34.4 * | −3.0 | −3.2 | −12.5 | −12.6 * |
| Sel_L145 | −33.3 * | −0.9 | −3.4 | −6.2 | 0.8 |
| Sel_L191 | −38.5 * | −4.5 * | −10.2 * | 5.7 | −10.6 * |
| Sel_L56 | −29.2 * | 2.6 | 11.1 * | −2.4 | 0.2 |
| Mean Sel_L | −33.7 | −0.9 | −0.8 | −1.0 | −3.2 |
| Sel_S213 | −31.0 * | −0.6 | −4.1 | −8.8 | −10.3 * |
| Sel_S37 | −23.1 | 1.5 | 0.6 | 4.6 | −6.6 |
| Sel_S46 | −34.5 * | −4.7 | 0.4 | −15.8 | −17.8 * |
| Mean Sel_S | −29.5 | −1.3 | −1.0 | −6.7 | −11.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timaeus, J.; Weedon, O.D.; Finckh, M.R. Combining Genetic Gain and Diversity in Plant Breeding: Heritability of Root Selection in Wheat Populations. Sustainability 2021, 13, 12778. https://doi.org/10.3390/su132212778
Timaeus J, Weedon OD, Finckh MR. Combining Genetic Gain and Diversity in Plant Breeding: Heritability of Root Selection in Wheat Populations. Sustainability. 2021; 13(22):12778. https://doi.org/10.3390/su132212778
Chicago/Turabian StyleTimaeus, Johannes, Odette Denise Weedon, and Maria Renate Finckh. 2021. "Combining Genetic Gain and Diversity in Plant Breeding: Heritability of Root Selection in Wheat Populations" Sustainability 13, no. 22: 12778. https://doi.org/10.3390/su132212778
APA StyleTimaeus, J., Weedon, O. D., & Finckh, M. R. (2021). Combining Genetic Gain and Diversity in Plant Breeding: Heritability of Root Selection in Wheat Populations. Sustainability, 13(22), 12778. https://doi.org/10.3390/su132212778






