Abstract
Fοllow up with our previous study on the extraction of saffron processing waste polyphenols using deep eutectic solvents, the objective of this examination was a comparative evaluation of pressurized liquid extraction (PLE), stirred-tank extraction (STE) and stirred-tank extraction with ultrasonication pretreatment (STE/UP) with respect to the recovery of pigments and antioxidant polyphenols from saffron processing waste. Aqueous solutions of citric and lactic acids at two different concentrations were used as green solvents. The extracts obtained under the specified conditions were analyzed for total pigment and total polyphenol yields as well as for their ferric-reducing power and antiradical activity. Furthermore, each produced extract was analyzed with liquid chromatography–mass spectrometry to profile its analytical polyphenolic composition. In all cases, PLE provided inferior results compared to the two other techniques, producing extracts with lower polyphenolic concentration and weaker antioxidant properties. On the other hand, no specific pattern was detected concerning the effect of ultrasonication, acid type and acid concentration. Hierarchical cluster analysis indicated that stirred-tank extraction with 1% (w/v) lactic acid and ultrasonication pretreatment might be the highest-performing combination, providing extracts with increased polyphenol and pigment concentration; however, it also enhanced antioxidant activity. It was also concluded that the significantly shorter extraction time when using PLE might be an important element in further optimizing the process, buttressing the use of this technique for the establishment of innovative and sustainable-by-design extraction methodologies.
1. Introduction
As the world’s population and industrial activity are rapidly expanding, resource depletion and environmental aggravation are challenges which need to be imminently addressed [1]. The agricultural and food industries are responsible for a large share of waste and byproducts generated as a result of farming practices and the harvesting and processing of raw materials. These side-streams are particularly rich in organic substances, and their uncontrolled dumping results in environmental pollution with detrimental consequences to the neighboring eco-systems and public health [2]. On the other hand, agri-food waste represents a vast pool of materials which can be used for the production of bio-based chemicals, bio-fuels, and high value-added substances [3]. Thus, in the framework of the circular economy, the rational utilization of agri-food waste biomass within a biorefinery concept may contribute to a fully sustainable agri-food sector.
Plant processing by-products consist mainly of peels, seeds, stems, flowers, and disfigured and undersized or damaged tissues. These residues are rich in a spectrum of bioactive compounds including polyphenols, carotenoids, oils, pectins, etc., and their promotion as cheap and abundant bioresources is state-of-the-art for the commercialization of commodities in the food, pharmaceutical, and cosmetics industry [4]. Thus, proper management of these byproducts should target their re-introduction to the production line as raw materials for the obtaining of novel products with health-related properties and added value through sustainable technologies of extraction [3]. The principles pertaining to the Green Chemistry concept are mainly focused on reducing waste and promoting efficient use of energy and resources. Concerning extraction processes, these principles may include (but are not limited to) the use of alternative solvents such as water or other bio-based solvents, reducing energy consumption by implementing innovative technologies, reducing unit operations for safer and more robust processes, and targeted generation of extracts with increased or improved bioactivity [2].
Pressurized liquid extraction (PLE) is also known as pressurized solvent extraction and accelerated solvent extraction. When water is used as the solvent, this technique is characterized as sub-critical water extraction, pressurized hot water extraction (PHWE), or superheated water extraction. It is based on liquid solvents used at a relatively high temperature and pressure, which enables improved extraction performance compared to techniques carried out at near-atmospheric temperature and pressure by boosting the solubility of the target molecules and increasing mass transfer [5]. PLE is considered a green extraction process and is performed mainly with non-toxic, non-volatile and reusable solvents such as water; it has been applied to the extraction of several plant matrices for the effective recovery of bioactive metabolites such as polyphenols, terpenes, oil, etc. [6,7].
In addition to replacing conventional extraction processes with more efficient green processes, pretreatment of samples prior to extraction with ultrasonication has also been shown to improve extraction performance. This effect has been demonstrated with the extraction of polyphenols from elderberry flowers [8], red grape pomace [9], and wheat bran [10]. Extraction enhancement by ultrasonication occurs through mechanisms involving mostly cavitation phenomena, which result in the generation of cavitation bubbles. These bubbles implode on the surface of the solid matrix, generating micro-jetting and other effects such as erosion, particle breakdown, surface peeling, micro-mixing and macro-turbulence [11].
Saffron (Crocus sativus, Iridaceae) is a herb acknowledged since the ancient time for its medicinal properties and culinary uses [12]. The stigma is the most valuable tissue of the plant; these are manually collected and dried to produce a highly appreciated spice. After separation, the remaining parts of the flower, composed of the tepals (undifferentiated petals and sepals), are rejected as processing waste. However, the evidence accumulated by recent studies has shown that saffron tepals may contain several bioactive substances, including flavonol glycosides and anthocyanins. Some of these phytochemicals have been reported to exhibit multiple beneficial bioactivities [13,14], and on this ground several extraction processes have been proposed with the aim of producing polyphenol-enriched extracts from saffron processing waste (SPW) [15,16].
Nevertheless, to the best of the authors’ knowledge there is only one extant examination of PLE extraction of SPW, which focused only on total polyphenols [17]. Moreover, aqueous organic acid solutions such as those tested on other plant materials [18,19] have never been used for the extraction of SPW polyphenols. This being the case, the study reported herein describes an investigation of the extraction of flavonols and anthocyanins from SPW employing PLE. This technique was compared to conventional batch stirred-tank extraction both with and without ultrasonication pretreatment. The differences in the extraction yield among the techniques tested were revealed by determining the analytical metabolite profile of each obtained extract.
2. Materials and Methods
2.1. Chemicals
For chromatographic determination, the solvents used were of HPLC grade. Pelargonin chloride (pelargonidin 3,5-di-O-glucoside) was from Extrasynthese (Genay, France). Kaempferol 3-O-glucoside, rutin (quercetin 3-O-rutinoside) (>94%), 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ), 2,2-diphenylpicrylhydrazyl (DPPH), and Folin-Ciocalteu reagent were from Sigma-Aldrich (St. Louis, MO, USA). Citric acid anhydrous and iron chloride hexahydrate were from Merck (Darmstadt, Germany). Ascorbic acid (99.5%), L-lactic acid, and sodium carbonate anhydrous (99%) were from Penta (Praha, Czechia). Gallic acid hydrate was from Panreac (Barcelona, Spain).
2.2. Plant Material
Saffron (Crocus sativus L.) processing waste (SPW) consisting of saffron tepals was collected from a processing plant in Kozani (West Macedonia, Greece) immediately after manual processing of the saffron flowers. The material was transferred to the laboratory within 24 h and freeze-dried in a Biobase BK-FD10P freeze-drier (Shandong, China) for 24 h. The dried tissue was then ground in a domestic blender to give a powder with an average particle diameter of 0.637 mm and stored in air-tight tubes at −18 °C until used.
2.3. Pressurized-Liquid Extraction (PLE)
The equipment used was a PLE system (Fluid Management Systems, Inc., Watertown, MA, USA). An analytical description of the device is given in Figure 1. For the purposes of this study, static extraction was performed using a 100-mL stainless steel cell. An amount of SPW (2 g) was loaded onto the extraction cell and capped with two filtration end fittings. The PLE system then automatically pressurized and heated the sample while pumping solvent into the chamber for a predetermined resident time and solvent flow. The specific settings used were: filling (with solvent) time, 1.3 min; pressurization, 0.5 min; preheating (at 120 °C) time, 5 min; extraction (at 120 °C) time, 10 min; cooling (at T < 50 °C), 7 min; depressurization time, 0.02 min; solvent flush, 1.3 min; nitrogen flush, 1 min. Under these conditions, the liquid-to-solid ratio was 40 mL g−1.

Figure 1.
The PLE system used in this study: 1. Touch screen (program algorithm display and control center); 2. Pressure gauge; 3. Chamber outlet; 4. Pressure relief valve (pressure control); 5. Extraction chamber; 6. Chamber heating jacket; 7. Chamber inlet; 8. Pump control; 9. Positive displacement pump; 10. Solvent dosing hose.
During heating, a temperature rise (overshooting) was observed which in no case exceeded 4 °C, while the temperature set was restored within 1–2 min. After the completion of the extraction, the extract was drained and transferred to a suitable vial for further processing or analysis. The choice of the extraction temperature and time was based on previous data [20,21,22], as well as preliminary experimentation. The solvents used were distilled water (pH = 5.61), 1% (w/v) citric acid (pH = 2.88), 5% (w/v) citric acid (pH = 2.42), 1% (w/v) lactic acid (pH = 2.71), and 5% (w/v) lactic acid (pH = 2.48).
2.4. Stirred-Tank Extraction in Batch Mode
An exact mass of 0.250 g of SPW was mixed with 10 mL of solvent (liquid-to-solid ratio 40 mL g−1) in a 25-mL Duran glass vial and stirred continuously at 500 rpm for 180 min at 80 °C into an oil bath heated by a thermostat-equipped hotplate (Witeg, Wertheim, Germany). After the extraction, all samples were centrifuged at 10,000× g for 10 min.
2.5. Batch Stirred-Tank Extraction with Ultrasonication Pretreatment
Prior to batch stirred-tank extraction samples were subjected to ultrasonication in pulse mode for 15 min in an Elma S 100 (H) heated ultrasonic bath (Elma Schmidbauer GmbH, Singen, Germany) at a frequency of 37 Hz and nominal power of 550 W. During this resident time, increases in the initial temperature (31 °C) in no case exceeded 6 °C. The actual ultrasonication power (P) dissipated into the system, as well as the acoustic energy density (AED), were determined using the following equations:
where m corresponds to the mass of the coupling liquid (water) contained in the ultrasonication bath (in g), Cp corresponds to the specific heat capacity of water (4.2 J g−1 K−1), and represents the temperature rise per s, which was calculated by fitting temperature change (dT) as measured by a thermocouple as a function of time [23]. P and AED were determined to be 159.6 W and 39.9 W L−1, respectively.
2.6. Determinations
Determination of total polyphenols was performed with Folin-Ciocalteu reagent and results were given as mg gallic acid equivalents (GAE) per g of dry mass (dm). The antiradical activity (AAR) was estimated with a DPPH radical probe using a stoichiometric assay, and results were given as μmol DPPH per g dm. Ferric-reducing power (PR) was determined using the TPTZ chromophore probe; results were expressed as μmol ascorbic acid equivalents (AAE) per g dm. The analytical protocols for all these methodologies have been previously reported in detail [24]. Total pigments were likewise determined with a protocol reported elsewhere [25].
2.7. Liquid Chromatography–Diode Array-Mass Spectrometry (LC-DAD-MS)
A published methodology was employed [24] using a Finnigan AQA mass spectrometer coupled to a Finnigan (San Jose, CA, USA) MAT Spectra System P4000 pump and a UV6000LP diode array detector. Chromatography was performed on a Fortis RP-18 column, 150 mm × 2.1 mm, 3 μm, at 40 °C, with a 10-μL injection loop. Μass spectra were acquired at 20 and 70 eV with electrospray ionization (ESI) in positive ion mode. Mass acquisition settings were as follows: temperature 250 °C, probe source voltage 25 V, detector voltage 450 V, and capillary voltage 4 kV. Elution was carried out with (A) 2% acetic acid and (B) methanol at a flow rate of 0.3 mL min−1, as follows: 0–30 min, 0–100% methanol; 30–40 min, 100% methanol.
2.8. High-Performance Liquid Chromatography (HPLC)
A Shimadzu CBM-20A liquid chromatograph (Shimadzu Europa GmbH, Duisburg, Germany) along with a Shimadzu SPD-M20A diode-array detector was used, interfacing with Shimadzu LC solution software. Analyses were run on a Phenomenex Luna C18(2) column (100 Å, 5 μm, 4.6 × 250 mm) (Phenomenex, Inc., Torrance, CA, USA) at 40 °C. Information concerning the elution program, calibration standards and calibration curves used for the quantification have been previously reported in detail [24].
2.9. Statistical Analyses
At least two individual extractions were accomplished for each treatment, and all determinations were performed in triplicate. The values reported are the average ± standard deviation (SD). Linear regressions were established using SigmaPlot™ 12.5 (Systat Software Inc., San Jose, CA, USA), and distribution analyses with JMP™ Pro 13 (SAS, Cary, NC, USA). All statistical analyses were performed with at least at a 95% significance level.
3. Results and Discussion
3.1. Yield in Total Polyphenols and Total Pigments
Figure 2A illustrates the yield in total polyphenols (YTP) achieved with the various solvents tested through deployment of pressurized liquid extraction (PLE), stirred-tank extraction (STE) and stirred-tank extraction with ultrasonication pretreatment (STE/UP). The highest YTP (38.24 mg GAE g−1 dm) was recorded for the STE/UP performed with 1% (w/v) lactic acid (LA). Extractions carried out with 5% (w/v) citric acid (CA) and 5% LA under the same conditions had comparable performance, since there was no statistical difference amongst the YTP obtained (p < 0.05). The same held true for the extractions carried out with water, 1% CA, 5% CA and 5% LA in stirred-tank mode without ultrasonication pretreatment. These findings show that there was no clear effect of either the type of the acid or its concentration on extraction efficiency. Furthermore, ultrasonication pretreatment offered virtually no advantage over simple stirred-tank extraction. On the other hand, all samples generated with PLE displayed lower YTP, which reached statistical significance (p < 0.05) irrespective of the acid used or its concentration.
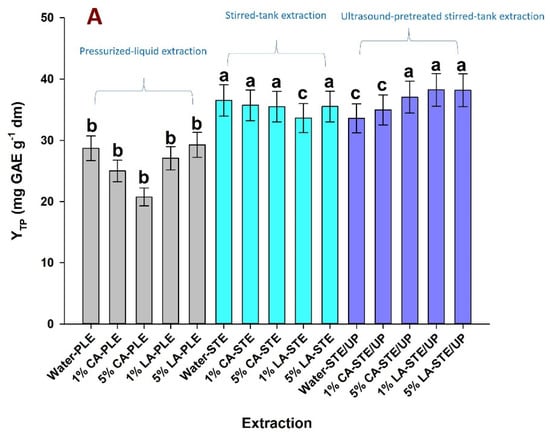
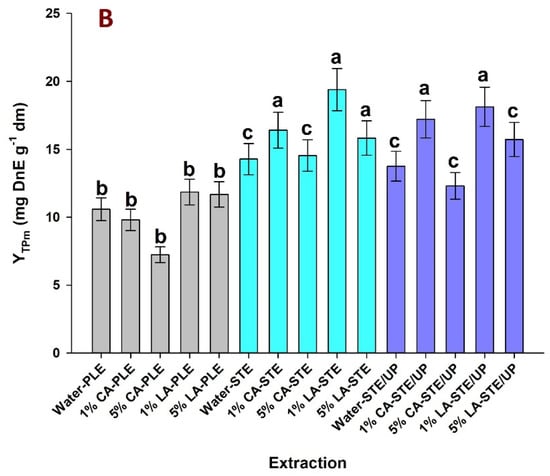
Figure 2.
Diagrams illustrating the efficiency of various solvents in recovering total polyphenols (A) and total pigments (B) from saffron processing waste. PLE, pressurized–liquid extraction; STE, stirred–tank extraction; STE/UP, stirred–tank extraction with ultrasonication pretreatment; CA and LA correspond to aqueous solutions of citric acid and lactic acid. Letters a, b, and c denote statistically different values (p < 0.05).
The results on the yield in total pigments (YTPm) provided a different image (Figure 2B). The extractions carried out with 1% CA, 1% LA and 5% LA in stirred-tank mode, and those with 1% CA and 1% LA in stirred-tank mode with ultrasonication pretreatment exhibited the highest and most statistically significant YTPm (p < 0.05). On the contrary, PLE performed with any of the extraction media tested showed significantly lower YTPm (p < 0.05). In this case as well, a distinction between CA and LA or between STE and STE/UP was not apparent. Taking into consideration both YTP and YTPm, the samples prepared with 1% CA-STE, 5% CA-STE, 5% LA-STE, and 1% LA STE/UP were those with the richest composition.
3.2. Effect on the Antioxidant Properties
The results on the determination of reducing power (PR) and antiradical activity (AAR) are depicted in Figure 3. Extracts produced with water, 5% CA and 5% LA in stirred-tank mode had significantly higher AAR (p < 0.05). The same was observed for the extract generated with 5% CA in stirred-tank mode after ultrasonication pretreatment. By contrast, all extracts produced with PLE showed lower AAR (p < 0.05) (Figure 3A). The pattern concerning PR was essentially similar, with the samples prepared with 1% CA-STE, 5% CA-STE, 5% LA-STE and 5% CA STE/UP showing the highest values. On the other hand, all PLE samples as well as 1% LA-STE/UP had significantly lower PR (Figure 3B). Considering both AAR and PR, the samples prepared with 5% CA-STE, 5% LA-STE and 5% CA-STE/UP were the most efficacious in terms of expressing antioxidant activity.
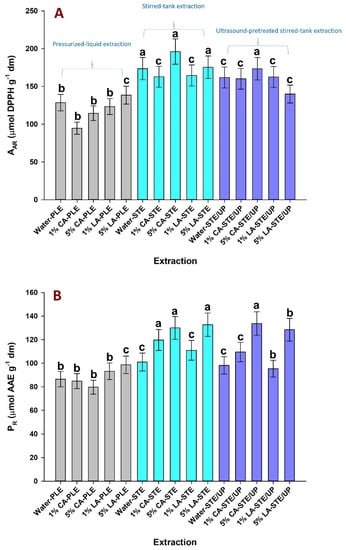
Figure 3.
Diagrams illustrating the effect of various solvents on the antiradical activity (A) and ferric–reducing power (B) of saffron processing waste extracts. PLE, pressurized–liquid extraction; STE, stirred–tank extraction; STE/UP, stirred-tank extraction with ultrasonication pretreatment; CA and LA correspond to aqueous solutions of citric acid and lactic acid. Letters a, b, and c denote statistically different values (p < 0.05).
3.3. Effect on the Flavonol and Anthocyanin Profile
The extract generated from each assay was subjected to HPLC analysis in order to portray the analytical polyphenolic composition and detect differences attributed to different extraction modes. Chromatograms were traced at both 360 and 520 nm; in all cases, seven principal compounds could be tentatively identified and quantified: four flavonol glycosides and three anthocyanin pigments (Figure 4). The tentative identification of these substances was based on mass spectral data, as previously described [24]; the quantitative data concerning flavonol and anthocyanin composition are given in Table 1 and Table 2, respectively.
Figure 4.
HPLC trace of the extract 1% LA-STE/UP, monitored at 360 (A) and 520 nm (B). Peak assignment: 1, kaempferol 3–O–sophoroside 7–O–glucoside; 2, quercetin 3–O–sophoroside; 3, kaempferol 3–O–sophoroside; 4, kaempferol 3–O–glucoside; 5, delphinidin 3,5–di–O–glucoside; 6, petunidin 3,5–di–O–glucoside; 7, delphinidin 3–O–glucoside.

Table 1.
Analytical flavonol composition of the extracts produced in this study; values reported are means ± standard deviation.

Table 2.
Anthocyanin pigment composition of the extracts produced in this investigation; values reported are means ± standard deviation.
The most abundant flavonol glycoside was kaempferol 3-O-sophoroside (KS), followed by kaempferol 3-O-sophoroside 7-O-glucoside (KSG), quercetin 3-O-sophoroside (QS) and kaempferol 3-O-glucoside (KG). This finding was in accordance with recent findings on SPW extraction with a deep eutectic solvent [24]. The pattern of flavonol composition in the samples produced with PLE was identical to those seen in samples generated with STE and STE/UP, which showing that there was no selectivity towards any SPW flavonol. The extraction with 1% LA-STE/UP was proven to be the most efficient for all flavonols, affording significantly higher yields (p < 0.05). On the contrary, extractions with 1% CA-PLE, 5% CA-PLE and 5% LA-PLE were the least efficient in this regard. Considering the total flavonol yield, the extractions with Water-STE, Water-STE/UP, 1% LA-STE/UP and 5% LA-STE/UP were of equivalent performance.
These findings suggest that ultrasonication pretreatment enabled the extraction of increased flavonol amounts. On the other hand, the use of citric acid appeared to disfavor flavonol recovery.
With respect to anthocyanins, extraction with 1% LA-STE/UP was once again the most efficacious, while 1% CA-STE, 5% CA-STE, 5% LA-STE and 5% LA-STE/UP were of comparable efficiency.
However, unlike flavonols, PLE afforded significantly higher levels of delphinidin 3,5-di-O-glucoside (DDG) compared to both STE and STE/UP. This outcome indicates that DDG recovery might be favored with PLE. On average, the most abundant anthocyanin was delphinidin 3-O-glucoside (DG), followed by petunidin 3,5-di-O-glucoside (PDG) and delphinidin 3,5-di-O-glucoside (DDG). These findings contrast with a recent investigation in which it was demonstrated that DDG was the predominant anthocyanin [24,26].
Taking into account the total anthocyanin yield, it was evident that STE and STE/UP were of higher efficiency compared to PLE; however, the distinction between STE and STE/UP was unclear. By jointly considering the yield in total flavonols and total anthocyanins, the highest-performing system was 1% LA-STE/UP. This was corroborated by the data on YTP, YTPm (Figure 2), AAR and PR (Figure 3). To confirm these observations, a hierarchical cluster analysis was performed including the yield in all individual polyphenols as well as AAR and PR. As can be seen in Figure 5, 1% LA-STE/UP was clustered separately, which could be considered sound evidence of its supremacy over all other extracts. Apart from Water-PLE, all other PLE samples were grouped together, which clearly points out their similarity in extraction yield and antioxidant properties.
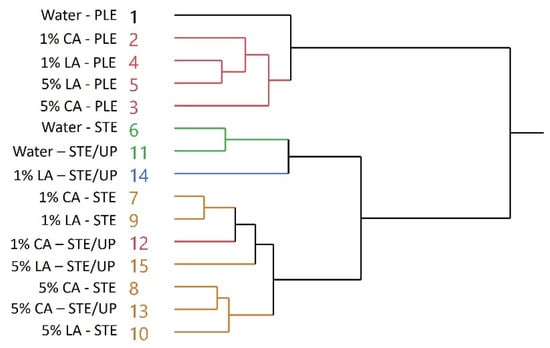
This outcome suggests that under the PLE conditions employed, the addition of citric acid or lactic acid does not foster extraction efficiency or antioxidant activity. Likewise, 5% CA-STE, 5% LA-STE, 5% CA-STE/UP and 5% LA-STE/UP were on the same cluster, an indication of their comparable efficiency. Thus, it can be supported that extraction with aqueous solution containing 5% of either citric or lactic acid showed no significant differences, while ultrasonication pretreatment offered no detectable statistically significant advantage. It is also to be noted that the categorization of Water-STE and Water-STE/UP in the same cluster provides additional evidence that ultrasonication pretreatment of SPW might not always provide a significant benefit in terms of increasing polyphenol extraction yield and enhancing antioxidant activity.
Such an outcome apparently contradicts recent examinations in which ultrasonication pretreatment significantly boosted polyphenol extraction using various means, including β-cyclodextrin [27], deep eutectic solvents [8], and hydroethanolic solutions [9]. However, negative effects have also been reported [28]. Therefore, it would be reasonable to presume that different plant matrices may behave in a different manner as a response to ultrasonication prior to performing stirred-tank extraction. Furthermore, the role of extraction media should also be taken into account. Early investigations highlighted the importance of the type and concentration of acid on the aqueous extraction of anthocyanins from red grape pomace [29]. In the same line, a more recent study has suggested that aqueous solutions of acetic acid are more efficient for flavanol extraction from red grape pomace compared to citric acid solution [30]. In that study, a significant role in the extraction performance was also attributed to acid concentration. Such an approach was more thoroughly carried out by deploying response surface methodology, where it was demonstrated that acidification with lactic acid provided a more effective means of recovering flavonoids from red grape pomace compared to acetic, tartaric and citric acids [31].
The efficacy of PLE compared to both conventional STE and STE implemented after ultrasonication pretreatment was lower, as judged by the YTP, AAR, PR, as well as the yield of major polyphenols. These results contrasted with previous studies where PLE outperformed both conventional and emerging techniques applied for anthocyanin extraction [32,33] and other polyphenols [22]. However, other investigations showed that these differences might be marginal [34]. Nevertheless, it should be emphasized that the yields attained using PLE cannot be overlooked considering the significantly shorter required extraction time. Furthermore, although PLE was carried out at 120 °C, considerably higher than the 80 °C used for STE and STE/UP, no alteration in the polyphenolic profile was observed. This may indicate that SPW polyphenols are rather stable under these conditions. Such evidence could be a key element in the future optimization of PLE methodology, which is anticipated to shed more light on the potential of PLE for obtaining high extraction yields and extracts with improved antioxidant properties from SPW. Generally, PLE processes have the advantage of providing important enhancements compared to conventional extraction procedures, including higher extraction yields and recoveries, faster extraction, and lower solvent volumes. The use of a high temperature results in an increase in the rate of mass transfer, enhancement of the solubility of the target compounds, and a decrease in solvent viscosity [7]. Moreover, the use of alternative solvents such as deep eutectic solvents should also be considered. The use of such a solvent composed of lactic acid and glycine has been demonstrated to significantly enhance the performance of polyphenol extraction from SPW compared to conventional solvents [24]. Thus, a combination of deep eutectic solvents with PLE might provide a highly effective means of polyphenol and pigment extraction from SPW.
4. Conclusions
Follow up with a previous study of ours on the use of deep eutectic solvents, in this study, pressurized-liquid extraction was compared to conventional stirred-tank extraction and stirred-tank extraction integrated by ultrasonication pretreatment, in order to obtain evidence regarding their suitability for antioxidant polyphenol and pigment extraction from saffron processing wastes (floral residues). The solvents used were green aqueous solutions of citric and lactic acids. The outcome of the investigation evidenced that stirred-tank extraction and stirred-tank extraction including ultrasonication pretreatment outperformed pressurized-liquid extraction under the conditions employed. This appraisal was based on the yields of total pigments and total polyphenols, as well as the antioxidant properties of the obtained extracts. Determination of the analytical polyphenolic composition also showed that the extracts generated with either stirred-tank extraction or stirred-tank extraction with ultrasonication pretreatment were richer in the major substances identified. On the other hand, the significantly shorter extraction time used for pressurized-liquid extraction should not be overlooked. It is suggested that optimization of the pressurized-liquid extraction process based on the data reported in this study might establish a green and efficient methodology for the recovery of polyphenols and pigments from saffron processing waste. Such an approach would contribute to more effective promotion of this precious residue.
Author Contributions
Conceptualization, D.P.M. and S.I.L.; methodology, V.M.P., D.P.M. and S.I.L.; validation, V.M.P.; formal analysis, V.M.P., V.A., K.P. and E.B.; investigation, V.M.P., V.A., D.P., writing—original draft preparation, D.P.M., S.I.L., V.M.P.; writing—review and editing, D.P.M., S.I.L., V.M.P.; supervision, D.P.M., S.I.L.; project administration, S.I.L., D.P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was co-financed by the European Union and the Hellenic Ministry of Economy and Development through the Operational Programme Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH—CREATE—INNOVATE (project code: T1EDK-05677).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are not to be shared.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Manzanares, P. The role of biorefinering research in the development of a modern bioeconomy. Acta Innov. 2020, 37, 47–56. [Google Scholar] [CrossRef]
- Herrero, M.; Ibañez, E. Green extraction processes, biorefineries and sustainability: Recovery of high added-value products from natural sources. J. Supercrit. Fluids 2018, 134, 252–259. [Google Scholar] [CrossRef] [Green Version]
- Osorio, L.L.D.R.; Flórez-López, E.; Grande-Tovar, C.D. The potential of selected agri-food loss and waste to contribute to a circular economy: Applications in the food, cosmetic and pharmaceutical industries. Molecules 2021, 26, 515. [Google Scholar] [CrossRef]
- Carpentieri, S.; Soltanipour, F.; Ferrari, G.; Pataro, G.; Donsì, F. Emerging green techniques for the extraction of antioxidants from agri-food by-products as promising ingredients for the food industry. Antioxidants 2021, 10, 1417. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef]
- Plaza, M.; Turner, C. Pressurized hot water extraction of bioactives. TrAC Trends Anal. Chem. 2015, 71, 39–54. [Google Scholar] [CrossRef] [Green Version]
- Arshadi, M.; Attard, T.M.; Lukasik, R.M.; Brncic, M.; da Costa Lopes, A.M.; Finell, M.; Geladi, P.; Gerschenson, L.N.; Gogus, F.; Herrero, M.; et al. Pre-treatment and extraction techniques for recovery of added value compounds from wastes throughout the agri-food chain. Green Chem. 2016, 18, 6160–6204. [Google Scholar] [CrossRef] [Green Version]
- Kaltsa, O.; Lakka, A.; Grigorakis, S.; Karageorgou, I.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D.P. A green extraction process for polyphenols from elderberry (Sambucus nigra) flowers using deep eutectic solvent and ultrasound-assisted pretreatment. Molecules 2020, 25, 921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, A.; Bhushan, B.; Rosales, A.; Turienzo, L.R.; Cortina, J. Valorisation potential of Cabernet grape pomace for the recovery of polyphenols: Process intensification, optimisation and study of kinetics. Food Bioprod. Proc. 2018, 109, 74–85. [Google Scholar] [CrossRef]
- Cherif, M.M.; Grigorakis, S.; Halahlah, A.; Loupassaki, S.; Makris, D.P. High-efficiency extraction of phenolics from wheat waste biomass (bran) by combining deep eutectic solvent, ultrasound-assisted pretreatment and thermal treatment. Environ. Proc. 2020, 7, 845–859. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Ghaffari, S.; Roshanravan, N. Saffron; an updated review on biological properties with special focus on cardiovascular effects. Biomed. Pharmac. 2019, 109, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Moratalla-López, N.; Bagur, M.J.; Lorenzo, C.; Martínez-Navarro, M.; Salinas, M.R.; Alonso, G.L. Bioactivity and bioavailability of the major metabolites of Crocus sativus L. Flower. Molecules 2019, 24, 2827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chichiriccò, G.; Ferrante, C.; Menghini, L.; Recinella, L.; Leone, S.; Chiavaroli, A.; Brunetti, L.; Di Simone, S.; Ronci, M.; Piccone, P.; et al. Crocus sativus by-products as sources of bioactive extracts: Pharmacological and toxicological focus on anthers. Food Chem. Toxicol. 2019, 126, 7–14. [Google Scholar] [CrossRef]
- Da Porto, C.; Natolino, A. Extraction kinetic modelling of total polyphenols and total anthocyanins from saffron floral bio-residues: Comparison of extraction methods. Food Chem. 2018, 258, 137–143. [Google Scholar] [CrossRef]
- Ahmadian-Kouchaksaraie, Z.; Niazmand, R. Supercritical carbon dioxide extraction of antioxidants from Crocus sativus petals of saffron industry residues: Optimization using response surface methodology. J. Supercrit. Fluids 2017, 121, 19–31. [Google Scholar] [CrossRef]
- Ahmadian-Kouchaksaraie, Z.; Niazmand, R.; Najafi, M.N. Optimization of the subcritical water extraction of phenolic antioxidants from Crocus sativus petals of saffron industry residues: Box-Behnken design and principal component analysis. Innov. Food Sci. Emerg. Technol. 2016, 36, 234–244. [Google Scholar] [CrossRef]
- Li, Y.; Han, L.; Ma, R.; Xu, X.; Zhao, C.; Wang, Z.; Chen, F.; Hu, X. Effect of energy density and citric acid concentration on anthocyanins yield and solution temperature of grape peel in microwave-assisted extraction process. J. Food Eng. 2012, 109, 274–280. [Google Scholar] [CrossRef]
- Benfica, J.; Morais, E.S.; Miranda, J.S.; Freire, M.G.; de Cássia, R.; de Sousa, S.; Coutinho, J.A.P. Aqueous solutions of organic acids as effective solvents for levodopa extraction from Mucuna pruriens seeds. Sep. Purif. Technol. 2021, 274, 119084. [Google Scholar] [CrossRef]
- Hossain, M.; Brunton, N.; Martin-Diana, A.; Barry-Ryan, C. Application of response surface methodology to optimize pressurized liquid extraction of antioxidant compounds from sage (Salvia officinalis L.), basil (Ocimum basilicum L.) and thyme (Thymus vulgaris L.). Food Funct. 2010, 1, 269–277. [Google Scholar] [CrossRef]
- Huamán-Castilla, N.L.; Campos, D.; García-Ríos, D.; Parada, J.; Martínez-Cifuentes, M.; Mariotti-Celis, M.S.; Pérez-Correa, J.R. Chemical properties of Vitis vinifera Carménère pomace extracts obtained by hot pressurized liquid extraction, and their inhibitory effect on type 2 diabetes mellitus related enzymes. Antioxidants 2021, 10, 472. [Google Scholar] [CrossRef] [PubMed]
- Katsinas, N.; Bento da Silva, A.; Enríquez-de-Salamanca, A.; Fernández, N.; Bronze, M.R.; Rodríguez-Rojo, S. Pressurized liquid extraction optimization from supercritical defatted olive pomace: A green and selective phenolic extraction process. ACS Sustain. Chem. Eng. 2021, 9, 5590–5602. [Google Scholar] [CrossRef]
- Kimura, T.; Sakamoto, T.; Leveque, J.-M.; Sohmiya, H.; Fujita, M.; Ikeda, S.; Ando, T. Standardization of ultrasonic power for sonochemical reaction. Ultrason. Sonochem. 1996, 3, S157–S161. [Google Scholar] [CrossRef]
- Lakka, A.; Grigorakis, S.; Karageorgou, I.; Batra, G.; Kaltsa, O.; Bozinou, E.; Lalas, S.; Makris, D.P. Saffron processing wastes as a bioresource of high-value added compounds: Development of a green extraction process for polyphenol recovery using a natural deep eutectic solvent. Antioxidants 2019, 8, 586. [Google Scholar] [CrossRef] [Green Version]
- Dourtoglou, V.G.; Mamalos, A.; Makris, D.P. Storage of olives (Olea europaea) under CO2 atmosphere: Effect on anthocyanins, phenolics, sensory attributes and in vitro antioxidant properties. Food Chem. 2006, 99, 342–349. [Google Scholar] [CrossRef]
- Goupy, P.; Vian, M.A.; Chemat, F.; Caris-Veyrata, C. Identification and quantification of flavonols, anthocyanins and lutein diesters in tepals of Crocus sativus by ultra performance liquid chromatography coupled to diode array and ion trap mass spectrometry detections. Ind. Crops. Prod. 2013, 44, 496–510. [Google Scholar] [CrossRef]
- Alibante, A.; Lakka, A.; Bozinou, E.; Chatzilazarou, A.; Lalas, S.; Makris, D.P. Integrated green process for the extraction of red grape pomace antioxidant polyphenols using ultrasound-assisted pretreatment and β-cyclodextrin. Beverages 2021, 7, 59. [Google Scholar] [CrossRef]
- Lakka, A.; Lalas, S.; Makris, D.P. Hydroxypropyl-β-cyclodextrin as a green co-solvent in the aqueous extraction of polyphenols from waste orange peels. Beverages 2020, 6, 50. [Google Scholar] [CrossRef]
- Metivier, R.; Francis, F.; Clydesdale, F. Solvent extraction of anthocyanins from wine pomace. J. Food Sci. 1980, 45, 1099–1100. [Google Scholar] [CrossRef]
- Tzima, K.; Kallithraka, S.; Kotseridis, Y.; Makris, D.P. Kinetic modelling for flavanol extraction from red grape (Vitis vinifera L.) pomace using aqueous organic acid solutions. Int. Food Res. J. 2014, 21, 1919. [Google Scholar]
- Tzima, K.; Kallithraka, S.; Kotseridis, Y.; Makris, D.P. A comparative evaluation of aqueous natural organic acid media for the efficient recovery of flavonoids from red grape (Vitis vinifera) pomace. Waste Biomass Valorization 2015, 6, 391–400. [Google Scholar] [CrossRef]
- Corrales, M.; Toepfl, S.; Butz, P.; Knorr, D.; Tauscher, B. Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: A comparison. Innov. Food Sci. Emerg. Technol. 2008, 9, 85–91. [Google Scholar] [CrossRef]
- Corrales, M.; García, A.F.; Butz, P.; Tauscher, B. Extraction of anthocyanins from grape skins assisted by high hydrostatic pressure. J. Food Eng. 2009, 90, 415–421. [Google Scholar] [CrossRef]
- Sumampouw, G.A.; Jacobsen, C.; Getachew, A.T. Optimization of phenolic antioxidants extraction from Fucus vesiculosus by pressurized liquid extraction. J. Appl. Phycol. 2021, 33, 1195–1207. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).