Abstract
We studied the impact of secondary succession in xerothermic grasslands on a population of Pulsatilla patens, a species of European Community interest. We established two permanent plots with a high number of individuals of P. patens in a xerothermic grassland in Southern Poland. We compared two areas, the first in open grassland (plot A), and the second with overgrowing vegetation (plot B). We assessed the population structure as well as the individual traits of the species. The total abundance of P. patens in the open xerothermic grassland was five times higher than in the overgrowing xerothermic grassland. A randomly clustering distribution was noted only in plot A; in plot B a random type of distribution occurred. The density structure of the rosettes was higher in plot A. The mean number of leaves in rosettes of P. patens as well as dimensions of intermediate stems and leaves of the species is strongly correlated with habitat conditions. The shadowing caused by shrubs and trees and high weeds observed in the overgrowing xerothermic grassland negatively impacted on the number of individuals, distribution, structure and morphology of P. patens.
1. Introduction
Light is a significant factor in the formation of varied plant communities [1,2], as well as playing a fundamental role for different plants species [3,4]. Light provides the energy for the production of organic matter and is responsible for growth effects [5], e.g., seed germination or organ elongation. One of the major factors affecting plant growth is light intensity [6]. The ecological effects of changes in light intensity are most clearly visible in non-forest semi-natural plant communities, i.e., meadows or grasslands [7]. In these communities, changes in access to light for herb species are correlated with secondary succession [8,9]. After the cessation of usage (mowing or grazing), the overgrowing secondary succession species like Rhamnus cathartica and Pinus sylvestris intensify the shadowing [10,11].
This phenomenon was observed in the xerothermic grassland communities that are characteristic of the Festuco-Brometea class. These communities have a high conservation value and they are included in the European Natura 2000 network [12]. In xerothermic grasslands, the amount of light significantly affects the species richness and changes in plant cover. The transformations of xerothermic grasslands are caused mainly by changes in the ways and the intensity of their use, especially the cessation of grazing [13]. Across Europe these communities have been endangered mainly by secondary succession [14], which causes, firstly, overgrowth of gaps, next, the appearance of high hardy perennial plants, and lastly, saplings of shrubs and trees. The overgrowing grassland species are forced to adapt to the changing quality and quantity of light.
The Eastern Pasque Flower is one of the heliophilous species which occurs in xerothermic grasslands characteristic of the Festuco-Brometea class [15,16]. Pulsatilla patens (L.) Mill. ssp. patens (exemplary synonyms: Pulsatilla patens (L.) Mill., Anemone patens L.) is a lowland species of boreo–meridional–continental and circumpolar distribution. Its range covers the sub-polar areas of Europe, Asia and North America [17,18]. In Europe, it occurs in the central and eastern parts. The western border runs across Germany and the Czech Republic [19]. In Poland, the localities of the species are concentrated in the north-eastern part, and its frequency decreases towards the west and south [20].
In many European countries it is a rare and threatened plant species. It has been included in Appendix I of the Bern Convention [21] and in Annexes II and IV to the Habitat Directive of EU [12]. It is also listed in the IUCN List of Threatened Species—DD category [22].
Within its geographical range, P. patens appears in different habitats and types of plant communities, but the species prefers habitats with good access to light—mainly open, dry coniferous forests and different types of grasslands [12,23]. Additionally, in forests, the most favourable conditions P. patens finds are disturbed areas, which allow for better exposure, e.g., gaps in stands, forest edges, near forest paths and clearances. Moreover, in its entire geographical range, many localities are found in well-exposed anthropogenic sites, i.e., pastures, at the edges of yard areas, near roadsides, on forest fringes and clearances in forests, and also on the edges of gravel pits and on almost bare rock pavements (primarily limestone pavements) [12].
Still, the constant disappearance of localities of the Eastern Pasque Flower is observed, as well the diminishing size of its populations [24,25], hence species conservation is of special concern. Due to this fact, it is very important to become acquainted with every ecological factor which can impact its decreasing populations.
Among the major factors threatening the species are the destruction and fragmentation of habitats, continuously decreasing forest areas, eutrophication of habitat and also cessation of grazing [23,26,27]. Sometimes the reasons for the decline are unknown and named as a ‘biocoenotic evolution’—but we still do not know which particular factor impacts this process and what are the symptoms of the disappearance of the species.
Considering this insufficient state of knowledge, the present investigations—aiming at an assessment of the impact of secondary succession on the population of this threatened species in a new, abundant location in Poland—were undertaken.
Our hypothesis is that secondary succession in the xerothermic grassland (connected with a reduction in the intensity of light) is one of the main factors that negatively impacts on the number of individuals, distribution, age, structure and morphology of Pulsatilla patens.
The main goal of the study was to show the impact of the habitat conditions (coverage, light intensity, species composition and selected soil characteristics) on the population and individual traits of the Eastern Pasque Flower occurring in permanent plots established within two different successional stages (open and overgrowing) in the xerothermic grassland.
2. Materials and Methods
2.1. Study Area
The study area is located on the Chęciny Hills, a region situated in Southern Poland (Central Europe). The climate of the region is temperate continental; the mean annual air temperature is 7.4 °C (the average temperature in January is 2.9 °C; in July, 17.2 °C). The annual number of days with snow cover varies from 60 to 84. The mean annual precipitation is 600 mm. This area is formed of Middle Devonian limestones and dolomites or Upper Jurassic limestones that cover the Lower Cambrian slates. The prevailing soil type is rendzina (shallow alkaline or neutral soils). The agricultural landscape predominates, however, in some parts of the area the mining landscape is also clearly marked. Semi-natural xerothermic grasslands of the Festuco-Brometea class have developed on the highest parts of deforested hills (mainly on the southern and south-western slopes) as a result of human agro-pastoral activity. After abandoning the traditional management practices, large areas of grasslands are overgrown by thermophilous shrubs communities, or they have been reforested with Pinus sylvestris. Small patches of xerothermic grasslands have been preserved in the vicinity of carbonate rock quarries [15].
Pulsatilla patens grows in a xerothermic grassland between two active limestone and dolomite quarries. This phytocoenosis covers about 0.2 ha and is surrounded by a pine forest. Halfway, the grassland has not been managed during at least 30 years (owner information), and it is overgrown with Pinus sylvestris, Rhamnus cathartica and Frangula alnus.
2.2. Field Sampling
Fieldwork was carried out in the growing season of the year 2020 in the xerothermic grassland (50°47’11” N, 20°33’51” E) where Pulsatilla patens ssp. patens occurs. In this habitat, two permanent study plots (with the dimensions 5 m × 5 m) were established in two different structures of this community: open xerothermic grassland (plot A) and overgrowing xerothermic grassland (plot B). Both plots were located next to each other (at a distance of 10 m), occupy the same geological background, and had the same exposure (south) and slope (15°).
In each plot, the following parameters about the population structure of P. patens were surveyed: (i) total abundance—number of individuals (leaf rosettes), (ii) distribution and density and (iii) age structure (share of juvenile, vegetative and generative individuals).
Then, we measured the following traits for every individual rosette: (i) the number of leaves, (ii) the height of the lowest and the highest leaf, (iii) the width of the largest and the smallest leaf blade and (iv) the length of the greatest and the lowest lobe of the leaf.
To determine the floristic composition, the structure and plant cover of the grassland in every plot, phytosociological relevés using the Braun-Blanquet method [28] were made (one in each permanent plot). In each relevé, the cover-abundance of each species was scored using the Braun-Blanquet scale (+ < 5%, 1 = 5%, 2 = 5–25%, 3 = 25–50%, 4 = 50–75% and 5 = 75–100%). In addition, the height of the neighbouring plants was studied in each plot on the basis of the 10 lowest and 10 highest stems [29].
Additionally, in the two study plots, the light conditions (illuminance) were measured in the centre of each plot using a hand-held light meter (Digital Lux Meter, Model: GM 1020L-50, Białystok, Poland), in lux (lx)—SI unit of illumination equal to a luminous flux of 1 lumen per square metre, where 1000 lx = 1 kilolux (klx). During the measurement, the light meter sensor was aligned directly upwards, detecting the amount of light reaching the sensor from the entire sky hemisphere [30]. Two locations were within a distance of 10 m, so the time between the light measurement did not exceed 5 minutes [31]. The measurements were done every week for four weeks. Three repetitive replicates were taken for each plot. In total, we collected 24 measurements for both plots.
Moreover, within the border of each plot, three substrate samples (mineral material) were taken from a depth of 2–30 cm (in the beginning, the middle and the end of each plot) using a soil auger (three soils for one plot for a total of six soil samples), in order to analyse the basic soil characteristics.
2.3. Data Analysis
Chemical analyses were made from the collected soil samples, using methods generally accepted in the field of soil science. We determined the following parameters: the pH, KCl and Ca (calcium), Mg (magnesium), K (potassium) and P (phosphorus) availability content.
The non-parametric Mann–Whitney U test was applied to check whether there were significant differences between two plots in terms of: i) studied individual traits of Pulsatilla patens ssp. patens, ii) height of stems of the highest and the lowest species growing in the vicinity of Pulsatilla patens ssp. patens, iii) intensity of light and iv) soil characteristics.
The chi-square test was applied to establish whether significant differences existed among the two study plots in terms of some parameters of the structure of the populations.
All the results of the conducted analyses were considered as statistically significant at p ≤ 0.05.
The nomenclature of the species was used according Mirek et al. [32], and syntaxa names according to Mucina et al. [33]. The analyses were carried out using Statistica 6.1 [34].
3. Results
3.1. The Habitat Conditions
The chemical analyses of soil samples showed that there were no differences between both plots in terms of the soil properties. The plots are located on the same soil type and the soil properties showed no difference between the two plots (Table 1). This suggests that the differences between the population structure and individual traits are connected with other factors (cf. Methods section).

Table 1.
Mean (range) of some habitat conditions (height of plants, light, properties of soil) in two study plots: open xerothermic grassland (A) and overgrowing xerothermic grassland (B).
The two studied plots were located in two different structures of xerothermic grassland. The statistical analysis (the Mann–Whitney U test) confirmed that the mean light conditions differed significantly between the study plots (U = 2.88, p ≤ 0.001). In the open xerothermic grassland (plot A), the light had almost 100 times higher value than in plot B (Table 1). Different light conditions may have influenced changes in the structure of the xerothermic grassland, i.e., percentage cover, floristic composition, as well as impacted the structure of the herbaceous plants (including Pulsatilla patens).
The analysis of the floristic composition of the communities indicated that it is xerothermic grassland of the Festuco-Brometea class (Table 2). However, the structure of the phytocoenosis differs in the two plots. Plot A showed a single herbaceous layer (Table 2, relevé 1) with 34 plant species. Some gaps in the cover (85%) are typical of xerothermic grasslands. Sixteen thermophilic species characteristics of the Festuco-Brometea and Trifolio medii-Geranietea sanguinei classes were noted in the investigated patch (Table 2, relevé 1). Pulsatilla patens occupied a 25% area of the whole plot.

Table 2.
Floristic composition of the two studied plots of Pulsatilla patens: open xerothermic grassland (relevé 1) and overgrowing xerothermic grassland (relevé 2). To show the percentage cover of species, the Braun-Blanquet scale is used (+ < 5%, 1 = 5%, 2 = 5–25%, 3 = 25–50%; Ch—species characteristic for respective syntaxa, All.—alliance, Ass.—association, Cl.—class, O.—order [28].
Plot B showed a different structure (Table 2, relevé 2). The phytocoenosis is structured in a three-layer community and follows an evident secondary succession process. The tree layer was built of Pinus sylvestris. The shrub layer occupied ca. 65% of the whole plot. In this layer, Rhamnus cathartica prevailed. In the herb layer, 32 species were noted (including 17 thermophilic species characteristics of the Festuco-Brometea and Trifolio medii-Geranietea sanguinei classes—Table 2, relevé 2). The total coverage of the herb layer was 100% and no gaps were observed. In this plot, Pulsatilla patens had the lowest percentage cover of all plots (ca. 5%).
The differences between the two studied plots were also noted in the mean height (range) of stems of the herbaceous plants (Table 1). The mean height of the stems of the highest herbaceous plants on plot A had a medium value—53.4 cm. On plot B, the value was the highest and amounted to 98.9 cm. Additionally, the mean height of the stems of the lowest herbaceous plants was different between the plots. The statistical analysis confirmed that the height of the herbaceous plants differed significantly between the study plots in the case of the tallest plant species, as well as the lowest ones (Table 1).
3.2. The Population Structure
The total abundance of individuals (leaf rosettes) in the open xerothermic grassland (plot A, 40) is five times higher than in the overgrowing xerothermic grassland (plot B, 8). The distribution of leaf rosettes in plot A is randomly clustering and in plot B it is a random type of distribution (Figure 1).
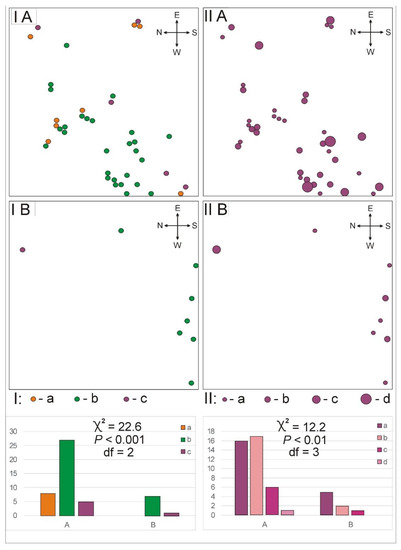
Figure 1.
Some parameters of the structure of population of Pulsatilla patens in two study plots: (A) open xerothermic grassland and (B) overgrowing xerothermic grassland. I—distribution and age structure of individuals (a—juvenile, b—vegetative, c—generative); II—distribution and density of leaf rosettes (number of leaves in rosettes: a—1–5, b—6–10, c—11–15, d—16–20).
Additionally, the density structure of the rosettes of Pulsatilla patens differed significantly between the study plots (χ2 = 12.2, p < 0.01, df = 3). Leaf rosettes showed from 6 to 10 leaves in plot A, whereas only from 1 to 5 leaves in plot B. Moreover, only in the open xerothermic grassland the species occurred in every four of the studied ranges (Figure 1).
The difference between the plots occurs also on the basis of the age structure of the P. patens. Juvenile individuals were only noted in plot A. The share of the juvenile, vegetative and generative individuals between A and B plot differed significantly (χ2 = 12.2, p < 0.01, df = 3) (Figure 1).
3.3. The Traits of Individuals
The mean number of leaves in the rosettes was higher in the open xerothermic grassland (plot A), than in the overgrowing xerothermic grassland (plot B), but the difference was not statistically significant (Table 3). The investigations of leaf dimensions showed significant differences between the two plots occurring in the differing structure of xerothermic grassland and they showed that a shadowing caused by a process of secondary succession brought increased height and width to the leaves (Table 3).

Table 3.
Mean (range) of traits of leaves of Pulsatilla patens in permanent plots established in the open xerothermic grassland (A) and overgrowing xerothermic grassland (B).
4. Discussion
Xerothermic grasslands are among the ecosystems currently undergoing the most profound transformation [35,36]. These phytocoenoses are threatened by advancing succession in many countries [36,37]. One of the results of succession is the disappearance of heliophilous species and the formation of shrubs and forest species more adapted to poor light conditions [38,39]. The overgrowing xerothermic grassland on plot B, where the lack of agricultural use was observed for more than 30 years, was transformed into a three-layer association dominated by tall herbaceous plants with shrubs and trees (Table 2). The emergence of shrub and trees resulted in shading, which reduced the amount of available light (Table 1), and at the same time impacted the herb layer.
In this grassland, the species characteristic of the Festuco-Brometea class still occur, but they are over two times higher compared to the open xerothermic grassland on plot A (Table 1). Additionally, the herb layer is more compacted compared to the herb layer from plot A. In plot B, there were no gaps which contributed to the recruitment and recovery of numerous grassland species because of better light conditions [40,41]. Lack of gaps and more compacted herb layer influences the structure of Pulsatilla patens—on overgrowing grassland, no juvenile individuals occurred; the total abundance of species was 5 times lower, compared with open grassland; in distribution type, no clusters were noted, and the leaf rosettes were smaller than in the open xerothermic grassland (Figure 1).
The reduced access of light also impacted the individual traits of Pulsatilla patens. The presence of significantly longer and wider leaves of Pulsatilla patens in the overgrowing xerothermic grassland is presumably caused by the greater height of the neighbouring plants (Table 1). Such a phenomenon has previously been observed by many authors—in different semi-natural phytocoenoses and with different species, e.g., Iris sibirica, Dianthus superbus or Gladiolus imbricatus [29,42,43]. All of these species show a similar scenario, allowing plants to outperform their neighbours and contributing to more effective light interception, as well as possibly improving flower visibility for pollinators and seed dispersal [29].
Pulsatilla patens in the open xerothermic grassland (plot A) had the most typical structure—distribution, age structure and density [44,45,46]—with the medium values of the investigated leaf and stem dimensions (Figure 1, Table 3).
The chemical analyses of soil samples showed that there were no statistical differences between both plots in terms of the soil properties (Table 1). However, it should be pointed out that in the overgrowing xerothermic grassland, a slightly lower pH was observed, which is probably caused by the presence of Pinus sylvestris, whose needles acidify the topsoils [47] and it may have influenced some characteristics of specimens in the population [48]. According to some scientists, the degree of soil acidification is the major variable determining the size of populations and size of the individuals [48,49].
The dominance of vegetative individuals of the Eastern Pasque Flower observed in our plots is similar to other localities from different areas where the vegetative individuals prevailed [50]. P. patens has a long life cycle that may last for several decades, which leads to the formation of compact clumps [46]. The great number of leaves in rosettes and the width of the leaf blades in clumps of our study population suggests a long cycle of life of species in the xerothermic grassland and that we have to deal with an old population that needs protection.
5. Conclusions
- The total abundance of Pulsatilla patens in the open xerothermic grassland is five times higher than in the overgrowing xerothermic grassland, (40:8 leaf rosettes, respectively).
- A randomly clustering distribution of P. patens was noted only in the open xerothermic grassland (in the overgrowing xerothermic grassland a random type of distribution occurred).
- The density structure of the rosettes of P. patens differed significantly between the study plots. Leaf rosettes showed from 6 to 10 leaves in the open xerothermic grassland, whereas only from 1 to 5 leaves in the overgrowing xerothermic grassland.
- The difference on the basis of age structure of the P. patens shows that only in the open xerothermic grassland juvenile individuals were noted.
- The mean number of leaves in the rosettes was higher in the open xerothermic grassland, but the leaf dimensions were higher in the overgrowing xerothermic grassland (a shadowing caused by a process of secondary succession increased the height and width of the leaves).
The long-term changes observed in an overgrowing xerothermic grassland (shadowing caused by shrubs and trees and high weeds, increased of percentage cover and the height of plants) impacted the structure and individual traits of the Pulsatilla patens.
Within the range of P. patens, abandoned grazing has caused overgrowth in several habitats and caused the species to be in alarming decline for decades, and now it is critically endangered. We hope that the results of our research—impact of the different successional stages of xerothermic grassland on the structure and individual traits of population of the Eastern Pasque Flower—can help us find a way to better protect the species.
Author Contributions
Conceptualization, M.P.; methodology, M.P.; formal analysis, M.P.; investigation, M.P. and G.Ł.; resources, M.P and G.Ł.; writing—original draft preparation, M.P. and G.Ł; writing—review and editing, M.P.; visualization, M.P.; supervision, M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Polish Ministry of Science and Higher Education (Research Project no. SUPB.RN.21.245). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bartemucci, P.; Messier, C.H.; Canham, C.H.D. Overstory influences on light attenuation patterns and understory plant community diversity and composition in southern boreal forests of Quebec. Can. J. For. Res. 2006, 36, 2065–2079. [Google Scholar] [CrossRef]
- Domke, G.M.; Caspersen, J.P.; Jones, T.A. Light attenuation following selection harvesting in northern hardwood forests. For. Ecol. Manag. 2007, 239, 182–190. [Google Scholar] [CrossRef]
- Kostrakiewicz, K. The influence of shadow created by adjacent plants on phenotypic plasticity of endangered species Trollius europaeus L. (Ranunculacae). Pol. J. Ecol. 2009, 57, 625. [Google Scholar]
- Zervoudakis, G.; Salahas, G.; Kaspiris, G.; Konstantopoulou, E. Influence of light intensity on growth and physiological characteristics of common sage (Salvia officinalis L.). Braz. Arch. Biol. Technol. 2012, 55, 89–95. [Google Scholar] [CrossRef]
- Navvab, M. Daylighting aspects for plant growth in interior environments. Light Eng. 2009, 17, 46–54. [Google Scholar]
- Rezazadeh, A.; Harkess, R.L.; Telmadarrehei, T. The Effect of light intensity and temperature on flowering and morphology of potted red firespike. Horticulturae 2018, 4, 36. [Google Scholar] [CrossRef]
- Li, J.; Zheng, Z.; Xie, H.; Zhao, N.; Gao, Y. Increased soil nutrition and decreased light intensity drive species loss after eight years grassland enclosures. Sci. Rep. 2017, 7, 44525. [Google Scholar] [CrossRef] [PubMed]
- Poptcheva, K.; Schwartze, P.; Vogel, A.; Kleinebecker, T.; Holzel, N. Changes in wet meadow vegetation after 20 years of different management in a field experiment (North-West Germany). Agric. Ecosyst. Environ. 2009, 134, 108–114. [Google Scholar] [CrossRef]
- Michalska-Hejduk, D.; Kopeć, D. Dynamics of semi-natural vegetation with a focus on Molinion meadows after 50 years of strict protection. Pol. J. Environ. Stud. 2012, 21, 1731–1741. [Google Scholar]
- Marriott, C.A.; Hood, K.; Fisher, J.M.; Pakeman, R.J. Long-term impacts of extensive grazing and abandonment on the species composition, richness, diversity and productivity of agricultural grassland. Agric. Ecosyst. Environ. 2009, 134, 190–200. [Google Scholar] [CrossRef]
- Ignatavičius, G.; Sinkevičius, S.; Ložytė, A. Effects of grassland management on plant communities. Ekologija 2013, 59, 99–110. [Google Scholar] [CrossRef]
- European Communities (Ed.) Council Directive 92/43/EEC of 21 May 1992 on the Conservation of Natural Habitats and of Wild Fauna and Flora. 2009. Available online: http://ec.europa.eu/environment/nature/legislation/habitatsdirective/index_en.htm (accessed on 15 August 2020).
- Liess, A.; Lange, K.; Schulz, F.; Piggott, J.J.; Matthaei, C.D.; Townsend, C.R. Light, nutrients and grazing interact to determine diatom species richness via changes to productivity, nutrient state and grazer activity. J. Ecol. 2009, 97, 326–336. [Google Scholar] [CrossRef]
- Poschlod, P.; Bakker, J.P.; Kahmen, S. Changing land use and its impact on biodiversity. Basic Appl. Ecol. 2005, 6, 93–98. [Google Scholar] [CrossRef]
- Łazarski, G. Protected, rare and endangered vascular plant species in the Chęciny Hills and Dyminy Range (Małopolska Upland)—part I. Grassland and thermophilous fringe species. Fragm. Florist. Geobot. Pol. 2019, 26, 49–73. [Google Scholar]
- Łazarski, G.; Podgórska, M. New and highly threatened locality of Pulsatilla patens subsp. patens (Ranunculaceae) in the Świętokrzyskie Mountains (Małopolska Upland). Fragm. Florist. Geobot. Pol. 2020, 27, 527–535. [Google Scholar]
- Meusel, H.; Jäger, E.; Weinert, E. Vergleichende Chorologie der Zentraleuropäischen Flora; Karten. Fischer: Jena, Germany, 1965. [Google Scholar]
- Hulten, E.; Fries, M. Atlas of North European Vascular Plants North of the Tropic of Cancer; Koeltz: Konigstein, Germany, 1986. [Google Scholar]
- Tutin, T.G.; Akeroyd, J.R. Pulsatilla Miller. In Flora Europaea, Vol. 1: Psilotaceae to Platanaceae, 2nd ed.; Tutin, T.G., Burges, N.A., Chater, A.O., Edmondson, J.R., Heywood, V.H., Moore, D.M., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1993; pp. 264–266. [Google Scholar]
- Zając, A.; Zając, M. Distribution Atlas of Vascular Plants in Poland; Laboratory of Computer Chorology, Institute of Botany, Jagiellonian University: Kraków, Poland, 2001. [Google Scholar]
- Council of Europe. Convention on the Conservation of European Wildlife and Natural Habitats. 1979. Available online: http://conventions.coe.int/Treaty/en/Treaties/Word/104.doc (accessed on 15 August 2020).
- Bilz, M. Pulsatilla patens. The IUCN Red List of Threatened Species 2011: E.T165908A6162193. 2011. Available online: https://www.iucnredlist.org/species/165908/6162193 (accessed on 15 August 2020).
- Kalliovirta, M.; Ryttäri, T.; Heikkinen, R.K. Population structure of a threatened plant, Pulsatilla patens, in boreal forests: Modelling relationships to overgrowth and site closure. Biodivers. Conserv. 2006, 15, 3095–3108. [Google Scholar] [CrossRef]
- Podgórska, M.; Bróż, E. The extinction of stations of Pulsatilla patens (L.) Mill. subsp. patens (American pasqueflower) on the Małopolska Upland. Acta Soc. Bot. Pol. Suppl. 2010, 79, 53. [Google Scholar]
- Łazarski, G.; Podgórska, M.; Bróż, E. Pasque flowers at the Natura 2000 Site Wzgórza Chęcińsko- Kieleckie and its vicinity—distribution and conservation status. Chrońmy Przyr. Ojczysta 2018, 74, 37–51. [Google Scholar]
- Uotila, P. Decline of Anemone patens (Ranunculaceae) in Finland. Acta Univ. Ups. Symb. Bot. Ups. 1996, 31, 205–210. [Google Scholar]
- Pilt, I.; Kukk, Ü. Pulsatilla patens and Pulsatilla pratensis (Ranunculaceae) in Estonia: Distribution and ecology. Proc. Nat. Acad. Sci. USA 2002, 51, 242–256. [Google Scholar]
- Braun-Blanquet, J. Pflanzensoziologie, Grundzüge der Vegetationskunde [Plant Sociology, Basics of Vegetation Science], 3rd ed.; Springer: Berlin/Heidelberg, Germany, 1964. [Google Scholar] [CrossRef]
- Kostrakiewicz-Gierałt, K.; Podgórska, M. Regeneration of the rare meadow species Iris sibirica in a postcultural land. Bot. Lett. 2020, 167, 331–339. [Google Scholar] [CrossRef]
- Jarzyna, K.; Podgórska, M.; Szwed, M.; Jóźwiak, M. A simple light meter as a device for studying the influence of seasonal changes of light conditions on the phenology of herbaceous undergrowth species in a fertile beach forest. Balt. For. 2018, 249, 148–157. [Google Scholar]
- Comeau, P.G. Measuring Light in a Forest; Extension Note 42; British Columbia Ministry of Forest, Research Branch: Vancouver, BC, Canada, 2000. [Google Scholar]
- Mirek, Z.; Piękoś-Mirkowa, H.; Zając, A.; Zając, M. Vascular Plants of Poland. An Annotated Checklist; W. Szafer Institute of Botany PAS: Kraków, Poland, 2020; p. 526. [Google Scholar]
- Mucina, L.; Bültmann, H.; Dierßen, K.; Theurillat, J.P.; Raus, T.; Čarni, A.; Šumberová, K.; Willner, W.; Dengler, J.; García, R.G.; et al. Vegetation of Europe: Hierarchical floristic classification system of vascular plant, bryophyte, lichen and algal communities. Appl. Veg. Sci. 2016, 19, 3–264. [Google Scholar] [CrossRef]
- StatSoft. STATISTICA (Data Analysis Software System), Version 6.1.; StatSoft, Inc.: Tulsa, OK, USA, 2003. [Google Scholar]
- Valkó, O.; Żmihorski, M.; Biurrun, I.; Loos, J.; Labadessa, R.; Venn, S. Ecology and conservation of steppes and semi-natural grasslands. Hacquetia 2016, 15, 5–14. [Google Scholar] [CrossRef]
- Valkó, O.; Venn, S.; Żmihorski, M.; Biurrun, I.; Labadessa, R.; Loos, J. The challenge of abandonment for the sustainable management of Palaearctic natural and semi-natural grasslands. Hacquetia 2018, 17, 5–16. [Google Scholar] [CrossRef]
- Dzwonko, Z. Effect of changes in land use during the 20th century on woodland and calcareous grassland vegetation in southern Poland. Acta Univ. Lodz Folia Biol. Oecologica 2011, 7, 27–48. [Google Scholar] [CrossRef]
- Bąba, W. Changes in the structure and floristic composition of the limestone grasslands after cutting trees and shrubs and mowing. Acta Soc. Bot. Pol. 2003, 72, 61–69. [Google Scholar] [CrossRef]
- Bąba, W. The species composition and dynamics in well-preserved and restored calcareous xerothermic grasslands (South Poland). Biol. Bratisl. 2004, 59, 447–456. [Google Scholar]
- Bąba, W.; Kompała-Bąba, A. Do small-scale gaps in calcareous grassland swards facilitate seedling establishment? Acta Soc. Bot. Pol. 2005, 74, 125–131. [Google Scholar] [CrossRef]
- Dzwonko, Z.; Loster, S. Changes in plant species composition in abandoned and restored limestone grasslands—the effects of tree and shrub cutting. Acta Soc. Bot. Pol. 2008, 77, 67–75. [Google Scholar] [CrossRef][Green Version]
- Kostrakiewicz-Gierałt, K. The size structure of ramets in Dianthus superbus L. in mosaic meadow vegetation. Acta Agrobot. 2013, 66, 23–30. [Google Scholar] [CrossRef][Green Version]
- Kubíková, P.; Zeidler, M. Habitat demands and population characteristics of the rare plant species Gladiolus imbricatus L. in the Frenštát region (NE Moravia, the Czech Republic). Čas. Slez. Zemského Muz. Sér. A 2011, 60, 154–164. [Google Scholar] [CrossRef][Green Version]
- Juśkiewicz-Swaczyna, B. Population structure of Pulsatilla patens in relation to the habitat quality. Tuexenia 2010, 30, 457–466. [Google Scholar]
- Ciosek, M.T.; Piórek, K.; Sikorski, R.; Trębicka, A. Population dynamics of Pulsatilla patens (L.) Mill. in a new locality in Poland. Biodivers. Res. Conserv. 2016, 41, 61–68. [Google Scholar] [CrossRef]
- Kricsfalusy, V. Variations in the life cycle of Anemone patens L. (Ranunculaceae) in wild populations of Canada. Plants 2016, 5, 29. [Google Scholar] [CrossRef]
- Podgórska, M. The long-term changes of forest communities as an effect of former iron-ore mining activities and current forest management: Importance for local biodiversity. Pol. J. Ecol. 2016, 64, 35–44. [Google Scholar] [CrossRef]
- Sienkiewicz, A.; Łaska, G. Application of Bayesian networks in evaluation of current status and protection of Pulsatilla patens (L.) Mill. Ecosphere 2021, 12, e03337. [Google Scholar] [CrossRef]
- Sultangazina, G.J.; Kuprijanov, O.A.; Kuprijanov, A.N.; Beyshov, R.S. Coenoflora Pulsatilla patens (L.) Mill. s. l. in northern Kazakhstan. Bull. Natl. Acad. Sci. Repub. Kazakhstan 2019, 380, 83–92. [Google Scholar] [CrossRef]
- Juśkiewicz-Swaczyna, B.; Choszcz, D. Effect of habitat quality on the structure of populations of Pulsatilla patens (L.) Mill. (Ranunculaceae)—Rare and endangered species in European flora. Pol. J. Ecol. 2012, 60, 567–576. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).