Red Mud-Blast Furnace Slag-Based Alkali-Activated Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection and Characterization of the Raw Materials
2.2. Samples Characterisation
2.3. Preparation of the Samples
3. Results
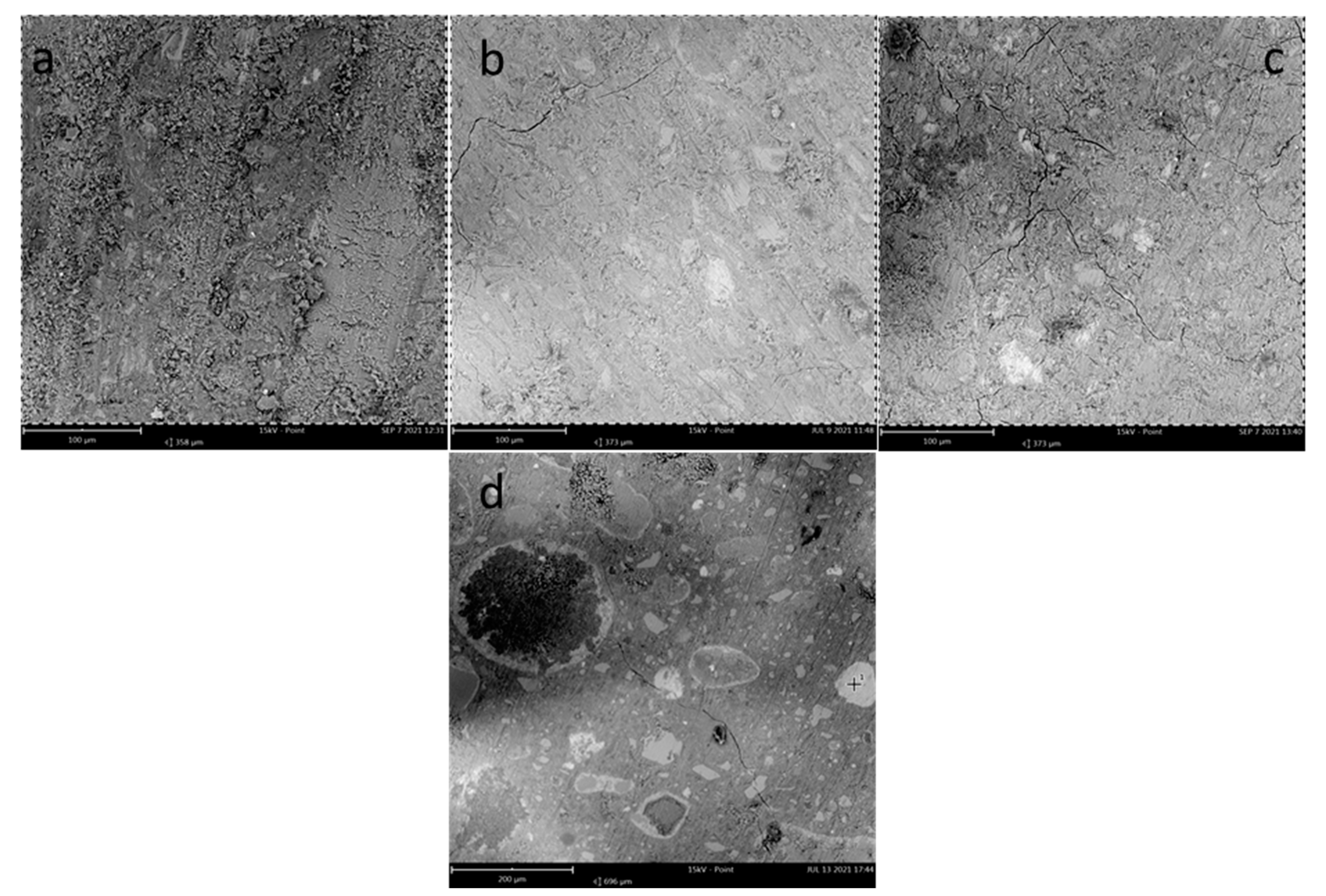
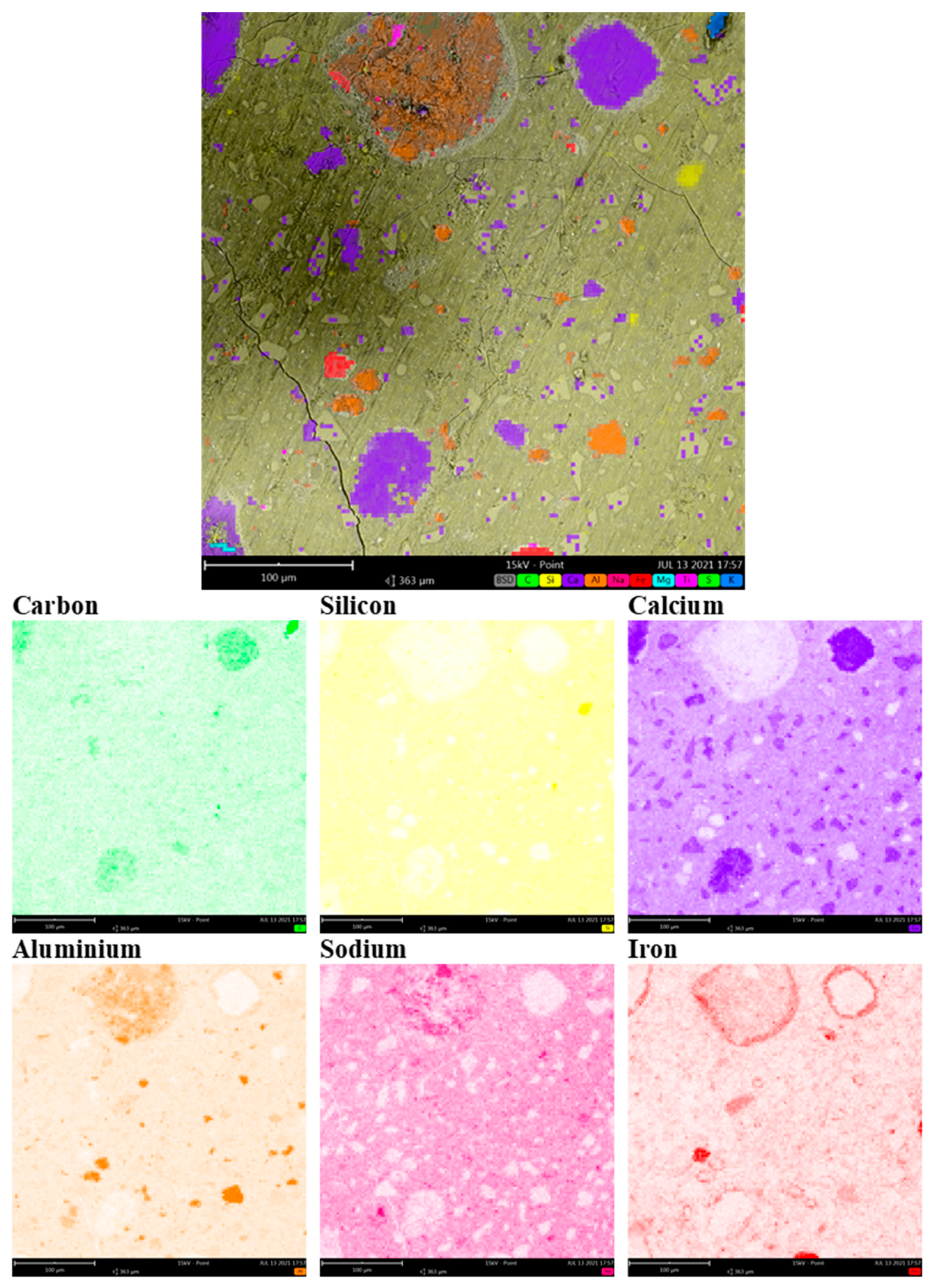
3.1. SEM/EDS Analysis
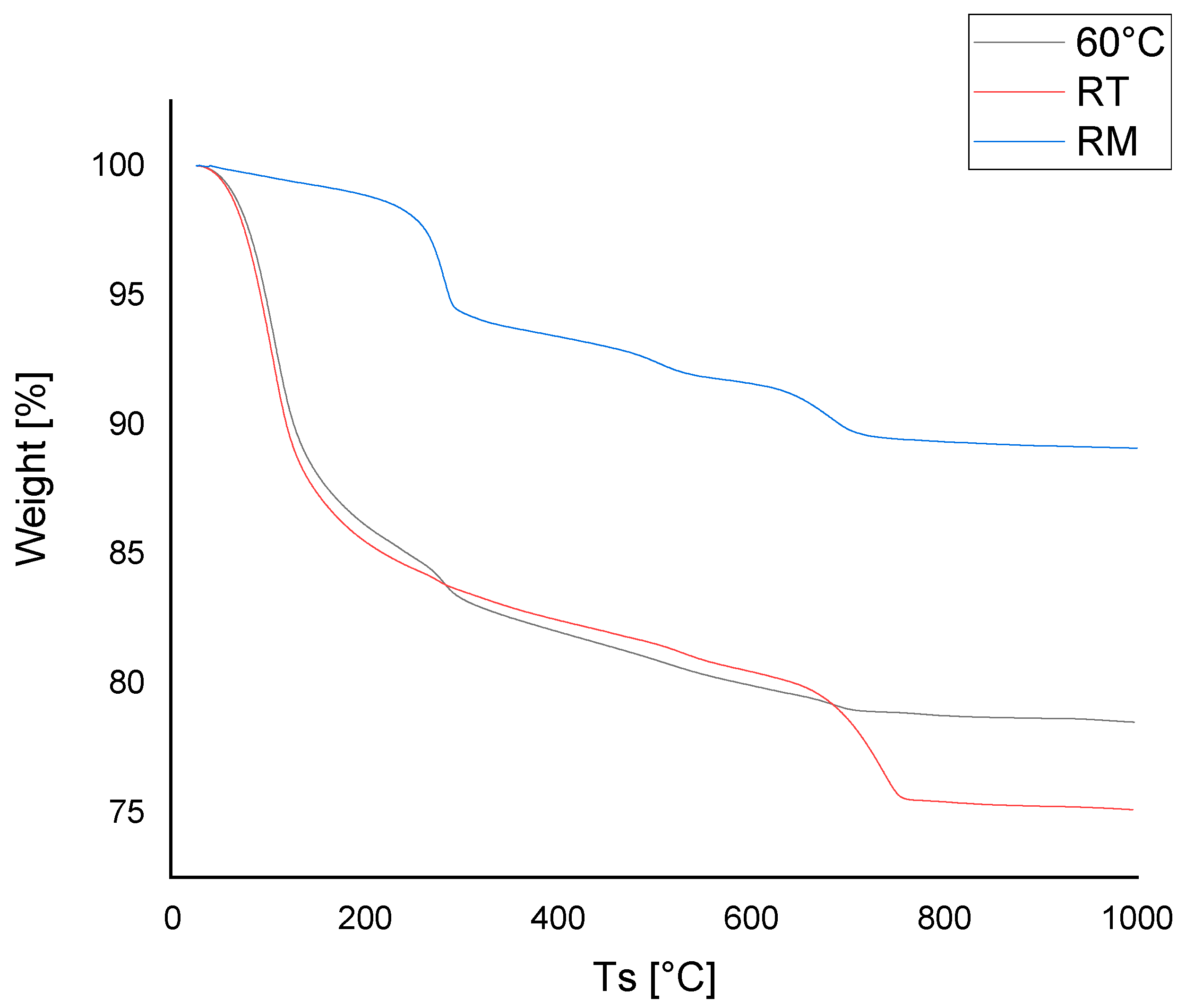
3.2. TGA
- The curing conditions did not significantly affect the behavior of the final product. In fact, the amount of water in the samples after 28 days was almost the same. In both cases, a weight loss of 14 to 15 wt.% (appreciable from the curves in the temperature range 25–220 °C) due to the water still present in the sample, with an inflection point around 105 °C, was observed;
- A second weight loss of approximately 2 wt.% was recorded in the temperature range 220–400 °C, centered at 283 °C, which could be attributed to the loss of crystallization water of the hydroxides. In this range of temperature goethite and gibbsite (see XRD pattern) can lose water to transform into hematite [35,36] and boehmite, respectively, as occurred also in dried RM sample;
- Further 2 wt.% in weight was lost in the temperature range 400–580 °C, centered at 500 °C in all the samples and is likely related to the boehmite transformation into corundum (Al2O3) [37];
- A significant difference between the two mixtures was due to a different percentage of carbonate species. A marked weight loss in the temperature range 600–800 °C, typical of calcination (inflection point at 735 °C), was observed in the sample cured at RT. This phenomenon was related to the presence of calcium/sodium hydroxides in excess which carbonate during the curing and drying period, reacting with atmospheric CO2. The sample cured at the higher temperature showed a lower carbonate amount compared to the RT treated sample. This suggested that in the sample cured at 60 °C the alkali–activation reaction involved a higher amount of hydroxides, creating a higher amount of amorphous phase.
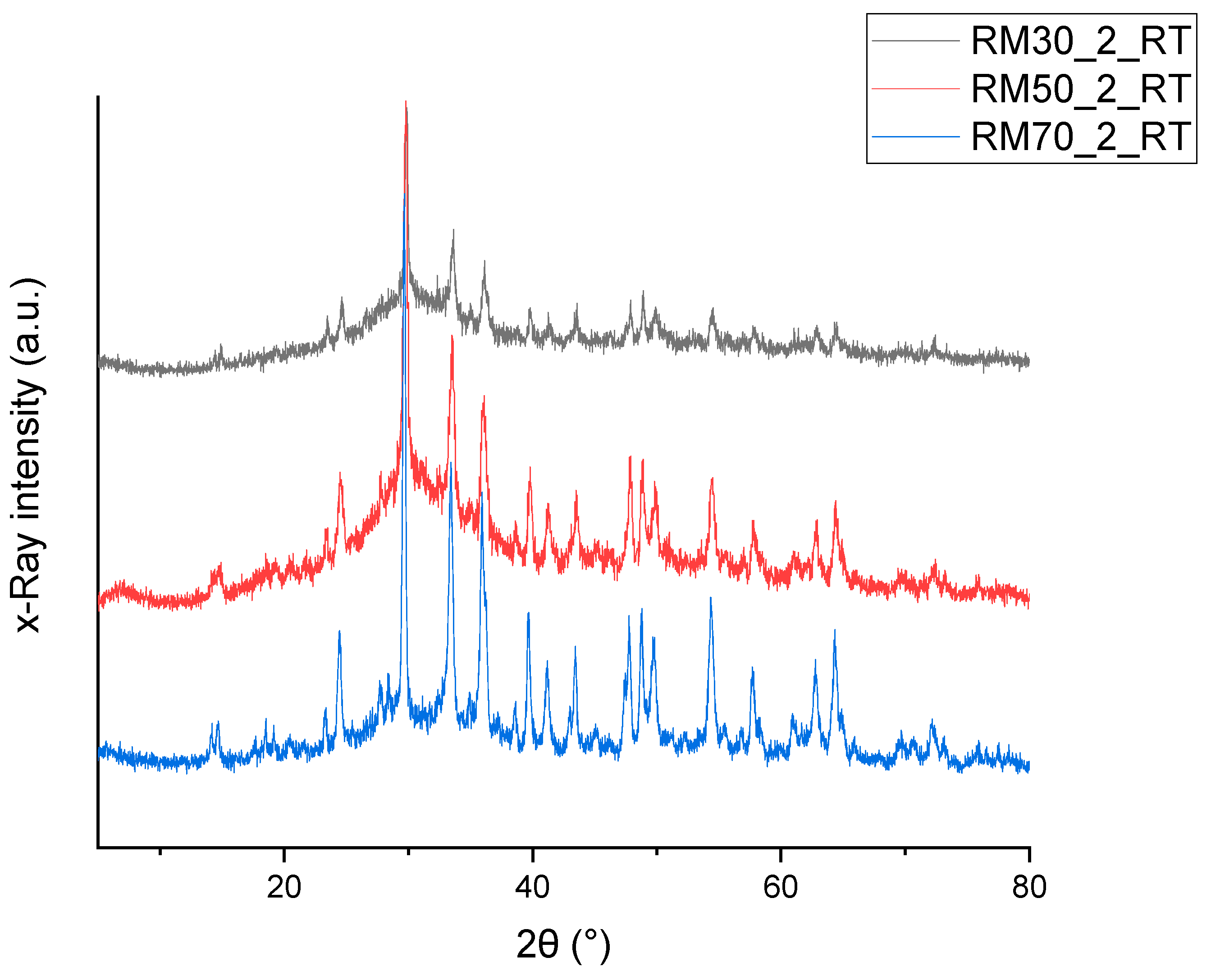
3.3. XRD
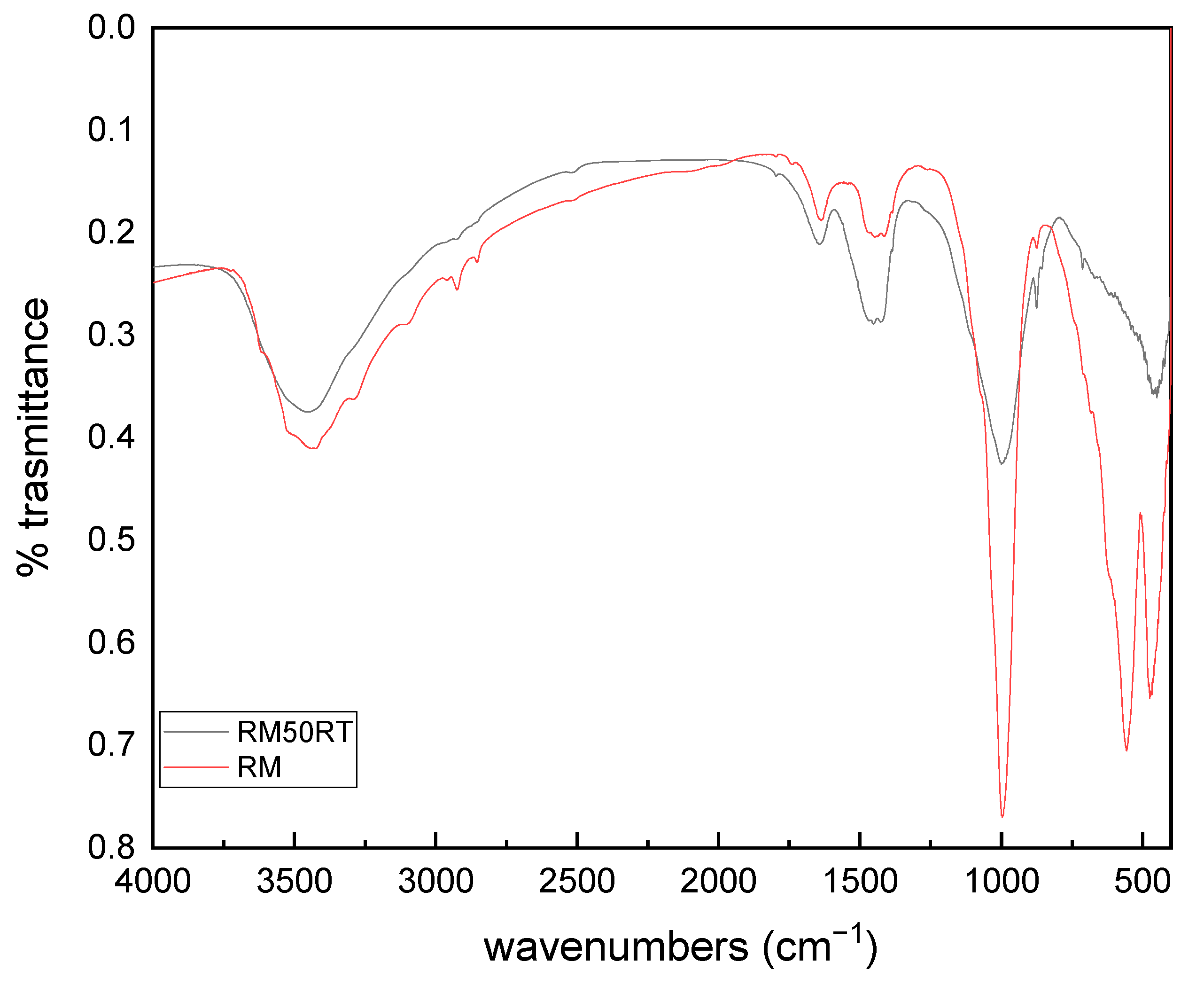
3.4. FTIR
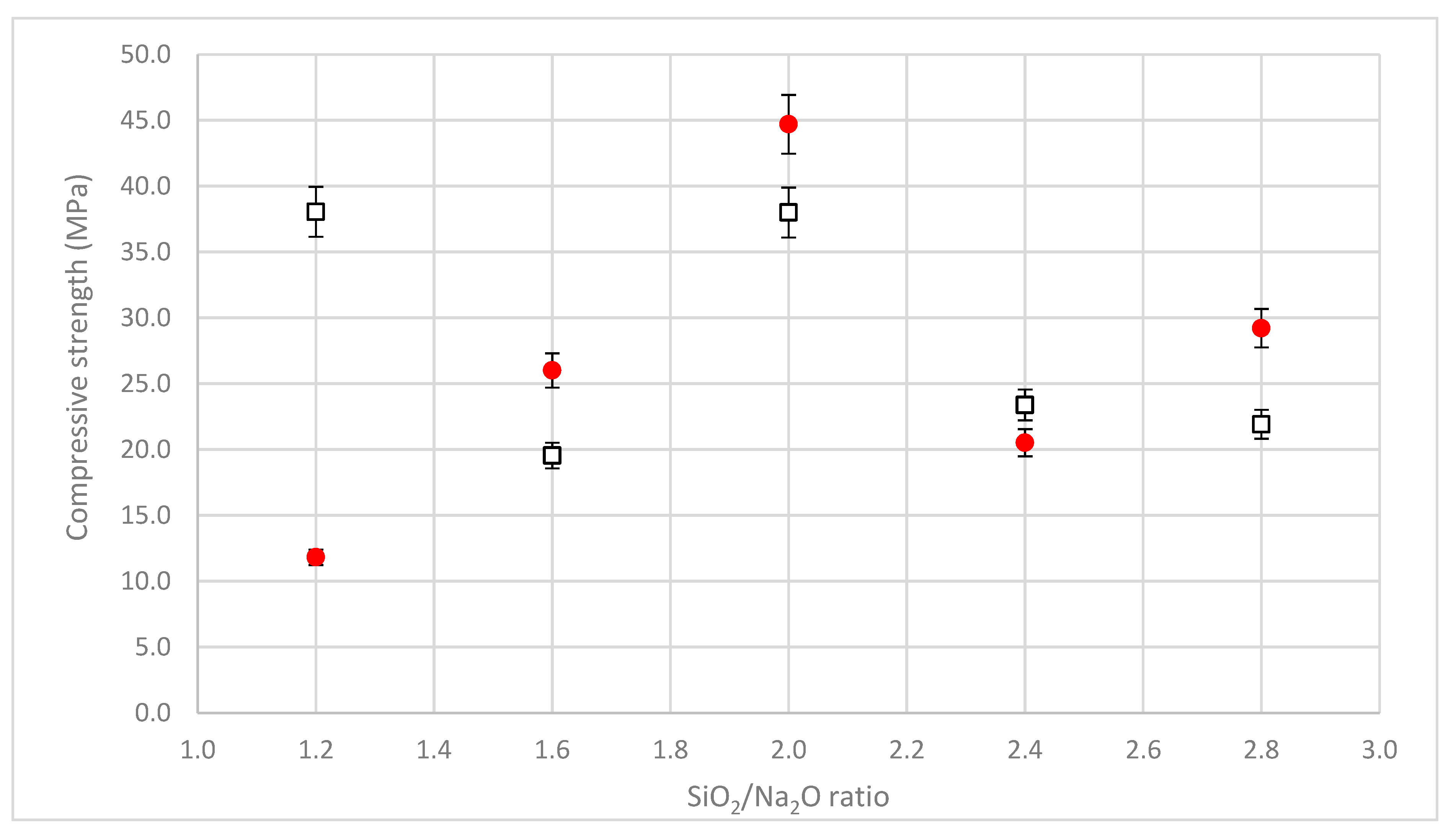
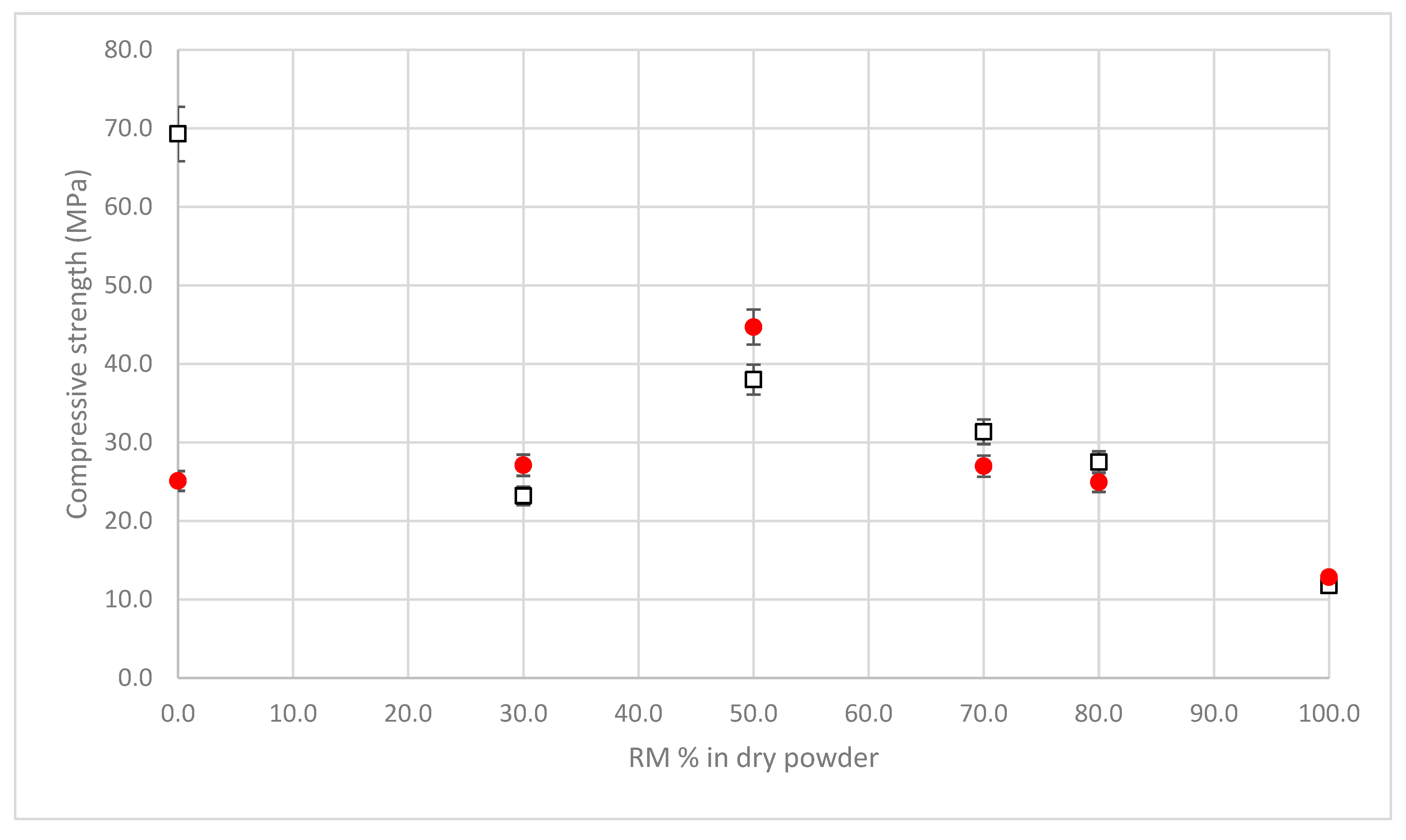
3.5. Compressive Strength
- The formation of C(A)SH gel, due to the BFS precursor;
- The formation of Si-O-Al and, in less part, of Si-O-Fe phases, due to the presence of amorphous silicon and aluminum oxides in the two precursors (BFS and RM), activated by SS and sodium hydroxide [42].
- With higher pH alkaline solution, the dissolution of silicon from the raw material and the formation of CSH or CASH gel was favored, limiting geopolymerization;
- Decreasing the amount of NaOH in the activating solution, the quantity of free calcium decreased [43] and consequently, the CSH phase amount decreased, probably forming the geopolymeric Si-O-Al phase;
- If R > 2, the low alkalinity reduced the Ca quantity in solution [43], and further decreased the solubilities of Si and Al [43,44]. In these conditions, there was not enough calcium in the system to form a diffused calcium–silicate–hydrate (CSH) phase and the small amount of geopolymer gel formed [32] was responsible for mechanical strength.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Aluminium. 2018. Available online: http://www.world-aluminium.org/statistics/alumina-production/ (accessed on 11 September 2020).
- Totten, G.E.; MacKenzie, D.S. Handbook of Alluminium. In Physical Metallurgy and Process; CRC Press: New York, NY, USA, 2003. [Google Scholar]
- Lima, M.S.S.; Thives, L.P.; Haritonovs, V.; Bajars, K. Red mud application in construction industry: Review of benefits and possibilities. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Riga, Latvia, 2017; Volume 251. [Google Scholar]
- Hind, A.R.; Bhargava, S.K.; Grocott, S.C. The surface chemistry of Bayer process solids: A review. Colloids Surf. A Physicochem. Eng. Asp. 1999, 146, 359–374. [Google Scholar] [CrossRef]
- Boskovic, I.V.; Nenadovic, S.S.; Kljajevic, L.M.; Vukanac, I.S.; Stankovic, N.G.; Lukovic, J.M.; Vukcevic, M.A. Radiological and physicochemical properties of red mud based geopolymers. Nucl. Technol. Radiat. Prot. 2018, 33, 188–194. [Google Scholar] [CrossRef]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Smelting of Bauxite Residue (Red Mud) in View of Iron and Selective Rare Earths Recovery. J. Sustain. Metall. 2016, 2, 28–37. [Google Scholar] [CrossRef] [Green Version]
- The International Aluminium Institute. Primary Aluminium Production. 2016. Available online: http://www.world-aluminium.org/statistics (accessed on 11 September 2020).
- Cardenia, C.; Balomenos, E.; Panias, D. Iron recovery from bauxite residue through reductive roasting process and wet magnetic separation. J. Sustain. Metall. 2018, 5, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Brown, T.J.; Idoine, N.E.; Raycraft, E.R.; Shaw, R.A.; Deady, E.A.; Hobbs, S.F.; Bide, T. World Mineral Production: 2013–2017; British Geological Survey: Notthingam, UK, 2019. [Google Scholar]
- Stanford, K. Red Mud—Addressing the Problem. In Alluminium Insider. 2016. Available online: https://aluminiuminsider.com/red-mud-addressing-the-problem/ (accessed on 11 September 2020).
- Ayres, R.U.; Holmberg, J.; Andersson, B. Materials and the Global Environment: Waste Mining in the 21st Century. MRS Bull. 2001, 26, 477–480. [Google Scholar] [CrossRef] [Green Version]
- Varnavas, S.; Ferentinos, G.; Collins, M. Dispersion of Bauxitic red mud in the Gulf of Corinth, Greece. Mar. Geol. 1986, 70, 211–222. [Google Scholar] [CrossRef]
- Power, G.; Gräfe, M.; Klauber, C. Bauxite residue issues: I. Current management, disposal and storage practices. Hydrometallurgy 2011, 108, 33–45. [Google Scholar] [CrossRef]
- Gelencser, A.; Kovats, N.; Turoczi, B.; Rostasi, A.; Hoffer, A.; Imre, K.; Nyiro-Kosa, I.; Csakberenyi-Malasics, D.; Toth, A.; Czitrovszky, A.; et al. The red mud accident in Ajka (Hungary): Characterization and potential health effect of fugitive dust. Environ. Sci. Technol. 2011, 45, 1608–1615. [Google Scholar] [CrossRef]
- Clark, M.W.; McConchie, D.; Berry, J.; Caldicott, W.; Davies-McConchie, F.; Castro, J. Bauxsol technology to treat acid and metals; Applications in the coal industry. In Proceedings of the Joint Conference of the American Society of Mining and Reclamation and the 25th West Virginia Surface Mine Drainage Task Force, Morgantown, WV, USA, 18–22 April 2004; pp. 292–313. [Google Scholar]
- Smith, P.H.T. Recovery of Soda from Bauxite Residue. Australia Patent WO 2012/145797 Al, 27 April 2012. [Google Scholar]
- World Aluminium. Available online: https://international-aluminium.org/resource/towards-sustainable-cities-series/ (accessed on 10 October 2021).
- Jitsangiam, P.; Nikraz, H.; Jamieso, E. Pozzolanic-stabilised Mixture (PSM) for red sand as road base materials. In Proceedings of the 4th International Conference on Structural Engineering and Construction, Melbourne, Australia, 26–28 September 2007; pp. 647–651. [Google Scholar]
- Klauber, C.; Gräfe, M.; Power, G. Bauxite residue issues: II. options for residue utilization. Hydrometallurgy 2011, 108, 11–32. [Google Scholar] [CrossRef]
- Tsakiridis, P.E.; Agatzini-Leonardou, S.; Oustadakis, P. Red mud addition in the raw meal for the production of Portland cement clinker. J. Hazard. Mater. 2004, 116, 103–110. [Google Scholar] [CrossRef]
- Vangelatos, I.; Angelopoulos, G.N.; Boufounos, D. Utilization of ferroalumina as raw material in the production of Ordinary Portland Cement. J. Hazard. Mater. 2009, 168, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.C.; Chang, F.C.; Lo, S.L.; Lee, M.L.; Wang, C.F.; Lin, J.D. Production of lightweight aggregates from mining residues, heavy metal sludge, and incinerator fly ash. J. Hazard. Mater. 2007, 144, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Nenadović, S.S.; Ferone, C.; Nenadović, M.T.; Cioffi, R.; Mirković, M.M.; Vukanac, I.; Kljajević, L.M. Chemical, physical and radiological evaluation of raw materials and geopolymers for building applications. J. Radioanal. Nucl. Chem. 2020, 325, 435–445. [Google Scholar] [CrossRef]
- Capasso, I.; Liguori, B.; Ferone, C.; Caputo, D.; Cioffi, R. Strategies for the valorization of soil waste by geopolymer production: An overview. J. Clean. Prod. 2021, 288, 125646. [Google Scholar] [CrossRef]
- Luukkonena, T.; Abdollahnejada, Z.; Yliniemia, J.; Kinnunena, P.; Illikainena, M. One-part alkali-activated materials: A review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Frattini, D.; Occhicone, A.; Ferone, C.; Cioffi, R. Fibre-reinforced geopolymer concretes for sensible heat thermal energy storage: Simulations and environmental impact. Materials 2021, 14, 414. [Google Scholar] [CrossRef]
- Roviello, G.; Ricciotti, L.; Molino, A.J.; Menna, C.; Ferone, C.; Asprone, D.; Cioffi, R.; Ferrandiz-Mas, V.; Russo, P.; Tarallo, O. Hybrid fly ash-based geopolymeric foams: Microstructural, thermal and mechanical properties. Materials 2020, 13, 2919. [Google Scholar] [CrossRef] [PubMed]
- Roviello, G.; Ricciotti, L.; Molino, A.J.; Menna, C.; Ferone, C.; Cioffi, R.; Tarallo, O. Hybrid geopolymers from fly ash and polysiloxanes. Molecules 2019, 24, 3510. [Google Scholar] [CrossRef] [Green Version]
- Ricciotti, L.; Occhicone, A.; Petrillo, A.; Ferone, C.; Cioffi, R.; Roviello, G. Geopolymer-based hybrid foams: Lightweight materials from a sustainable production process. J. Clean. Prod. 2020, 250, 119588. [Google Scholar] [CrossRef]
- Liguori, B.; Aprea, P.; Roviello, G.; Ferone, C. Self-supporting zeolites by Geopolymer Gel Conversion (GGC). Microporous Mesoporous Mater. Vol. 2019, 286, 125–132. [Google Scholar] [CrossRef]
- Schollbach, K.; van Hoek, C.; van der Laan, S.; de Wolf, T.; Brouwers, H.J.H. In-depth mineralogical quantification of MSWI bottom ash phases and their association with potentially toxic elements. Waste Manag. 2019, 87, 1–12. [Google Scholar]
- Boskovic, I.; Vukcevic, M.; Nenadovic, S.; Mirkovic, M.; Stojmenovic, M.; Pavlovic, V.; Kljajevic, L. Characterization of Red Mud/Metakaolin-based geopolymers as modified by Ca(OH)2. Mater. Technol. 2019, 53, 341–348. [Google Scholar]
- Roviello, G.; Chianese, E.; Ferone, C.; Ricciotti, L.; Roviello, V.; Cioffi, R.; Tarallo, O. Hybrid Geopolymeric Foams for the Removal of Metallic Ions from Aqueous Waste Solutions. Materials 2019, 12, 4091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidovits, J. Geopolymer Chemistry & Applications, 3rd ed.; Joseph Davidovits: Saint-Quentin, France, 2011. [Google Scholar]
- Hill, M.; Bastow, T.; Celotto, S.; Hill, A. Integrated Study of the Calcination Cycle from Gibbsite to Corundum. Chem. Mater. 2007, 19, 2877–2883. [Google Scholar] [CrossRef]
- Gialanella, S.; Girardi, F.; Ischia, G.; Lonardelli, I.; Mattarelli, M.; Montagna, M. On the goethite to hematite phase transformation. J. Therm. Anal. Calorim. 2010, 102, 867–873. [Google Scholar] [CrossRef]
- Atasoy, A. An investigation on characterization and thermal analysis of the Aughinish red mud. J. Therm. Anal. Calorim. 2015, 81, 357–361. [Google Scholar] [CrossRef]
- Singha, S.; Aswathb, M.U.; Das Biswasb, R.; Ranganathc, R.V.; Choudharyd, H.K.; Kumard, R.; Sahoo, B. Role of iron in the enhanced reactivity of pulverized Red mud: Analysis by Mössbauer spectroscopy and FTIR spectroscopy. Case Stud. Constr. Mater. 2019, 11, e00266. [Google Scholar]
- Barbosa, V.F.F.; Mackenzie, K.J.D.; Thaumaturgo, C. Syntesis and characterization of materials based on inorganic polymers of alumina and silica: Sodium polysialate polymers. Int. J. Inorg. Mater. 2000, 2, 309–317. [Google Scholar] [CrossRef]
- Roviello, G.; Ricciotti, L.; Ferone, C.; Colangelo, F.; Cioffi, R.; Tarallo, O. Synthesis and Characterization of Novel Epoxy Geopolymer Hybrid Composites. Materials 2013, 6, 3943–3962. [Google Scholar] [CrossRef] [Green Version]
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Hosseini, S.R.; Bonyani, M.; Leonardi, S.G.; Bonavita, A.; Neri, G. Synthesis and characterization of mesoporous α-Fe2O3 and investigation of electrical properties of fabricated thick films. Process. Appl. Ceram. 2016, 10, 209–217. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry & Applications; Institut-Géopolymér: Saint-Quentin, France, 2008. [Google Scholar]
- Lemougna, P.N.; Wang, K.; Tang, Q.; Cui, X. Study on the development of inorganic polymers from red mud and slag system: Application in mortar and lightweight materials. Constr. Build. Mater. 2017, 156, 486–495. [Google Scholar] [CrossRef]
- Zivica, V. Effects of type and dosage of alkaline activator and temperature on the properties of alkali-activated slag mixtures. Constr. Build. Mater. 2007, 21, 1463–1469. [Google Scholar] [CrossRef]


| Oxides (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Material | SiO2 | Al2O3 | Na2O | Fe2O3 | TiO2 | MgO | CaO | K2O |
| RM | 15.5 ± 0.1 | 24.0 ± 0.4 | 8.1 ± 0.1 | 45.0 ± 0.2 | 5.4 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.1 | 0.30 ± 0.05 |
| BFS | 35.7 ± 0.7 | 11.2 ± 0.1 | In trace | 0.3 ± 0.1 | 0.5 ± 0.1 | 6.5 ± 0.1 | 43.9 ± 0.8 | 0.31 ± 0.07 |
| Name | RM (%) | BFS (%) | Solid/Liquid | R (SiO2/NaO2) | Curing Temperature (°C) |
|---|---|---|---|---|---|
| RM50_1.2_RT | 50 | 50 | 2.22 | 1.2 | 25 |
| RM50_1.2_60 | 50 | 50 | 2.22 | 1.2 | 60 |
| RM50_1.6_RT | 50 | 50 | 2.22 | 1.6 | 25 |
| RM50_1.6_60 | 50 | 50 | 2.22 | 1.6 | 60 |
| RM50_2_RT | 50 | 50 | 2.22 | 2 | 25 |
| RM50_2_60 | 50 | 50 | 2.22 | 2 | 60 |
| RM50_2.4_RT | 50 | 50 | 2.22 | 2.4 | 25 |
| RM50_2.4_60 | 50 | 50 | 2.22 | 2.4 | 60 |
| RM50_2.8_RT | 50 | 50 | 2.22 | 2.8 | 25 |
| RM50_2.8_60 | 50 | 50 | 2.22 | 2.8 | 60 |
| RM0_2_RT | 0 | 100 | 2.22 | 2 | 25 |
| RM0_2_60 | 0 | 100 | 2.22 | 2 | 60 |
| RM30_2_RT | 30 | 70 | 2.22 | 2 | 25 |
| RM30_2_60 | 30 | 70 | 2.22 | 2 | 60 |
| RM70_2_RT | 70 | 30 | 2.22 | 2 | 25 |
| RM70_2_60 | 70 | 30 | 2.22 | 2 | 60 |
| RM80_2_RT | 80 | 20 | 2.22 | 2 | 25 |
| RM80_2_60 | 80 | 20 | 2.22 | 2 | 60 |
| RM100_2_RT | 100 | 0 | 2.22 | 2 | 25 |
| RM100_2_60 | 100 | 0 | 2.22 | 2 | 60 |
| Material | C | CaO | SiO2 | Fe2O3 | Na2O | Al2O3 | MgO | TiO2 |
|---|---|---|---|---|---|---|---|---|
| RM30_2_RT | 13.9 ± 1.1 | 22 ± 2 | 33 ± 2 | 5.2 ± 1.0 | 8.5 ± 0.7 | 10.2 ± 1.2 | 4.1 ± 0.8 | 0.86 ± 0.15 |
| RM50_2_RT | 15.0 ± 1.2 | 20.5 ± 1.1 | 34 ± 2 | 8.8 ± 0.9 | 8.1 ± 0.9 | 10.5 ± 1.7 | 3.4 ± 0.4 | 0.92 ± 0.18 |
| RM70_2_RT | 16.5 ± 1.3 | 20.2 ± 1.5 | 28 ± 2 | 11.1 ± 1.2 | 8.3 ± 0.6 | 11.1 ± 1.5 | 2.7 ± 0.7 | 1.26 ± 0.13 |
| RM50_2_RT(spot) | 3.52 | 6.46 | 14.25 | 58.05 | 3.4 | 5.35 | 1.19 | 7.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Occhicone, A.; Vukčević, M.; Bosković, I.; Ferone, C. Red Mud-Blast Furnace Slag-Based Alkali-Activated Materials. Sustainability 2021, 13, 11298. https://doi.org/10.3390/su132011298
Occhicone A, Vukčević M, Bosković I, Ferone C. Red Mud-Blast Furnace Slag-Based Alkali-Activated Materials. Sustainability. 2021; 13(20):11298. https://doi.org/10.3390/su132011298
Chicago/Turabian StyleOcchicone, Alessio, Mira Vukčević, Ivana Bosković, and Claudio Ferone. 2021. "Red Mud-Blast Furnace Slag-Based Alkali-Activated Materials" Sustainability 13, no. 20: 11298. https://doi.org/10.3390/su132011298
APA StyleOcchicone, A., Vukčević, M., Bosković, I., & Ferone, C. (2021). Red Mud-Blast Furnace Slag-Based Alkali-Activated Materials. Sustainability, 13(20), 11298. https://doi.org/10.3390/su132011298







