Hydrogeochemical Characteristics of Uranium and Radon in Groundwater from the Goesan Area of the Ogcheon Metamorphic Belt (OMB), Korea
Abstract
1. Introduction
2. Materials and Methods
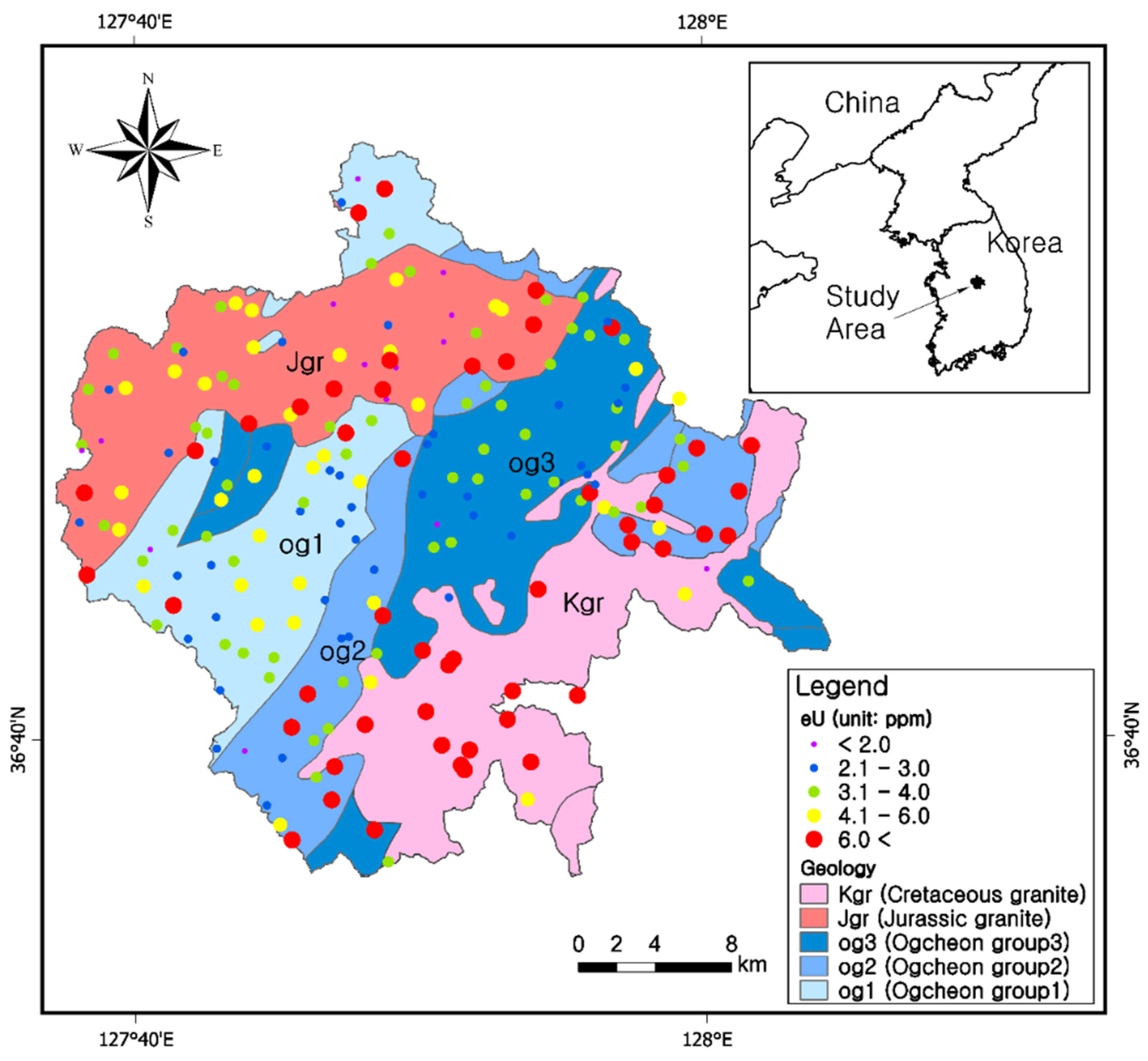
2.1. Geology of the Study Area
2.2. Sampling and Analytical Methods
3. Results and Discussion
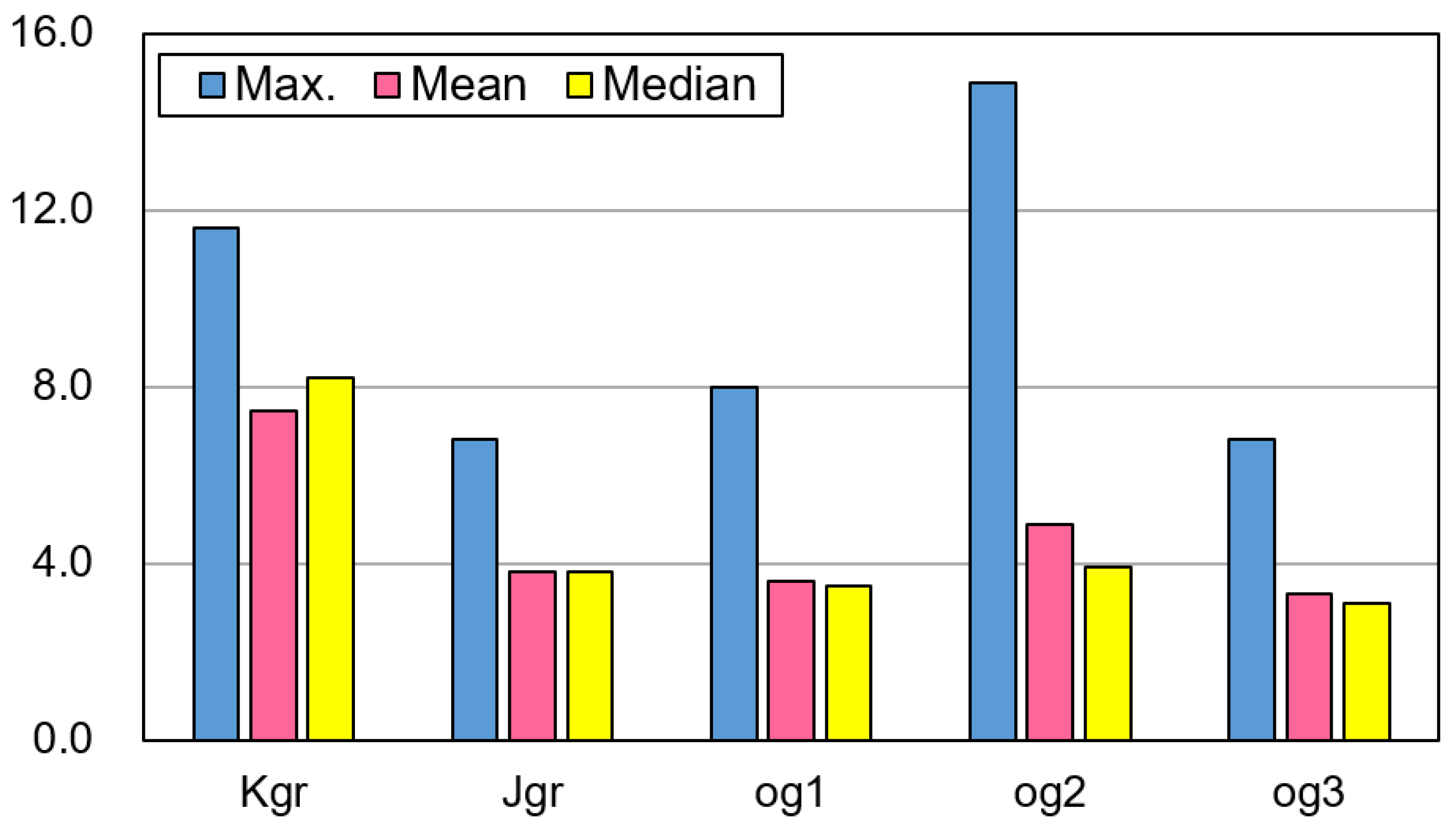
3.1. Uranium Concentrations (eU) in the Five Bedrock Types
3.2. Physicochemical Properties of Groundwater and Water Types
| Depth (m) | T (°C) | pH | EC (μS/cm) | Eh (mV) | DO | K | Na | Ca | Mg | SiO2 | Sr | F | Cl | NO3 | SO4 | HCO3 | Rn (Bq/L) | U (μg/L) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Max. | 122.0 | 16.9 | 8.22 | 756 | 352 | 10.80 | 8.12 | 36.50 | 41.90 | 6.91 | 35.50 | 0.19 | 3.59 | 29.70 | 137.0 | 19.80 | 170.8 | 1540.9 | 293.0 | |
| Kgr | Min. | 10.0 | 12.6 | 6.05 | 61.0 | −335 | 0.80 | 0.09 | 1.39 | 2.62 | 0.03 | 6.24 | 0.01 | 0.03 | 1.70 | 0.06 | 0.28 | 18.3 | 18.9 | 0.21 |
| (25) | Mean | 76.3 | 14.2 | 7.25 | 220 | 221 | 6.92 | 1.26 | 11.16 | 19.11 | 2.15 | 21.94 | 0.08 | 1.27 | 6.38 | 17.21 | 7.05 | 60.4 | 404.8 | 26.0 |
| Med. | 70.0 | 14.3 | 7.45 | 172 | 218 | 7.10 | 0.82 | 8.90 | 15.10 | 2.04 | 22.00 | 0.06 | 1.23 | 5.82 | 4.92 | 6.10 | 51.9 | 345.9 | 4.13 | |
| Std. | 29.8 | 1.0 | 0.55 | 179 | 59 | 1.89 | 1.63 | 8.23 | 12.11 | 1.52 | 7.28 | 0.05 | 0.89 | 5.53 | 30.72 | 5.19 | 42.3 | 381.0 | 67.0 | |
| Max. | 200.0 | 17.7 | 8.31 | 740 | 310 | 10.10 | 6.81 | 32.50 | 99.60 | 22.90 | 35.50 | 1.61 | 3.77 | 61.30 | 75.20 | 176.0 | 219.6 | 523.9 | 32.1 | |
| Jgr | Min. | 10.0 | 13.0 | 6.21 | 93.0 | −110 | 1.20 | 0.49 | 3.43 | 7.68 | 1.57 | 10.70 | 0.07 | 0.05 | 1.70 | 0.06 | 2.54 | 29.0 | 3.3 | 0.02 |
| (56) | Mean. | 85.6 | 15.2 | 7.17 | 259 | 198 | 5.53 | 2.00 | 12.31 | 26.39 | 7.84 | 22.96 | 0.47 | 0.41 | 11.26 | 19.51 | 15.38 | 98.0 | 107.9 | 4.37 |
| Med. | 100.0 | 15.4 | 7.18 | 247 | 207 | 5.65 | 1.49 | 10.30 | 22.45 | 6.49 | 22.35 | 0.31 | 0.23 | 7.20 | 16.90 | 11.00 | 85.4 | 84.4 | 2.32 | |
| Std. | 40.6 | 1.0 | 0.51 | 113 | 84 | 2.09 | 1.48 | 6.42 | 15.26 | 4.97 | 6.24 | 0.40 | 0.62 | 11.68 | 17.51 | 22.96 | 42.9 | 90.7 | 5.83 | |
| Max. | 160.0 | 18.0 | 8.36 | 625 | 366 | 9.20 | 4.36 | 24.00 | 67.40 | 15.00 | 29.30 | 1.52 | 2.10 | 44.60 | 54.30 | 42.00 | 213.5 | 128.4 | 16.4 | |
| og1 | Min. | 15.0 | 11.1 | 6.50 | 75.0 | 70 | 1.64 | 0.36 | 1.39 | 7.97 | 1.23 | 8.15 | 0.02 | 0.04 | 1.79 | 0.42 | 0.28 | 19.8 | 5.2 | 0.02 |
| (43) | Mean | 74.2 | 14.4 | 7.23 | 222 | 219 | 6.13 | 1.63 | 6.60 | 25.15 | 4.84 | 15.19 | 0.19 | 0.24 | 7.79 | 14.68 | 10.58 | 82.5 | 62.5 | 1.19 |
| Med. | 90.0 | 14.3 | 7.21 | 201 | 214 | 6.23 | 1.48 | 5.15 | 22.80 | 4.01 | 13.90 | 0.10 | 0.10 | 6.14 | 10.90 | 7.50 | 67.4 | 59.6 | 0.26 | |
| Std. | 46.8 | 1.4 | 0.48 | 116 | 59 | 1.94 | 0.79 | 4.63 | 12.50 | 3.18 | 5.00 | 0.29 | 0.42 | 7.10 | 12.05 | 9.00 | 40.7 | 27.7 | 3.23 | |
| Max. | 150.0 | 16.9 | 9.04 | 770 | 336 | 10.20 | 9.92 | 170.0 | 130.0 | 13.30 | 43.60 | 0.48 | 10.30 | 40.30 | 186.0 | 47.50 | 344.7 | 927.6 | 21.5 | |
| og2 | Min. | 20.0 | 13.3 | 6.22 | 76.0 | −10 | 1.15 | 0.39 | 2.13 | 2.62 | 0.39 | 7.83 | 0.03 | 0.03 | 1.84 | 0.66 | 1.48 | 21.4 | 1.9 | 0.02 |
| (38) | Mean | 76.4 | 14.7 | 7.41 | 277 | 230 | 5.94 | 2.23 | 12.16 | 32.14 | 5.69 | 22.08 | 0.13 | 0.60 | 9.80 | 23.64 | 18.02 | 100.6 | 83.2 | 2.03 |
| Med. | 80.5 | 14.6 | 7.47 | 225 | 251 | 6.08 | 1.70 | 7.17 | 25.80 | 4.82 | 21.10 | 0.10 | 0.20 | 7.15 | 16.50 | 13.50 | 82.4 | 38.1 | 0.45 | |
| Std. | 36.3 | 0.9 | 0.62 | 167 | 71 | 2.17 | 1.94 | 26.80 | 27.49 | 3.10 | 7.13 | 0.10 | 1.68 | 8.83 | 32.30 | 12.47 | 65.5 | 163.6 | 4.59 | |
| Max. | 220.0 | 20.0 | 9.65 | 673 | 296 | 9.50 | 11.60 | 38.50 | 102.0 | 20.30 | 51.60 | 0.94 | 4.63 | 100.0 | 89.30 | 73.80 | 311.1 | 167.2 | 3.54 | |
| og3 | Min. | 21.0 | 13.4 | 6.60 | 101 | −335 | 0.80 | 0.09 | 1.93 | 4.79 | 0.03 | 10.10 | 0.04 | 0.06 | 1.99 | 0.93 | 2.02 | 32.0 | 1.8 | 0.03 |
| (38) | Mean | 93.4 | 15.7 | 7.65 | 307 | 155 | 5.26 | 2.98 | 8.23 | 43.59 | 9.78 | 25.42 | 0.16 | 0.61 | 13.93 | 20.63 | 18.90 | 140.5 | 33.2 | 1.12 |
| Med. | 100.0 | 15.5 | 7.60 | 275 | 172 | 5.24 | 1.88 | 6.10 | 40.60 | 9.75 | 21.50 | 0.12 | 0.19 | 9.53 | 14.70 | 15.30 | 135.7 | 17.9 | 0.80 | |
| Std. | 43.0 | 1.3 | 0.52 | 133 | 124 | 2.41 | 3.05 | 6.45 | 22.59 | 4.94 | 12.31 | 0.18 | 0.93 | 16.64 | 18.97 | 14.55 | 58.1 | 37.7 | 1.01 |
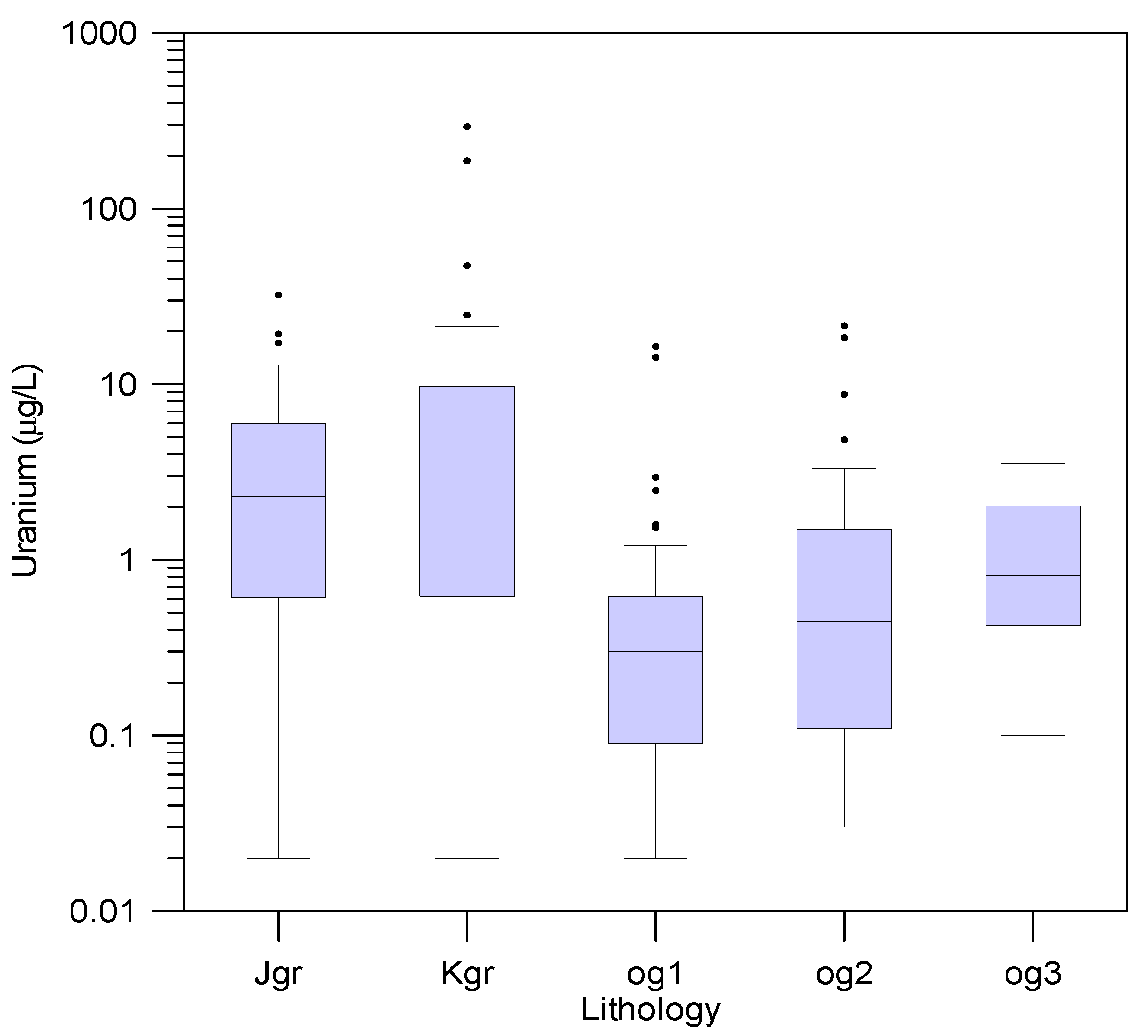
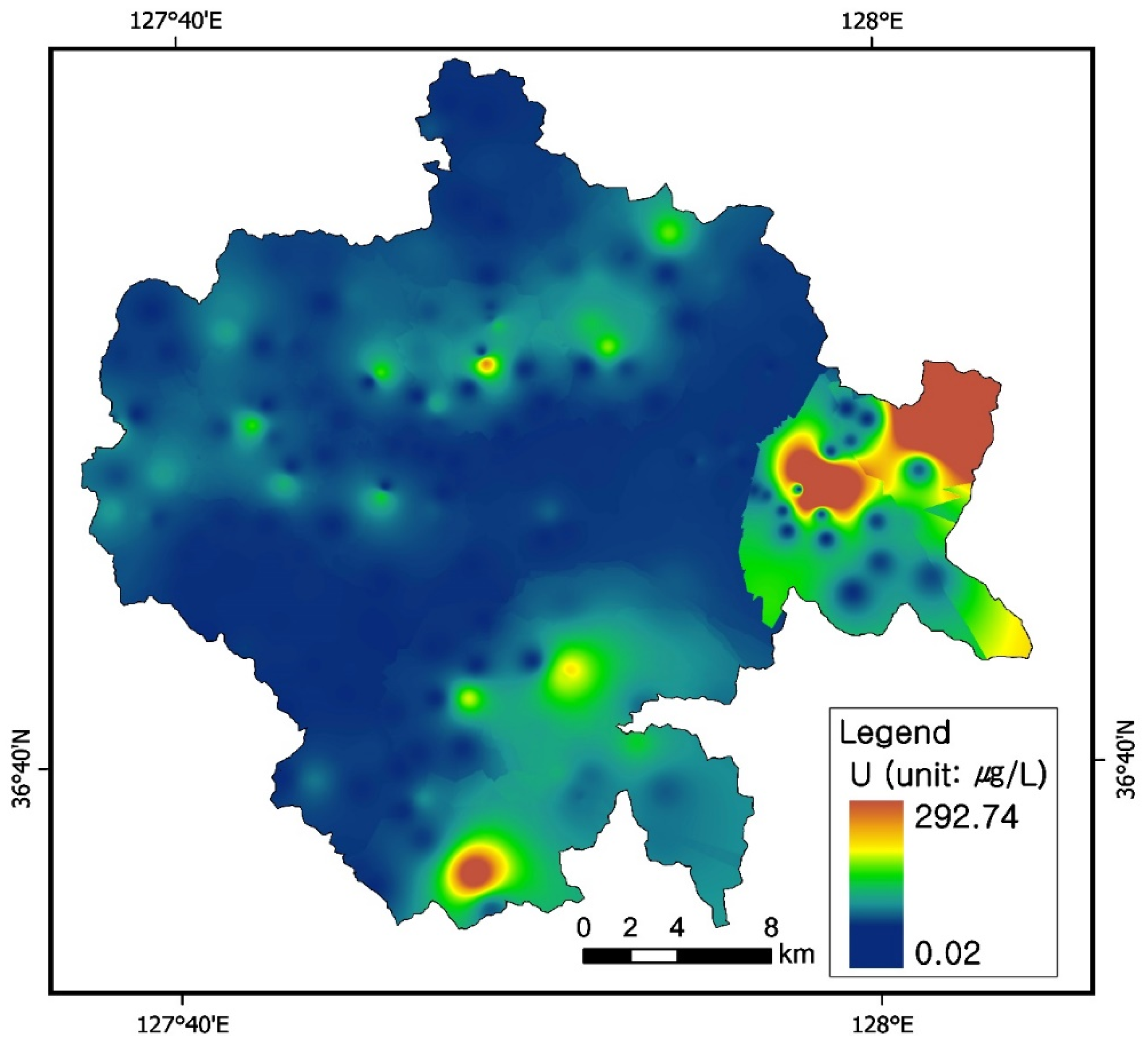
3.3. Uranium Concentrations in Groundwater
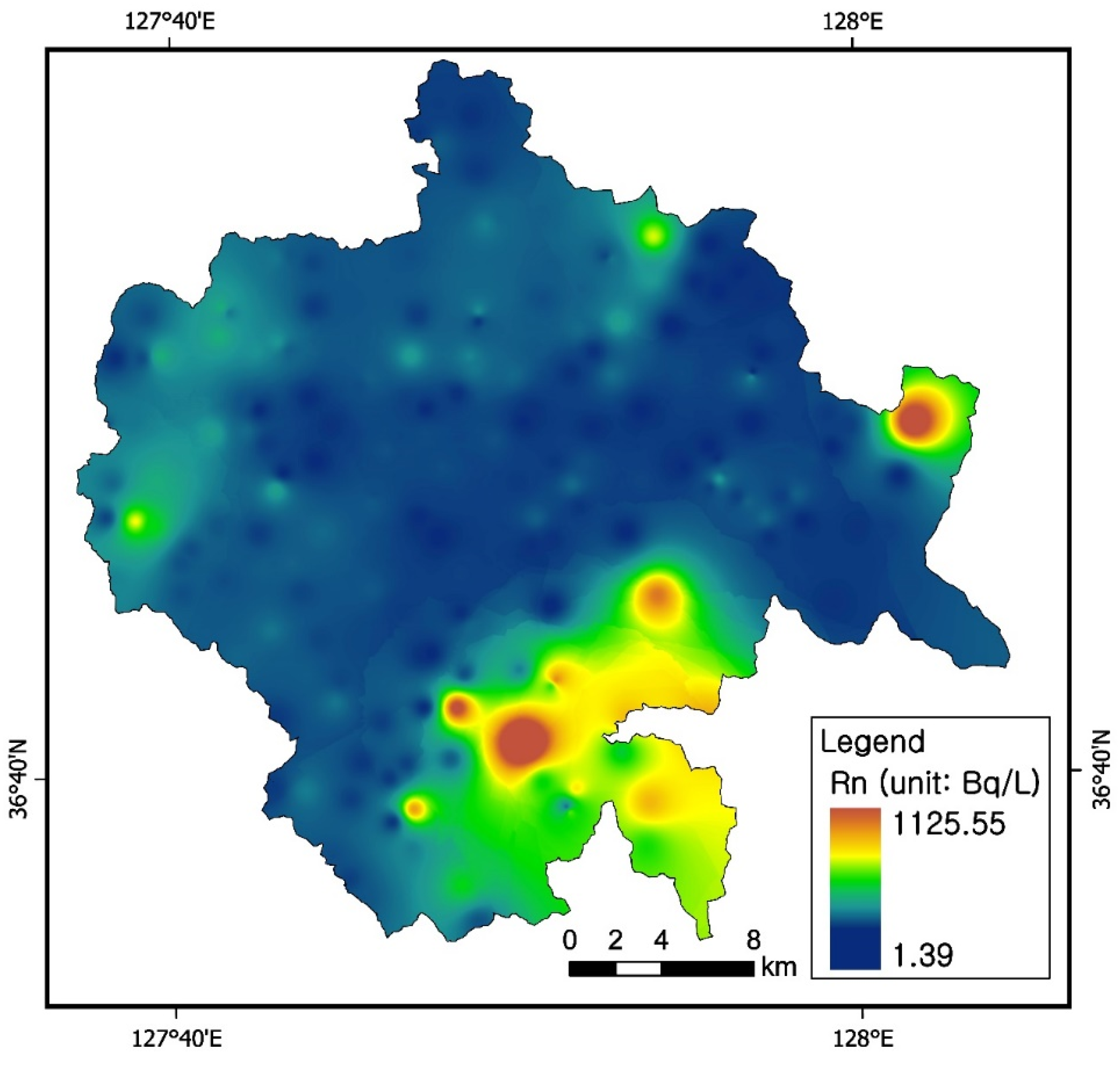
3.4. Radon Concentrations in Groundwater
3.5. Correlation Coefficients of Physicochemical Components in Groundwater
| Comp. | pH | EC | Eh | DO | K | Na | Ca | Mg | SiO2 | Sr | F | Cl | NO3 | SO4 | HCO3 | Rn | U |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1.00 | ||||||||||||||||
| EC | −0.02 | 1.00 | |||||||||||||||
| Eh | −0.43 | −0.18 | 1.00 | ||||||||||||||
| DO | −0.18 | −0.17 | 0.29 | 1.00 | |||||||||||||
| K | −0.12 | 0.27 | 0.06 | 0.04 | 1.00 | ||||||||||||
| Na | 0.21 | 0.33 | −0.31 | −0.23 | −0.04 | 1.00 | |||||||||||
| Ca | 0.02 | 0.64 | 0.01 | −0.08 | 0.40 | −0.09 | 1.00 | ||||||||||
| Mg | −0.03 | 0.50 | −0.10 | −0.13 | 0.54 | −0.02 | 0.62 | 1.00 | |||||||||
| SiO2 | −0.04 | 0.11 | −0.02 | −0.01 | −0.13 | −0.01 | 0.17 | 0.19 | 1.00 | ||||||||
| Sr | 0.07 | 0.28 | −0.19 | −0.11 | 0.16 | 0.19 | 0.19 | 0.47 | −0.06 | 1.00 | |||||||
| F | 0.43 | 0.15 | −0.41 | −0.21 | −0.22 | 0.81 | −0.19 | −0.27 | 0.05 | 0.02 | 1.00 | ||||||
| Cl | −0.11 | 0.48 | −0.04 | −0.03 | 0.42 | 0.25 | 0.54 | 0.56 | 0.18 | 0.20 | 0.05 | 1.00 | |||||
| NO3 | −0.36 | 0.45 | 0.11 | 0.12 | 0.31 | 0.00 | 0.48 | 0.30 | 0.21 | 0.04 | −0.20 | 0.35 | 1.00 | ||||
| SO4 | −0.01 | 0.52 | −0.10 | −0.16 | 0.32 | 0.19 | 0.58 | 0.50 | 0.04 | 0.36 | −0.01 | 0.44 | 0.27 | 1.00 | |||
| HCO3 | 0.29 | 0.60 | −0.22 | −0.28 | 0.32 | 0.34 | 0.73 | 0.64 | 0.07 | 0.32 | 0.20 | 0.35 | 0.07 | 0.39 | 1.00 | ||
| Rn | −0.09 | −0.13 | 0.04 | 0.04 | −0.22 * | 0.31 * | −0.26 * | −0.30 * | 0.11 | −0.12 | 0.36 * | −0.03 | −0.03 | −0.12 | −0.18 | 1.00 | |
| U | 0.08 | 0.04 | −0.04 | 0.03 | −0.06 | 0.20 * | 0.03 | −0.06 | −0.01 | 0.05 | 0.30 * | 0.01 | −0.07 | 0.04 | 0.12 | 0.26 | 1.00 |
3.6. Uranium Speciation and Saturation Indices in Groundwater
| Minerals | 1 G7 | 2 G29 | 2 G36 | 2 G37 | 3 G44 | 2 G46 | 4 G52 | 2 G86 | 4 G109 | 4 G124 | 4 G151 | 4 G155 | 4 G157 | 3 G163 | 2 G181 | 4 G182 | 1 G195 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anhydrite | −2.21 | −2.93 | −3.24 | −3.19 | −3.73 | −3.72 | −3.51 | −2.92 | −2.23 | −3.30 | −3.16 | −3.08 | −1.52 | −3.23 | −3.08 | −2.40 | |
| Aragonite | 0.63 | −0.56 | −0.72 | −0.88 | 0.21 | −1.11 | −0.59 | −0.14 | 0.14 | −0.31 | −1.62 | −0.42 | −0.62 | −0.78 | −3.40 | −1.40 | −0.07 |
| Brucite | −4.32 | −6.86 | −6.00 | −6.28 | −4.07 | −6.56 | −5.90 | −5.35 | −5.29 | −4.89 | −7.31 | −5.86 | −6.57 | −5.93 | −6.30 | −7.37 | −5.32 |
| Calcite | 0.78 | −0.41 | −0.58 | −0.73 | 0.35 | −0.96 | −0.45 | 0.01 | 0.28 | −0.16 | −1.48 | −0.27 | −0.47 | −0.64 | −3.25 | −1.26 | 0.08 |
| Dolomite | 1.21 | −2.01 | −1.71 | −2.15 | 0.26 | −2.63 | −1.61 | −0.62 | 0.19 | −0.15 | −3.02 | −1.21 | −1.33 | −1.74 | −7.17 | −2.93 | −0.01 |
| Gypsum | −1.96 | −2.68 | −2.99 | −2.94 | −3.48 | −3.47 | −3.26 | −2.67 | −1.98 | −3.05 | −2.91 | −2.83 | −1.27 | −2.98 | −2.83 | −2.15 | |
| Chrysotile | −0.89 | −7.71 | −5.12 | −5.66 | 0.14 | −6.66 | −4.83 | −3.25 | −3.15 | −2.02 | −8.99 | −4.82 | −6.57 | −4.95 | −6.15 | −8.97 | −3.48 |
| Magnesite | −0.72 | −2.75 | −2.29 | −2.56 | −1.24 | −2.82 | −2.31 | −1.78 | −1.25 | −1.14 | −2.70 | −2.09 | −2.00 | −2.25 | −5.07 | −2.83 | −1.23 |
| Fluorite | −2.85 | −0.44 | −0.84 | −0.89 | −0.60 | −1.04 | −0.77 | −0.91 | −2.30 | −3.34 | −2.65 | −1.38 | −2.45 | −1.99 | −0.52 | −2.64 | −2.18 |
| Celestite | −2.29 | −3.41 | −3.88 | −3.90 | −3.48 | −4.36 | −3.46 | −3.25 | −2.26 | −2.88 | −3.05 | −2.52 | −1.47 | −2.96 | −3.41 | −1.98 | |
| Cristobalite | −0.16 | 0.23 | 0.24 | 0.40 | −0.03 | 0.31 | 0.24 | 0.20 | 0.16 | 0.12 | 0.28 | 0.18 | 0.37 | 0.22 | 0.17 | 0.36 | 0.04 |
| Strontianite | −0.78 | −2.37 | −2.68 | −2.92 | −0.86 | −3.08 | −1.87 | −1.80 | −1.21 | −1.21 | −2.83 | −1.18 | −1.89 | −1.84 | −5.05 | −2.81 | −0.98 |
| Chalcedony | 0.04 | 0.43 | 0.44 | 0.60 | 0.17 | 0.51 | 0.44 | 0.40 | 0.36 | 0.32 | 0.48 | 0.38 | 0.57 | 0.42 | 0.37 | 0.56 | 0.24 |
| Quartz | 0.49 | 0.88 | 0.89 | 1.05 | 0.62 | 0.96 | 0.89 | 0.85 | 0.81 | 0.77 | 0.93 | 0.83 | 1.02 | 0.87 | 0.82 | 1.01 | 0.69 |
| SiO2(am) | −0.77 | −0.38 | −0.37 | −0.21 | −0.64 | −0.30 | −0.37 | −0.41 | −0.45 | −0.49 | −0.33 | −0.43 | −0.24 | −0.39 | −0.44 | −0.25 | −0.57 |
| Schoepite | −7.09 | −4.78 | −4.47 | −4.10 | −6.45 | −4.00 | −4.85 | −4.48 | −6.40 | −5.45 | −3.89 | −4.91 | −5.61 | −4.41 | 1.64 | −4.23 | −6.16 |
| UO2(NO3)2(s) | −37.43 | −34.68 | −34.46 | −32.96 | −41.32 | −34.18 | −34.96 | −33.03 | −34.83 | −36.42 | −31.02 | −36.16 | −32.81 | −33.70 | −27.30 | −29.46 | −35.49 |
| UO2(NO3)2:2H2O(s) | −30.13 | −27.38 | −27.17 | −25.66 | −34.03 | −26.88 | −27.66 | −25.73 | −27.53 | −29.12 | −23.73 | −28.87 | −25.51 | −26.41 | −20.01 | −22.16 | −28.19 |
| UO2(NO3)2:3H2O(s) | −28.67 | −25.92 | −25.71 | −24.20 | −32.56 | −25.42 | −26.20 | −24.27 | −26.07 | −27.66 | −22.27 | −27.41 | −24.05 | −24.95 | −18.55 | −20.70 | −36.73 |
| UO2(NO3)2:6H2O(s) | −27.32 | −24.58 | −24.36 | −22.86 | −31.22 | −24.08 | −24.86 | −22.92 | −24.73 | −26.32 | −20.92 | −26.06 | −22.71 | −23.60 | −17.21 | −19.36 | −25.39 |
| UO2(OH)2 | −7.32 | −5.00 | −4.69 | −4.32 | −4.32 | −4.22 | −5.07 | −4.70 | −6.62 | −5.67 | −4.11 | −5.14 | −5.83 | −4.63 | 1.42 | −4.45 | −6.38 |
| UO3(s) | −9.40 | −7.09 | −6.78 | −6.31 | −6.41 | −6.31 | −7.16 | −6.79 | −8.71 | −7.76 | −6.20 | −7.22 | −7.92 | −6.72 | −0.67 | −6.54 | −8.47 |
3.7. Uranium and Radon Behavior in Groundwater in Relation to Bedrocks
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurttio, P.; Auvinen, A.; Salonen, L.; Saha, H.; Pekkanen, J.; Mäkeläinen, I.; Väisänen, S.B.; Penttilä, I.M.; Komulainen, H. Renal effects of uranium in drinking water. Environ. Health Perspect. 2002, 110, 337–342. [Google Scholar] [CrossRef]
- NRC (National Research Council). Risk Assessment of Radon in Drinking Water; National Academy Press: Washington, DC, USA, 1999; p. 279. [Google Scholar]
- National Institute of Environmental Research (NIER). Study on the Radionuclide Concentrations in Groundwater (I); KIGAM Report; Ministry of Environment of Korea: Seoul, Korea, 1998; p. 338.
- National Institute of Environmental Research (NIER). A Detailed Study on the Radionuclide Concentrations in the Groundwater (II); NIER Report; Ministry of Environment of Korea: Seoul, Korea, 2009; p. 273.
- National Institute of Environmental Research (NIER). Study on the Naturally Occurring Radionuclides in Groundwater of the Two High Potential Areas; KIGAM Report; Ministry of Environment of Korea: Seoul, Korea, 2011; p. 253.
- National Institute of Environmental Research (NIER). Study on the Naturally Occurring Radionuclides in Groundwater of the High Potential Area; KIGAM Report; Ministry of Environment of Korea: Seoul, Korea, 2012; p. 245.
- National Institute of Environmental Research (NIER). Studies on the Naturally Occurring Radionuclides in Groundwater; KIGAM Report; Ministry of Environment of Korea: Sejong, Korea, 2016; p. 213.
- Yun, U.; Kim, T.S.; Kim, H.K.; Kim, M.S.; Cho, S.Y.; Choo, C.O.; Cho, B.W. Natural radon reduction rate of the community groundwater system in South Korea. Appl. Radiat. Isot. 2017, 126, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.-W.; Choo, C.O.; Kim, M.S.; Hwang, J.; Yun, U.; Lee, S. Spatial relationships between radon and topographical, geological, and geochemical factors and their relevance in all of South Korea. Environ. Earth Sci. 2015, 74, 5155–5168. [Google Scholar] [CrossRef]
- Cho, B.W.; Kim, H.K.; Kim, M.S.; Hwang, J.H.; Yoon, U.; Cho, S.Y.; Choo, C.O. Radon concentrations in the community groundwater system of South Korea. Environ. Monit. Assess. 2019, 191, 189. [Google Scholar] [CrossRef]
- Cuney, M.; Friederich, M. Physicochemical and crystal-chemical controls on accessory mineral paragenesis in granitoids: Implications for uranium metallogenesis. Bull. Miner. 1987, 110, 235–247. [Google Scholar] [CrossRef]
- Loomis, D.P. Radon-222 Concentration and Aquifer Lithology in North Carolina. Ground Water Monit. Remediat. 1987, 7, 33–39. [Google Scholar] [CrossRef]
- Förster, H.-J. The chemical composition of uraninite in Variscan granites of the Erzgebirge, Germany. Miner. Mag. 1999, 63, 239–252. [Google Scholar] [CrossRef]
- Skeppström, K.; Olofsson, B. Uranium and radon in groundwater. Eur. Water 2007, 17–18, 51–62. [Google Scholar]
- Brindha, K.; Elango, L. Occurrence of uranium in groundwater of a shallow granitic aquifer and its suitability for domestic use in southern India. J. Radioanal. Nucl. Chem. 2012, 295, 357–367. [Google Scholar] [CrossRef]
- Boekhout, F.; Gérard, M.; Kanzari, A.; Michel, A.; Déjeant, A.; Galoisy, L.; Calas, G.; Descostes, M. Uranium migration and retention during weathering of a granitic waste rock pile. Appl. Geochem. 2015, 58, 123–135. [Google Scholar] [CrossRef]
- Kanzari, A.; Gérard, M.; Boekhout, F.; Galoisy, L.; Calas, G.; Descostes, M. Impact of incipient weathering on uranium migration in granitic waste rock piles from former U mines (Limousin, France). J. Geochem. Explor. 2017, 183, 114–126. [Google Scholar] [CrossRef]
- Rajaraman, H.S.; Bisht, B.S.; Jeyagopal, A.V.; Verma, M.B. Uranium Mineralization in Leucogranite of Mastipuram Area, Wanaparti District, Telangana—A New Find. J. Geol. Soc. India 2019, 93, 487–491. [Google Scholar] [CrossRef]
- Kim, J.W. Geochemistry and genesis of the Guryongsan (Ogcheon) uraniferous black slate. J. Korean Inst. Min. Geol. 1989, 22, 35–63. [Google Scholar]
- Chi, S.J.; Yun, U.; Hong, Y.K.; Kim, T.G.; Kim, K.H. Surface intensity of radioactivity and uranium contents according to geology in Ogcheon Belt. In Proceedings of the 38th Meeting of the Korea Society of Economic and Environmental Geology, Seoul, Korea, 21–22 April 2005; pp. 55–59. [Google Scholar]
- Kim, Y.; Seo, J.; Kang, S.-A.; Choi, S.-G.; Lee, Y.J. Geochemistry and uranium mineralogy of the black slate in the Okcheon Metamorphic Belt, South Korea. Geochem. J. 2015, 49, 443–452. [Google Scholar] [CrossRef]
- Lee, D.J. Mineralogy of low-grade uranium ores in the black slate of the Ogcheon Group, Korea. J. Korean Inst. Min. Geol. 1986, 19, 133–146. [Google Scholar]
- Jeong, G.Y. Mineralogy and geochemistry of metalliferous black slates in the okcheon metamorphic belt, Korea: A metamorphic analogue of black shales in the South China block. Miner. Depos. 2006, 41, 469–481. [Google Scholar] [CrossRef]
- Shin, D.-B.; Kim, S.-J. Geochemical Characteristics of Black Slate and Coaly Slate from the Uranium Deposit in Deokpyeong Area. Econ. Environ. Geol. 2011, 44, 373–386. [Google Scholar] [CrossRef][Green Version]
- Choo, C.O. Characteristics of uraniferous minerals in Daebo granite and significance of mineral species. J. Miner. Soc. Korea 2002, 15, 11–21. [Google Scholar]
- Hwang, J. Occurrence of U-minerals and Source of U in Groundwater in Daebo Granite, Daejeon Area. J. Eng. Geol. 2013, 23, 399–407. [Google Scholar] [CrossRef]
- Moon, S.H.; Hwang, J.; Lee, J.Y.; Hyun, S.P.; Bae, B.K.; Park, Y. Establishing the Origin of Elevated Uranium Concentrations in Groundwater near the Central Ogcheon Metamorphic Belt, Korea. J. Environ. Qual. 2013, 42, 118–128. [Google Scholar] [CrossRef]
- Yun, U.; Kim, M.S.; Jeong, D.H.; Hwang, J.H.; Cho, B.W. Uranium and radon concentrations in groundwater of the Daejeon granite area: Comparison with other granite areas. J. Eng. Geol. 2018, 28, 631–643. [Google Scholar]
- Cho, B.W.; Choo, C.O. Geochemical Behavior of Uranium and Radon in Groundwater of Jurassic Granite Area, Icheon, Middle Korea. Water 2019, 11, 1278. [Google Scholar] [CrossRef]
- Hwang, J. Hydrochemistry of Groundwater in the Uraniferous Sedimentary Rocks of the Ogcheon Belt, Republic of Korea. J. Korean Earth Sci. Soc. 2010, 31, 205–213. [Google Scholar] [CrossRef]
- Shin, I.; Kim, S.; Shin, D. Mineralogy and sulfur isotope compositions of the uraniferous black slates in the Ogcheon Metamorphic Belt, South Korea. J. Geochem. Explor. 2016, 169, 1–12. [Google Scholar] [CrossRef]
- Lee, B.J.; Kim, D.H.; Choi, H.I.; Kee, W.S.; Park, K.H. 1: 250,000 Explanatory Note of the Daejeon Sheet; Korea Institute of Geology, Mining and Materials: Daejeon, Korea, 1996; p. 59.
- Lee, C.H.; Kim, J.H. Geological Report of the Goesan Sheet (1:50,000), Korea; Geological and Mining Institute Korea: Seoul, Korea, 1972; pp. 1–22. [Google Scholar]
- International Atomic Energy Agency (IAEA). Gamma Ray Surveys in Uranium Exploration; Technical Reports Series No. 186; IAEA: Vienna, Austria, 1979; p. 91. [Google Scholar]
- International Atomic Energy Agency (IAEA). Guidelines for Radioelement Mapping using Gamma Ray Spectrometry Data; Technical Reports Series, No. 1363; IAEA: Vienna, Austria, 2003; p. 173. [Google Scholar]
- Adabanija, M.A.; Anie, O.; Oladunjoye, M.A. Radioactivity and gamma ray spectrometry of basement rocks in Okene area, southwestern Nigeria. NRIAG J. Astron. Geophys. 2020, 9, 71–84. [Google Scholar] [CrossRef]
- Chiozzi, P.; De Felice, P.; Pasquale, V.; Russo, D.; Verdoya, M. Field γ-ray spectrometry on the Vulcano island (Aeolian Arc, Italy). Appl. Radiat. Isot. 1999, 51, 247–253. [Google Scholar] [CrossRef]
- Correia, G.G.; Duarte, L.V.; Pereira, A.; Silva, R.L. Outcrop gamma-ray spectrometry: Applications to the Sinemurian–Pliensbachian organic-rich facies of the Lusitanian Basin (Portugal). J. Iber. Geol. 2013, 38. [Google Scholar] [CrossRef]
- Korun, M.; Modec, P.M.; Vodenik, B.; Zorko, B. Uranium-induced background of germanium gamma-ray spectrometers. Appl. Radiat. Isot. 2012, 70, 1480–1484. [Google Scholar] [CrossRef]
- Bartier, P.M.; Keller, C. Multivariate interpolation to incorporate thematic surface data using inverse distance weighting (IDW). Comput. Geosci. 1996, 22, 795–799. [Google Scholar] [CrossRef]
- Gustafsson, J.P. Visual MINTEQ; Version 3.1; Department of Land and Water Resources Engineering, KTH Institute of Technology: Stockholm, Sweden, 2013; Available online: https://vminteq.lwr.kth.se (accessed on 1 March 2020).
- Appleton, J.; Miles, J. A statistical evaluation of the geogenic controls on indoor radon concentrations and radon risk. J. Environ. Radioact. 2010, 101, 799–803. [Google Scholar] [CrossRef]
- National Institute of Environmental Research (NIER). Studies on the Naturally Occurring Radionuclides in Groundwater in Multi-Geologic Areas; KIGAM Report; Ministry of Environment of Korea: Seoul, Korea, 2015; p. 203.
- Salonen, L. 238U series radionuclides as a source of increased radioactivity in groundwater originating from Finnish bedrock. In Future Groundwater Resources at Risk, Proceedings of the Helsinki Conference, Helsinki, Finland, 13–16 June 1994; IAHS (International Association of Hydrological Sciences): Wallingford, UK, 1994; Volume 222, pp. 71–84. [Google Scholar]
- Scheib, C.; Appleton, J.; Miles, J.; Hodgkinson, E. Geological controls on radon potential in England. Proc. Geol. Assoc. 2013, 124, 910–928. [Google Scholar] [CrossRef]
- Pryzlibski, T.; Gorecka, J. 222Rn activity concentration differences in groundwater of three Variscan granitoid massifs in the Sudetes (NE Borhemian Massif, SW Poland). J. Environ. Radioact. 2014, 134, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Yang, J.H.; Jeong, C.H.; Kim, H.K.; Kim, D.W.; Jo, B.U. Geochemical Origins and Occurrences of Natural Radioactive Materials in Borehole Groundwater in the Goesan Area. J. Eng. Geol. 2014, 24, 535–550. [Google Scholar] [CrossRef]
- Jeong, C.-H.; Kim, N.-W.; Kim, M.-S.; Lee, Y.-J.; Kim, T.-S.; Han, J.-S.; Jo, B.-U. Occurrence of Natural Radioactive Materials in Borehole Groundwater and Rock Core in the Icheon Area. J. Eng. Geol. 2012, 22, 95–111. [Google Scholar] [CrossRef]
- Atkins, M.L.; Santos, I.R.; Perkins, A.; Maher, D.T. Dissolved radon and uranium in groundwater in a potential coal seam gas development region (Richmond River Catchment, Australia). J. Environ. Radioact. 2016, 154, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Murphy, W.M.; Shock, E.L. Environmental aqueous geochemistry of actinides. In Uranium: Mineralogy, Geochemistry and the Environment; Mineralogical Society of America: Chantilly, VA, USA, 1995; Volume 38, pp. 221–253. [Google Scholar]
- Vinson, D.; Vengosh, A.; Hirschfeld, D.; Dwyer, G.S. Relationships between radium and radon occurrence and hydrochemistry in fresh groundwater from fractured crystalline rocks, North Carolina (USA). Chem. Geol. 2008, 260, 159–171. [Google Scholar] [CrossRef]
- Veeger, A.; Ruderman, N. Hydrogeologic Controls on Radon-222 in a Buried Valley-Fractured Bedrock Aquifer System. Groundwater 1998, 36, 596–604. [Google Scholar] [CrossRef]
- Langmuir, D. Actinides and their daughter and fission products. In Aqueous Environmental Geochemistry; Prentice Hall Inc.: Upper Saddle River, NJ, USA, 1997; pp. 486–557. [Google Scholar]
- Katsoyiannis, I.A. Carbonate effects and pH-dependence of uranium sorption onto bacteriogenic iron oxides: Kinetic and equilibrium studies. J. Hazard. Mater. 2007, 139, 31–37. [Google Scholar] [CrossRef]
- Maher, K.; Bargar, J.R.; Brown, J.G.E. Environmental Speciation of Actinides. Inorg. Chem. 2012, 52, 3510–3532. [Google Scholar] [CrossRef]
- Abdelouas, A. Uranium Mill Tailings: Geochemistry, Mineralogy, and Environmental Impact. Elements 2006, 2, 335–341. [Google Scholar] [CrossRef]
- Kelly, S.D.; Rasbury, T.; Chattopadhyay, S.; Kropf, A.J.; Kemner, K.M. Evidence of a Stable Uranyl Site in Ancient Organic-Rich Calcite. Environ. Sci. Technol. 2006, 40, 2262–2268. [Google Scholar] [CrossRef] [PubMed]
- Porcelli, D.; Swarzenski, P.W. The behavior of U- and Th-series nuclides in groundwater. In Uranium-Series Geochemistry; Mineralogical Society of America: Chantilly, VA, USA, 2003; Volume 52, pp. 317–361. [Google Scholar]
- Finch, R.; Murakami, T. Systematics, Paragenesis of U minerals. In Uranium: Mineralogy, Geochemistry and the Environment; Mineralogical Society of America: Chantilly, VA, USA, 1999; Volume 38, pp. 91–180. [Google Scholar]
- Finch, R.; Ewing, R.C. The corrosion of uraninite under oxidizing conditions. J. Nucl. Mater. 1992, 190, 133–156. [Google Scholar] [CrossRef]
- Plášil, J. Oxidation-hydration weathering of uraninite: The current state-of-knowledge. J. Geosci. 2014, 59, 99–114. [Google Scholar] [CrossRef]
- Nolan, J.O. Mobilization of Naturally Occurring Uranium in Sediment into Groundwater. Ph.D. Thesis, University of Nebraska, Lincoln, NE, USA, July 2016. [Google Scholar]
- Woo, N.; Choi, M.; Lee, K. Assessment of Groundwater Quality and Contamination from Uranium-Bearing Black Shale in Goesan–Boeun Areas, Korea. Environ. Geochem. Health 2002, 24, 264–273. [Google Scholar] [CrossRef]
- Hsi, C.-K.D.; Langmuir, D. Adsorption of uranyl onto ferric oxyhydroxides: Application of the surface complexation site-binding model. Geochim. Cosmochim. Acta 1985, 49, 1931–1941. [Google Scholar] [CrossRef]
- Campbell, K.; Gallegos, T.; Landa, E. Biogeochemical aspects of uranium mineralization, mining, milling, and remediation. Appl. Geochem. 2015, 57, 206–235. [Google Scholar] [CrossRef]
- Asikainen, M.; Kahlos, H. Anomalously high concentrations of uranium, radium and radon in water from drilled wells in the Helsinki region. Geochim. Cosmochim. Acta 1979, 43, 1681–1686. [Google Scholar] [CrossRef]
- Bhoopathi, V.; Kumar, S.N.; Sastry, R.S.N.; Srinivas, B. Surface radioactive anomalies at Srisailam sub basin, Cuddapah basin, India. Chin. J. Geochem. 2015, 34, 265–271. [Google Scholar] [CrossRef]
- Focazio, M.J.; Tipton, D.; Shapiro, S.D.; Geiger, L.H. The Chemical Quality of Self-Supplied Domestic Well Water in the United States. Ground Water Monit. Remediat. 2006, 26, 92–104. [Google Scholar] [CrossRef]
- Bottrell, S.H. Redistribution of uranium by physical processes during weathering and implications for radon production. Environ. Geochem. Health 1993, 15, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Przylibski, T.A. Estimating the radon emanation coefficient from crystalline rocks into groundwater. Appl. Radiat. Isot. 2000, 53, 473–479. [Google Scholar] [CrossRef]
- Wanty, R.B.; Lawrence, E.P.; Gundersen, L.C.S. A theoretical model for the flux of radon from rock to ground water. In Geologic Controls on Radon; Geological Society of America: Boulder, CO, USA, 1992; pp. 73–78. [Google Scholar] [CrossRef]

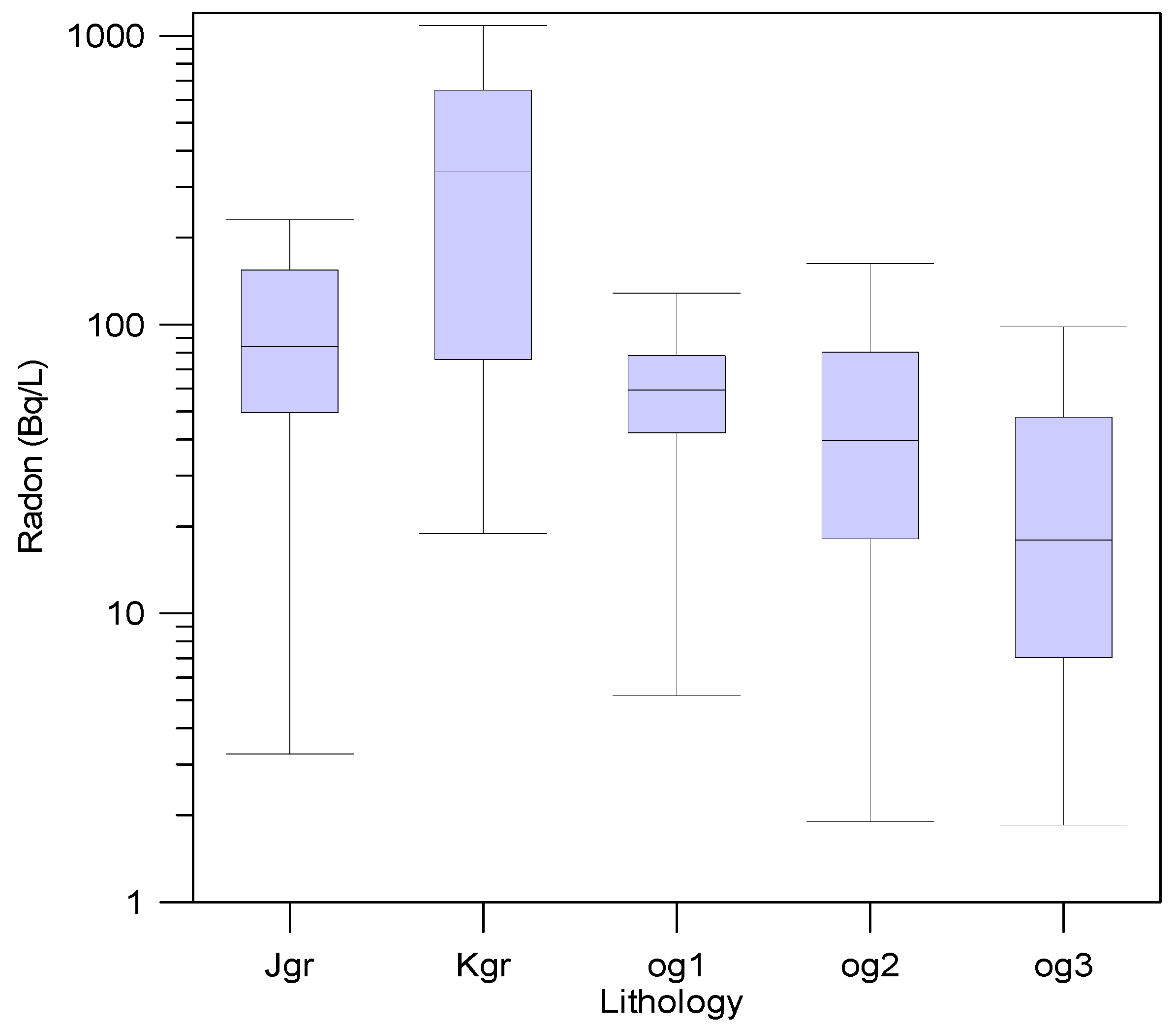
| U (μg/L) | Jgr | Kgr | og1 | og2 | og3 | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| <1.0 | 18 | 32.1 | 7 | 28.0 | 34 | 79.1 | 22 | 57.9 | 23 | 60.5 | 104 | 52.0 |
| 1.1–3.0 | 17 | 30.4 | 1 | 4.0 | 7 | 16.3 | 11 | 28.9 | 12 | 31.6 | 48 | 24.0 |
| 3.1–10.0 | 14 | 25.0 | 11 | 44.0 | 0 | 0.0 | 3 | 7.9 | 3 | 7.9 | 31 | 15.5 |
| 10.1–30.0 | 6 | 10.7 | 3 | 12.0 | 2 | 4.7 | 2 | 5.3 | 0 | 0.0 | 13 | 6.5 |
| 30.1< | 1 | 1.8 | 3 | 12.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 4 | 2.0 |
| Total | 56 | 100 | 25 | 100 | 43 | 100 | 38 | 100 | 38 | 100 | 200 | 100 |
| Rn (Bq/L) | Jgr | Kgr | og1 | og2 | og3 | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| <11.1 | 4 | 7.1 | 0 | 0.0 | 1 | 2.3 | 4 | 10.5 | 13 | 34.2 | 22 | 11.0 |
| 11.2–37.0 | 5 | 8.9 | 3 | 12.0 | 8 | 18.6 | 14 | 36.8 | 13 | 34.2 | 43 | 21.5 |
| 37.1–148 | 33 | 58.9 | 7 | 28.0 | 34 | 79.1 | 17 | 44.7 | 11 | 28.9 | 102 | 51.0 |
| 148.1–300.0 | 12 | 21.4 | 0 | 0.0 | 0 | 0.0 | 1 | 2.6 | 1 | 2.6 | 14 | 7.0 |
| 300.1< | 2 | 3.6 | 15 | 60.0 | 0 | 0.0 | 2 | 5.3 | 0 | 0.0 | 19 | 9.5 |
| Total | 56 | 100 | 25 | 100 | 43 | 100 | 38 | 100 | 38 | 100 | 200 | 100 |
| Species | 1 G7 | 2 G29 | 2 G36 | 2 G37 | 3 G44 | 2 G46 | 4 G52 | 2 G86 | 4 G109 |
|---|---|---|---|---|---|---|---|---|---|
| Ca2UO2(CO3)3 (aq) | 71.20 | 59.47 | 51.80 | 51.19 | 4.95 | 42.33 | 48.27 | 60.67 | 74.48 |
| CaUO2(CO3)32− | 28.42 | 38.92 | 45.73 | 45.58 | 66.12 | 51.78 | 49.37 | 38.38 | 25.13 |
| UO2(CO3)22− | 0.04 | 0.82 | 1.40 | 2.00 | 0.24 | 3.86 | 1.13 | 0.31 | 0.11 |
| UO2(CO3)34− | 0.35 | 0.66 | 0.94 | 0.95 | 28.70 | 1.39 | 1.17 | 0.63 | 0.28 |
| UO2CO3 (aq) | 0.06 | 0.12 | 0.24 | 0.57 | 0.06 | ||||
| UO2H3SiO4+ | 0.01 | 0.02 | |||||||
| UO2OH+ | |||||||||
| UO2(OH)2 (aq) | 0.01 | 0.03 | |||||||
| (UO2)2CO3(OH)3− | 0.01 | ||||||||
| Species | 4 G124 | 4 G151 | 4 G155 | 4 G157 | 3 G163 | 2 G181 | 4 G182 | 1 G195 | |
| Ca2UO2(CO3)3 (aq) | 47.98 | 41.72 | 57.69 | 74.14 | 48.01 | 59.53 | 54.32 | 64.86 | |
| CaUO2(CO3)32− | 50.09 | 41.13 | 40.95 | 24.86 | 48.95 | 39.02 | 36.65 | 34.36 | |
| UO2(CO3)22− | 0.59 | 9.97 | 0.62 | 0.60 | 1.72 | 0.05 | 5.37 | 0.24 | |
| UO2(CO3)34− | 1.33 | 0.99 | 0.71 | 0.30 | 1.17 | 0.73 | 0.69 | 0.54 | |
| UO2CO3 (aq) | 0.02 | 5.88 | 0.03 | 0.10 | 0.14 | 0.68 | 2.80 | ||
| UO2H3SiO4+ | 0.15 | 0.10 | |||||||
| UO2OH+ | 0.07 | 0.04 | |||||||
| UO2(OH)2 (aq) | 0.04 | 0.02 | |||||||
| (UO2)2CO3(OH)3− | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, B.-W.; Kim, D.-S.; Kim, M.-S.; Hwang, J.-H.; Choo, C.-O. Hydrogeochemical Characteristics of Uranium and Radon in Groundwater from the Goesan Area of the Ogcheon Metamorphic Belt (OMB), Korea. Sustainability 2021, 13, 11261. https://doi.org/10.3390/su132011261
Cho B-W, Kim D-S, Kim M-S, Hwang J-H, Choo C-O. Hydrogeochemical Characteristics of Uranium and Radon in Groundwater from the Goesan Area of the Ogcheon Metamorphic Belt (OMB), Korea. Sustainability. 2021; 13(20):11261. https://doi.org/10.3390/su132011261
Chicago/Turabian StyleCho, Byong-Wook, Dong-Soo Kim, Moon-Su Kim, Jae-Hong Hwang, and Chang-Oh Choo. 2021. "Hydrogeochemical Characteristics of Uranium and Radon in Groundwater from the Goesan Area of the Ogcheon Metamorphic Belt (OMB), Korea" Sustainability 13, no. 20: 11261. https://doi.org/10.3390/su132011261
APA StyleCho, B.-W., Kim, D.-S., Kim, M.-S., Hwang, J.-H., & Choo, C.-O. (2021). Hydrogeochemical Characteristics of Uranium and Radon in Groundwater from the Goesan Area of the Ogcheon Metamorphic Belt (OMB), Korea. Sustainability, 13(20), 11261. https://doi.org/10.3390/su132011261






