Intensified Interspecific Competition for Water after Afforestation with Robinia pseudoacacia into a Native Shrubland in the Taihang Mountains, Northern China
Abstract
1. Introduction
2. Materials and Methods
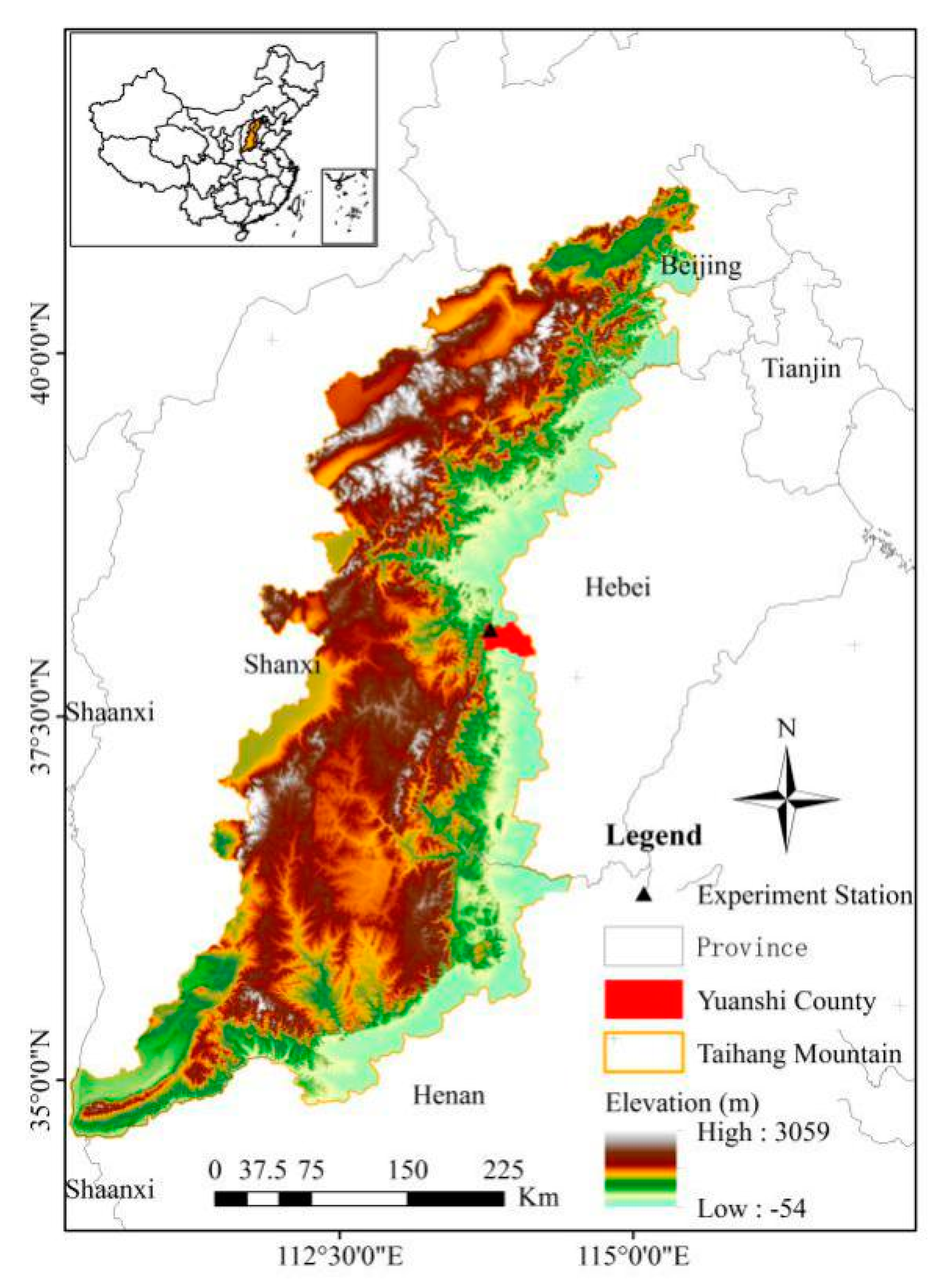
2.1. Study Sites and Target Plant Species
2.2. Sample Collection and Stable Isotope Analyses
2.3. Quantifying the Proportion of Water Use Sources
2.4. Species Niche Overlap of Water Use
2.5. Statistical Analysis
3. Results
3.1. Variation in Soil Water Content
3.2. Isotopic Values of All Water Samples
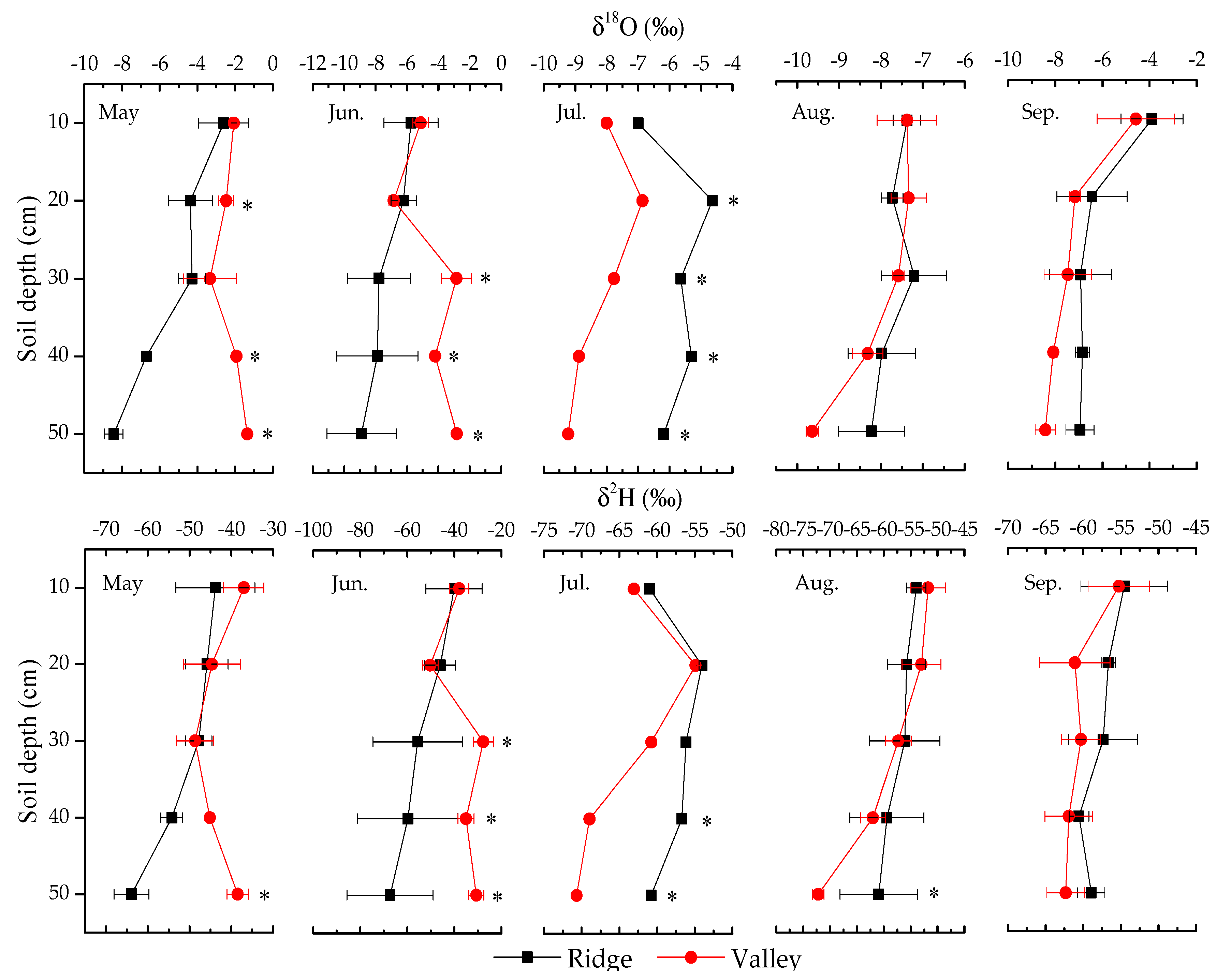
3.2.1. Variation in Soil Water Isotopic Values
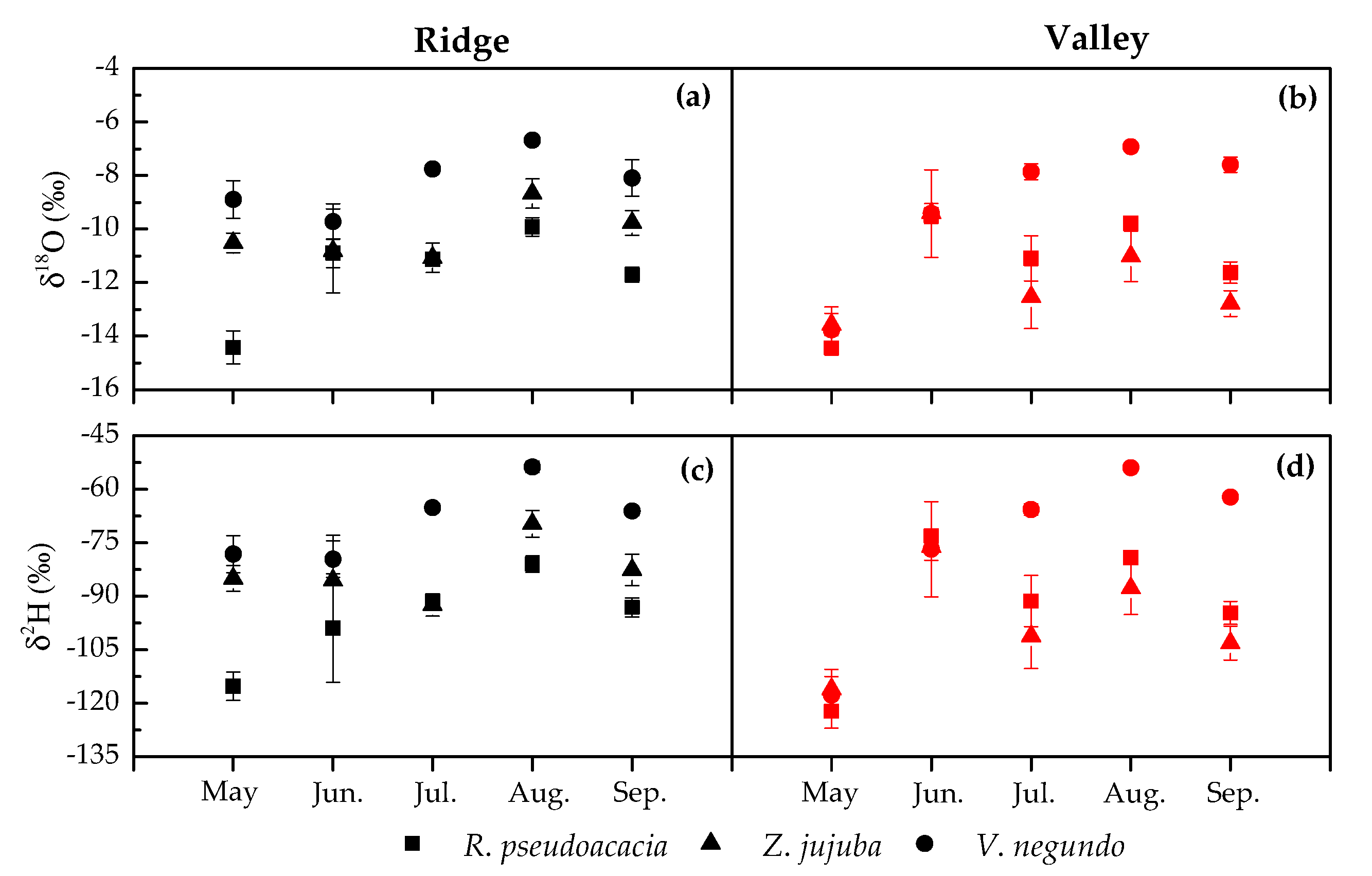
3.2.2. Variation in Plant Xylem Water Isotopic Values
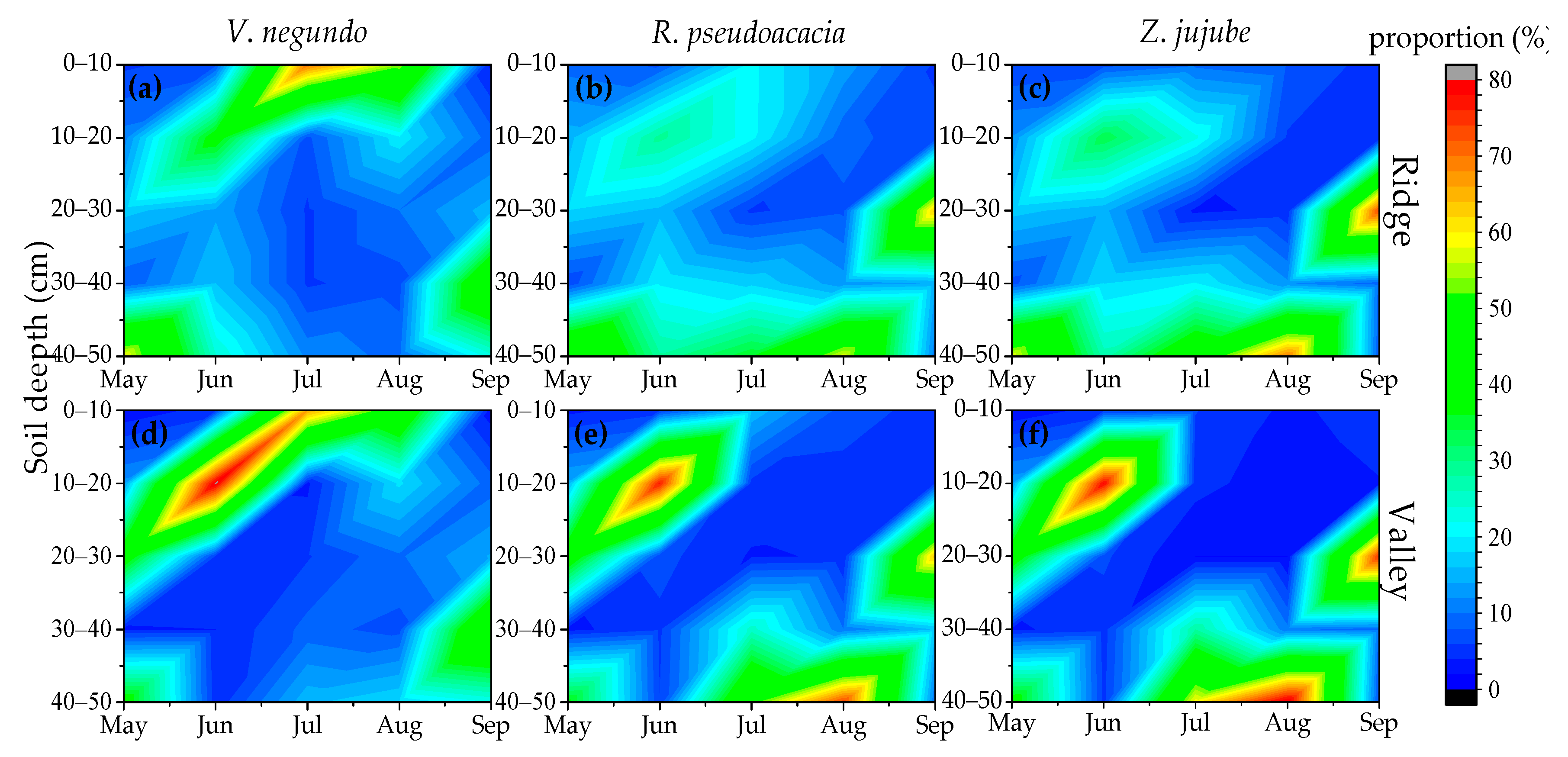
3.3. Proportion of Plant Water Source Utilization
3.4. Interspecific Niche Overlap
4. Discussion
4.1. Soil Water Content and Its Isotopic Composition
4.2. Seasonal Variation of Water Source Use in Different Species
4.3. Niche Overlap in Water Source Use and Interspecies Relationship
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nunez-Mir, G.C.; Iannone, B.V.; Curtis, K.; Fei, S. Evaluating the evolution of forest restoration research in a changing world: A “big literature” review. New For. 2015, 46, 669–682. [Google Scholar] [CrossRef]
- Li, W.H. Degradation and restoration of forest ecosystems in China. For. Ecol. Manag. 2004, 201, 33–41. [Google Scholar] [CrossRef]
- Wang, G.; Innes, J.L.; Lei, J.; Dai, S.; Wu, S.W. Ecology—China’s forestry reforms. Science 2007, 318, 1556–1557. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Dong, G.; Zhang, Y.; Zhang, F.; Wang, M. Patterns of species and phylogenetic diversity of Pinus tabuliformis forests in the eastern Loess Plateau, China. For. Ecol. Manag. 2017, 394, 42–51. [Google Scholar] [CrossRef]
- Yang, B.; Wen, X.; Sun, X. Seasonal variations in depth of water uptake for a subtropical coniferous plantation subjected to drought in an East Asian monsoon region. Agric. For. Meteorol. 2015, 201, 218–228. [Google Scholar] [CrossRef]
- Cao, J.; Liu, C.; Zhang, W.; Han, S. Using temperature effect on seepage variations as proxy for phenological processes of basin-scale vegetation communities. Hydrol. Process. 2013, 27, 360–366. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, W.; Yang, F.; Zhou, X.; Liu, Z.; Qu, F.; Lian, S.; Wang, C.; Tang, X. Changes in vegetation–environment relationships over long-term natural restoration process in Middle Taihang Mountain of North China. Ecol. Eng. 2012, 49, 193–200. [Google Scholar] [CrossRef]
- Fu, B.J.; Chen, L.D. Agricultural landscape spatial pattern analysis in the semi-arid hill area of the Loess Plateau, China. J. Arid Environ. 2000, 44, 291–303. [Google Scholar] [CrossRef]
- McVicar, T.R.; Van Niel, T.G.; Li, L.; Wen, Z.; Yang, Q.; Li, R.; Jiao, F. Parsimoniously modelling perennial vegetation suitability and identifying priority areas to support China’s re-vegetation program in the Loess Plateau: Matching model complexity to data availability. For. Ecol. Manag. 2010, 259, 1277–1290. [Google Scholar] [CrossRef]
- Liu, X.; Lu, Y.; Xie, Y.; Xue, Y. The positive interaction between two nonindigenous species, Casuarina (Casuarina equisetifolia) and Acacia (Acacia mangium), in the tropical coastal zone of south China: Stand dynamics and soil nutrients. Trop. Conserv. Sci. 2015, 8, 598–609. [Google Scholar] [CrossRef]
- Cao, S.; Tian, T.; Chen, L.; Dong, X.; Yu, X.; Wang, G. Damage caused to the environment by reforestation policies in arid and semi-arid areas of China. Ambio 2010, 39, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Chen, L.; Shankman, D.; Wang, C.; Wang, X.; Zhang, H. Excessive reliance on afforestation in China’s arid and semi-arid regions: Lessons in ecological restoration. Earth-Sci. Rev. 2011, 104, 240–245. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Z.; Dang, P.; Zhu, H.; Gao, Y.; Ha, V.N.; Zhao, Z. Response of soil microbial community dynamics to Robinia pseudoacacia L. afforestation in the Loess Plateau: A chronosequence approach. Plant Soil 2017, 423, 327–338. [Google Scholar] [CrossRef]
- Yang, X.; Yan, D.; Liu, C. Natural regeneration of trees in three types of afforested stands in the Taihang Mountains, China. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Kou, M.; Garcia-Fayos, P.; Hu, S.; Jiao, J. The effect of Robinia pseudoacacia afforestation on soil and vegetation properties in the Loess Plateau (China): A chronosequence approach. For. Ecol. Manag. 2016, 375, 146–158. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, W.; Zhong, Z.; Guo, S.; Han, X.; Yang, G.; Ren, C.; Chen, Z.; Dai, Y.; Qiao, W. Vegetation restoration alters the diversity and community composition of soil nitrogen-fixing microorganisms in the Loess Hilly Region of China. Soil Sci. Soc. Am. J. 2019, 83, 1378–1386. [Google Scholar] [CrossRef]
- Zhang, Q.; Wei, W.; Chen, L.; Yang, L. The joint effects of precipitation gradient and afforestation on soil moisture across the Loess Plateau of China. Forests 2019, 10, 285. [Google Scholar] [CrossRef]
- Bowling, D.R.; Schulze, E.S.; Hall, S.J. Revisiting streamside trees that do not use stream water: Can the two water worlds hypothesis and snowpack isotopic effects explain a missing water source? Ecohydrology 2016, 10, e1771. [Google Scholar] [CrossRef]
- Eggemeyer, K.D.; Awada, T.; Harvey, F.E.; Wedin, D.A.; Zhou, X.; Zanner, C.W. Seasonal changes in depth of water uptake for encroaching trees Juniperus virginiana and Pinus ponderosa and two dominant C4 grasses in a semiarid grassland. Tree Physiol. 2009, 29, 157–169. [Google Scholar] [CrossRef]
- Grossiord, C.; Sevanto, S.; Dawson, T.E.; Adams, H.D.; Collins, A.D.; Dickman, L.T.; Newman, B.D.; Stockton, E.A.; McDowell, N.G. Warming combined with more extreme precipitation regimes modifies the water sources used by trees. New Phytol. 2017, 213, 584–596. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, X.; Jia, G.; Jia, J.; Lou, Y.; Lu, W. Contrasting water sources of evergreen and deciduous tree species in rocky mountain area of Beijing, China. Catena 2017, 150, 108–115. [Google Scholar] [CrossRef]
- Nippert, J.B.; Knapp, A.K. Soil water partitioning contributes to species coexistence in tallgrass prairie. Oikos 2007, 116, 1017–1029. [Google Scholar] [CrossRef]
- Sun, S.J.; Meng, P.; Zhang, J.S.; Wan, X. Variation in soil water uptake and its effect on plant water status in Juglans regia L. during dry and wet seasons. Tree Physiol. 2011, 31, 1378–1389. [Google Scholar] [CrossRef] [PubMed]
- Tao, F.; Zhang, Z. Adaptation of maize production to climate change in North China Plain: Quantify the relative contributions of adaptation options. Eur. J. Agron. 2010, 33, 103–116. [Google Scholar] [CrossRef]
- Gao, X.; Wu, P.; Zhao, X.; Shi, Y.; Wang, J.; Zhang, B. Soil moisture variability along transects over a well-developed gully in the Loess Plateau, China. Catena 2011, 87, 357–367. [Google Scholar] [CrossRef]
- Moreno-de las Heras, M.; Espigares, T.; Merino-Martín, L.; Nicolau, J.M. Water-related ecological impacts of rill erosion processes in Mediterranean-dry reclaimed slopes. Catena 2011, 84, 114–124. [Google Scholar] [CrossRef]
- Guderle, M.; Bachmann, D.; Milcu, A.; Gockele, A.; Bechmann, M.; Fischer, C.; Roscher, C.; Landais, D.; Ravel, O.; Devidal, S.; et al. Dynamic niche partitioning in root water uptake facilitates efficient water use in more diverse grassland plant communities. Funct. Ecol. 2017, 32, 214–227. [Google Scholar] [CrossRef]
- Levine, J.M.; HilleRisLambers, J. The importance of niches for the maintenance of species diversity. Nature 2009, 461, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.; Wiegand, K.; Getzin, S. Walter’s two-layer hypothesis revisited: Back to the roots! Oecologia 2013, 172, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zheng, X.J.; Tang, L.S.; Li, Y. Stable oxygen isotopes reveal distinct water use patterns of two Haloxylon species in the Gurbantonggut Desert. Plant Soil 2015, 389, 73–87. [Google Scholar] [CrossRef]
- Rossatto, D.R.; da Silveira Lobo Sternberg, L.; Franco, A.C. The partitioning of water uptake between growth forms in a neotropical savanna: Do herbs exploit a third water source niche? Plant Biol. 2013, 15, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, H.; Zheng, X.J.; Li, Y.; Tang, L.S. Seasonal changes in the water use strategies of three co-occurring desert shrubs. Hydrol. Process. 2014, 28, 6265–6275. [Google Scholar] [CrossRef]
- Yang, H.; Auerswald, K.; Bai, Y.; Han, X. Complementarity in water sources among dominant species in typical steppe ecosystems of Inner Mongolia, China. Plant Soil 2011, 340, 303–313. [Google Scholar] [CrossRef]
- Zunzunegui, M.; Boutaleb, S.; Diaz Barradas, M.C.; Esquivias, M.P.; Valera, J.; Jauregui, J.; Tagma, T.; Ain-Lhout, F. Reliance on deep soil water in the tree species Argania spinosa. Tree Physiol. 2018, 38, 678–689. [Google Scholar] [CrossRef]
- David, T.S.; Henriques, M.O.; Kurz-Besson, C.; Nunes, J.; Valente, F.; Vaz, M.; Pereira, J.S.; Siegwolf, R.; Chaves, M.M.; Gazarini, L.C.; et al. Water-use strategies in two co-occurring Mediterranean evergreen oaks: Surviving the summer drought. Tree Physiol. 2007, 27, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Ehleringer, J.R.; Dawson, T.E. Water uptake by plants: Perspectives from stable isotope composition. Plant Cell Environ. 1992, 15, 1073–1082. [Google Scholar] [CrossRef]
- Ohte, N.; Koba, K.; Yoshikawa, K.; Sugimoto, A.; Matsuo, N.; Kabeya, N.; Wang, L. Water utilization of natural and planted trees in the semiarid desert of Inner Mongolia, China. Ecol. Appl. 2003, 13, 337–351. [Google Scholar] [CrossRef]
- Valentini, R.; Mugnozza, G.E.S.; Ehleringer, J.R. Hydrogen and carbon isotope ratios of selected species of a Mediterranean Macchia Ecosystem. Funct. Ecol. 1992, 6, 627. [Google Scholar] [CrossRef]
- Williams, D.G.; Ehleringer, J.R. Intra- and interspecific variation for summer precipitation use in Pinyon-Juniper Woodlands. Ecol. Monogr. 2000, 70, 517–537. [Google Scholar] [CrossRef]
- Wu, H.; Li, X.Y.; Jiang, Z.; Chen, H.; Zhang, C.; Xiao, X. Contrasting water use pattern of introduced and native plants in an alpine desert ecosystem, Northeast Qinghai-Tibet Plateau, China. Sci. Total Environ. 2016, 542, 182–191. [Google Scholar] [CrossRef]
- Walter, H. Ecology of Tropical and Subtropical Vegetation; Oliver & Boyd: Edinburgh, UK, 1971; Volume 28, p. 165. [Google Scholar] [CrossRef]
- Penna, D.; Oliviero, O.; Assendelft, R.; Zuecco, G.; van Meerveld, I.; Anfodillo, T.; Carraro, V.; Borga, M.; Dalla Fontana, G. Tracing the water sources of trees and streams: Isotopic analysis in a small pre-alpine catchment. In Proceedings of the Four Decades of Progress in Monitoring and Modeling of Processes in the Soil-Plant-Atmosphere System: Applications and Challenges, Italy, 19–21 June 2013; Romano, N., Durso, G., Severino, G., Chirico, G.B., Palladino, M., Eds.; Elsevier Procedia Environmental Sciences: Amsterdam, The Netherlands, 2013; Volume 19, pp. 106–112. [Google Scholar]
- White, J.C.; Smith, W.K. Seasonal variation in water sources of the riparian tree species Acer negundo and Betula nigra, southern Appalachian foothills, USA. Botany 2015, 93, 519–528. [Google Scholar] [CrossRef]
- Barbeta, A.; Mejia-Chang, M.; Ogaya, R.; Voltas, J.; Dawson, T.E.; Penuelas, J. The combined effects of a long-term experimental drought and an extreme drought on the use of plant-water sources in a Mediterranean forest. Glob. Chang. Biol. 2015, 21, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.F.; Fang, J.; Liu, S.; Zhao, X.Y.; Li, S.G. Stable isotopic observation of water use sources of Pinus sylvestris var. mongolica in Horqin Sandy Land, China. Trees-Struct. Funct. 2013, 27, 1249–1260. [Google Scholar] [CrossRef]
- De Deurwaerder, H.; Herve-Fernandez, P.; Stahl, C.; Burban, B.; Petronelli, P.; Hoffman, B.; Bonal, D.; Boeckx, P.; Verbeeck, H. Liana and tree below-ground water competition-evidence for water resource partitioning during the dry season. Tree Physiol. 2018, 38, 1071–1083. [Google Scholar] [CrossRef]
- Antunes, C.; Díaz-Barradas, M.C.; Zunzunegui, M.; Vieira, S.; Máguas, C.; Paruelo, J. Water source partitioning among plant functional types in a semi-arid dune ecosystem. J. Veg. Sci. 2018, 29, 671–683. [Google Scholar] [CrossRef]
- Guo, J.S.; Hungate, B.A.; Kolb, T.E.; Koch, G.W. Water source niche overlap increases with site moisture availability in woody perennials. Plant Ecol. 2018, 219, 719–735. [Google Scholar] [CrossRef]
- Higgins, S.I.; Delgado-Cartay, M.D.; February, E.C.; Combrink, H.J. Is there a temporal niche separation in the leaf phenology of savanna trees and grasses? J. Biogeogr. 2011, 38, 2165–2175. [Google Scholar] [CrossRef]
- Saha, A.K.; da Silveira Lobo O’Reilly Sternberg, L.; Miralles-Wilhelm, F. Linking water sources with foliar nutrient status in upland plant communities in the Everglades National Park, USA. Ecohydrology 2009, 2, 42–54. [Google Scholar] [CrossRef]
- Antunes, C.; Díaz Barradas, M.C.; Zunzunegui, M.; Vieira, S.; Pereira, Â.; Anjos, A.; Correia, O.; Pereira, M.J.; Máguas, C.; Llorens, L. Contrasting plant water-use responses to groundwater depth in coastal dune ecosystems. Funct. Ecol. 2018, 32, 1931–1943. [Google Scholar] [CrossRef]
- Drake, P.L.; Franks, P.J. Water resource partitioning, stem xylem hydraulic properties, and plant water use strategies in a seasonally dry riparian tropical rainforest. Oecologia 2003, 137, 321–329. [Google Scholar] [CrossRef]
- Máguas, C.; Rascher, K.G.; Martins-Loução, A.; Carvalho, P.; Pinho, P.; Ramos, M.; Correia, O.; Werner, C. Responses of woody species to spatial and temporal ground water changes in coastal sand dune systems. Biogeosciences 2011, 8, 3823–3832. [Google Scholar] [CrossRef]
- Si, J.; Feng, Q.; Cao, S.; Yu, T.; Zhao, C. Water use sources of desert riparian Populus euphratica forests. Environ. Monit. Assess. 2014, 186, 5469–5477. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Deng, L.; Yan, W.M.; Shangguan, Z.P. Interaction of soil water storage dynamics and long-term natural vegetation succession on the Loess Plateau, China. Catena 2016, 137, 52–60. [Google Scholar] [CrossRef]
- Han, S.; Yang, Y.; Fan, T.; Xiao, D.; Moiwo, J.P. Precipitation-runoff processes in Shimen hillslope micro-catchment of Taihang Mountain, north China. Hydrol. Process. 2012, 26, 1332–1341. [Google Scholar] [CrossRef]
- Liang, H.; Xue, Y.; Li, Z.; Wang, S.; Wu, X.; Gao, G.; Liu, G.; Fu, B. Soil moisture decline following the plantation of Robinia pseudoacacia forests: Evidence from the Loess Plateau. For. Ecol. Manag. 2018, 412, 62–69. [Google Scholar] [CrossRef]
- Liu, X.P.; Zhang, W.J.; Hu, C.S.; Tang, X.G. Soil greenhouse gas fluxes from different tree species on Taihang Mountain, North China. Biogeosciences 2014, 11, 1649–1666. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, W.; Shen, H.; Cao, J.; Zhao, X. Soil respiration response in different vegetation types at Mount Taihang, China. Catena 2014, 116, 78–85. [Google Scholar] [CrossRef]
- Martí-Gómez, P.; Serrano, L.; Ferrio, J.P. Short-term dynamics of evaporative enrichment of xylem water in woody stems: Implications for ecohydrology. Tree Physiol. 2017, 37, 511–522. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, W.; Currell, M.; Yang, Y.; Zhao, H.; Lv, M. Relationship between land-use and sources and fate of nitrate in groundwater in a typical recharge area of the North China Plain. Sci. Total Environ. 2017, 609, 607–620. [Google Scholar] [CrossRef]
- Parnell, A.C.; Inger, R.; Bearhop, S.; Jackson, A.L. Source partitioning using stable isotopes: Coping with too much variation. PLoS ONE 2010, 5, e9672. [Google Scholar] [CrossRef]
- Stock, B.C.; Semmens, B.X. Unifying error structures in commonly used biotracer mixing models. Ecology 2016, 97, 2562–2569. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, N.; Fu, B. Inter-comparison of stable isotope mixing models for determining plant water source partitioning. Sci. Total Environ. 2019, 666, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.E.; Pate, J.S. Seasonal water uptake and movement in root systems of Australian phraeatophytic plants of dimorphic root morphology: A stable isotope investigation. Oecologia 1996, 107, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fu, B.; Lu, N.; Zhang, L. Seasonal variation in water uptake patterns of three plant species based on stable isotopes in the semi-arid Loess Plateau. Sci. Total Environ. 2017, 609, 27–37. [Google Scholar] [CrossRef]
- Stock, B.C.; Semmens, B.X. MixSIAR User Manual, version 3.1; A framework for Bayesian mixing models in R; GitHub: San Francisco, CA, USA, 2013. [Google Scholar]
- Trogisch, S.; Salmon, Y.; He, J.S.; Hector, A.; Scherer-Lorenzen, M. Spatio-temporal water uptake patterns of tree saplings are not altered by interspecific interaction in the early stage of a subtropical forest. For. Ecol. Manag. 2016, 367, 52–61. [Google Scholar] [CrossRef]
- Hoekstra, N.J.; Finn, J.A.; Hofer, D.; Lüscher, A. The effect of drought and interspecific interactions on depth of water uptake in deep- and shallow-rooting grassland species as determined by δ18O natural abundance. Biogeosciences 2014, 11, 4493–4506. [Google Scholar] [CrossRef]
- Gazis, C.; Feng, X. A stable isotope study of soil water: Evidence for mixing and preferential flow paths. Geoderma 2004, 119, 97–111. [Google Scholar] [CrossRef]
- Tang, K.; Feng, X. The effect of soil hydrology on the oxygen and hydrogen isotopic compositions of plants’ source water. Earth Planet. Sci. Lett. 2001, 185, 355–367. [Google Scholar] [CrossRef]
- Deng, W.; Yu, X.X.; Jia, G.; Li, Y.; Liu, Y. An analysis of characteristics of hydrogen and oxygen stable isotopes in Jiufeng Mountain areas of Beijing. Adv. Water Sci. 2013, 24, 642–650. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, S.P.; Han, X.G.; Lin, G.H. Effects of long-term grazing on the morphological and functional traits of Leymus chinensis in the semiarid grassland of Inner Mongolia, China. Ecol. Res. 2009, 24, 99–108. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Zhao, S.; Ma, H.; Qi, G.; Guo, S. The depth of water taken up by walnut trees during different phenological stages in an irrigated arid hilly area in the Taihang Mountains. Forests 2019, 10, 121. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Q.R.; Fan, W.; Song, G.H. The relationship between secondary forest and environmental factors in the Southern Taihang Mountains. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.P.; Chen, H.S.; Wang, K.L.; Tan, W.; Deng, P.Y.; Yang, J. Seasonal water use patterns of woody species growing on the continuous dolostone outcrops and nearby thin soils in subtropical China. Plant Soil 2011, 341, 399–412. [Google Scholar] [CrossRef]
- Burgess, S.S.; Adams, M.A.; Turner, N.C.; Ong, C.K. The redistribution of soil water by tree root systems. Oecologia 1998, 115, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Hultine, K.R.; Williams, D.G.; Burgess, S.S.; Keefer, T.O. Contrasting patterns of hydraulic redistribution in three desert phreatophytes. Oecologia 2003, 135, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Midwood, A.J.; Boutton, T.W.; Archer, S.R.; Watts, S.E. Water use by woody plants on contrasting soils in a savanna parkland: Assessment with delta H-2 and delta O-18. Plant Soil 1998, 205, 13–24. [Google Scholar] [CrossRef]
- Zou, C.B.; Barnes, P.W.; Archer, S.; McMurtry, C.R. Soil moisture redistribution as a mechanism of facilitation in savanna tree-shrub clusters. Oecologia 2005, 145, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Monaco, T.A.; Johnson, D.A.; Creech, J.E. Morphological and physiological responses of the invasive weed Isatis tinctoria to contrasting light, soil-nitrogen and water. Weed Res. 2005, 45, 460–466. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, J.; Lu, Z.; Li, J.; Sun, J. Water-use strategies of coexisting shrub species in the Yellow River Delta, China. Can. J. For. Res. 2018, 48, 1099–1107. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Zhao, B.; Zhu, A.; Zhang, H.; Huang, P.; Li, X. Coupling a two-tip linear mixing model with a δD–δ18O plot to determine water sources consumed by maize during different growth stages. Field Crop. Res. 2011, 123, 196–205. [Google Scholar] [CrossRef]
- Dawson, T.E.; Mambelli, S.; Plamboeck, A.H.; Templer, P.H.; Tu, K.P. Stable isotopes in plant ecology. Annu. Rev. Ecol. Syst. 2002, 33, 507–559. [Google Scholar] [CrossRef]
- Ma, H.; Zhu, Q.; Zhao, W. Soil water response to precipitation in different micro-topographies on the semi-arid Loess Plateau, China. J. For. Res. 2018, 31, 245–256. [Google Scholar] [CrossRef]
- Araya, Y.N.; Silvertown, J.; Gowing, D.J.; McConway, K.J.; Linder, H.P.; Midgley, G. A fundamental, eco-hydrological basis for niche segregation in plant communities. New Phytol. 2011, 189, 253–258. [Google Scholar] [CrossRef]
- Silvertown, J. Plant coexistence and the niche. Trends Ecol. Evol. 2004, 19, 605–611. [Google Scholar] [CrossRef]
- Mazer, S.J.; Dudley, L.S.; Hove, A.A.; Emms, S.K.; Verhoeven, A.S. Physiological performance in Clarkia sister taxa with contrasting mating systems: Do early-flowering autogamous taxa avoid water stress relative to their pollinator-dependent counterparts? Int. J. Plant Sci. 2010, 171, 1029–1047. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, L.; Jiang, J. Vertical root distribution and root cohesion of typical tree species on the Loess Plateau, China. J. Arid Land 2014, 6, 601–611. [Google Scholar] [CrossRef]
- Li, L.; Gao, X.; Wu, P.; Zhao, X.; Li, H.; Ling, Q.; Sun, W. Soil water content and root patterns in a rain-fed jujube plantation across stand ages on the Loess Plateau of China. Land Degrad. Dev. 2017, 28, 207–216. [Google Scholar] [CrossRef]
- Mei, X.; Zhu, Q.; Ma, L.; Zhang, D.; Wang, Y.; Hao, W. Effect of stand origin and slope position on infiltration pattern and preferential flow on a Loess hillslope. Land Degrad. Dev. 2018, 29, 1353–1365. [Google Scholar] [CrossRef]
- Nicolescu, V.-N.; Redei, K.; Mason, W.L.; Vor, T.; Poeetzelsberger, E.; Bastien, J.-C.; Brus, R.; Bencat, T.; Dodan, M.; Cvjetkovic, B.; et al. Ecology, growth and management of black locust (Robinia pseudoacacia L.), a non-native species integrated into European forests. J. For. Res. 2020, 31, 1081–1101. [Google Scholar] [CrossRef]

| Microtopographic Site | Site Characteristic | Species | Life Form | Mean Height (m) | Average DBH (cm) |
|---|---|---|---|---|---|
| Ridge | On the top of slope, and the condition of solar radiation was the strongest | R. pseudoacacia | Arbor | 3.54 ± 0.20 b | 5.04 ± 0.22 b |
| Z. jujuba | Semi-arbor/ shrub | 4.00 ± 0.21 a | 5.21 ± 0.44 a | ||
| V. negundo | Shrub | 2.90 ± 0.13 a | 2.82 ± 0.15 a | ||
| Valley | On the bottom of slope, which connect with the flat terrain | R. pseudoacacia | Arbor | 5.35 ± 0.25 a | 8.04 ± 0.54 a |
| Z. jujuba | Semi-arbor/ shrub | 3.83 ± 0.28 b | 4.08 ± 0.42 b | ||
| V. negundo | Shrub | 2.79 ± 0.09 a | 2.35 ± 0.10 a |
| Fixed Effect | df | Soil Water Content (%) | δ18Osoil (‰) | δ2Hsoil (‰) | |||
|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | ||
| Microtopographic site | 1 | 82.978 | <0.001 | 10.428 | 0.002 | 7.487 | 0.007 |
| Periods of season | 4 | 191.476 | <0.001 | 77.205 | <0.001 | 33.773 | <0.001 |
| Soil depth | 4 | 75.515 | <0.001 | 13.471 | <0.001 | 7.703 | <0.001 |
| Microtopographic site × periods of season | 4 | 1.860 | 0.119 | 46.974 | <0.001 | 14.326 | <0.001 |
| Microtopographic site × soil depth | 4 | 1.659 | 0.161 | 3.122 | 0.019 | 1.571 | 0.189 |
| Soil depth × periods of season | 16 | 7.431 | <0.001 | 4.007 | <0.001 | 1.057 | 0.408 |
| Soil depth × periods of season × microtopographic site | 16 | 1.052 | 0.404 | 5.147 | <0.001 | 3.222 | <0.001 |
| Fixed Effect | df | δ18Oxylem (‰) | δ2Hxylem (‰) | ||
|---|---|---|---|---|---|
| F | p | F | p | ||
| Microtopographic site | 1 | 3.348 | 0.073 | 5.285 | 0.025 |
| Species | 2 | 29.098 | <0.001 | 32.173 | <0.001 |
| Periods of season | 4 | 14.104 | <0.001 | 23.995 | <0.001 |
| Species × microtopographic site | 2 | 4.270 | 0.019 | 4.362 | 0.018 |
| Microtopographic site × periods of season | 4 | 3.788 | 0.009 | 6.067 | <0.001 |
| Species × periods of season | 8 | 1.813 | 0.095 | 2.713 | 0.014 |
| Species × periods of season × microtopographic site | 8 | 1.073 | 0.396 | 1.005 | 0.443 |
| Periods of Season | Species | Ridge | Valley | ||||
|---|---|---|---|---|---|---|---|
| R. pseudoacacia | Z. jujuba | V. negundo | R. pseudoacacia | Z. jujuba | V. negundo | ||
| Dry season | R. pseudoacacia | 100% | 100% | ||||
| Z. jujuba | 94.20% | 100% | 97.73% | 100% | |||
| V. negundo | 90.43% | 96.13% | 100% | 96.38% | 98.55% | 100% | |
| Rainy season | R. pseudoacacia | 100% | 100% | ||||
| Z. jujuba | 82.20% | 100% | 83.98% | 100% | |||
| V. negundo | 23.58% | 6.43% | 100% | 18.95% | 3.38% | 100% | |
| Whole season | R. pseudoacacia | 100% | 100% | ||||
| Z. jujuba | 76.40% | 100% | 81.70% | 100% | |||
| V. negundo | 14.00% | 2.55% | 100% | 15.33% | 1.93% | 100% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; Li, W.; Shi, P.; Cao, J.; Zong, N.; Geng, S. Intensified Interspecific Competition for Water after Afforestation with Robinia pseudoacacia into a Native Shrubland in the Taihang Mountains, Northern China. Sustainability 2021, 13, 807. https://doi.org/10.3390/su13020807
Zhu W, Li W, Shi P, Cao J, Zong N, Geng S. Intensified Interspecific Competition for Water after Afforestation with Robinia pseudoacacia into a Native Shrubland in the Taihang Mountains, Northern China. Sustainability. 2021; 13(2):807. https://doi.org/10.3390/su13020807
Chicago/Turabian StyleZhu, Wanrui, Wenhua Li, Peili Shi, Jiansheng Cao, Ning Zong, and Shoubao Geng. 2021. "Intensified Interspecific Competition for Water after Afforestation with Robinia pseudoacacia into a Native Shrubland in the Taihang Mountains, Northern China" Sustainability 13, no. 2: 807. https://doi.org/10.3390/su13020807
APA StyleZhu, W., Li, W., Shi, P., Cao, J., Zong, N., & Geng, S. (2021). Intensified Interspecific Competition for Water after Afforestation with Robinia pseudoacacia into a Native Shrubland in the Taihang Mountains, Northern China. Sustainability, 13(2), 807. https://doi.org/10.3390/su13020807







