Valorization of Agro-Industrial Residues: Bioprocessing of Animal Fats to Reduce Their Acidity
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Fat Characterization
2.2. Reactants and Enzymes
- Lipozyme® TL 100 L, a 1,3 lipase originating from Thermomyces lanuginosus provided in liquid formulation. It is recommended by the producer as a very effective catalyst for transesterification, interesterification, ester hydrolysis, and desymmetrisation of esters, exhibiting a high degree of substrate selectivity.
- Lipozyme® CALB is a lipase from Candida antarctica B provided in liquid formulation. It is stable over a broad pH range, especially alkaline, exhibiting a high degree of substrate specificity.
- Novozym® 435 is a CALB lipase from Candida antarctica B immobilized on a hydrophobic carrier, an acrylic resin Lewatit VP OC 160. The physical appearance is white spherical beads with a particle diameter in the range of 252–687 μm.
- Lecitase® Ultra, a phospholipase A1 from Thermomyces lanuginosus provided in liquid formulation. It has inherent activity towards both phospholipid and triglyceride structures and is commonly applied for degumming and reducing the vegetable oil phosphatides.
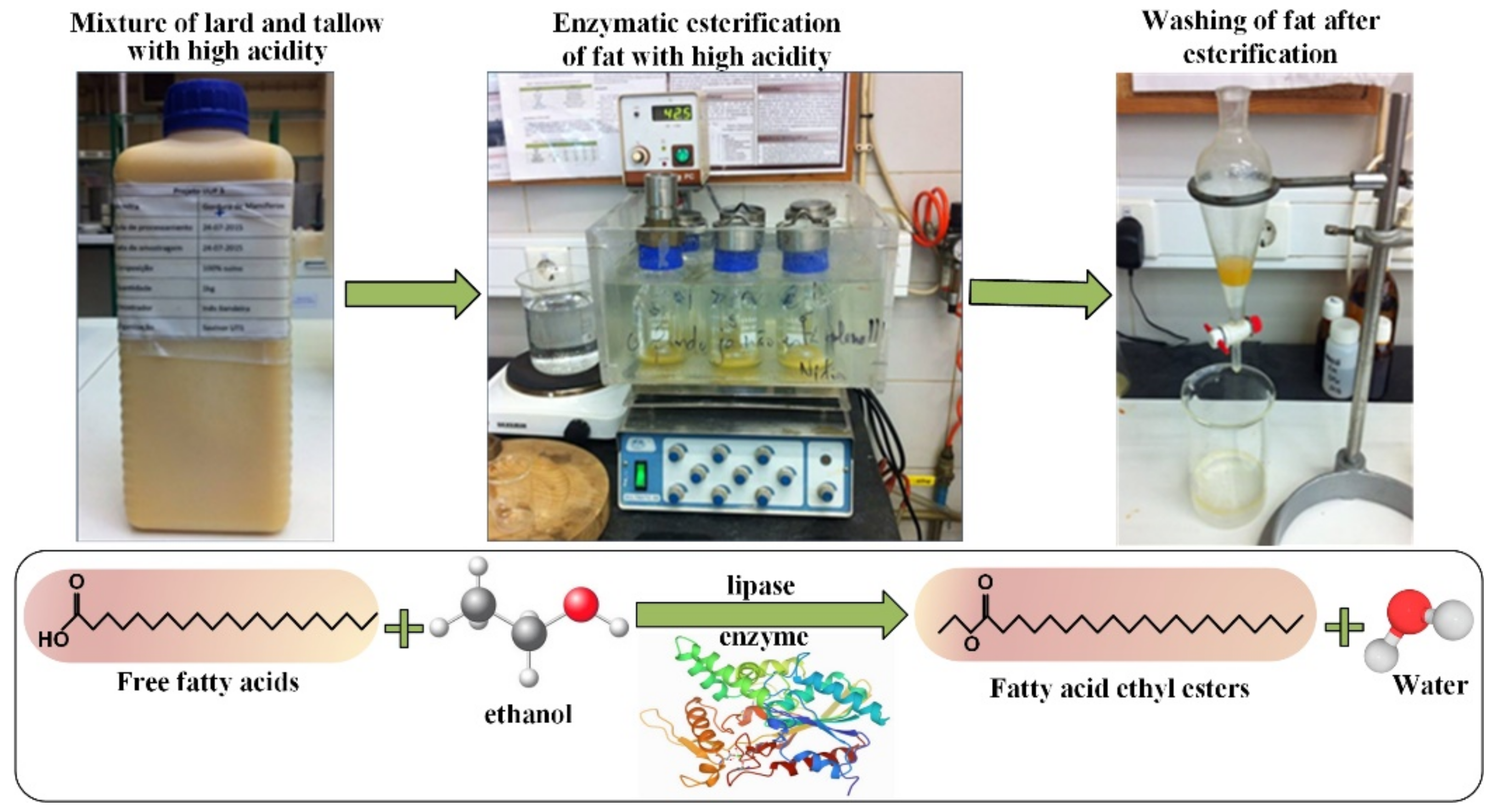
2.3. Procedure for the Esterification Assays
2.4. Parametric Study Experimental Strategy
- (i)
- First, the best enzyme was identified from a set of four commercial lipases.
- (ii)
- Then, for the selected enzyme, the most adequate operating conditions to perform the esterification were evaluated, aiming to maximize the acidity reduction.
- (iii)
- Finally, the reaction kinetics were determined, focusing on using simple reaction rate models, more suitable to design industrial scale processes, where the reactant mixtures are very complex and difficult to characterize.
- Enzyme/fat mass ratio,
- Alcohol/FFA mass ratio, and
- Reaction temperature.
- (1)
- Thus, a first set of six experimental assays was carried out at different reaction temperatures, keeping fixed the alcohol/FFA and the enzyme/fat mass ratios.
- (2)
- Then, a second set of four experiments was performed, varying the alcohol/FFA mass ratio and fixing the temperature and the enzyme/fat mass ratio, for which the percentage of acidity reduction determined in the previous set of experimental assays was higher.
- (3)
- Finally, a third set of four experiments was performed, varying the enzyme/fat mass ratio and keeping fixed the temperature and alcohol/FFA mass ratio, for which the percentage of acidity reduction determined in the previous sets of experimental assays was higher.
2.5. Kinetic Modeling
- First-order kinetics, a function of , taking into account that the concentration of alcohol varies little in the course of the reaction and that, after a reaction time of three hours, the chemical equilibrium is still far away.
- Second-order kinetic function of , again considering the small variation of the alcohol concentration during the assays and the possibility that the equilibrium impacts of the acidity reduction are negligible.
- Second-order kinetics function of oleic acid and ethanol concentration, , where is the ratio between the initial concentrations of oleic acid and ethanol, to better account for reaction stoichiometry and the potential influence of alcohol concentration in the reaction kinetics.
- Michaelis-Menten kinetics:, function of , where is the maximum reaction velocity, is the Michaelis-Menten constant, and , to verify if the reaction kinetics flow.
- The second-order reversible rate law is expressed as in Equation (7).where and represent the water and ester concentrations, respectively, and and represent the kinetic constant of the forward and reverse reactions, respectively. In this work, the concentration of oleic acid, , is limiting, and assuming that, at , the concentration of ester and water in the solution are very small and can be considered to be zero, Equation (7) can be re written in the form of Equation (8).where is the oleic acid conversion, directly related to the acidity reduction and defined by the relation , and is the initial concentration of oleic acid that can be determined directly from the sample initial acidity. To use the rate law defined by Equation (2), the general mass balance must the written in terms of , in the form of Equation (9).
3. Results
3.1. Fat Characterization
- 4.43 mg KOH/g fat of acid value
- 0.15 wt % of moisture content
- 2.2% of acidity or FFA content
- 49.8 mm2/s kinematic viscosity at 40 °C
- 920 kg/m3 density at 20 °C
- 94.5 g I2/100 g fat of iodine value.
3.2. Enzyme Selection
3.3. Reaction Conditions Parametric Study
3.4. Kinetic Rate Laws
4. Discussion
4.1. Adequacy of the Homogenous Media Assumption
4.2. Considerations on the Economic Feasibility of the New Process
4.3. Enzyme-Catalyzed Esterification vs. Other Approaches
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alao, B.O.; Falowo, A.B.; Chulayo, A.; Muchenje, V. The potential of animal by-products in food systems: Production, prospects and challenges. Sustainability 2017, 9, 1089. [Google Scholar] [CrossRef]
- Mata, T.M.; Pinto, F.; Caetano, N.; Martins, A.A. Economic and environmental analysis of animal fats acidity reduction by enzymatic esterification. J. Clean. Prod. 2018, 184, 481–489. [Google Scholar] [CrossRef]
- A Farm to Fork Strategy for a Fair, Healthy and Environmentally-Friendly Food System; COM (2020) 381 Final; European Commission: Brussels, Belgium, 2020; p. 19.
- Zagklis, D.; Konstantinidou, E.; Zafiri, C.; Kornaros, M. Assessing the economic viability of an animal byproduct rendering plant: Case study of a slaughterhouse in Greece. Sustainability 2020, 12, 5870. [Google Scholar] [CrossRef]
- Santagata, R.; Viglia, S.; Fiorentino, G.; Liu, G.; Ripa, M. Power generation from slaughterhouse waste materials. An emergy accounting assessment. J. Clean. Prod. 2019, 223, 536–552. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Valorization of waste frying oils and animal fats for biodiesel production. In Advanced Biofuels and Bioproducts; Lee, J., Ed.; Springer: New York, NY, USA, 2013; pp. 671–693. [Google Scholar]
- Shahzad, K.; Narodoslawsky, M.; Sagir, M.; Ali, N.; Ali, S.; Rashid, M.I.; Ismail, I.M.I.; Koller, M. Techno-economic feasibility of waste biorefinery: Using slaughtering waste streams as starting material for biopolyester production. Waste Manag. 2017, 67, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Muro-Small, M.L.; Neckers, D.C. A green route to petroleum feedstocks: Photochemistry of fats and oils. ACS Sustain. Chem. Eng. 2013, 1, 1214–1217. [Google Scholar] [CrossRef]
- Lasekan, A.; Abu Bakar, F.; Hashim, D. Potential of chicken by-products as sources of useful biological resources. Waste Manag. 2013, 33, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Vamvuka, D.; Dermitzakis, S.; Pentari, D.; Sfakiotakis, S. Valorization of Meat and Bone Meal through pyrolysis for soil amendment or lead adsorption from wastewaters. Food Bioprod. Process. 2018, 109, 148–157. [Google Scholar] [CrossRef]
- Zhang, C.; Garcia, R.A.; Piazza, G.J. Solubilization of meat & bone meal protein by dilute acid hydrolysis for the production of bio-based flocculant. Food Bioprod. Process. 2017, 102, 362–366. [Google Scholar] [CrossRef]
- Kazemi-Bonchenari, M.; Alizadeh, A.R.; Javadi, L.; Zohrevand, M.; Odongo, N.E.; Salem, A.Z.M. Use of poultry pre-cooked slaughterhouse waste as ruminant feed to prevent environmental pollution. J. Clean. Prod. 2017, 145, 151–156. [Google Scholar] [CrossRef]
- Etemadian, Y.; Ghaemi, V.; Shaviklo, A.R.; Pourashouri, P.; Sadeghi Mahoonak, A.R.; Rafipour, F. Development of animal/plant-based protein hydrolysate and its application in food, feed and nutraceutical industries: State of the art. J. Clean. Prod. 2021, 278, 123219. [Google Scholar] [CrossRef]
- UN SDG. United Nations Sustainable Development Goals. Available online: https://www.un.org/sustainabledevelopment/ (accessed on 22 June 2021).
- Gregg, J.S.; Jürgens, J.; Happel, M.K.; Strøm-Andersen, N.; Tanner, A.N.; Bolwig, S.; Klitkou, A. Valorization of bio-residuals in the food and forestry sectors in support of a circular bioeconomy: A review. J. Clean. Prod. 2020, 267, 122093. [Google Scholar] [CrossRef]
- Lauridsen, C.; Bruun Christensen, T.; Halekoh, U.; Krogh Jensen, S. Alternative fat sources to animal fat for pigs. Lipid Technol. 2007, 19, 156–159. [Google Scholar] [CrossRef]
- Mata, T.M.; Mendes, A.M.; Caetano, N.S.; Martins, A. Properties and Sustainability of Biodiesel from Animal Fats and Fish Oil. Chem. Eng. Trans. 2014, 38, 175–180. [Google Scholar] [CrossRef]
- Mata, T.M.; Trovisco, I.; Pinto, A.; Matos, E.; Martins, A.A.; Caetano, N.S. Enzymatic esterification for acidity reduction of poultry fat. Chem. Eng. Trans. 2017, 57, 2005–2010. [Google Scholar] [CrossRef]
- Mata, T.M.; Correia, D.; Pinto, A.; Andrade, S.; Trovisco, I.; Matos, E.; Martins, A.A.; Caetano, N.S. Fish oil acidity reduction by enzymatic esterification. Energy Procedia 2017, 136, 474–480. [Google Scholar] [CrossRef]
- Abdelmoez, W.; Mostafa, N.A.; Mustafa, A. Utilization of oleochemical industry residues as substrates for lipase production for enzymatic sunflower oil hydrolysis. J. Clean. Prod. 2013, 59, 290–297. [Google Scholar] [CrossRef]
- Caetano, N.S.; Teixeira, J.M.I.; Mata, T.M. Enzymatic catalysis of vegetable oil with ethanol in the presence of co-solvents. Chem. Eng. Trans. 2012, 26, 81–86. [Google Scholar] [CrossRef]
- Martins, A.A.; Pinto, F.; Caetano, N.S.; Mata, T.M. Acidity reduction in animal fats by enzymatic esterification: Economic and environmental analysis. Energy Procedia 2017, 136, 308–315. [Google Scholar] [CrossRef]
- Mata, T.M.; Andrade, S.; Correia, D.; Matos, E.; Martins, A.A.; Caetano, N.S. Acidity reduction of mammalian fat by enzymatic esterification. Energy Procedia 2017, 136, 290–295. [Google Scholar] [CrossRef]
- Mata, T.M.; Correia, D.; Andrade, S.; Casal, S.; Ferreira, I.M.P.L.V.O.; Matos, E.; Martins, A.A.; Caetano, N.S. Fish Oil Enzymatic Esterification for Acidity Reduction. Waste Biomass Valoriz. 2020, 11, 1131–1141. [Google Scholar] [CrossRef]
- Mata, T.M.; Sousa, I.R.B.G.; Caetano, N.S. Transgenic Corn Oil for Biodiesel Production Via Enzymatic Catalysis with Ethanol. Chem. Eng. Trans. 2012, 27, 19–24. [Google Scholar] [CrossRef]
- Caetano, N.S.; Caldeira, D.; Martins, A.A.; Mata, T.M. Valorisation of Spent Coffee Grounds: Production of Biodiesel via Enzymatic Catalysis with Ethanol and a Co-solvent. Waste Biomass Valoriz. 2017, 8, 1981–1994. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, K.; Sun, S. Simultaneous esterification and transesterification of waste phoenix seed oil with a high free fatty acid content using a free lipase catalyst to prepare biodiesel. Biomass Bioenergy 2021, 144, 105930. [Google Scholar] [CrossRef]
- Mata, T.M.; Sousa, I.R.B.G.; Vieira, S.S.; Caetano, N.S. Biodiesel production from corn oil via enzymatic catalysis with ethanol. Energy Fuels 2012, 26, 3034–3041. [Google Scholar] [CrossRef]
- Chai, M.; Tu, Q.; Lu, M.; Yang, Y.J. Esterification pretreatment of free fatty acid in biodiesel production, from laboratory to industry. Fuel Process. Technol. 2014, 125, 106–113. [Google Scholar] [CrossRef]
- Dias, J.M.; Alvim-Ferraz, M.C.M.; Almeida, M.F. Production of biodiesel from acid waste lard. Bioresour. Technol. 2009, 100, 6355–6361. [Google Scholar] [CrossRef] [PubMed]
- Pollardo, A.A.; Lee, H.-S.; Lee, D.; Kim, S.; Kim, J. Solvent effect on the enzymatic production of biodiesel from waste animal fat. J. Clean. Prod. 2018, 185, 382–388. [Google Scholar] [CrossRef]
- Cesarini, S.; Javier Pastor, F.I.; Nielsen, P.M.; Diaz, P. Moving towards a competitive fully enzymatic biodiesel process. Sustainability 2015, 7, 7884–7903. [Google Scholar] [CrossRef]
- Berrios, M.; Siles, J.; Martín, M.A.; Martín, A. A kinetic study of the esterification of free fatty acids (FFA) in sunflower oil. Fuel 2007, 86, 2383–2388. [Google Scholar] [CrossRef]
- Sun, J.; Liu, S.Q. Ester Synthesis in Aqueous Media by Lipase: Alcoholysis, Esterification and Substrate Hydrophobicity. J. Food Biochem. 2015, 39, 11–18. [Google Scholar] [CrossRef]
- Scrimgeour, C. Chemistry of fatty acids. In Bailey’s Industrial Oil and Fact Products; Shahidi, F., Ed.; Jonh Wiley and Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Saw, M.H.; Siew, W.L. The effectiveness of immobilized lipase Thermomyces lanuginosa in catalyzing interesterification of palm olein in batch reaction. J. Oleo Sci. 2014, 63, 295–302. [Google Scholar] [CrossRef][Green Version]
- Sousa, R.R.; Silva, A.S.; Fernandez-Lafuente, R.; Ferreira-Leitão, V.S. Solvent-free esterifications mediated by immobilized lipases: A review from thermodynamic and kinetic perspectives. Catal. Sci. Technol. 2021, 11, 5696–5711. [Google Scholar] [CrossRef]
- Alenezi, R.; Leeke, G.A.; Winterbottom, J.M.; Santos, R.C.D.; Khan, A.R. Esterification kinetics of free fatty acids with supercritical methanol for biodiesel production. Energy Convers. Manag. 2010, 51, 1055–1059. [Google Scholar] [CrossRef]
- Hussain, Z.; Kumar, R. Esterification of free fatty acids: Experiments, kinetic modeling, simulation & optimization. Int. J. Green Energy 2018, 15, 629–640. [Google Scholar] [CrossRef]
- Tesser, R.; Casale, L.; Verde, D.; Di Serio, M.; Santacesaria, E. Kinetics of free fatty acids esterification: Batch and loop reactor modeling. Chem. Eng. J. 2009, 154, 25–33. [Google Scholar] [CrossRef]
- Gofferjé, G.; Stäbler, A.; Herfellner, T.; Schweiggert-Weisz, U.; Flöter, E. Kinetics of enzymatic esterification of glycerol and free fatty acids in crude Jatropha oil by immobilized lipase from Rhizomucor miehei. J. Mol. Catal. B Enzym. 2014, 107, 1–7. [Google Scholar] [CrossRef]
- Ning, Z.; Zhang, H.; Li, W.; Zhang, R.; Liu, G.; Chen, C. Anaerobic digestion of lipid-rich swine slaughterhouse waste: Methane production performance, long-chain fatty acids profile and predominant microorganisms. Bioresour. Technol. 2018, 269, 426–433. [Google Scholar] [CrossRef]
- Radzi, S.M.; Mohamad, R.; Basri, M.; Salleh, A.B.; Ariff, A.; Rahman, M.B.A.; Abdul Rahman, R.N.Z.R. Kinetics of enzymatic synthesis of liquid wax ester from oleic acid and oleyl alcohol. J. Oleo Sci. 2010, 59, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, M.S.; Safinski, T.; Nelson, M.I.; Sidhu, H.S.; Adesina, A.A. Kinetic analysis of oleic acid esterification using lipase as catalyst in a microaqueous environment. Ind. Eng. Chem. Res. 2010, 49, 1071–1078. [Google Scholar] [CrossRef]
- Enser, M.; Hallett, K.; Hewitt, B.; Fursey, G.A.J.; Wood, J.D. Fatty acid content and composition of English beef, lamb and pork at retail. Meat Sci. 1996, 42, 443–456. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical Reaction Enginnering, 3rd ed.; John Wiley and Sons: Hoboken, NJ, USA, 1999. [Google Scholar]
- Rajendran, A.; Palanisamy, A.; Thangavelu, V. Brazilian Archives of Biology and Technology Lipase Catalyzed Ester Synthesis for Food Processing Industries. Braz. Arch. Biol. Technol. 2009, 52, 207–219. [Google Scholar] [CrossRef]
- Novozymes. Immobilized Lipases for Biocatalysis for Smarter Chemical Synthesis. Available online: https://www.novozymes.com/-/media/Project/Novozymes/Website/website/document-library/Advance-your-business/Pharma/Biocatalysis_brochure_Immobilised_Lipases.pdf (accessed on 26 June 2021).
- Oliveira, A.C.; Rosa, M.F.; Aires-Barros, M.R.; Cabral, J.M.S. Enzymatic esterification of ethanol and oleic acid—A kinetic study. J. Mol. Catal. B Enzym. 2001, 11, 999–1005. [Google Scholar] [CrossRef]
- Ancheyta, J. Chemical Reaction Kinetics: Concepts, Methods and Case Studies; John Wiley and Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Murad, P.C.; Hamerski, F.; Corazza, M.L.; Luz, L.F.L.; Voll, F.A.P. Acid-catalyzed esterification of free fatty acids with ethanol: An assessment of acid oil pretreatment, kinetic modeling and simulation. React. Kinet. Mech. Catal. 2018, 123, 505–515. [Google Scholar] [CrossRef]
- Rani, K.N.P.; Neeharika, T.S.V.R.; Kumar, T.P.; Satyavathi, B.; Sailu, C.; Prasad, R.B.N. Kinetics of enzymatic esterification of oleic acid and decanol for wax ester and evaluation of its physico-chemical properties. J. Taiwan Inst. Chem. Eng. 2015, 55, 12–16. [Google Scholar] [CrossRef]
- Chapra, S.C.; Canale, R.P. Numerical Methods for Engineers, 6th ed.; Mac-Graw Hill: New York, NY, USA, 2010. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017. [Google Scholar]
- Fogler, H.S. Elements of Chemical Reaction Engineering, 5th ed.; Prentice Hall: Hoboken, NJ, USA, 2016. [Google Scholar]
- Chen, B.; Miller, E.M.; Miller, L.; Maikner, J.J.; Gross, R.A. Effects of macroporous resin size on candida antarctica lipase B adsorption, fraction of active molecules, and catalytic activity for polyester synthesis. Langmuir 2007, 23, 1381–1387. [Google Scholar] [CrossRef]
- Morais, W.G.; Pacheco, T.F.; Corrêa, P.S.; Martins, A.A.; Mata, T.M.; Caetano, N.S. Acid pretreatment of sugarcane biomass to obtain hemicellulosic hydrolisate rich in fermentable sugar. Energy Rep. 2020, 6, 18–23. [Google Scholar] [CrossRef]
- Morais Junior, W.G.; Gorgich, M.; Corrêa, P.S.; Martins, A.A.; Mata, T.M.; Caetano, N.S. Microalgae for biotechnological applications: Cultivation, harvesting and biomass processing. Aquaculture 2020, 528, 735562. [Google Scholar] [CrossRef]
- Caetano, N.S.; Moura, R.F.; Meireles, S.; Mendes, A.M.; Mata, T.M. Bioethanol from brewer’s spent grains: Acid pretreatment optimization. Chem. Eng. 2013, 35, 1021–1026. [Google Scholar] [CrossRef]
- Mata, T.M.; Tavares, T.F.; Meireles, S.; Caetano, N.S. Bioethanol from Brewers’ Spent Grain: Pentose Fermentation. Chem. Eng. Trans. 2015, 43, 241–246. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Bio-refinery approach for spent coffee grounds valorization. Bioresour. Technol. 2018, 247, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Caetano, N.S.; Mata, T.M.; Martins, A.A.; Felgueiras, M.C. New Trends in Energy Production and Utilization. Energy Procedia 2017, 107, 7–14. [Google Scholar] [CrossRef]
- Gandhi, N.N.; Patil, N.S.; Sawant, S.B.; Joshi, J.B.; Wangikar, P.P.; Mukesh, D. Lipase-Catalyzed Esterification. Catal. Rev. Sci. Eng. 2000, 42, 439–480. [Google Scholar] [CrossRef]

| Enzyme | Formulation | Optimum Temperature | Activity 1 |
|---|---|---|---|
| Lipozyme® CALB L | liquid | 30–60 °C | 5000 LU/g |
| Lipozyme® TL 100 L | liquid | 20–50 °C | 100,000 LU/g |
| Novozym® 435 | immobilized | 30–60 °C | 10,000 PLU/g |
| Lecitase® Ultra | liquid | 35–60 °C | 10,000 LU/g |
| Fatty Acids | g/100 g fat sample |
|---|---|
| SFA, of which | 39.8 ± 0.9 |
| C14:0 | 1.6 ± 0.1 |
| C16:0 | 23.0 ± 0.9 |
| C18:0 | 14.2 ± 0.2 |
| C20:0 | 0.1 ± 0.0 |
| MUFA, of which | 43.9 ± 0.6 |
| C16:1 | 2.9 ± 0.1 |
| C18:1 | 36.7 ± 0.5 |
| C20:1 | 0.7 ± 0.0 |
| PUFA, of which | 14.2 ± 0.3 |
| C18:2n6 | 11.9 ± 0.2 |
| C18:3n3 | 0.7 ± 0.0 |
| C20:2n6 | 0.5 ± 0.0 |
| C20:4n6 | 0.6 ± 0.0 |
| C20:5n3 | n.d. |
| C22:5n3 | 0.1 ± 0.0 |
| C22:6n3 | 0.1 ± 0.0 |
| TFA | 1.7 ± 0.1 |
| Unsaponifiables | 1.3 ± 0.1 |
| Enzyme/Fat Mass Ratio | % Acidity Reduction, 2 h Reaction Time | % Acidity Reduction, 3 h Reaction Time |
|---|---|---|
| Novozym 435 | ||
| 0.0010 | 24% | 27% |
| Lecitase Ultra | ||
| 0.0011 | 10% | 2% |
| 0.0113 | 9% | 10% |
| Lipozyme CALB L | ||
| 0.0100 | 68% | 64% |
| 0.0200 | 67% | 72% |
| Lipozyme TL 100 L * | ||
| 0.0050 | −90% | −55% |
| 0.0100 | −133% | −146% |
| Experimental Assays | Temperature (°C) | Alcohol/FFA Mass Ratio | Enzyme/Fat Mass Ratio | % Acidity Reduction |
|---|---|---|---|---|
| 1st set | ||||
| 1 | 32.5 | 3.25 | 0.0060 | 32 |
| 2 | 35.0 | 3.25 | 0.0060 | 49 |
| 3 | 40.0 | 3.25 | 0.0060 | 62 |
| 4 | 42.5 | 3.25 | 0.0060 | 62 |
| 5 | 45.0 | 3.25 | 0.0060 | 67 |
| 6 | 47.5 | 3.25 | 0.0060 | 63 |
| 2nd set | ||||
| 7 | 45.0 | 2.44 | 0.0060 | 42 |
| 8 | 45.0 | 4.06 | 0.0060 | 38 |
| 9 | 45.0 | 4.88 | 0.0060 | 43 |
| 10 | 45.0 | 6.50 | 0.0060 | 36 |
| 3rd set | ||||
| 11 | 45.0 | 3.25 | 0.0030 | 39 |
| 12 | 45.0 | 3.25 | 0.0048 | 55 |
| 13 | 45.0 | 3.25 | 0.0090 | 58 |
| 14 | 45.0 | 3.25 | 0.0120 | 65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, A.A.; Andrade, S.; Correia, D.; Matos, E.; Caetano, N.S.; Mata, T.M. Valorization of Agro-Industrial Residues: Bioprocessing of Animal Fats to Reduce Their Acidity. Sustainability 2021, 13, 10837. https://doi.org/10.3390/su131910837
Martins AA, Andrade S, Correia D, Matos E, Caetano NS, Mata TM. Valorization of Agro-Industrial Residues: Bioprocessing of Animal Fats to Reduce Their Acidity. Sustainability. 2021; 13(19):10837. https://doi.org/10.3390/su131910837
Chicago/Turabian StyleMartins, António A., Soraia Andrade, Daniela Correia, Elisabete Matos, Nídia S. Caetano, and Teresa M. Mata. 2021. "Valorization of Agro-Industrial Residues: Bioprocessing of Animal Fats to Reduce Their Acidity" Sustainability 13, no. 19: 10837. https://doi.org/10.3390/su131910837
APA StyleMartins, A. A., Andrade, S., Correia, D., Matos, E., Caetano, N. S., & Mata, T. M. (2021). Valorization of Agro-Industrial Residues: Bioprocessing of Animal Fats to Reduce Their Acidity. Sustainability, 13(19), 10837. https://doi.org/10.3390/su131910837









