The Synergic Effect of Whey-Based Hydrogel Amendment on Soil Water Holding Capacity and Availability of Nutrients for More Efficient Valorization of Dairy By-Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hydrogel Preparation and Analysis
2.2. Artificial Soil Mixture
2.3. Hydrogel Application in a Pot Experiment
2.4. Soil Analysis
2.4.1. Chemical Properties
| Method | Reference | Accuracy (% rel.) | |
|---|---|---|---|
| pH | Determination of pH | ISO 10390 [38] | 4–5 |
| C | Oxidimetric method | ISO 14235 [39] | 10–15 |
| N | Modified Kjeldahl method | ISO 11261 [40] | 15–20 |
| P | Mehlich III solution | Mehlich (1984) [41] | 20 |
| K | Mehlich III solution | Mehlich (1984) [41] | 20 |
| Ca | Mehlich III solution | Mehlich (1984) [41] | 20 |
| Mg | Mehlich III solution | Mehlich (1984) [41] | 20 |
| CEC | Barium chloride solution | ISO 13536 [42] | 20 |
2.4.2. Soil Physical Properties
2.4.3. Statistical Analysis
3. Results
3.1. Effect of Hydrogel on Chemical Soil Properties
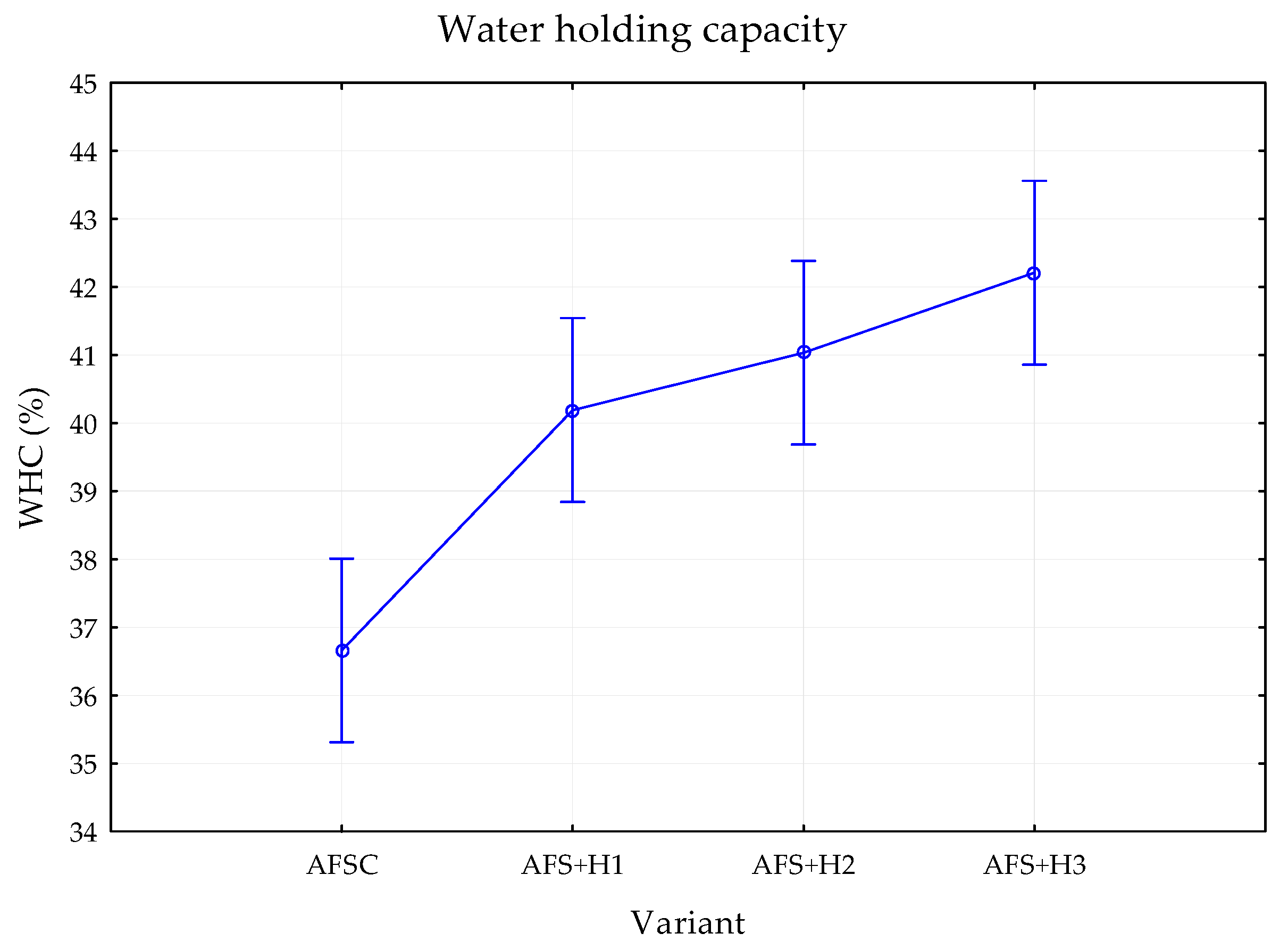
3.2. Effect of Hydrogel on Physical Soil Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schaller, N. The concept of agricultural sustainability. Agric. Ecosyst. Environ. 1993, 46, 89–97. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I. Applications of absorbent polymers for sustainable plant protection and crop Yield. Sustainability 2021, 13, 3253. [Google Scholar] [CrossRef]
- Montesano, F.F.; Parentea, A.; Santamaria, A.; Sannino, A.; Serio, F. Biodegradable superabsorbent hydrogel increases water retention properties of growing media and plant growth. Agric. Agric. Sci. Procedia 2015, 4, 451–458. [Google Scholar]
- Zhang, J.; Li, A.; Wang, A. Study on superabsorbent composite XVI. Synthesis, characterization and swelling behaviors of poly(sodium acrylate)/vermiculite superabsorbent composites. Eur. Polym. J. 2007, 43, 1691–1698. [Google Scholar] [CrossRef]
- Chang, C.; Duan, B.; Cai, J.; Zhang, L. Superabsorbent hydrogels based on cellulose for smart swelling and controllable delivery. Eur. Polym. J. 2010, 46, 92–100. [Google Scholar] [CrossRef]
- Klein, M.; Poverenov, E. Natural biopolymer-based hydrogels for use in food and agriculture. J. Sci. Food. Agric. 2010, 100, 2337–2347. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Metters, A.T. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv. Drug. Delivery Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef]
- Tomášková, I.; Svatoš, M.; Macků, J.; Vanická, H.; Resnerová, K.; Čepl, J.; Holuša, J.; Hosseini, S.M.; Dohrenbusch, A. Effect of different soil treatments with hydrogel on the performance of drought-sensitive and tolerant tree species in a semi-arid region. Forests 2020, 11, 211. [Google Scholar] [CrossRef] [Green Version]
- Miller, V.S.; Naeth, M.A. Hydrogel and organic amendments to increase water retention in anthroposols for land reclamation. Appl. Environ. Soil Sci. 2019, 2019, 11. [Google Scholar] [CrossRef]
- Sim, D.H.H.; Tan, I.A.W.; Lim, L.L.P.; Hameed, B.H. Encapsulated biochar-based sustained release fertilizer for precision agriculture: A review. J. Clean. Prod. 2021, 303, 127018. [Google Scholar] [CrossRef]
- Ibrahim, M.; Abd-Eladl, M.; Abou-Baker, N.H. Lignocellulosic biomass for the preparation of cellulose-based hydrogel and its use for optimizing water resources in agriculture. J. Appl. Polym. Sci. 2015, 132, 42. [Google Scholar] [CrossRef]
- Michalik, R.; Wandzik, I. A mini-review on chitosan-based hydrogels with potential for sustainable agricultural applications. Polymers 2020, 12, 2425. [Google Scholar] [CrossRef] [PubMed]
- Wilske, B.; Bai, M.; Lindenstruth, B.; Bach, M.; Rezaie, Z.; Frede, H.G.; Breuer, L. Biodegradability of a polyacrylate superabsorbent in agricultural soil. Environ. Sci. Pollut. Res. 2014, 21, 9453–9460. [Google Scholar] [CrossRef] [PubMed]
- Cannazza, G.; Cataldo, A.; De Benedetto, E.; Demitri, C.; Madaghiele, M.; Sannino, A. Experimental assessment of the use of a novel superabsorbent polymer (SAP) for the optimization of water consumption in agricultural irrigation process. Water 2014, 6, 2056–2069. [Google Scholar] [CrossRef] [Green Version]
- Chang, L.; Xu, L.; Liu, Y.; Qiu, D. Superabsorbent polymers used for agricultural water retention. Polym. Test. 2021, 94, 107021. [Google Scholar] [CrossRef]
- Shahid, S.A.; Ansar, A.A.; Anwar, F.; Ullah, I.; Rashid, U. Improvement in the water retention characteristics of sandy loam soil using a newly synthesized poly(acrylamide-co-acrylic acid)/AlZnFe2O4 superabsorbent hydrogel nanocomposite material. Molecules 2021, 17, 9397–9412. [Google Scholar] [CrossRef]
- El-Rehim, H.A.; Hegazy, E.S.A.; El-Mohdy, H.A. Radiation synthesis of hydrogels to enhance sandy soils water retention and increaseplant performance. J. Appl. Polym. Sci. 2004, 93, 1360–1371. [Google Scholar] [CrossRef]
- Kapłan, M.; Lenart, A.; Klimek, K.; Borowy, A.; Wrona, D.; Lipa, T. Assessment of the possibilities of using cross-linked polyacrylamide (Agro Hydrogel) and preparations with biostimulation in building the quality potential of newly planted apple trees. Agronomy 2021, 11, 125. [Google Scholar] [CrossRef]
- Rudzinski, W.E.; Dave, A.M.; Vaishnav, U.H.; Kumbar, S.G.; Kulkarni, A.R.; Aminabhavi, T.M. Hydrogels as controlled release devices in agriculture. Des. Monomers Polym. 2002, 5, 39–65. [Google Scholar] [CrossRef]
- Demitri, C.; Scalera, F.; Madaghiele, M.; Sannino, A.; Maffezzoli, A. Potential of cellulose-based superabsorbent hydrogels as water reservoir in agriculture. Int. J. Polym. Sci. 2013, 12, 1–6. [Google Scholar] [CrossRef]
- Billah, S.M.R.; Mondal, M.I.H.; Somoal, S.H.; Pervez, M.N.; Haque, M.O. Enzyme-responsive hydrogels. In Cellulose-Based Superabsorbent Hydrogels; Mondal, M., Ed.; Cellulose Polymers and Polymeric Composites: A Reference Series; Springer: Berlin/Heidelberg, Germany, 2019; pp. 309–330. [Google Scholar]
- Guilherme, M.R.; Aouada, F.A.; Fajardo, A.R.; Martins, A.F.; Paulino, A.T.; Davi, M.F.T.; Rubira, A.F.; Muniz, E.C. Superabsorbent hydrogels based on polysaccharides for application in agriculture as soil conditioner and nutrient carrier: A review. Eur. Polym. J. 2015, 72, 365–385. [Google Scholar] [CrossRef] [Green Version]
- Warkar, S.G.; Kumar, A. Synthesis and assessment of carboxymethyl tamarind kernel gum based novel superabsorbent hydrogels for agricultural applications. Polymer 2019, 182, 7. [Google Scholar]
- Kruif, C.G.; Anema, S.G.; Zhu, C.; Havea, P. Water holding capacity and swelling of casein hydrogels. Food Hydrocoll. 2015, 44, 372–379. [Google Scholar] [CrossRef]
- Mariotti, M.; Fratini, F.; Cerri, D.; Andreuccetti, V.; Giglio, R.; Angeletti, F.G.S.; Turchi, B. Use of fresh scotta whey as an additive for alfalfa silage. Agronomy 2020, 10, 365. [Google Scholar] [CrossRef] [Green Version]
- Zotta, T.; Solieri, L.; Iacumin, L.; Picozzi, C.; Gullo, M. Valorization of cheese whey using microbial fermentations. Appl. Microbiol. Biotechnol. 2020, 104, 2749–2764. [Google Scholar] [CrossRef]
- Akay, A.; Sert, D. The effects of whey application on the soil biological properties and plant growth. Eurasian Soil Sci. 2020, 9, 349–355. [Google Scholar] [CrossRef]
- Papademas, P.; Kotsaki, P. Technological utilization of whey towards sustainable exploitation. J. Adv. Dairy Res. 2021, 7, 231. [Google Scholar]
- Rocha-Mendoza, D.; Kosmerl, E.; Krentz, A.; Zhang, L.; Badiger, S.; Miyagusuku-Cruzado, G.; Mayta-Apaza, A.; Giusti, M.; Jimenez-Flores, R.; Garcia-Cano, I. Acid whey trends and health benefits. J. Dairy Sci. 2021, 104, 1262–1275. [Google Scholar] [CrossRef]
- Zadona, E.; Blažić, M.; Jambrak, A.R. Whey utilization: Sustainable uses and environmental Approach. Food Technol. Biotechnol. 2021, 59, 147–161. [Google Scholar] [CrossRef]
- Peterson, A.E.; Walker, W.G.; Watson, K.S. Effect of whey applications on chemical properties of soils and crops. J. Agric. Food. Chem. 1979, 27, 654–658. [Google Scholar] [CrossRef]
- Watson, K.S.; Peterson, A.E.; Powell, R.D. Benefits of spreading whey on agricultural land. J. Water Pollut. Control Fed. 1977, 49, 24–34. [Google Scholar]
- Grosu, L.; Fernandez, B.; Grigoras, C.G.; Patriciu, O.I.; Grig-Alexa, I.C.; Nicuta, D.; Ciobanu, D.; Gavrila, L.; Finaru, A.L. Valorization of whey from diary industry for agricultural use as fertilizer: Effects on plant germination and growth. Environ. Eng. Manag. J. 2012, 11, 2203–2210. [Google Scholar] [CrossRef]
- Bielská, L.; Hovorková, I.; Kuta, J.; Machát, J.; Hofman, J. The variability of standard artificial soils: Cadmium and phenanthrene sorption measured by a batch equilibrium method. Ecotoxicol. Environ. Saf. 2017, 135, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Halecki, W.; Stachura, T. Evaluation of soil hydrophysical parameters along a semiurban small river: Soil ecosystem services for enhancing water retention in urban and suburban green areas. Catena 2021, 196, 104910. [Google Scholar] [CrossRef]
- Durpekova, S.; Filatova, K.; Cisar, J.; Ronzova, A.; Kutalkova, E.; Sedlarik, V. A novel hydrogel based on renewable materials for agricultural application. Int. J. Polym. Sci. 2020, 2020, 13. [Google Scholar] [CrossRef]
- International Organization for Standardization. Soil Quality—Effects of Pollutants on Earthworms—Part 1: Determination of Acute Toxicity to Eisenia Fetida/Eisenia Andrei. Available online: https://www.iso.org/standard/53527.html (accessed on 15 September 2021).
- International Organization for Standardization. Soil Quality—Determination of pH. Available online: https://www.iso.org/standard/40879.html (accessed on 15 September 2021).
- International Organization for Standardization. Soil Quality—Determination of Organic Carbon by Sulfochromic Oxidation. Available online: https://www.iso.org/standard/23140.html (accessed on 15 September 2021).
- International Organization for Standardization. Soil Quality—Determination of Total Nitrogen—Modified Kjeldahl Method. Available online: https://www.iso.org/standard/19239.html (accessed on 15 September 2021).
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- International Organization for Standardization. Soil Quality—Determination of the Potential Cation Exchange Capacity and Exchangeable Cations Using Barium Chloride Solution Buffered at pH = 8.1. Available online: https://www.iso.org/standard/22180.html (accessed on 15 September 2021).
- European Standard. Soil Improvers and Growing Media—Determination of Physical Properties—Dry Bulk Density, Air Volume, Water Volume, Shrinkage Value and Total Pore Space. Available online: https://standards.iteh.ai/catalog/standards/cen/b487afb1-b8ce-4151-a8d8-fdb7959b2e3d/en-13041-2011 (accessed on 15 September 2021).
- International Organization for Standardization. Soil Quality—Determination of Particle Density. Available online: https://standards.iteh.ai/catalog/standards/cen/a540c124-0bd3-4c72-8152-b4d18b7c397c/en-iso-11508-2017 (accessed on 15 September 2021).
- International Organization for Standardization. Soil Quality—Determination of Dry Bulk Density. Available online: https://www.iso.org/standard/68255.html (accessed on 15 September 2021).
- Skrzypczak, D.; Mikula, K.; Kossińska, N.; Widera, B.; Warchoł, J.; Moustakas, K.; Chojnacka, K.; Witek-Krowiak, A. Biodegradable hydrogel materials for water storage in agriculture—Review of recent research. Desalin. Water Treat. 2020, 194, 324–332. [Google Scholar] [CrossRef]
- Pillai, C.K.S. Challenges for natural monomers and polymers: Novel design strategies and engineering to develop advanced polymers. Des. Monomers Polym. 2010, 13, 87–121. [Google Scholar] [CrossRef]
- Ni, B.; Liu, M.; Lü, S.; Xie, L.; Wang, Y. Environmentally friendly slow-release nitrogen fertilizer. J. Agric. Food. Chem. 2011, 59, 10169–10175. [Google Scholar] [CrossRef]
- Agaba, H.; Orikiriza, L.J.B.; Esegu, J.F.O.; Obua, J.; Kabasa, J.D.; Hüttermann, A. Effects of hydrogel amendment to different soils on plant available water and survival of trees under drought conditions. Clean Soil Air Water 2010, 38, 328–335. [Google Scholar] [CrossRef]
- Akhter, J.; Mahmood, M.; Malik, K.A.; Mardan, A.; Ahmad, M.; Iqbal, M.M. Effects of hydrogel amendment on water storage of sandy loam and loam soils and seedling growth of barley, wheat and chickpea. Plant Soil Environ. 2004, 50, 463–469. [Google Scholar] [CrossRef] [Green Version]
- Ritonga, H.; Basri, M.I.; Rembon, F.S.; Ramadhan, L.O.A.N.; Nurdin, M. High performance of chitosan-co-polyacrylamide-TiO2 crosslinked glutaraldehyde hydrogel as soil conditioner for soybean plant (Glycine max). Soil Sci. Ann. 2020, 71, 194–204. [Google Scholar] [CrossRef]
- Song, B.; Liang, H.; Sun, R.; Peng, P.; Jiang, Y.; She, D. Hydrogel synthesis based on lignin/sodium alginate and application in agriculture. Int. J. Biol. Macromol. 2020, 144, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Graber, E.R.; Fine, P.; Levy, G.J. Soil stabilization in semiarid and arid land agriculture. J. Mater. Civ. Eng. 2006, 18, 190–205. [Google Scholar] [CrossRef]
- Robbins, C.W.; Lehrsch, G.L. Cottage cheese whey effects on sodic soils. Arid Soil Res. Rehab. 1992, 6, 127–134. [Google Scholar] [CrossRef]
- Berry, R.A. The production, composition and utilisation of whey. J. Agric. Sci. 1923, 13, 192–239. [Google Scholar] [CrossRef]
- Radford, J.B.; Galpin, D.B.; Parkin, M.F. Utilization of whey as a fertilizer replacement for dairy pasture. N. Zealand J. Dairy Sci. 1986, 21, 65–72. [Google Scholar]
- Sharratt, W.J.; Peterson, A.E.; Caibek, H.E. Effect of whey on soil and plant growth. Agron. J. 1962, 54, 359–361. [Google Scholar] [CrossRef]
- Ketterings, Q.; Czymmek, K.; Gami, S.; Godwin, G.; Ganoe, K. Guidelines for Land Application of Acid Whey. Available online: http://nmsp.cals.cornell.edu/publications/files/AcidWheyGuidelines2017.pdf (accessed on 3 August 2021).
- Robbins, C.W.; Hansen, C.L.; Roginske, M.F.; Sorensen, D.L. Bicarbonate extractable K and Soluble Ca, Mg, Na, and K status of two calcareous soils treated with whey. J. Environ. Qual. 1996, 25, 791–795. [Google Scholar] [CrossRef] [Green Version]
- Östberg, T.L.; Jonsson, A.P.; Bylund, D.; Lundström, U.S. The effects of carbon sources and micronutrients in fermented whey on the biodegradation of n-hexadecane in diesel fuel contaminated soil. Int. Biodeterior. Biodegrad. 2007, 60, 334–341. [Google Scholar] [CrossRef]
- Konzen, E.R.; Navroski, M.C.; Friederichs, G.; Ferrari, L.H.; Pereira, M.D.O.; Felippe, D. The use of hydrogel combined with appropriate substrate and fertilizer improve quality and growth performance of Mimosa scabrella benth seedlings. Cerne 2017, 23, 473–482. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Liu, M. Preparation and properties of chitosan-coated NPK compound fertilizer with controlled-release and water-retention. Carbohydr. Polym. 2008, 72, 240–247. [Google Scholar] [CrossRef]
- Noppakundilograt, S.; Pheatcharat, N.; Kiatkamjornwong, S. Multilayer-coated NPK compound fertilizer hydrogel with controlled nutrient release and water absorbency. J. Appl. Polym. Sci. 2015, 132, 41249. [Google Scholar] [CrossRef]
- Abdallah, A. The effect of hydrogel particle size on water retention properties and availability under water stress. Int. Soil Water Conserv. Res. 2019, 7, 275–285. [Google Scholar] [CrossRef]
- Johnson, M.S. Effect of soluble salts on water absorption by gel-forming soil conditioners. J. Sci. Food Agric. 1984, 35, 1196–1200. [Google Scholar] [CrossRef]
- Abedi-Koupai, J.; Sohrab, F.; Swarbrick, G. Evaluation of hydrogel application on soil water retention characteristics. J. Plant Nutr. 2008, 31, 317–331. [Google Scholar] [CrossRef]
- Lentz, R.D. Polyacrylamide and biopolymer effects on flocculation, aggregate stability and water seepage in a silt loam. Geoderma 2015, 241, 289–294. [Google Scholar] [CrossRef]





| Hydrogel Characteristics | ||
|---|---|---|
| Properties | ||
| pHH2O | 4.17 | |
| C | % | 40.1 |
| N | % | 1.26 |
| P | mg·kg−1 | 6683 |
| K | mg·kg−1 | 13,059 |
| Ca | mg·kg−1 | 6093 |
| Mg | mg·kg−1 | 806 |
| CEC | mmol·100 g−1 | 82.2 |
| Artificial Soil Characteristics | ||
|---|---|---|
| Soil Properties | ||
| pHH2O | 7.65 | |
| Cox | % | 2.54 |
| N | % | 0.075 |
| P | mg·kg−1 | 18.0 |
| K | mg·kg−1 | 73.5 |
| Ca | mg·kg−1 | 3204 |
| Mg | mg·kg−1 | 487 |
| CEC | mmol·100 g−1 | 9.35 |
| pHH2O | |||
|---|---|---|---|
| Variant | 24 | 7 | 3 |
| AFS control | 7.65 | 7.75 | 7.62 |
| AFS+H1 | 7.56 ** | 7.73 | 7.57 |
| AFS+H2 | 7.67 | 7.62 | 7.62 |
| AFS+H3 | 7.52 ** | 7.57 | 7.51 |
| Variant | 24 | 7 | 3 | |
|---|---|---|---|---|
| N | AFS control | 755 | 800 | 700 |
| AFS+H1 | 785 | 805 | 745 | |
| AFS+H2 | 780 | 885 | 670 | |
| AFS+H3 | 760 | 915 | 740 | |
| P | AFS control | 18 | 17 | 19 |
| AFS+H1 | 21 | 24 | 27 ** | |
| AFS+H2 | 27 ** | 28 ** | 29 ** | |
| AFS+H3 | 29 ** | 35 ** | 44 ** | |
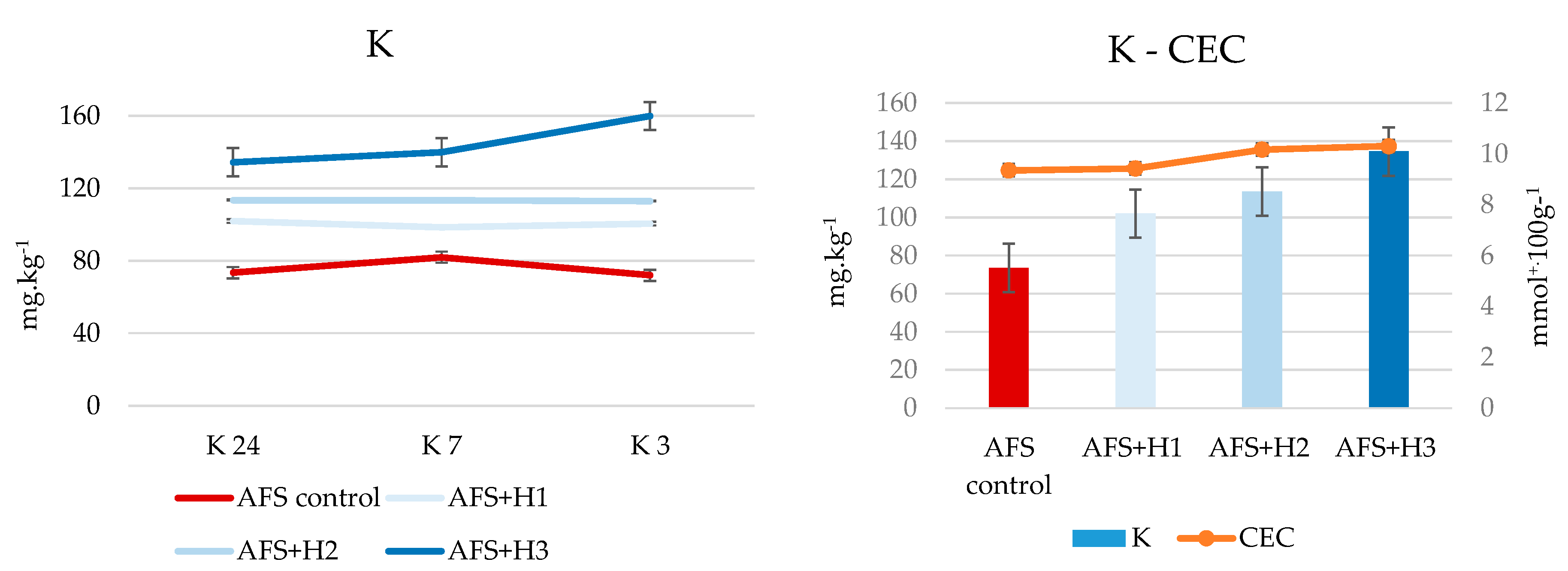
| K | AFS control | 74 | 82 | 72 |
| AFS+H1 | 102 | 99 ** | 101 | |
| AFS+H2 | 114 ** | 114 ** | 113 ** | |
| AFS+H3 | 135 ** | 140 ** | 160 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čechmánková, J.; Skála, J.; Sedlařík, V.; Duřpeková, S.; Drbohlav, J.; Šalaková, A.; Vácha, R. The Synergic Effect of Whey-Based Hydrogel Amendment on Soil Water Holding Capacity and Availability of Nutrients for More Efficient Valorization of Dairy By-Products. Sustainability 2021, 13, 10701. https://doi.org/10.3390/su131910701
Čechmánková J, Skála J, Sedlařík V, Duřpeková S, Drbohlav J, Šalaková A, Vácha R. The Synergic Effect of Whey-Based Hydrogel Amendment on Soil Water Holding Capacity and Availability of Nutrients for More Efficient Valorization of Dairy By-Products. Sustainability. 2021; 13(19):10701. https://doi.org/10.3390/su131910701
Chicago/Turabian StyleČechmánková, Jarmila, Jan Skála, Vladimír Sedlařík, Silvie Duřpeková, Jan Drbohlav, Alexandra Šalaková, and Radim Vácha. 2021. "The Synergic Effect of Whey-Based Hydrogel Amendment on Soil Water Holding Capacity and Availability of Nutrients for More Efficient Valorization of Dairy By-Products" Sustainability 13, no. 19: 10701. https://doi.org/10.3390/su131910701
APA StyleČechmánková, J., Skála, J., Sedlařík, V., Duřpeková, S., Drbohlav, J., Šalaková, A., & Vácha, R. (2021). The Synergic Effect of Whey-Based Hydrogel Amendment on Soil Water Holding Capacity and Availability of Nutrients for More Efficient Valorization of Dairy By-Products. Sustainability, 13(19), 10701. https://doi.org/10.3390/su131910701





