1. Introduction
Over the last thirty years, the scientific community has been reporting that the main source of greenhouse gases and global warming is the massive use of fossil fuels, such as coal, oil, and natural gas [
1,
2]. Various proposals have been put forward to mitigate their effects [
3], prominent among which is the use of alternative fuels to reduce emissions [
4].
In the case of cement factories, the substitution of traditional fossil fuels not only reduces emissions, particularly CO
2, but also provides a use for wastes such as sludge, municipal solid waste, and tires, yielding energy savings and environmental benefits [
5,
6,
7,
8], with several particular case studies analyzed in this regard [
9].
Cement companies emit pollutants coming from scattered point sources into the atmosphere. Emissions from nonpoint sources are neither discharged through specific structures, nor are they associated with combustion, grinding, or drying processes. Instead, they arise from simple operations, such as the intermittent loading of lorries by diggers, stored piles of limestone, and the circulation of vehicles on unpaved tracks. Basically, the only pollutants such nonpoint sources emit into the atmosphere are solid particles. Hence, the overall emissions have to be strictly controlled to avoid adverse conditions for the environment and the inhabitants close to the emission sources [
10].
On the other hand, emissions from point sources are produced during the production process by the kiln, the homogenization system, grinding, silos, and the thermal processing of materials, and are discharged through ducts and chimneys [
11]. The most important source of emissions into the atmosphere by chimney is the clinker kiln. The main pollutant compounds generated are solid particles, nitrogen oxides (NO
x), and sulfur dioxide (SO
2). Depending on the characteristics of the process, other compounds may also be emitted from the same sources, including carbon monoxide (CO), carbon dioxide (CO
2), hydrofluoric acid (HF), volatile organic compounds, etc. [
12]. The emission of these pollutants is normally very low, but their mandatory control ensures the correct operation of the kiln in waste treatment activities [
13,
14].
The cement industry is very vulnerable to fuel price fluctuations. Consequently, an important effort has been made to improve energy efficiency over time, with a reduction of around 30% in the energy consumed to produce clinker since the 1970s [
15]. In addition, the use of alternative fuels yields economic savings for companies in this sector [
16] and it is an interesting approach to tackle the environmental impact generated [
17].
The goals of this study are: (a) compare how the gases emitted during the clinker manufacture vary in a cement plant using two types of fuel: petroleum coke and unusable tires (UTs), and (b) evaluate the introduction of UTs into the system from economic and environmental points of view. In the first part of this study, several pollutants generated in a cement company are described (CO2, CO, NOx, and SO2), as well as how the fuel variation used to manufacture the clinker affects them, depending on the use of only petroleum coke, or a combination with unusable tires. Other possible contaminants are beyond the scope of this study. The second part of this study analyzes possible variations in the quality of the product, taking into account the parameters of the production process. Finally, an economic comparison is made between both types of fuel.
1.1. Clinker
The four major oxides that make up the chemical composition of the typical Portland clinker are CaO (67%), SiO2 (22%), Al2O3 (5%), and Fe2O3 (3%). The clinker also contains 3% minority elements, such as Na+, K+, Mg2+, Ti4+, and S2−. The production of Portland cement clinker is the result of a process of heating minerals, such as clay and limestone (CaCO3), at 1450 °C. As warming progresses, a series of transformations occur:
From 70 °C to 110 °C, the free water of the raw materials evaporates.
From 400 °C to 600 °C, the clay starts to break down into its oxide-like components, mainly SiO2 and Al2O3.
In the next step, from 600 °C to 1000 °C, the limestone reacts with silicon dioxide to form dicalcium or belite silicate (Ca2SiO4), while excess CaCO3 decomposes into calcium oxide (CaO) and carbon dioxide (CO2).
Finally, from 1100 °C to 1450 °C, partial fusion occurs, and belite reacts with calcium oxide to form tricalcium silicate or alite (Ca3SiO5).
These reactions, together with the burning of fuel to heat the mixture, are primarily responsible for the emission of carbon dioxide (CO
2) during cement manufacturing [
18].
The typical final composition of a Portland cement clinker primarily consists of the most abundant oxides: CaO and SiO2, which are the main components of the predominant phases, alite and belite. Other oxides are present in small, but not insignificant, amounts and correspond to the mineral phases, such as tricalcium aluminate (C3A), and ferrite (C2AxF1-x with 0 <x <0.7).
The mineralogical composition of the clinker studied presented different phases: Alite (C3S), or tricalcium silicate (Ca3SiO5), formed the principal compound in Portland clinker above 70%, accounting for the highest percentage by weight; belite (C2S), or dicalcium silicate (Ca2SiO4), presented a mean value of 7% by weight of the clinker analyzed in the case study; while aluminate (C3A), or tricalcium aluminate (Ca3Al2O6), presented a mean percentage of 8.5% by weight of the clinker. On the other hand, tetra-calcium aluminoferrite (C4AF) [Ca2(AlFe)2O5] presented a mean percentage of 8.85% by weight of the clinker. Other minor phases, such as free CaO, were also detected.
1.2. Emissions
Table 1 gives emissions data for kilns operating in the European Union, including the lowest and highest values allowed according to the current legislation and analyzed in the study [
19].
Data are based on emissions from 2000 m
3/t of clinker and the production of one million tons of clinker/year. Emissions intervals are annual averages and represent indicative values based on various measurement techniques. The O
2 content is typically around 10%. The volume of gases emitted by clinker kilns usually ranges between 1700 and 2500 m
3 per ton of clinker (dry gas, 101.3 kPa, 273 K). Kiln systems with a preheater and precalciner normally produce gas volumes of around 2000 m
3/t of clinker (dry gas, 101.3 kPa, 273 K) [
19].
CO
2 emissions in dry cement manufacturing have a double origin. Around 60% are generated by the decarbonation of the main raw material (limestone), which is chemically broken down into calcium oxide and CO
2. These emissions are called process and, currently, they cannot be reduced. The other 40% of the emissions comes from the fuels necessary to carry out the clinkerization process, which is the part that can be improved. In the last two decades, measures have been taken to reduce emissions and improve manufacturing techniques and the uses of alternative fuels [
20]. CO
2 emissions have been reduced from 540 t of CO
2/t of clinker in 1990 to 525 t of CO
2/t of clinker in 2014 [
19].
The emission of CO is related to the content of organic matter in the raw materials and to the conditions of the manufacturing process, although it can also be caused by incomplete combustion when the control of the feed of solid fuels is not optimal. Depending on the characteristics of the quarries, between 1.5 g and 6 g of organic carbon per kg of clinker from raw materials are contributed to the process [
21].
Assays performed with raw materials from various sources have shown that between 85% and 95% of the organic compounds present in the raw materials are completely oxidized to CO
2 in the presence of 3% excess oxygen, while between 5% and 15% is partially oxidized to CO, which in some particular raw materials, may exceed 2000 mg/Nm
3 [
22].
Nitrogen oxide and nitrogen dioxide (usually presented as NOx) are the predominant nitrogen oxides in the gases emitted by the cement kiln (NO > 90% of the nitrogen oxides). There are two main sources for the production of NOx: the first (the so-called "thermal" source) is produced by the reaction of the combustion air nitrogen with oxygen to form nitrogen oxides; and the second is due to the fuel, where the nitrogen compounds present in the fuel react with oxygen to form nitrogen oxides. Thermal NOx is mainly produced in the kiln’s clinkerization zone, which is related to the temperature and oxygen content. The higher the excess oxygen is, the greater the thermal NOx formation. In addition, NOx formation may be influenced by the shape of the flame and its temperature, the combustion chamber geometry, the reactivity and nitrogen content of the fuel, the presence of moisture, the reaction time, and the design of the fuel burner. The range of nitrogen oxide emissions in European kilns is between 200 mg and 3000 mg NOx/m
3. On average, European cement kilns emit about 1300 mg NOx/m
3, while the range of nitrogen oxide emissions in Spanish kilns is between 400 mg and 2800 mg NOx/m
3 [
23].
Sulfur dioxide (SO2) emissions from cement factories are directly related to the content of volatile sulfur compounds in raw materials. Kilns that use raw materials with low volatile sulfur compounds have very low SO2 emissions and are, in some cases, below the detection limits. If inorganic compounds of sulfur (FeS) are used, the emissions of SO2 content will be high. Hydrogen sulphide (H2S) can also be generated. Sulfides, and organic sulfur that exists in raw materials, evaporate when the oil temperature begins to rise. 30% is emitted into the atmosphere or takes the raw mill, from the first stage of the cyclone exchanger, where the material is prepared, in terms of granulometry and drying, for the kiln process. The feed to the mill is by means of a dosing device.
Sulfur present in fuels used to heat kilns with a preheater does not generate significant SO2 emissions because of the strongly alkaline environment in the sintering zone, the calcining zone, and in the lowest preheater stage, remaining trapped in the clinker. In this regard, previous studies done by the Environmental Protection Agency (EPA) and the Portland Cement Association (PCA) have determined that the emission of particles when using UTs is 35% lower, including dioxins and furans.
Oxygen excess (from 1% to 3% of O
2 maintained in the kiln to obtain good quality cement) oxidizes the sulfur compounds released, converting them into SO
2. Emissions from European and Spanish kilns range from below the detection limit up to values of 3500 mg/Nm
3 [
24].
1.3. Usage of Tires as Fuel
The number of cars in Spain has grown exponentially since the 1960s, and this has generated an equivalent increase in used tires, nowadays reaching almost 300,000 t/year, making them one of the most promising fuel alternatives for the future [
25]. All the Spanish companies that use alternative fuels to manufacture clinker and cement have environmental authorization by the competent body, which in the case study is the Ministry of Development and Environment from the Community of Castilla y León, where they have adapted to the Best Available Techniques (BATs) and the State Plan for Waste Management Framework (PEMAR) 2016–2022, in accordance with Directive 2010/75/EU on industrial emissions. Therefore, the data obtained and used in this research is based on the current regulations and conditions established by law.
One of the advantages of recycling UTs in clinker kilns is that it is not necessary to remove the metal reinforcements in the tires as they serve to partially replace the ferric corrector, which is used to form the tetra-calcium aluminoferrite (C4AF) in the mineralogical phases of clinker. Thus, one of the obvious benefits of using UTs in clinker kilns is the cost of preparing this alternative fuel, which is lower because of the metal reinforcements within the raw material.
When introducing alternative fuels, in addition to the emissions shown in
Table 1, many other compounds can be found, such as polychlorinated dibenzodioxins (PCDDs), dibenzofurans (PCDFs), as well as metals and their nonvolatile, semivolatile, and volatile compounds. In combustion processes, the presence of chlorine and organic compounds can lead to the formation of dioxins and furans (PCDDs and PCDFs) if certain retention time and temperature conditions are met. Several studies performed in Europe show that cement production is not a major source of furans and dioxins because the gases are placed for a long enough time in a high-temperature sintering kiln [
26], despite the fuel used in the production process.
The measurements done indicate that cement kilns emit less than 0.1 mg of toxicity equivalents (TE/Nm
3), which it is the limit value in European legislation for waste incineration and co-incineration plants [
27]. Since 2000, cement companies in Spain have contributed to compile the Spanish Ministry of the Environment’s Inventory of Dioxins and Furans. Around 40 measurements in 29 clinker kilns had been obtained by late 2001, all of them below the limit value of 0.1 mg TE/Nm
3 [
21].
Environmental legislation and the operation of factories usually focus on three emissions: nitrogen oxides (NOx), sulfur dioxide (SO2), and particles with an emission limit value of 500 mg/m3, 400 mg/m3 and 20 mg/m3, respectively, when residues are co-incinerated. The concentrations are considered under normal conditions of pressure and temperature (101.3 kPa, 273º K) on a dry basis and for combustion gases standardized at 10% O2.
2. Materials and Methods
This study was carried out in a cement factory in the north of Spain. It commercializes cement and clinker depending on the demand, with a capacity to produce 3200 t/day of clinker.
All the data, materials, and specific equipment are taken from the case study, including the descriptions of the clinker manufacturing process, the raw material for cement manufacturing, the type of rotary kiln, the characteristics of the UTs used as fuel, and the most relevant parameters for the process.
2.1. Materials for Clinker Manufacturing
The main chemical components for clinker manufacturing are lime, silica, alumina, and iron oxide. The rocks and minerals used are, among others, limestone, marls, and clay. They provide mineral compounds, such as calcium carbonate (CaCO
3), which is a source of lime in the manufacture of clinker. The percentage of the materials milled that enter the kiln are 96% limestone + clay, 1.2% Fe mineral, and 2.3% sand. The total organic carbon (TOC) found in the material introduced into the kiln is 0.2% in weight, which is within the acceptable range defined by the Standard UNE-EN 13639:2019.
Table 2 displays the components of the material introduced into the kiln, based on X-ray fluorescence analysis. The calcination loss is the loss of weight when the material is heated within a range of 900–1000 ºC, consisting of CO
2 and H
2O.
2.2. Rotary Kiln
The POLRO rotary kiln (dry process) is an important element of the process, together with the Dopol towers, preheaters, cyclones, combustion chambers, and calciners. Boiling takes place in the rotary kiln and the raw flour detailed in
Table 2 becomes clinker, which has been preheated in the preheater, and decarbonized in the calciner. The material in the kiln moves against the flow of the hot combustion gases, reducing the emission of pollutants since it acts as a circulating fluid bed. Many compounds produced by the combustion and transformation of raw materials into clinker remain in the gas stream until they are absorbed, retained, or condensed by the countercurrent flow of raw materials [
28,
29]. The capacity of the material absorption depends on the area of the kiln where this material is and varies with the chemical composition and physical parameters, such as the sintering temperature or the kiln rotation speed. The temperature increases as the material and gases progress through the kiln, rising to a maximum of 1450 °C for the material, and 2100 °C for the gases. The material leaving the calcination (decarbonation) stage of a kiln has a high CaO content and, therefore, a high capacity to absorb (neutralization) acids such as HCl, HF, and SO
2.
Table 3 gives the characteristics of the rotary kiln used in the case study.
The sintering temperature is the highest temperature reached in the kiln during the clinkering process and the most important parameter in production [
30,
31]. The temperature is related to the stabilization zones of tricalcium silicate or alite (C
3S), and the content of free Cal (CaO) [
32]. Theoretically, the area with the highest amount of C
3S is between the minimum temperature of 1250 °C and the maximum of 1450 °C. Therefore, it is necessary to work at sintering temperatures within this range.
2.3. UTs and Conventional Fuel
Car tires consist of various materials, including steel, textile fibers, and elastomers. The percentage composition of materials relative to the total mass of tires is: 48% natural and synthetic rubber, 22% carbon black, 15% steel wires, 5% textile cables, 1% zinc oxide, 1% sulfur, and 8% other chemical products in minor proportions. These average values are adjusted to those used in the case studied.
Some 70% of the tire mass consists of hydrocarbon derivatives. These substrates are suitable for obtaining fuels and chemicals via thermochemical transformation. The minimum calorific value of UTs is around 7100 kcal/kg.
Carbon and oxygen account for 88% of a tire. Its complete destruction is ensured at temperatures above 800 °C and with gas retention at high temperatures, such as in clinker kilns. This complete destruction prevents the formation of intermediate products resulting from incomplete combustion, such as black smoke and odors. Sulfur (average of 1.3% by weight of tires) is neutralized as sulfate, which is lower than in traditional fuel. This transformation is due to the highly alkaline nature of the material being fused in clinker manufacture. Moreover, the metal component of the tires can partially replace the iron additions used as a flux in the composition of cement raw mix. The characteristics of the petroleum coke used are as follows: a superior calorific value of 8442 kcal/g with 6.56% of humidity, 0.56% of ashes, and 10.84% of volatile particles.
The facilities for the reception of used tires consist of two silos of 335 m3 each, where the tires in powdered form are stored, with no further process done to the UTs. Two hoppers are fed by a crane bridge with a grapple of 1.5 m3 capacity. Under each of these hoppers, there is a band dosing machine for the control of the material provided to the kiln and the preheater. Regarding the dosing system, the material is transported by conveyor belt to the secondary burner in the precalciner zone. The storage of UTs follows the regulations established in the Royal Decree 1619/2005 of December 30 on the management of unusable tires.
2.4. Emission Control
An emission control was carried out at the cement plant. The results are stored in the company’s database and the Air Pollution Emission Registration Book, as required by the current legislation. In these books, the results of the emission measures of pollutants into the atmosphere are recorded, as well as any incident that could occur during the installation with reference to the atmospheric environment. The values registered in the different focuses must be below the emission limits established by current environmental authorization (ORDER FYM/787/2017).
More than 40 values are registered and stored in the company database. For this study, the following were considered in the output of the rotary kiln: O2, CO, and NOx. The secondary air temperature, sintering temperature, O2 analysis in the preheater, CO analysis in the preheater, and the SO2 output in the chimney were also analyzed. Alite and free lime were also determined as quality parameters. These last parameters are fundamental for the production process of Portland clinker. Alite is a major compound of clinker, having the largest percentage of weight, while free lime is the fraction that has not reacted during the sintering process and does not mix in the crystallographic phases of the clinker. Ideally, the percentage should be as close to zero as possible. However, this would mean that the clinkering temperature would have been very high, as well as the residence time in the kiln. Under these conditions, the energy consumption would be too high. The values of free lime range from the lower limit of 0% to the upper limit of 3%.
The parameters of the rotary kiln and clinker quality are identified and recorded in the database for different periods of time. The quality parameters are analyzed every two hours, and those from the kiln every five minutes, making averages of the hours and comparisons between them. The values analyzed were those recorded in the cement factory database for thirty days: 160 values for each type of fuel, producing 320 values in total. These data are analyzed to determine the variations that exist when 100% petroleum coke is used, and when 40% of alternative fuels are used (in this case, unusable tires) [
18].
Hence, the parameters analyzed were the gases emitted by the kiln and the preheater (CO, O
2, and NOx), and the SO
2 in the chimney. Initially, only petroleum coke was used. Subsequently, a percentage of UTs (40%) and petroleum coke (60%), were used. [
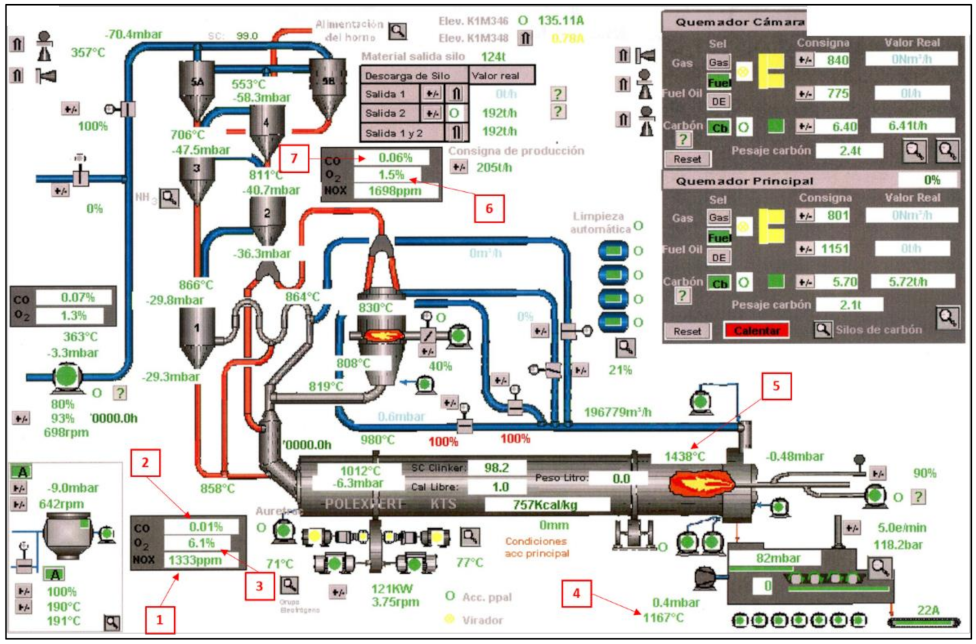
33]. Most of the alternative fuels were introduced into the preheater, where the consumption of UTs is around 80% of all the fuel that is introduced, compared to the 20% that is used in the kiln. This fact is due to the temperature requirements at each stage. The entire production process is regulated through a control center (
Figure 1). The data obtained were stored with the quality parameters [
34]. The kiln parameters illustrated in
Figure 1 are the following: (1) O
2 emissions; (2) CO emissions; (3) NOx emissions; (4) secondary air temperature; (5) sintering temperature; (6) analysis of O
2 in the preheater; and (7) analysis of CO in the preheater.
The sintering temperature is the highest temperature reached in the kiln during the clinkering process, and it is measured by a pyrometer sited close to the fuel outlet nozzle between 1–2 m from the end and directed towards the clinker [
35]. This value was recorded every five minutes, and hourly averages were calculated for comparison with quality values obtained from diffraction diagrams and X-ray fluorescence [
36].
The hot air in the clinker cooler, heated by the cooling material and the external temperature, is distributed in three lines: the temperature of the clinker going to the silos; the temperature of the dust collected by the filter, which is taken to the cooler; and the temperature of the secondary and tertiary air going into the kiln. The secondary air temperature cooler input parameter is the secondary air temperature from the cooler as it enters the kiln, which is recorded by a temperature probe.
Continuous monitoring was used, at selected points established by the current regulations, to obtain the emissions. Among others, the variables used in this study were: SO2, NOx, and CO. The percentage of NOx that contains the material at the entrance of the kiln was analyzed by means of an extractive probe. Subsequently, a sample was taken, cooled, dusted, and then analyzed.
3. Results and Discussion
Table 4 allows for a comparison of the results obtained using petroleum coke and 40% UTs as fuels. It shows: (a) the sintering temperature, (b) the clinker composition (% of alite and free CaO); and (c) the concentration of O
2, CO, NOx, and SO
2 emitted in the furnace, preheater, and stack. Oxygen is kept at the minimum necessary for combustion to be complete.
3.1. Emissions from the Kiln
The mean CO emission calculated with petroleum coke is 3.92 × 10
−2%, and the addition of tires as fuel is 5.16 × 10
−2% (
Table 4). There is a decrease in O
2 and an increase in CO. This increase in CO is due to the carbon monoxide generated by the incomplete combustion of the fuel produced by a lack of oxygen. This can happen because of the inaccurate regulation of the oxygen by the staff in the control room.
With regard to NOx emissions, a decrease of 17% is observed using 40% UTs. The data ranges from an average of 905.69 ppm with petroleum coke, to 753.74 ppm in the case with tires. Experimentally, it has been found that nitrogen oxide produced in the sintering kiln is released as a result of: flame temperature and shape, excess oxygen, gas retention time in the combustion zone, and loading temperature and load retention time in the combustion zone. The amount of NOx produced by the secondary burner depends on the nitrogen content of the fuel, the excess oxygen, and the flame temperature. These parameters increase with temperature, and more nitrogen in the combustion air leads to an increase in nitrogen oxide and alite content.
Nitrogen oxides emissions decreased when 40% UTs were added as fuel. This effect was related to a reduction in the sintering temperature and a lower oxygen content at the extreme end of the kiln, with the result that less NOx was generated. However, O2 content must be balanced against the increases in CO and SO2 that occur when the percentage of oxygen drops. The formation of a reducing atmosphere in parts of the kiln can also exert a substantial influence. In the cement manufacturing process, NO may be oxidized to NO2 at lower temperatures but, normally, NO accounts for more than 90% of NOx emissions.
Despite the reduction in some pollutants, it must be pointed out that there are still large amounts of contaminants emitted that should be reduced in terms of global warming and human toxicity indicators, as seen in previous studies in the field [
8]. However, several studies state that low emissions of NOx, SOx, CO, PM10, persistent organic compounds, and heavy metals are achieved. Furthermore, the potential impact of this practice on human health due to the release of carcinogenic chemicals is also low [
37,
38].
3.2. O2 and CO in the Preheater
When UTs were used as fuel, the preheater required more oxygen than when petroleum coke was used (
Table 4). The mean oxygen input into the preheater was 2.23 ppm, whereas it was 6.15 ppm when UTs were used. As can be seen in the same table, a smaller amount of carbon monoxide was detected in the preheater. Moreover, the mean CO into the preheater was 0.15 ppm with petroleum coke, whereas it was 5.93 10−2 ppm for UTs, which means CO was 60% lower using 40% UTs. Hence, the O
2 inlet is increased for the correct combustion of the UTs, which means less CO formation at the preheater outlet.
3.3. SO2 Output from the Chimney
The basic raw material, limestone, already has a high sulfur content. As can be seen in
Table 4, there was a reduction in SO
2 when UTs were added as a percentage of the fuel. The usage of only petroleum coke gave a mean value of 407.16 ppm, whereas it was reduced to 294.46 ppm using UTs. The following points, in order of importance, are the main reason for this difference:
Petroleum coke contains around 6% sulfur and the tires had 1.3%, which implies a decrease in the raw material input.
The reduced volatility of SO2 at lower flame and combustion temperatures.
The oxidizing atmosphere in the kiln, together with stable kiln operation.
3.4. Sintering Temperature
The sintering temperature varied during the process. When petroleum coke was used as fuel, the mean operational temperature was 1360 °C, whereas when a percentage of UTs were used, the mean temperature was 1292 °C. This temperature difference means a reduction in CO
2 and energy savings (
Table 5). It has been estimated in previous studies that a decreasing sintering temperature of 50 °C means a reduction in CO
2 emissions of more than four thousand tons a year [
35,
39] and this is in accordance with other studies that discuss the fact that incineration in cement factories has the greatest environmental impact, together with the production of artificial lawn [
8], and that have carried out an extensive LCA analysis to determine all the implications of UTs as fuel [
40].
3.5. Clínker Quality Parameters
With regard to the quality parameters, some changes occur in alite and free lime when adding a part of the tires as fuel while maintaining the same quality of the clinker.
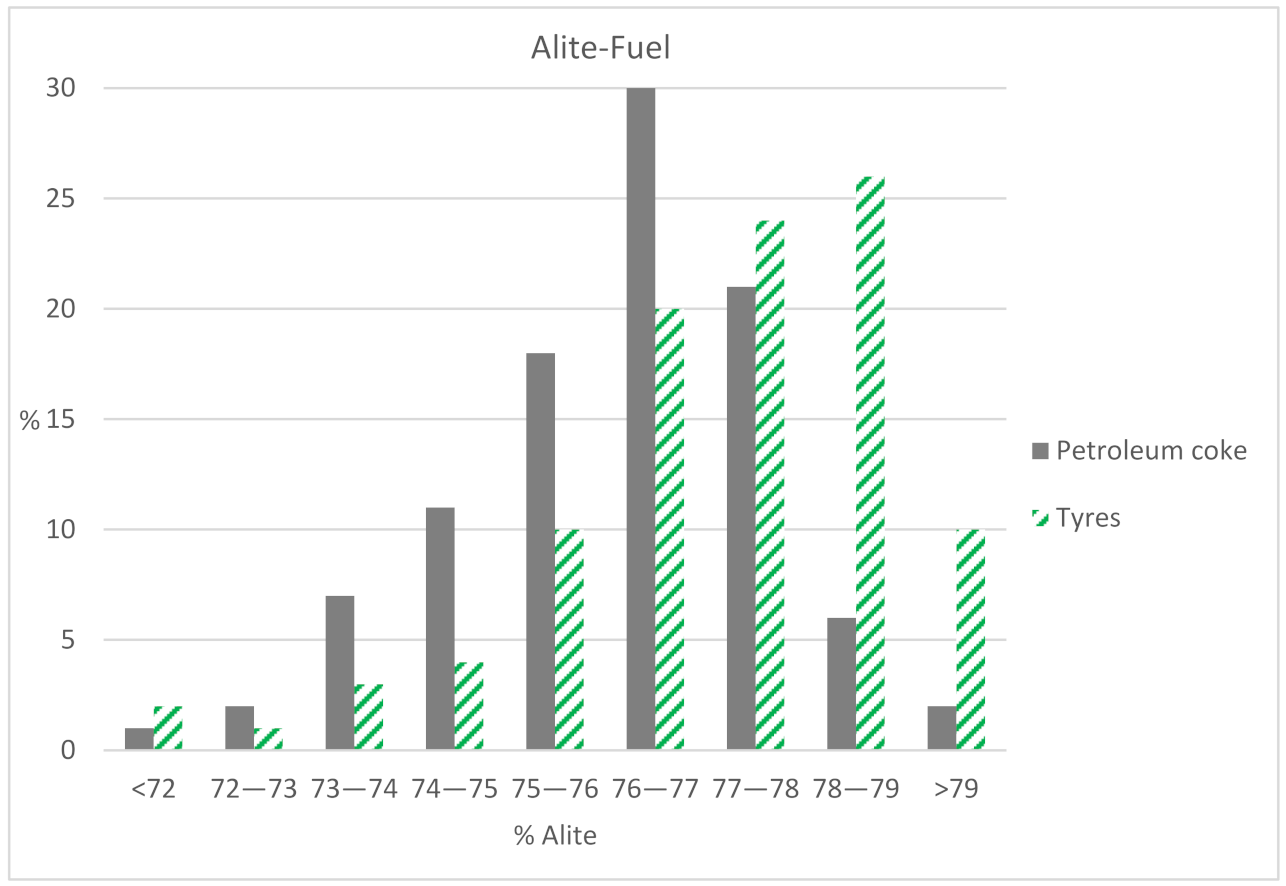
In this cement factory, values of more than 70% are considered optimal for the alite majority phase. All the results obtained from the factory exceed this value, regardless of the type of fuel. In
Figure 2, an interval study of the C
3S results is carried out depending on the type of fuel. It is observed that 87% of the values using petroleum coke are between 74% and 79% of C
3S. For the same interval, the alite using alternative fuels reaches 84% of the values. Hence, it is noted that the quality values are adequate for both fuels.
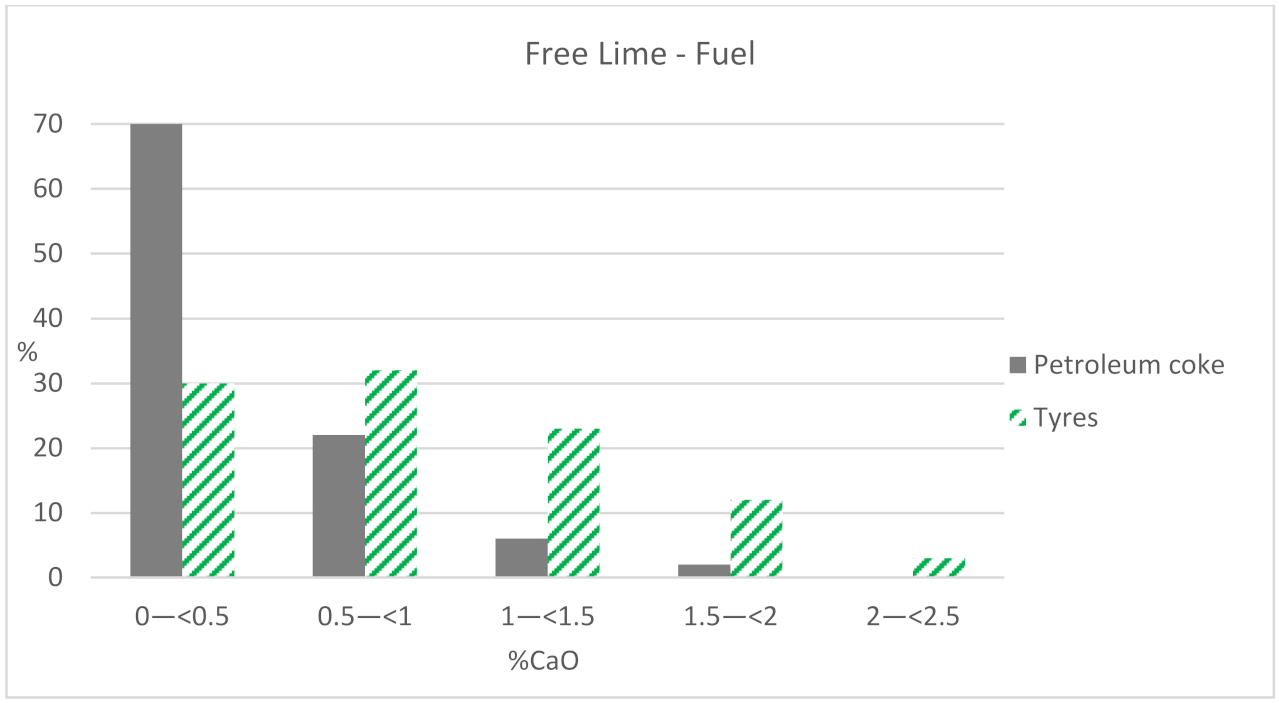
When using petroleum coke, 70% of the samples have values lower than 0.5% of the CaO phase, while the results of free lime are more distributed using tires as fuel, although always with values lower than 2.5% of CaO (
Figure 3). Therefore, the higher the kiln sintering temperature, the lower the amount of free lime the clinker will have. The sintering temperature is higher when only petroleum coke is used as fuel, instead of using the mix (60% petroleum coke + 40% UTs).
Additionally, it has been proven that the usage of tires is a residue-free option because the slag and ashes are included in the cement without significantly modifying its physicochemical properties [
41,
42].
3.6. Economic Assessment
Based on real economic and consumption data obtained from the case study, the price of petroleum coke is about 80 €/t, whereas the average cost of used tires is 25 €/t. Assuming an annual consumption of 100,000 t with a calorific value of 8200 kcal/t coke, the exclusive use of petroleum coke as fuel to manufacture clinker would cost 8,000,000 €/year. On the other hand, using 40% alternative fuel (UTs with a calorific value of 7200 kcal/tUTs) combined with 60% petroleum coke would cost 5,938,000 €/year (
Table 6). Thus, using a percentage of alternative fuel for clinker production would yield economic savings of around 25%. As the average cost of fuel in a cement factory represents 30% of the total cost, the economic savings using UTs represents 8% of the whole process studied. Moreover, a decrease of 50 °C in the sintering temperature supposes energy savings of 175 kg/hour for petroleum coke and, therefore, cost savings of 126,000 €/year.
The usage of both fuels does not require any major additional investment. The case study only had to install two additional continuous analyzers in the chimney, storage silos for the powdered used tires, and the corresponding feeding system.
4. Conclusions
The results obtained provide an interesting insight into the usage and comparison between petroleum coke and alternative fuels (used tires) from a real case study analyzing the emissions generated, the technical operability, and the economic implications of both fuel options. This study displays real values showing the benefits of an alternative fuel. Despite the fact that the usage of alternative fuels is common, it is very difficult to find real continuous data of the two types of fuel, petroleum coke and used tires, in the same cement plant.
In this regard, it has been proven that energy recovery from used tires yields economic and environmental benefits. The use of 40% UTs as fuel reduced the amount of NOx, CO, and SO2 generated in the production process with respect to the use of 100% petroleum coke. At the exit of the kiln, using UTs as 40% of the fuel, the amount of NOx decreased by 17% with respect to the use of petroleum coke, and SO2 also decreased from 407.16 ppm to 294.46 ppm. The sintering temperature of the kiln was 5% higher when using only petroleum coke as fuel than when using 40% UTs. The usage of UTs also requires a greater amount of oxygen so that the combustion can be complete, and it created lower quantities of CO: 60% lower in the preheater than when using petroleum coke. Further research should be done to fully determine all the potential environmental implications of using tires as fuel.
Moreover, the quality parameters and clinker remained the same. The main clinker compound, alite, was maintained, and 84% of the UT data show values above 74% for C3S, while it was 87% for petroleum coke. The behavior of free lime was within the established limits, not reaching 2.5% of free CaO in both cases. A slightly better result was observed with petroleum coke. From an economic perspective, UT fuel is 25% cheaper than petroleum coke, another important reason for its usage in place of traditional fuels.