Abstract
Biomass is an important renewable energy resource which primarily contributes to heating and cooling end use sectors. It is also a promising alternative source of biofuels to replace the depleting supply of fossil fuels. Surprisingly, few writers have been able to draw on the feedstock significance for oil palm empty fruit bunch (EFB) as the biomass resource for biofuels compared to the other types of biomass waste. Therefore, this paper presents a comprehensive review of EFB as a biomass resource presented in four major parts. First, the introduction covers the demand for bio-oil and describes the different kinds of feedstock, the relevance and potential of EFB biomass. Second, the characteristics of biomass are explained before it is upgraded as biofuel, drawing similarities and contrasts between EFB and other sources of biomass. Pyrolysis processes and reactors used for EFB conversion are described, and the factors affecting the bio-oil yield and quality are discussed. Major reactor parameters are summarized and reactor optimization is discussed. Third, comparison on the properties of the bio-oil vs. petroleum in transportation, power generation, and heating are compared followed by prioritizing the bio-oil properties from the most to least critical, revealing the most promising methods for upgrading. Fourth, the environmental impact, including CO2 emission, of the use of EFB as a promising renewable energy resource and a cleaner alternative fuel is recommended. This paper has comprehensively reviewed the conversion of oil palm empty fruit bunches into biofuels, including the similarities and differences between biomasses, the best reactors, its comparison with fossil fuels, and bio-oil upgrading methods. The upgrading mapping matrix is created to present the best upgrading strategies for the optimum quality of biofuels. This paper serves as a one-stop center for EFB conversion into biofuels.
1. Introduction
Increasing energy consumption, limited fuel reserves and climate change are major challenges of the 21st century. In 2016, the contribution of crude oil to global energy consumption was 32%, followed by coal at 27% and natural gas at 21% [1]. This indicates that approximately 80% of the energy consumed was supplied by fossil fuels. In 2017, oil provided the largest increment to energy consumption at 77 million tons of oil equivalent (mtoe), followed by natural gas (57 mtoe) and renewable power (53 mtoe) [2]. The current petroleum reserves are estimated to last for less than 50 years.
Researchers are continuously exploring sustainable and cheaper substitutes of fossil fuels. One of the most viable resources identified to replace the depleting petroleum reserves is renewable energy resources. The Renewable Energy Policy Network for the 21st Century (REN21) 2016 report indicates that an estimated 19.2% of the net global energy consumption in the year 2014 was provided by renewable energy, 78.3% by fossil fuels and 2.5% by nuclear power. Known renewable energy sources are biomass, hydropower, geothermal, ocean energy, solar, and wind [3]. The share of biomass in the net final energy consumption by end-use sector is 14%, which comprises of heating and cooling at 12.6%, transportation at 0.8% and electricity at 0.4% [3]. This shows that biomass is a potential resource to replace conventional energy sources such as fossil fuel, natural gas and coal. Bioenergy, the energy derived from biomass, is the highest contributor to renewable energy supply.
Biomass is any organic matter obtained from plant or animal tissue, which includes agricultural resources, forest resources, municipal solid waste, industrial waste and other wastes, that can be used as an energy source [4]. Some well-established commercial processes commonly applied to the biomass industry are combustion for heat or electricity and methane for heat or power generation, and chemical conversion of sugar and starch to produce bioethanol and vegetable oils to biodiesel [3]. Alternatively, liquid fuels or biofuels can be produced from cellulosic materials by biological or thermochemical conversion processes such as pyrolysis, liquefaction, and gasification.
The potential of biomass as biofuel is undeniable, although there are some critical aspects to be considered. The impacts of EFB bio-oil has been presented by reflecting its properties compared to petroleum-based fuel in transportation, power or electric generation, and heating by boiler and furnace are classified as the main three usages of bio-oil. This paper presents the simplest and most concise way of making decisions for biomass conversion into biofuels such as alternative biomass waste, reactor types, comparison of most critical to least critical properties of bio-oil, and bio-oil refinery for the most economical and effective upgrading techniques. The paper ends with the environmental impact of biofuels compared to other types of biomass waste conversion, especially in reducing the emission of carbon dioxide into the atmosphere.
1.1. Biofuels
There are three main reasons for the need for biofuel as alternative fuels. First, the limited fuel reserves which require the development of alternative sources of fuel in the future. Second, alternative fuels are needed in order to minimize the greenhouse effects generated from combustion of fossil fuel that cause climate change. The greenhouse gasses (GHGs) in the atmosphere are water vapor (H2O), carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), ozone (O3), chlorofluorocarbons (CFCs), and hydrocholorofluorocarbons (HCFCs) [5]. Human activities have caused a 40% increase in the atmospheric concentration of CO2 from 280 ppm in the year 1750 to 406 ppm in early 2017 [6]. The principal contributor to CO2 emissions arises from the combustion of coal, oil, and natural gas with minor contributions from deforestation, changes in land use, soil erosion and agriculture [7,8]. Biofuels are environmentally friendly and potentially reduce greenhouse gas and carbon dioxide emissions [9].
Third, due to air pollution which consists of SOx, NOx and particulate matter (PM) [10], arising from combustion of fossil fuels, a cleaner alternative fuel is needed. Bio-oil from biomass has high oxygen and low nitrogen content with trace amounts of sulfur, and potentially demonstrates cleaner combustion that results in lower emission of SOx and NOx [5,10,11]. Emissions of unburned hydrocarbons (HC), carbon dioxide, carbon monoxide, sulfates, polyaromatic hydrocarbons (PAHs), nitrated PAHs and ozone-forming HC and PM are reduced by biodiesel in a conventional diesel engine. Rice bran fatty oil methyl esters [12] are suitably converted into biodiesel for a compression ignition (CI) engine. Biodiesel from rice bran [13] produced cleaner and lower emission of CO, CO2, HC, and NOx emission from the vehicle’s CI engine.
The exhaust emissions of PM from biodiesel are estimated to be 30% lower than overall PM emissions from petroleum diesel [10]. Therefore, replacing fossil fuels with biofuels derived from biomass resources is more sustainable and has the potential to mitigate the adverse environmental impact caused by fossil fuel. In the midst of efforts to develop biofuels as alternative transportation fuels, a shift has been seen toward electric vehicles that are superior in terms of saving of primary energy (energy derived directly from natural sources), security of energy supply and lower local emissions. On the other hand, there is a need for biofuels to replace fossil fuels in aircraft due to the weight factor (i.e., the energy density per unit weight) [14], thus emphasizing the importance of continuous research in biofuels.
1.2. Generation of Biofuels
Fuel derived from biomass is classified in four different generations depending on its feedstock [15]. First-generation biofuel usually refers to biofuel produced from raw materials or traditional plant biomass which [16] mainly comprises of corn, oilseed, sugarcane and other oil-containing food and animal feed crops [15,17] and may also be consumed by human and animals. The most common first-generation biofuels include biodiesel, ethanol and biogas [18]. Biodiesel is extracted from vegetable oil, with or without esterification, from seeds of plants such as soybean, rapeseed (canola) and sunflower. The increase in food process caused by the first generation of biomass has motivated research on the second-generation biomass conversion which focuses on lignocellulosic biomass resources.
Second-generation biofuel is bioethanol or biodiesel that is produced from low cost crops, non-food crops, forest residue and other lignocellulosic biomass waste such as empty fruit bunch, rice husk and rice bran [12,13]. Lignocellulosic biomass comprises of wood, grass and municipal solid wastes that are non-starch, non-edible and non-food feedstocks [15]. Commercial scale-up of second-generation biofuels is challenging, and will require advances in biomass-to-fuel conversion technologies.
Third-generation biofuels are derived from microbes and involve the utilization of microbial growth to convert cellulosic or starchy biomass feedstock into lipids [15]. Microalgae are ideal for this purpose because of their rapid growth rate, ability to fix greenhouse gas and production of high amount of lipids [19]. A further benefit of algae as biofuel is that the resulting product can be manufactured into a wide range of fuels such as biodiesel, butanol, gasoline, methane, ethanol, vegetable oil and jet fuel [20].
Fourth-generation biofuel uses genetically modified organisms for higher CO2 capture and lipid production [15]. Fourth-generation biofuels are produced by (i) designing photosynthetic microorganisms to produce photobiological solar fuels, (ii) a combination of photovoltaics and electro biofuels or (iii) tailored production of synthetic fine chemicals (synthetic cell factories) biofuels [21]. The production of synthetic fine chemicals involves capture and sequestration of CO2 which promotes carbon negative source of fuel [19,20].
1.3. Relevance of Oil Palm EFB
The major biofuel crops are switchgrass, wheat, sunflower, cottonseed oil, soy, jatropha, palm oil, sugarcane, canola and corn [22,23]. The chosen feedstock is based on its availability of supplies and costs [24]. Table 1 shows the feedstock used by countries to produce biofuels and its total production costs.

Table 1.
Feedstock used by countries for the production of biofuels. Adapted with permission from Aziz Elbehri (2008) [25].
In 2020, the world’s largest producer of palm oil at 43.5 million tons was Indonesia, followed by Malaysia at 19.9 million tons [24]. In the form of empty fruit bunch; EFB, 1.07 ton of EFB is produced in every ton of palm oil. Consequently, the palm oil industry generates 1.07 ton of EFB for every ton of palm oil produced [26]. Therefore, the amount of EFB generated in Malaysia is approximately 21.3 million tons per year. Global production of EFB in 2018 was approximately 80 million tons [26]. This amount is not insignificant compared to other types of biomass: corn straw (128.02 million tons), rice husk (120 million ton) [27], sugarcane bagasse (180.7 million tons) and wheat straw (354.34 million tons) [28].
Due to the large amount of biomass produced by Malaysia and Indonesia (85% of global palm oil production), it is considered an appropriate feedstock to produce bio-oil. As oil palm biomass is available in large quantities in Malaysia and Indonesia, which constitutes 85% of the global palm oil production, the oil palm biomass is considered an appropriate feedstock to produce bio-oil. As seen in Table 1, fresh oil palm fruit bunch (FFB) contributed the highest quantity of agricultural produce in Malaysia in 2007 at 81,920 kilo tons [25].
As indicated in Table 2, in addition to biomass, a large amount of EFB residue is produced, which might be used as a feedstock for second-generation biofuels [15] (Figure 1 and Table 2). Mesocarp fiber, shell, empty fruit bunch (EFB), frond, trunk and palm oil mill effluent are also found in palm oil wastes (POME). POME (palm oil mill effluent) is an oily wastewater produced by palm oil processing mills [29].

Table 2.
Components of oil palm residues in Malaysia which have potential of generating energy. Adapted with permission from Saad Mekhilef (2011) [30].
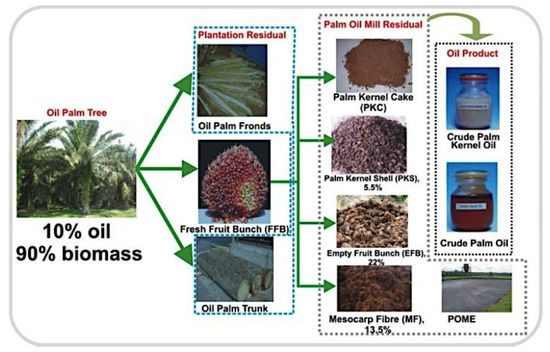
Figure 1.
Plantation and palm oil mill residual in production of crude palm oil from biomass. Adapted with permission from Salman Zafar (2018) [31].
Although oil palm EFB is abundant in South East Asia, there is a relative lack of research on conversion of oil palm EFB to bio-oil. It is not commonly used as feedstock compared to other kinds of biomass. As a result, technological advancements are needed to fully utilize this renewable energy source. The focus of this paper is on oil palm EFB as a resource for bio-oil production. The paper explains the application, principles, processes and reactor design for conversion of oil palm EFB to biofuels. In addition, consideration for biofuel refinement, methods, and environmental impact are discussed.
Table 3 The first category comprises papers on the methodology of biomass conversion into bio-oil [32,33,34,35,36,37,38,39,40,41]. It covers the pyrolysis and liquefaction process in producing bio-oil. The second category is bio-oil characterization and compound analysis [42,43,44,45]. The third category covers the conversion process, experimental design and optimization, including optimal design of reactors to achieve a high production yield, design of experiments, Taguchi Method and other methodologies involved in optimizing the reactors [46,47,48,49,50,51,52,53,54,55]. The fourth category covers bio-oil upgrading for fuel applications [56,57]. Finally, the fifth category comprises studies on the impact of oil palm EFB on the environment and its energy value compared to fossil fuels [30,58,59,60,61,62,63,64,65,66,67].

Table 3.
Summary of EFB literature.
2. Conversion of Oil Palm EFB to Bio-Oil: Principles and Processes
2.1. Oil Palm EFB Characterization and Compound Analysis
Oil palm EFB is a plant biomass which is also called lignocellulosic material. It is largely composed of various oxygen-containing organic polymers. Lignocellulosic materials may be divided into six classes: crop residues, hardwood, softwood, cellulose wastes, herbaceous biomass and organic solid wastes [60]. The chemical components of lignocellulosic biomass are cellulose (polymer glucosan), hemicelluloses (polyose), lignin, organic extractives and inorganic minerals [52,69].
The fibrous material in oil palm EFB is cellulose. Cellulose is the fibrous material in EFB. It forms the primary cell wall of green plants. Cellulose as shown in Figure 2a is a polysaccharide chain of glucose monomers. Cellulose is a linear polymer made of 106 or more β-(1→4)-D-glucose units in the 4C1 conformation. It is probably the most abundant organic molecule comprising 40–60% volume of biomass. Cellulose chains interact with each other via hydrogen bonds and make microfibrils, which constitute the basic unit of complex fibers.
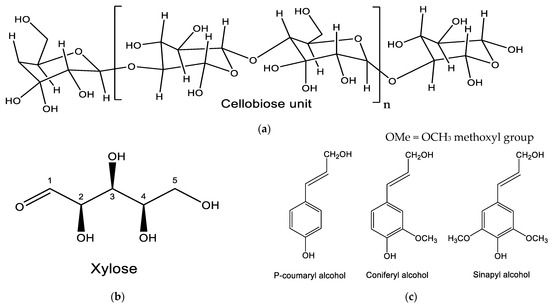
Figure 2.
Chemical Structure of (a) cellulose component: Cellobiose. Adapted with permission from Mohd Azri Sukiran (2008) [69]; (b) hemicellulose component: xylose and (c) lignin components: P-coumaryl alcohol, coniferyl alcohol and sinapyl alcohol. Adapted from [70] (OA).
Hemicellulose, also known as polyose, is a polymer of monosaccharides which are glucose, mannose, galactose, xylose, arabinose, 4-O-methyl glucuronic acid and galacturonic acid [71]. Unlike cellulose, hemicellulose is branched and consists of shorter chains: 500–3000 sugar units as opposed to 7000–15,000 glucose molecules per polymer.
Lignin is the second most abundant natural polymer, and is rich in aromatic benzene ring compound (Figure 2c), making up 10–25% of lignocellulosic biomass. Lignin is insoluble in water, chemically stable and acts as the “glue” that connects cellulose and hemicellulose. Lignin is a three-dimensional, highly cross-linked macromolecule composed of three types of substituted phenols-coniferyl, sinapyl, and p-coumaryl alcohols (Figure 2b) formed by enzymatic polymerization [70].
Inorganic minerals and organic extractives are two minor components of plant biomass. Inorganic compounds, especially of potassium, calcium, sodium, silicon, phosphorus, and chlorine are the main constituents of the ash in biomass feedstocks [69]. Major and minor elements in biomass, in decreasing order of abundance, are commonly C, O, H, N, Ca, K, Si, Mg, Al, S, Fe, P, Cl, Na, Mn and Ti [72].
The chemical composition of EFB fibers is similar to other biomass, but differs in the relative proportion of compounds. Table 4 provides the proportions of the major compositions in different kinds of biomass, including EFB. Interestingly, it shows that pine has the most similar composition with EFB. Compared to some other common biomass such as rice husk, corn cob, and soybean, EFB has a relatively high content of lignin. Figure 3 shows the ternary scatter plot of three main components: cellulose, hemicellulose and lignin content of EFB (labelled as 1) compared to various biomass species. EFB composition is mostly similar to pine (labelled as 2), followed by coconut shell (labelled as 7).

Table 4.
Comparison of components composition (%) in EFB with various biomass species. Adapted with permission from John Wiley and Sons (2014) [71].
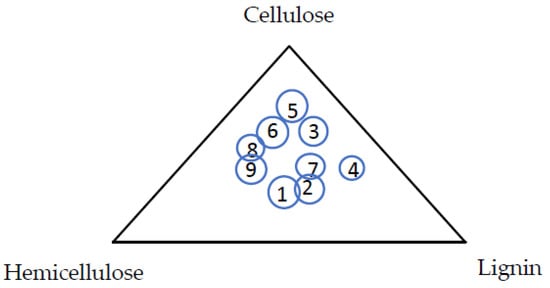
Figure 3.
Ternary plot of cellulose, hemicellulose, and lignin in other types listed in Table 4.
2.2. Pyrolysis Process
Pyrolysis is thermal degradation of biomass in the absence of oxygen [73]. It is one of the most promising processes for conversion of biomass to bio-oil, char and gases. Pyrolysis is classified based on heating rate: slow or fast. During slow pyrolysis, the biomass particles are subjected to heating rates of about 100 K/s while in fast pyrolysis it is in the range of 1300 K/s [71]. The typical products of pyrolysis are liquid, solid and gaseous fractions of C, H and O. Slow pyrolysis provides char or solid fractions and produces coke as a solid fuel. Slow pyrolysis is also called carbonization. Its operating temperature of 400 ± 10 °C is lower than fast pyrolysis [71], and it involves longer residence times, normally greater than 5 s. It has slower heating rates, for example, about 0.1 to 2 °C per second depending on the system, and moderately long, solid-and-vapor residence time. Biomass is slowly devolatilized to produce tar and char.
Up to 80% yield is produced by fast pyrolysis [74]. Fast pyrolysis process provides higher liquid fractions from biomass. Essential features of fast pyrolysis are very high heating and heat transfer rate that require finely ground biomass feed, carefully controlled temperature around 300 °C to 500 °C and rapid cooling of the pyrolysis vapors [71]. Fast pyrolysis occurs on timescales of only a few seconds. Subsequent condensation of the vapor on cooling yields dark brown liquid bio-oil. High heating and fast heat transfer rate at the reaction interface are important factors to produce bio-oil. It also requires a finely ground biomass feed for a larger surface area, good control of the reaction temperature between 450–600 °C and vapor temperature between 400–450 °C, vapor residence time (often <2 s), followed by rapid cooling of the vapor in a condenser.
The fast pyrolysis process can be subdivided into non-catalytic and catalytic fast pyrolysis. The catalytic fast pyrolysis is a promising route to yield higher liquid of bio-oil. Catalysis is added for many reasons, to upgrade pyrolysis vapor to obtain a bio-oil product with decreased oxygen in order to increase its heating value and thermal stability. Catalytic pyrolysis converts biomass into hydrocarbons and higher-value chemicals in a single step. The yields containing five major components, which are benzene, toluene, xylene, ethylene and propylene, are increased with catalytic fast pyrolysis. Besides, the catalytic pyrolysis reactions take place in an inert atmosphere. It is performed with a shorter residence time that is less than 10 s to minimize secondary reactions [75]. The catalyzed pyrolysis oil has lower oxygen content and increased hydrogen to carbon ratio. Thus, the energy content of bio-oil is increased, lower oxygen content leads to lower acidic components and thus a less corrosive nature compared to non-catalytic pyrolysis oil. The catalytic pyrolysis also enables a lower reaction temperature, therefore reducing energy consumption and process cost [75,76].
2.2.1. EFB Pyrolysis Reactions
The non-catalytic fast pyrolysis is a thermal cracking process to produce bio-oil from EFB without using any catalyst. Non-catalytic fast pyrolysis was applied on EFB water washing in order to reduce the ash content and consequently increase the bio-oil yield [42]. Non-catalytic fast pyrolysis produces oxygenated compounds and a small amount of hydrocarbons [77].
Catalytic pyrolysis of EFB is the main method for removing the oxygenated components in order to refine bio-oil with improved quality, stability and yield. Catalytic fast pyrolysis on various feedstocks has been extensively studied [78,79,80,81]. The catalyst facilitates breakage of the C-O, dehydration, decarboxylation and decarbonylation, resulting in improved characteristics of the products [53,82]. Examples of catalysts that have been applied previously include zeolites (HZSM-5, HY and Al-MCM-41), Co-Mo, Ni-Mo [83] and boric oxide [84]. Chang reported that Al-MCM-41 catalyst provides a high yield of phenolic compounds and low yield (<5%) of undesirable olefins, carboxylic acids, ketones and aldehydes [42]. Use of boric oxide as a catalyst, as reported by Lim et al., successfully reduced hydroxyl and methoxy groups by 50–80% [84]. Crystalline zeolites A and Y; synthesized from rice husk ash (RHA) were applied as heterogeneous catalysts [85]. The catalytic conversion of oil palm EFB to bio-oil was conducted at a temperature range of 320–400 °C with zeolite A catalyst loadings of 0.6–3.0 wt%. Four types of zeolite catalysts—Y, ZSM-5, Y-ZSM-5 hybrid, and Y/ZSM-5 composite—were carried out for catalytic conversion of palm oil to produce jet biofuel with high amounts of alkanes and low amounts of aromatic hydrocarbons [86]. Zeolite Y-ZSM-5 hybrid catalyst (99%) produced the highest conversion of palm oil to hydrocarbon compounds followed by zeolite Y/ZSM-5 composites (96%), zeolite Y (91%), and zeolite ZSM-5 (74%).
Three catalysts’ (K2CO3, Ca(OH)2 and MgO) applicability in the pyrolysis of palm oil empty fruit bunch (EFB) was investigated and Ca(OH)2 catalyzed pyrolysis showed greater effect in improving the quantity of the targeted bio-oil product [87]. This study involved evaluation of the fixed-bed operating factors’ impact on the product yield which included pyrolysis temperature, sweeping gas (N2) flow rate and Ca(OH)2 catalyst weight percent. The GC–MS analysis of the Ca(OH)2 catalyzed EFB pyrolysis bio-oil revealed a higher percentage (>10%) of desirable phenolic compounds and lower undesirable acidic groups (>35%) when compared with the non-catalyzed EFB pyrolysis bio-oil analysis. Inexpensive catalysts such as Calcium Oxide has been introduced into fast pyrolysis of palm empty fruit brunch (EFB) biomass [88]. The effect of Ni concentrations (1, 3, 5 and 7 wt.%) on the characteristics of the catalyst Ni/Silica-Alumina and the performance test for the catalytic cracking of bio-oil has been studied [89]. The presence of the HZSM-5 catalyst has also increased the yields of non-condensable gas, water and coke, while decreasing the liquid and char yields from corn cob pyrolysis of the fluidized bed reactor [89]. The elemental analysis revealed that the oxygen content of the collected liquid in the second condenser with HZSM-5 was reduced by more than 25% when compared to that without the catalyst. In the collected liquid with the catalyst, the H/C, O/C molar ratios, and higher heating value of the oil fraction were 1.511, 0.149, and 34.6 MJ/kg, respectively. Overall, catalytic fast pyrolysis of various feedstocks typically yields bio-oil of better quality.
2.2.2. Feedstock Preparation and Pretreatment
Differences in the type of biomass feedstock such as softwood, hardwood and agricultural are associated with changes in the content of the three major structural components that are hemicellulose, cellulose and lignin. These differentiations have led to the investigation of various methods and technologies for the efficient pretreatment of biomass before entering the pyrolysis reactor.
Pretreatment is essential to remove EFB residue, the leftovers produced at palm oil mills upon removing the fruit using rotary threshers [42]. EFB is not uniform in shape, with a typical weight of 3.5 kg and dimensions varying from 170–300 mm in length by 250–350 mm in width [42].
In order to produce high yield and quality bio-oil, EFB needs to be pretreated. Pretreatment can be performed by submerging bundles of EFB in NaOH to expose the fibrous materials [76]. In bioethanol production, the main role of pretreatment is the disruption of the recalcitrant lignocellulose structure, enabling easier access of enzymes to cellulose for a more effective hydrolysis toward fermentable sugars [90]. Pretreatment reduces the crystallinity of cellulose, removes hemicellulose, improves the permeability of lignin seals, delignification and increases the surface area, all of which facilitate the subsequent pyrolysis reactions and make it more accessible to catalysts [46,74,91]. Then, EFB is sun-dried to remove the remaining water content and further ground into fine fibrous biomass. The particle size of the dried EFB is reduced with a shredder and is sieved to a particle size of smaller than 500 µm.
2.3. Slow Pyrolysis Reactor
Slow pyrolysis is typically used to modify the solid material, minimizing the oil produced. Fast pyrolysis and ultra-fast (flash) pyrolysis maximize the gases and oil produced. One of the applications of slow pyrolysis is on producing biofuels on diesel engine performance, combustion and emission characteristics [92]. The pyrolysis process was carried out at 650 °C, with a particle size of 250 µm and 3 h of reaction time. Martynia annua seed pyrolysis oil can replace the petrodiesel up to 40% in an unmodified diesel engine without any major variation in performance and emissions and 40% solid biochar from slow pyrolysis of coffee silverskin [93]. A high amount of solid material from coffee silverskin has been studied as an adsorbent of organic pollutants in water, using methylene blue (MB) and methyl orange (MO) as model compound with 400 °C biochar giving the highest removal values at 98 MB and 40% MO. The slow pyrolysis process has a higher yield of biochar in comparison with fast pyrolysis. The biochar has a potential to be used for soil improvement and act as a carbon sink. The oil and gas products from the pyrolysis process can be used for power and heat.
2.4. Fast Pyrolysis Reactor
Pyrolysis of finely ground materials of several micrometer sizes occurs in a short vapor residence times of 1 to 2 s with high cooling rates in order to minimize thermal decomposition of the vapors after pyrolysis in the absence of, or less, oxygen [71]. Pyrolysis is an endothermic process which requires a source of heat. Heat can be supplied to the reactor by various methods depending on the reactor configuration. These methods are heat transfer through the reactor wall, a regenerable heat carrier transfer between a heat source and the reactor, preheated gases, hot tubes (inline heater), steam or a combination of one or more of these methods. Heat is required to remove the moisture content and transform the solid biomass into vapor. The biomass vapor is channeled out into a condenser. The rapid cooling in the condenser transforms the vapor into a liquid bio-oil and is collected at the condenser outlet.
2.4.1. Types of Reactors
The reactor is a critical component of fast pyrolysis. Therefore, most of the research is concerned with studying various reactor configurations and different feedstocks. For EFB, some examples are fluidized bed, spouted fluidized bed, transported bed, circulating fluid bed, rotating cone, vortex ablative, augur or screw, entrained flow, microwave, fixed bed, hydropyrolysis and vacuum reactor. The most commonly reported reactors for EFB pyrolysis are bubbling fluid beds [38,40,69,94], circulating fluid beds and transported bed [39,69,95,96], ablative pyrolysis [46,51,97], microwave pyrolysis [98], rotating cone reactor [99] and hydropyrolysis [100]. Table 5 lists the many types of pyrolysis reactors available. Fluid beds and circulating fluid beds are the most popular configurations for biomass pyrolysis because of their ease of operation and scale-up-ready modular designs [101], which are listed in the first column with references, types of pyrolysis reactors, operational characteristics, advantages and disadvantages of various types of pyrolysis reactors

Table 5.
Types of pyrolysis reactors, operational characteristics, advantages and disadvantages.
2.4.2. Pyrolysis Parameters and Their Optimization
Gas, liquid bio-oil and bio char are three major products of pyrolysis. The relative amount of product depend on the operating parameters, properties of biomass and types of pyrolysis. Usually, decomposition of biomass at medium temperature (400–550 °C) favors the production of liquid oils with short residence times. At higher temperatures, its ability to produce gaseous products increases. Meanwhile, at low temperatures, char is the dominant product [97]. Figure 4 shows the temperature-dependence of pyrolysis product yield.
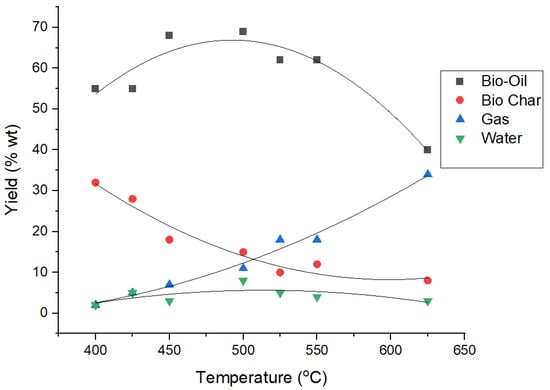
Figure 4.
Effect of pyrolysis temperature on yield of pyrolysis products (using Origin Lab). Adapted with permission from John Tustin (2006) [111].
For optimization purposes, it is therefore helpful to understand the effect of process parameters on yield and characteristics of bio-oil as the following:
- (a)
- Temperature, residence time, and heating rate: Javaid Akhtar et al. [97] reviewed temperature as the most influential parameter in the biomass pyrolysis. If the purpose is to maximize the yield of bio-oil, a low temperature, high heating rate and short vapor residence time are desirable. In order to maximize fuel gas, a high temperature and low heating rate with long vapor residence time is required. Lower temperature with low heating rate maximizes bio char production [112]. Although very short vapor residence times (few seconds to minutes) are recommended, short residence times may lead to incomplete conversion due to insufficient heat transfer into the particle;
- (b)
- Feed size: fine particle sizes of feedstock are preferred to enable rapid and uniform heating. Large particles have heat transfer limitations [53]. Some recommendations of particle size provided are less than 200 μm for rotating cone pyrolysis, less than 2 mm for fluid bed systems, less than 6 mm for circulating fluid beds and less than 10 mm for rotary disc ablative processes [102]. For fixed bed pyrolysis of rapeseed, ideal particle size between 0.85 to 200 μm were reported to yield 60% w/w bio-oil [113];
- (c)
- Sweeping gas: Some reactors use a sweeping gas and steam for fast purging of pyrolysis vapors. The sweeping gas (nitrogen) facilitates quick removal of the hot vapor. Quick cooling of the vapor is necessary to stop secondary reactions.
- (d)
- Biomass type: Different types of biomass contains different amounts of cellulose and hemicellulose which affects the amount of volatiles that form in bio-oil. [99]. Lignin seal is difficult to break at higher temperature which results char residue. Therefore, pretreatment is required in order to delignify the biomass hence maximize the bio-oil yield;
- (e)
- Moisture content: EFB needs to be dried thoroughly before being fed into the reactor, containing at most 30% moisture (as compared to 50–60% moisture in green biomass). Water content in EFB contributed by the pretreatment process can affect the heating rate during pyrolysis since energy is required to evaporate the moisture [114]. Lowering of heating rate due to moisture, reduces the bio-oil yield.
There are many methods to optimize the parameters. Thermochemical process can be optimized through experimental design methods namely response surface method, design of experiment, Taguchi method, numerical, stochastic and simulations [46,47,48,49,50,51,52,53,56]. The optimization of process parameters such as pyrolysis temperature, biomass particle size and holding time using ANOVA and Central Composite Design (CCD) are examples of commonly used methods that have been reported to improve the yield of bio-oil. Bio-oil yield is predicted by reaction kinetic simulation of pretreatment, fast pyrolysis, product collection and upgrading sections [58]. Kinetic modeling of pyrolysis describes practical conversion processes and optimizes the design of efficient reactors [105,115]. The reported EFB pyrolysis of bio-oil yield is summarized in Table 6.

Table 6.
Summary of reported EFB pyrolysis bio-oil yield.
Table 6 shows the average yield of bio-oil production by thermochemical pyrolysis is ~40%. The estimated bio-oil produced from EFB is 3 million tons/year [64]; which is equivalent to 7% of the current crude oil consumption in Malaysia. The crude oil consumption of Malaysia in 2016 is 283 million barrel per year which is equivalent to 45 million tons.
3. Bio-Oil Refinement
3.1. The Uses of Bio-Oil and the Need for Upgrading
By 2030, global demand for transportation fuel is expected to grow significantly, by up to 55% [109]. Due to limited reserves, high costs and increasing demand from the transport sector, bio-oil is a promising alternative to petrol fuel [74,118]. Furthermore, bio-oil provides more efficient combustion and cleaner emissions [10,11]. However, there are currently recognizable drawbacks of bio-oil, or the pyrolysis process that produces it. Some of the drawbacks of bio-oil compared to conventional fuels are low heating value, thermal instability and high corrosivity, as shown in Table 7. Thus, bio-oil needs to be upgraded so it is suitable as transportation fuel. Methods to upgrade bio-oil, such as water extraction, catalytic cracking and hydrotreating, must be investigated in order to improve the properties of bio-oil [74].
Besides being used in transportation, bio-oil can be substituted in gas turbine or steam-based power plants for electricity generation [73]. There are five types of power plants: oil-fired, coal-fired, gas-fired, hydroelectric power and others (biomass, biogas and solar) [119]. Gas turbines are used to drive electric power generators and provide power to aircraft [120] which mostly operate on petroleum distillates and gas fuel. However, gas turbines can essentially burn bio-oil if it is properly designed [120]. However, the use of bio-oil in turbines results in accumulation of deposits in the combustion chamber and turbine blades. Therefore, the application of bio-oil for heat and power generation is possible with minor modifications of the existing equipment.
Approximately three-quarters of the global energy used for heat is fossil fuel-based [3]. Bio-oil can be used as a substitute for fossil fuel oil or diesel in boilers and furnaces [73]. Furnaces heat air and distribute the heated air through the house using ducts. Therefore, biomass from lignocellulosic material such as EFB is a promising alternative for heating applications using boilers and furnaces to replace fossil fuels. Boilers and furnaces are used for industrial and home heating [121]. Boilers heat water and provide either hot water or steam for heating. There are two major types of boiler and furnace; gas-fired and oil-fired. Gas-fired boilers and furnaces use natural gas or propane. Oil-fired boilers and furnaces can be fueled by either petroleum fuel oil, or with renewable bio-oil with simple modifications considering the different characteristics of the fuels [122]. Another product of bio-oil is chemicals such as polyphenols for resins with formaldehyde, phenol for wood adhesives, molded plastics and foam insulations [112], calcium and magnesium acetate for biodegradable deicers, fertilizers [120], levoglucosan, hydroxyacetaldehyde and a range of food flavorings and essences for the food industry [123]. Table 7 summarizes the comparison of the properties, effects and impacts between bio-oil and fossil fuels:


Table 7.
Comparison of the properties, effects and impacts of bio-oil and fossil fuels.
Table 7.
Comparison of the properties, effects and impacts of bio-oil and fossil fuels.
| Properties | Bio-Oil Derived from EFB Fast Pyrolysis | Bio-Oil Derived from Other Lignocellulosic Material | Petroleum Fuel Oil (Diesel/Light Fuel Oil) | Effects of Difference in Characteristics Compared to Petroleum-Based Fuels | Impacts on the Uses of Bio-Oil | ||
|---|---|---|---|---|---|---|---|
| Transportation | Power/Electric Generation | Heating by Boiler and Furnace | |||||
| Moisture (%) | 6.66–24.3 | 14–31 | 0.025 | High water content leads to low ignition, reduces heating value [40], improves the flow characteristic of oil and reduces NOx emission [121] | Very critical—high moisture content makes ignition difficult [74,124] Solution: Pyrolysis parameters are modified | Tolerable | Tolerable |
| Ash, alkali metals (%) | 0.37–3.22 | 0.01–2 | 0.01 | Smoky smell alkaline metal leads to solid deposition during combustion [123]; high ashes reduce heating value | Very critical—solid residues and tar droplets [125] | Tolerable—damage for turbine with coated blades Solution: Hot vapor filtration, pretreat feedstock | Not relevant |
| Solids, Char (%) | 24.1 | NA | - | Bio char/residue in the oil | Critical—impurities in fuel, equipment blockage Solution: hot vapor filtration, liquid filtration [123,126] | Tolerable—impurities in fuel, equipment blockage Solution: Hot vapor filtration, liquid filtration, feedstock size reduction [125,127] | Not relevant |
| Oxygen (%) | 20.02–57.02 | 35–50 | 0.03 | Thermal instability, limited storage stability [42]. Higher combustion efficiency, less CO, CO2, HC and particulate matter (PM) emission [11] | Very critical—high combustion efficiency in transportation engine [11] | Very critical, high combustion efficiency in power generation [11] | Very critical, high combustion efficiency in heating [11] |
| Nitrogen (%) | 0.0113–2.74 | <0.4 | 0.04–0.20 | N2O emission is reduced [11] | Cleaner transportation emission | Cleaner flue gas emission | Cleaner gas emission |
| Sulfur (%) | <0.2 | 0–0.05 | 0.11–0.18 | SO2 emission is reduced [11] | Cleaner transportation emission | Cleaner flue gas emission | Cleaner gas emission |
| Heating Value (HV) (MJ/kg) | 20.23–36.06 | 13–18 | 40.3–45.8 | Low Heating Value (LHV); less energy usage [116,127] | Very critical—less heat of combustion lead to difficult ignition [70] Solution: water content reduction and solvent addition [126] | Tolerable Solution: solvent addition [126] | Tolerable Solution: solvent addition [126] |
| Kinematic Viscosity (mm2/s) | 38.4 at 25 °C | 13.5–128 | 3–7.5 at 40 °C | Viscosity of bio-oil is increased by aldehydes and phenols in it which can polymerize easily, particularly in acidic conditions [79] | Very critical—High pressure drop in pipelines, pipe leakage, pipe rupture [125] | Critical—high pressure drop in pipelines, pipe leakage, pipe rupture Solution: low temperature heating [123] | Tolerable—high pressure drop in pipelines, pipe leakage, pipe rupture [123] |
| pH and total acid number, KOH (g/kg) | 2.33–3.4, 67.75–110 | 2–8 | Neutral, 0.451 | Acidic pH of bio-oil leads to corrosion of aluminum, iron and steel [128] Acidity results from high concentration of carboxylic acids, particularly lauric, palmitic and acetic acids [45] | Very critical—corrosion of vessels and pipework, transportation engine and piping part modification | Tolerable—piping part modification Solution: Use of compatible materials such as stainless steel or olefin polymers [123] | Tolerable—piping part modification Solution: use of compatible materials such as stainless steel or olefin polymers [123] |
3.2. Methods for Bio-Oil Refinement
The application of bio-oil as transportation, turbines for power generation and boilers requires upgrading of the bio-oil to minimize undesired properties as mentioned in Table 7. Bio-oil upgrading methods are classified into physical (P) and chemical (C) upgrading methods [73]. Physical methods involve physical processes such as distillation, molecular distillation, water extraction, hot vapor filtration, solvent addition and emulsification. Chemical methods involve chemical reactions, typically aided by catalysts. Chemical upgrading includes steam reforming, hydrogenation, hydrodeoxygenation (HDO), esterification, one-step-hydrogenation-esterification (OHE) and catalytic cracking.
3.2.1. Physical Upgrading
- (i)
- Distillation is a conventional method for separation which includes atmospheric, vacuum, flash and steam distillation. Steam distillation is commonly used to refine bio-oil by introducing steam into a distilling vessel, followed by heating the bio-oil and decreasing its viscosity [129]. However, due to the thermal sensitivity and susceptibility to reactions such as decomposition, polymerization, and oxygenation, it is difficult to separate bio-oil using conventional distillation methods;
- (ii)
- Molecular distillation—so-called open-path or short-path high vacuum distillation—is performed in a vacuum, where the evaporating surface and condensing surface are in close proximity, smaller than the mean free path [130]. Since there is no atmospheric pressure, it does not involve boiling and evaporation and condensation occur whenever there is a temperature difference. It is very suitable for thermally unstable bio-oil. Due to its low operating temperature and short heating time, it offers high bio-oil separation efficiency [131] with three categories of bio-oil fractions: light, middle and heavy [132]. Molecular distillation consumes more energy than conventional distillation [79]. Shurong Wang [129] proposed a scheme of the process which is a combination of the molecular distillation separation with various methods. Xiangping Li et al. [98] introduced the applications of noble metal and transition catalysts supported on molecular sieves in HDO of lignin-derived bio-oil;
- (iii)
- Water extraction is a simple fractionation and inexpensive method that separates bio-oil constituents and extracts acidic compounds based on polarity and solubility in water [133]. Water is added into bio-oil, or vice versa, with continuous agitation to reach equilibrium, followed by centrifuging of the mixture. The solution forms two phases; the water-soluble fraction (WSF) contains more polar constituents such as acids and ketones. The water-insoluble fraction (WIF) contains hydrophobic compounds that originate from lignin, which may react to produce chemicals such as phenol-formaldehyde resins. Parameters in water extraction include water temperature, stirring time and water:bio-oil ratio. The acidity of pyrolytic lignin was reported to decrease significantly and yield was increased as water:bio-oil ratio was varied from 5:1 to 15:1 [134]. The upgraded bio-oil using water extraction has lower acidity, a higher heating value and more stability due to removal of carboxylic acids, alcohols, sugars, ketones, and other reactive compounds [133];
- (iv)
- Hot vapor filtration (HVF) is a technique to reduce inorganic content in bio-oil. The char particulates are removed by HVF at high temperature before condensation of the vapors [135]. Hot-vapor filtration reduces the ash content of the oil to less than 0.01%, the alkali content to less than 10 ppm and separates char from the pyrolysis gas or vapor steam [70]. However, the yield of bio-oil is low and oxygen and water content are high;
- (v)
- Emulsification is a method of upgrading bio-oil by using surfactants [79] that can favorably improve the ignition characteristics, but cetane number, corrosivity and heating value are not improved. Emulsification requires significant energy input; currently, most studies have reported emulsifying less than 400 mL of bio-oil [79]. Increasing the efficiency of fuel pumps, and testing, production and design of injectors are highly needed. In addition, the cost of surfactants is high;
- (vi)
- Solvent addition is a process of adding polar solvents, especially methanol, into bio-oil to homogenize oil stability and reduce the viscosity [73]. It is an economical and simple way of bio-oil upgrading. The solvents can be expensive and organic solvent addition is not really practical for commercialization due to its high cost [76].
3.2.2. Chemical Upgrading
- (i)
- Steam reforming is a chemical process that occurs between the range of 600–800 °C, and high space velocities with an Ni-based catalyst [74]. The output is syn-gas, which can be converted into methanol and alkanes [74]. It is an endothermic two-step reaction [94]. The first step involves reformation of natural gas to produce hydrogen and carbon monoxide. In the second step, known as water gas shift reaction, carbon monoxide gas produced in the first reaction reacts with steam over a catalyst to form hydrogen and carbon dioxide. A significant advantage of steam reforming is its ability to simultaneously upgrade bio-oil while producing renewable hydrogen gas [79]. As discussed in Section 3.2.1, bio-oils separate into an aqueous and organic fraction by water extraction. The water-soluble fraction can be converted into syn-gas by steam reforming [74]. However, deactivation of the catalyst due to coke is a challenge in steam reforming [74,79]; coking can be partially mitigated by hydrogenating or hydrotreating the bio-oil. The hydrogen produced may be recycled for hydrogenation purposes;
- (ii)
- Hydrogenation is a process of saturating the double bonds through catalytic addition of hydrogen in a reactor [136]. Hydrogenation is the type of reaction which occurs from hydrotreating of pyrolysis bio-oil, under specific conditions, such as high pressure (10–20 Mpa), optimized temperature and hydrogen flow rate as well as an appropriate catalyst [79]. Catalysts such as Al2O3 based catalysts [137,138] and Ru/SBA-15 catalysts [139] have been found to be effective. Hydrogenation reduces water content, increases pH and H element, improves stability, decreases viscosity and organic, carboxylic acid [112], aldehydes and reactive compounds’ contents. In addition, it is an efficient method to convert phenolic compounds into alcohol and alkanes [139] which leads to higher heating value and stability;
- (iii)
- Hydrotreatment is a chemical process of upgrading bio-oil, where hydrogen is added to the carbonyl group with the addition of a catalyst. The hydrogen may be obtained from the biomass itself if the system is integrated with a biorefinery [74]. Hydrotreating, when performed to minimize the oxygen level of the hydrocarbon, is called hydrodeoxygenation (HDO). In HDO, ideally the oxygen-containing compounds are converted to oxygen-free hydrocarbons and water using a hydrogen-using catalyst. Hydrotreatment is carried out at high pressure (up to 200 bars) and temperature up to 400 °C and requires a hydrogen supply [140]. Hydrotreating processes remove sulfur as well as other chemical compounds which are undesirable and detrimental to the stability and performance specifications of the product. The catalyst used is cobalt, molybdenum and nickel finely distributed on alumina extrudates [141]. HDO removes oxygen under high pressure of hydrogen with moderate temperature (>400 °C) in the presence of a heterogenous catalyst [142]. Sulfided Co-Mo and Ni-Mo-based catalysts are commonly used in petrochemical industry for removal of sulfur, nitrogen and oxygen from hydrocarbons. Pt/SiO–Al2O3, vanadium nitride and Ru have also been used for HDO [74,142]. HDO improves ignition characteristics by increasing the heating value, increases miscibility with hydrocarbon and improves stability by decreasing organic acid, aldehydes and reactive compounds. However, HDO of bio-oil is very costly, and requires high hydrogen consumption of 400 nitrogen per kg bio-oil [57];
- (iv)
- Esterification is performed by heating a mixture of bio-oil (carboxylic acid) with alcohol and acid catalyst. Esterification converts carboxyl acid in bio-oil into their corresponding esters; reduces the acid number, decreases water content, improves viscosity, fluidity [79], stability, engine ignition, corrosion [143] and promotes ozone oxidation [144]. Zhou et al. [80] found that esterification removed char particles to improve the homogeneity of dispersed organic droplets in bio-oil. However, large molecules are still present in the upgraded bio-oil [79]. Esterification does not reduce the oxygen content in bio-oil. Recently, hydrogenation and esterification were combined in one step, called One Step Hydrogenation-Esterification (OHE), to convert furfural and acetic acid into furfuryl alcohol and ester over a bifunctional catalyst. OHE reduces the acid number and forms a combustible bio-oil by improving its stability and heating value [79,145]. OHE is a promising method to upgrade bio-oil due to its capability to reduce larger oxygen content that limits many bio-oil applications.
- (v)
- Catalytic cracking breaks down long-chain alkanes in bio-oil into smaller alkanes and alkenes under hydrogen flow using catalysts and a temperature range above 350 °C with high pressure. Catalytic cracking produces solid coke, liquid and combustible gas. Oxygen level in bio-oil is mostly converted to H2O at lower temperatures, and to CO and CO2 at higher temperatures. Catalysts such as aluminum silicate, HZSM-5 and bifunctional catalysts under supercritical conditions can convert the majority of acids into esters [79], which reduces the density and viscosity and increases the pH and higher heating value. Bio-oil has a high content of carboxylic acids that make it corrosive and impart it with a lower pH and high acid number; the removal of acids from the bio-oil could make it more stable and less corrosive. Shurong Wang et al. used catalytic cracking with nitrogen purging to convert the carboxylic acids of bio-oil into liquid hydrocarbons [78]. Catalytic cracking is typically performed using solid acid catalysts such as aluminosilicates or zeolites [57]. Catalytic cracking of bio-oil using zeolites can improve bio-oil stability and lower oxygen content. However, the main challenges in catalytic cracking of bio-oil are coke formation (8–25%) [83] due to thermally unstable components [74] and low yield of upgraded liquid oil. Coke can be produced through homogeneous reaction in the gas phase and heterogeneous reaction over the catalyst. Zhu et al. [146] studied catalytic cracking using MCM-41, an acid cracking catalyst that was able to remove almost all acid in bio-oil to obtain an upgraded bio-oil yield of 22.3% and coke yield of 20.5%. One of the ways to reduce coking is to mix crude bio-oil with gas oil (C1–C4). Zhang et al. [147] investigated cracking of crude bio-oil and butanol mixture using ZSM-5 catalyst, which leads to an upgraded bio-oil and coke yield of 28.8 and 12.8%, respectively. Sunarno et al. [57] obtained an improved yield of bio-oil (45.42%) and a very low yield of coke (4.34%) using silica alumina as catalyst in a series tubular reactor under atmospheric pressure. Catalytic cracking improved bio-oil properties with higher heating value, lower density, and lower viscosity at 35.48 MJ/kg, 938.5 kg/m3 and 5.95 mm2/s, respectively [57].
3.3. Comparative Analysis of Upgrading Methods
Table 8 summarizes the undesired properties of bio-oil along with ways to remedy them by suitable upgrading methods. It is shown in Table 8 that the decision for suitable method of upgrading depends on the specific application needs. For example, deoxygenation and conventional refining is suitable for upgrading bio-oil for use as alternative for transportation fuels [73,79,112]. The degree of expensiveness is indicated by the quantity of symbol $. The least value of $ indicates the cheapest and $$$$$ is the most expensive value.

Table 8.
Effect of upgrading treatments on bio-oil properties.
The major effect to improve bio-oil quality can be mapped from six upgrading methods as listed in Table 9. The six methods are selected based on the four to at most six negative properties of bio-oil that can be overcome.

Table 9.
Major effect of bio-oil upgrading method summary.
In conclusion for upgrading method, hydrodeoxygenation (HDO) seems to be a promising method for making bio-oil compatible with conventional fuel. High impact on lowering oxygen content is mostly considered as this factor relates strongly with acidity, corrosiveness, heating value and thermal instability [74,120]. However, the cost for setting up the HDO process is quite high and difficult to maintain and commercialize. Water extraction is another good upgrading method to consider due to its lowest cost; the chemical compounds in the insoluble fraction can be used for other applications. As shown in Table 7, in selecting appropriate methods, the properties of bio-oil are mapped onto the critical requirements of various applications. In transportation fuels, properties such as water content, alkali metal, hydrogen and oxygen content are crucial for smooth ignition, minimizing blockage in equipment and ensuring high combustion efficiency. Many properties are desirable but not critical, whereas others are more important. Oxygen content is very critical to ensure high combustion efficiency for transportation, power generation and heating applications. pH is another critical property to be considered as it leads to corrosion of vessels and pipework. These properties can be overcome by HDO and water extraction as shown in Table 9. Water extraction is considered a promising method in upgrading bio-oil since it extracts acidic compounds, thus increasing its pH and reducing oxygen content. This also increases thermal stability and reduces viscosity in bio-oil. Compared to HDO, a water extraction method is less expensive, adding to its potential [134,151]. Some challenges still exist for HDO such as catalyst development and refinement, understanding the coke formation, and effect of impurities on bioactivity and performance [153]. Although the benefits outweigh the challenges, it is important to optimize the process and practical for industrial utilization. Continuous improvements are also seen for other methods in bio-oil upgrading research. As for catalyst development, Co-MoS2 system or metal catalysts have been extensively studied, but due to high carbon emission and the high prices of raw materials for the noble metals, alternatives are desired.
4. Environmental Impact
In order to reduce pollution and create sustainability in the palm oil industry, several efforts have been focused on biomass recycling involving the use of oil palm empty fruit bunches (EFB) [154]. Gases that trap heat in the atmosphere are called greenhouse gases (GHG) and include carbon dioxide, methane, nitrous oxide and fluorinated gases such as hydrofluorocarbons (HFC) and nitrogen trifluoride. The processing of EFB into bio-fuel and value-added materials is classified into seven categories (Table 10) to compare their environmental impact, including greenhouse gas emission [62]. Processes that produce fuel are production of ethanol, methane, briquettes and biofuel for use in combined heat and power (CHP) plants. Processes which create useful material are composting, medium density fiberboard (MDF) production, and production of pulp and paper [62]. Saswattecha et al. [154] considered six options for oil palm EFB treatment (mulching, composting plant, combustion, pellet production, ethanol production and gasification), and concluded that combustion was the most effective option as it reduced acidification by 20–238% and human toxicity by 30–92%, whereas mulching was the most cost-effective [154].

Table 10.
Environmental impact of commercial processes of oil palm EFB.
One ton of EFB is estimated to yield 94.7 kg ethanol, 79 m3 of methane, 0.33 t briquette, 0.43 MWh of electricity, 0.4 t compost, 0.1 m3 MDF and 0.2 t pulp and paper [62]. Pulp and paper production requires about 4.2 GJ to process a ton of EFB and is the highest among these processes. Ethanol production requires 1.3 GJ, and MDF requires 1.07 GJ. In addition to the conversion processes, pretreatment of EFB such as shredding, washing, and drying that is required to obtain the raw material in suitable form also requires energy. Composting requires less than 100 MJ per ton, consumed by the required heavy equipment. Methane, CHP plant and briquette production require 520, 360 and 166 MJ per ton energy consumption, respectively.
As seen in Table 10, composting is the best commercial process, it is environmentally friendly and it scores well on all aspects except toxicity for aquatic and terrestrial environments, in which it is outperformed by briquette and methane production. However, methane production has a relatively high potential for eutrophication. With respect to global warming, use of EFB as biofuel in CHP plants reduces GHG emission by 218.6 kg CO2 equivalent; the reduction for composting is 176.5 kg CO2 equivalent and for methane production is 154.6 kg CO2 equivalent. GHG emissions are not reduced in pulp and paper or MDF production. In terms of CO2 emission, composting releases the least CO2 < briquette < biofuel for CHP plant < MDF board < biofuel for CHP plant < methane production < ethanol production < pulp and paper [61]. However, for GHG reduction, biofuels for the CHP plant gives the most significant impact > composting > methane recovery [62]. Based on this analysis, biofuels production from EFB imposes a significant environmental impact compared to other materials. Table 10 summarizes the environmental impact of seven commercial processes of oil palm EFB.
5. Limitations and Way Forward
Biomass has been identified as one of the potential sources of renewable energy, involving a conversion of solid waste into biofuels by pyrolysis. It is favorable because of the abundant supply of biomass and process feasibility. This review underlined the current state of oil palm EFB as a second-generation biomass for conversion into bio-oil and its potential to substitute for the depleting fossil fuels. The chemical composition of oil palm EFB is compared with other forms of biomass to comply with the required characteristics and properties of an alternative fuel. Chemical composition and physical properties of lignocellulosic components of EFB materials are important criteria in determining the product derived from it. The lack of a rigorous and uniform methodology for biomass pretreatment [90,155] is one of the key issues connected with the first biomass pretreatment process [90,155]. Physical pretreatment methods such as reduction in size, densification, torrefaction and the chemical pretreatment processes such as alkaline, acid, steam explosion, hydrothermal and wet torrefaction have been studied and reported but there is no study relating to the application of ionic liquids pretreated lignocellulosic biomass in pyrolysis. Ionic liquids may positively impact the process of pyrolysis and facilitate improved efficiency. Fast pyrolysis is presented as an effective method for thermochemical biomass conversion into bio-oil. Circulating fluidized bed is the most widely used reactor for EFB due to its simplicity and high liquid yield, followed by fixed-bed and ablative systems. Although a fixed fluid bed reactor is a simple reactor with excellent temperature control, it has a high requirement for rapid char separation to prevent vapor cracking. The fundamental challenge in the circulating fluid bed is that the hydrodynamics are quite complex and must be thoroughly investigated.
The barrier includes greater concentrations of oxygen and moisture in the bio-oil, thus it is not suitable for direct use as a combustion fuel in automotive engines. Therefore, a suitable method has to be developed to eliminate the water content and oxygen in order to yield a high-grade bio-oil equivalent to the existing petroleum fuels. Several upgraded technologies for bio-oil have been reported so far, which includes in this paper, such as solvent extraction, hydrodeoxygenation, emulsification, catalytical approaches and steam reforming. However, the yield of the bio-oil in these processes is highly erratic, making it another limiting factor for bio-oil production. Bio-oil products must be improved while yields are maintained. Another problem is the lack of understanding of the relationship between the preliminary precursors (raw material) and the overall operation of the pyrolysis plant.
Understanding the pyrolysis process parameters in a reactor system is important in producing higher yield and best quality bio-oil. Three parameters, namely temperature, residence time and heating rates, are the major parameters that affect the output of products. A temperature range between 400 °C to 550 °C is effective in maximizing yield of liquid oil. It is established that a short vapor residence time with a high heating rate maximizes bio-oil yield, and long vapor residence time with a low heating rate maximizes yield of fuel gas. Finally, it is observed that char is the dominant product when pyrolysis occurs at low temperature with lower heating rate.
Oil palm EFB is an excellent non-conventional source of biomass energy. Deriving alternative jet fuels from oil palm EFB is an important strategy towards achieving green transportation, especially in Southeast Asia. Extraction or fractionation is a simple and inexpensive bio-oil refinement method. It improves the properties of bio-oil by extracting corrosive and thermally unstable acidic compounds by using their greater polarity and preferential solubility in water. Parameters in water extraction are investigated to remove high polarity compounds such as formic acid, acetic acid, ketone and reactive compounds, resulting in refined bio-oil with low pH for acidity, a higher heating value and more stability. The CO2 emissions from oil palm EFB processes can be reduced significantly by converting oil palm EFB into ethanol, briquette, biofuels for power plants and composting. Oil palm EFB as raw material for energy applications has the potential to minimize greenhouse gas emissions and pollution. Advances are needed to make pyrolysis as an efficient process and to find economical and effective means to upgrade bio-oil into biofuels. Ongoing research to commercialize biomass-derived bio-oil is still needed to provide a reliable and sustainable source of biomass-derived fuels.
6. Conclusions
Biomass is an attractive clean energy resource which can be converted into biofuels through biochemical and thermochemical technologies. It is favorable because of the abundant supply of biomass and process feasibility. This paper underlines the current status of oil palm EFB as the second-generation biomass for conversion into bio-oil and its potential to substitute the depleting fossil fuels or petroleum with biofuels. The review has presented important findings in oil palm empty fruit bunch conversion into biofuels that covers the similarities and contrast between biomasses, the best reactors followed by upgrading techniques for biomass conversion into biofuels. The biggest challenge is the incompatible properties of biofuels including low heating value, thermal instability, and high corrosivity that necessitates upgrading. In this paper, the comparison of the properties, effects and impacts of bio-oil and petroleum-based fuels are reflected to the main three uses of bio-oil that are transportation, power or electric generation, and heating by boiler and furnace. The difficulties in overcoming the incompatibility for making biofuels through bio-oil upgrading has been summarized in a mapping table arranged with the degree of criticality of the properties. Besides properties, the cost of these upgrading methods and its environmental impact which seldom been discussed in any reviews has been added to optimize the selection criteria. A broad spectrum of biomass-to-biofuels is reviewed concisely which is found to be unique to this paper.
Author Contributions
A huge appreciation to all authors in producing this manuscript successfully. R.D. as the main and corresponding author, contributions are writing the original draft, reviewing and editing, investigation of the mapping matrix, and funding acquisition. To all co-authors: R.K. who contributed in supervising, visualization, reviewing and editing, validation of methodology, and conceptualization of this paper. H.H. who contributed in writing the original draft together, editing of the paper, and assisted the analysis. All authors have read and agreed to the published version of the manuscript.
Funding
The research work is supported by the Malaysia Institute for Innovative Nanotechnology (NanoMITe) Research Consortium and research grant LRGS from the Malaysia Ministry of Higher Education R.K130000.7840.4L823, UTM Business Entity grant S.K130000.0456.4Y253, GUP Tier 2 grant Q.K130000.2656.15J85, Collaborative Research Grant R.K130000.7356.4B555, Contract Research Grant from L’Oréal For Women in Science Award 2020, R.K130000.7656.4C443 and Industry-International Incentive Grant. Q.K130000.3656.03M04. The work is done at Microfluidics and Nanofluidics Research Laboratory, Department of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, USA and Universiti Teknologi Malaysia, UTM Malaysia.
Institutional Review Board Statement
Ethical review and approval were waived for this study, for studies not involving humans or animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data source is provided in the References.
Acknowledgments
The authors fully acknowledge the Malaysia Ministry of Higher Education (MOHE), Massachusetts Institute of Technology (MIT), Cambridge USA, Universiti Teknologi Malaysia (UTM), Malaysia, and Naglus Industries Sdn. Bhd. (1359337-D) for the support and scholarship given in making this important research viable and effective.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Energy Consumption Statistics|Enerdata. Available online: https://yearbook.enerdata.net/total-energy/world-consumption-statistics.html (accessed on 25 August 2017).
- BP. BP Statistical Review of World Energy 2017. Br. Pet. 2017, 66, 8–11. [Google Scholar]
- Renewables 2016 Global Status Report. Available online: https://www.ren21.net/wp-content/uploads/2019/05/REN21_GSR2016_FullReport_en_11.pdf (accessed on 9 September 2021).
- Ben-Iwo, J.; Manovic, V.; Longhurst, P. Biomass resources and biofuels potential for the production of transportation fuels in Nigeria. Renew. Sustain. Energy Rev. 2016, 63, 172–192. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, S.E.; Wahid, M.A.; Aghili, N. The scenario of greenhouse gases reduction in Malaysia. Renew. Sustain. Energy Rev. 2013, 28, 400–409. [Google Scholar] [CrossRef]
- Monitoring Division—Global Greenhouse Gas Reference Network GGGRN. Available online: http://www.esrl.noaa.gov/gmd/ccgg/ (accessed on 9 September 2021).
- Global Greenhouse Gas Emissions Data. Available online: https://www.epa.gov/ghgemissions/global-greenhouse-gas-emissions-data (accessed on 5 January 2018).
- Intergovernmental Panel on CLIMATE CHANGE. Available online: https://www.nobelprize.org/prizes/peace/2007/ipcc/facts/ (accessed on 5 January 2018).
- Demirbas, A. Fuel Properties of Pyrolysis Oils from Biomass. Energy Sources Part A Recovery Util. Environ. Eff. 2009, 31, 412–419. [Google Scholar] [CrossRef]
- Demirbas, A. Importance of biodiesel as transportation fuel. Energy Policy 2007, 35, 4661–4670. [Google Scholar] [CrossRef]
- Demirbas, A. Political, economic and environmental impacts of biofuels: A review. Appl. Energy 2009, 86, S108–S117. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Dharmaraja, J.; Shobana, S.; Sundaram, A.; Chang, S.W.; Kumar, G.; Shin, H.; Saratale, R.G.; Saratale, G.D. Transesterification and fuel characterization of rice bran oil: A biorefinery path. Fuel 2019, 253, 975–987. [Google Scholar] [CrossRef]
- Dharmaraja, J.; Nguyen, D.D.; Shobana, S.; Saratale, G.D.; Arvindnarayan, S.; Atabani, A.E.; Chang, S.W.; Kumar, G. Engine performance, emission and bio characteristics of rice bran oil derived biodiesel blends. Fuel 2019, 239, 153–161. [Google Scholar] [CrossRef]
- Adams, E. Electric Airplanes Need Better Batteries—Which Should Arrive in 30 Years. Available online: https://www.wired.com/2017/05/electric-airplanes-2/ (accessed on 5 January 2018).
- Dutta, K.; Daverey, A.; Lin, J.G. Evolution retrospective for alternative fuels: First to fourth generation. Renew. Energy 2014, 69, 114–122. [Google Scholar] [CrossRef]
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Ghosh, S.K. Biomass & Bio-waste Supply Chain Sustainability for Bio-energy and Bio-fuel Production. Procedia Environ. Sci. 2016, 31, 31–39. [Google Scholar]
- Open Cleantech. Biofuels: 1st, 2nd and 3rd Generation. Available online: http://resources.opencleantech.com/our-blog/biofuels-1st-2nd-and-3rd-generation (accessed on 13 December 2017).
- Alam, F.; Mobin, S.; Chowdhury, H. Third generation biofuel from Algae. Procedia Eng. 2014, 105, 763–768. [Google Scholar] [CrossRef]
- Yang, S.I.; Hsu, T.C.; Wu, C.Y.; Chen, K.H.; Hsu, Y.L.; Li, Y.H. Application of biomass fast pyrolysis part II: The effects that bio-pyrolysis oil has on the performance of diesel engines. Energy 2014, 66, 172–180. [Google Scholar] [CrossRef]
- Aro, E.M. From first generation biofuels to advanced solar biofuels. Ambio 2016, 45, 24–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Zhou, J.; Zhang, Q.; Zhu, X. Evaluation methods and research progresses in bio-oil storage stability. Renew. Sustain. Energy Rev. 2014, 40, 69–79. [Google Scholar] [CrossRef]
- Biomass Energy. Available online: https://www.nationalgeographic.org/encyclopedia/biomass-energy/ (accessed on 12 December 2017).
- Average Prices for Palm Oil Worldwide from 2014 to 2025. Available online: https://www.statista.com/statistics/675813/average-prices-palm-oil-worldwide/ (accessed on 9 September 2021).
- Coyle, W.; Dohlman, E.; Elbheri, A. The Economics of Biomass Feedstocks in the United States: A Review of the Literature; Technical Report; U.S. Department of Agriculture USDA: Washington, DC, USA, January 2008. [Google Scholar]
- Asia Biomass Office. Import of EFB (Empty Fruit Bunch) of Palm Is Swelling/Asia Biomass Energy Cooperation Promotion Office—Asia Biomass Office. Available online: https://www.asiabiomass.jp/english/topics/1001_03.html (accessed on 19 September 2018).
- Abbas, A.; Ansumali, S. Global Potential of Rice Husk as a Renewable Feedstock for Ethanol Biofuel Production. Bioenergy Res. 2010, 3, 328–334. [Google Scholar] [CrossRef]
- Sarkar, N.; Ghosh, S.K.; Bannerjee, S.; Aikat, K. Bioethanol production from agricultural wastes: An overview. Renew. Energy 2012, 37, 19–27. [Google Scholar] [CrossRef]
- What Is POME|BioEnergy Consult. Available online: https://www.bioenergyconsult.com/tag/what-is-pome/ (accessed on 24 September 2018).
- Mekhilef, S.; Saidur, R.; Safari, A.; Mustaffa, W.E.S.B. Biomass energy in Malaysia: Current state and prospects. Renew. Sustain. Energy Rev. 2011, 15, 3360–3370. [Google Scholar] [CrossRef]
- Zafar, S. Bioenergy Developments in Malaysia. Available online: https://www.bioenergyconsult.com/bioenergy-developments-malaysia/ (accessed on 25 July 2017).
- Rachel-Tang, D.Y.; Islam, A.; Taufiq-Yap, Y.H. Bio-oil production via catalytic solvolysis of biomass. RSC Adv. 2017, 7, 7820–7830. [Google Scholar] [CrossRef] [Green Version]
- Sunarno; Herman, S.; Rochmadi; Mulyono, P.; Budiman, A. Effect of support on catalytic cracking of bio-oil over Ni/silica-alumina. AIP Conf. Proc. 2017, 1823, 020089. [Google Scholar] [CrossRef] [Green Version]
- Zainab, H.; Nurfatirah, N.; Norfaezah, A.; Othman, H. Green bio-oil extraction for oil crops. In Materials Science and Engineering; IOP Conference Series; IOP Publishing Ltd.: Bristol, UK, 2016; Volume 133, p. 012053. [Google Scholar]
- Sarwono, R.; Pusfitasari, E.D.; Ghozali, M. Hydrothermal liquefaction of palm oil empty fruit bunch (EFB) into bio-oil in different organic solvents. AIP Conf. Proc. 2016, 1737, 060015. [Google Scholar]
- Pogaku, R.; Hardinge, B.S.; Vuthaluru, H.; Amir, H.A. Production of bio-oil from oil palm empty fruit bunch by catalytic fast pyrolysis: A review. Biofuels 2016, 7, 647–660. [Google Scholar] [CrossRef]
- Awalludin, M.F.; Sulaiman, O.; Hashim, R.; Nadhari, W.N.A.W. An overview of the oil palm industry in Malaysia and its waste utilization through thermochemical conversion, specifically via liquefaction. Renew. Sustain. Energy Rev. 2015, 50, 1469–1484. [Google Scholar] [CrossRef]
- Sembiring, K.C.; Rinaldi, N.; Simanungkalit, S.P. Bio-oil from Fast Pyrolysis of Empty Fruit Bunch at Various Temperature. Energy Procedia 2015, 65, 162–169. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.W.; Koo, B.S.; Ryu, J.W.; Lee, J.S.; Kim, C.J.; Lee, D.H.; Kim, G.R.; Choi, S. Bio-oil from the pyrolysis of palm and Jatropha wastes in a fluidized bed. Fuel Process. Technol. 2013, 108, 18–124. [Google Scholar] [CrossRef]
- Isahak, W.N.R.W.; Hisham, M.W.M.; Yarmo, M.A.; Hin, T.Y.Y. A review on bio-oil production from biomass by using pyrolysis method. Renew. Sustain. Energy Rev. 2012, 16, 5910–5923. [Google Scholar] [CrossRef]
- Yusoff, S. Renewable energy from palm oil - innovation on effective utilization of waste. J. Clean. Prod. 2006, 14, 87–93. [Google Scholar] [CrossRef]
- Chang, S.H. An overview of empty fruit bunch from oil palm as feedstock for bio-oil production. Biomass Bioenergy 2014, 62, 174–181. [Google Scholar] [CrossRef]
- Nazirah, Z.S.; Ridzuan, M.J.M.; Hafis, S.M.; Mohamed, A.R.; Azduwin, K. Viscosity Analysis of Empty Fruit Bunch (EFB) Bio-Oil. J. Mech. Eng. Sci. 2013, 5, 623–630. [Google Scholar] [CrossRef]
- Abdullah, N.; Sulaiman, F.; Gerhauser, H. Characterisation of oil palm empty fruit bunches for fuel application. J. Phys. Sci. 2011, 22, 1–24. [Google Scholar]
- Khor, K.H.; Lim, K.O.; Zainal, Z.A.; Pinang, P.; Tebal, N. Characterization of Bio-Oil: A By-Product from Slow Pyrolysis of Oil Palm Empty Fruit Bunches School of Physics, School of Mechanical Engineering, Engineering Campus. Am. J. Appl. Sci. 2009, 6, 1647–1652. [Google Scholar]
- Dolah, R.; Hamdan, H.; Muhid, N.; Yahaya, H.; Rashidi, K.A.; Rashidi, S.B. Second Generation Catalytic Pyrolysis of Oil Palm Empty Fruit Bunches into Bio Oil. In Proceedings of the NanoMITe Annual Symposium (NMAS 2016), Kuala Lumpur, Malaysia, 28 September 2016. [Google Scholar]
- Rahman, A.A.; Sulaiman, F.; Abdullah, N. Effect of temperature on pyrolysis product of empty fruit bunches. AIP Conf. Proc. 2015, 1657, 040011. [Google Scholar] [CrossRef]
- Mohamed, A.R.; Hamzah, Z.; Daud, M.Z.M. Optimization of the pyrolysis process of empty fruit bunch (EFB) in a fixed-bed reactor through a central composite design (CCD). AIP Conf. Proc. 2014, 1605, 1172–1177. [Google Scholar]
- Alias, N.; Ibrahim, N.; Kamaruddin, M.; Hamid, A. Design and Fabrication of Bench-Scale Flash Pyrolysis Reactor for Bio-Fuel Production. Chem. Eng. 2014, 39, 943–948. [Google Scholar]
- Abdullah, N.; Gerhauser, H.; Sulaiman, F. Fast pyrolysis of empty fruit bunches. Fuel 2010, 89, 2166–2169. [Google Scholar] [CrossRef]
- Hew, K.L.; Tamidi, A.M.; Yusup, S.; Lee, K.T.; Ahmad, M.M. Catalytic cracking of bio-oil to organic liquid product (OLP). Bioresour. Technol. 2010, 101, 8855–8858. [Google Scholar] [CrossRef] [PubMed]
- Sukiran, M.A.; Kartini, N.O.R.; Bakar, A.B.U.; Chin, C.M.E.E. Optimization of Pyrolysis of Oil Palm Empty Fruit Bunches Optimization of Pyrolysis of Oil Palm Empty Fruit Bunches. Am. J. Appl. Sci. 2009, 21, 653–658. [Google Scholar]
- Misson, M.; Haron, R.; Fadhzir, M.; Kamaroddin, A.; Aishah, N.; Amin, S. Bioresource Technology Pretreatment of empty palm fruit bunch for production of chemicals via catalytic pyrolysis. Bioresour. Technol. 2009, 100, 2867–2873. [Google Scholar] [CrossRef]
- Rozzeta, D.; Hassan, M.Z.; Krishnan, S.; Ramlie, F.; Din, M.F.M.; Jamaludin, K.R. Development of F-N-C-O Taguchi Method for Robust Measurement System using a Case Study of T-Peel Test on Adhesion Strength. Appl. Sci. 2020, 10, 6203. [Google Scholar]
- Dolah, R.; Miyagi, Z. Effect of Peel Side on Optimum Condition for Measuring Flexible Film Peel Strength in T-Peel Adhesion Test. J. Test. Eval. 2014, 42, 50–62. [Google Scholar] [CrossRef]
- Abdullah, N.; Gerhauser, H. Bio-oil derived from empty fruit bunches. Fuel 2008, 87, 2606–2613. [Google Scholar] [CrossRef] [Green Version]
- Sunarno; Rochmadi; Mulyono, P.; Budiman, A. Catalytic cracking of the top phase fraction of bio-oil into upgraded liquid oil. In AIP Conference Proceedings; AIP Publishing LLC: New York, NY, USA, 2015; Volume 1737, p. 060008. [Google Scholar]
- Mabrouki, J.; Abbassi, M.A.; Guedri, K.; Omri, A.; Jeguirim, M. Simulation of biofuel production via fast pyrolysis of palm oil residues. Fuel 2015, 159, 819–827. [Google Scholar] [CrossRef]
- Do, T.X.; Lim, Y.I. Techno-economic comparison of three energy conversion pathways from empty fruit bunches. Renew. Energy 2016, 90, 307–318. [Google Scholar] [CrossRef]
- Do, T.X.; Lim, Y.; Jang, S.; Chung, H.J. Hierarchical economic potential approach for techno-economic evaluation of bioethanol production from palm empty fruit bunches. Bioresour. Technol. 2015, 189, 224–235. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.F.; Rahim, N.A. Palm Oil EFB: Green Energy Source in Malaysia. Appl. Mech. Mater. 2014, 619, 376–380. [Google Scholar] [CrossRef]
- Chiew, Y.L.; Shimada, S. Current state and environmental impact assessment for utilizing oil palm empty fruit bunches for fuel, fiber and fertilizer e A case study of Malaysia. Biomass Bioenergy 2013, 51, 109–124. [Google Scholar] [CrossRef]
- Luk, H.T.; Lam, T.Y.G.; Oyedun, A.O.; Gebreegziabher, T.; Hui, C.W. Drying of biomass for power generation: A case study on power generation from empty fruit bunch. Energy 2013, 63, 205–215. [Google Scholar] [CrossRef]
- Harsono, S.S.; Grundman, P.; Lau, L.H.; Hansen, A.; Salleh, M.A.M.; Meyer-Aurich, A.; Idris, A.; Ghazi, T.I.M. Energy balances, greenhouse gas emissions and economics of biochar production from palm oil empty fruit bunches. Resour. Conserv. Recycl. 2013, 77, 108–115. [Google Scholar] [CrossRef]
- Sulaiman, F.; Abdullah, N.; Gerhauser, H.; Shariff, A. An outlook of Malaysian energy, oil palm industry and its utilization of wastes as useful resources. Biomass Bioenergy 2011, 35, 3775–3786. [Google Scholar] [CrossRef]
- Tan, K.T.; Lee, K.T.; Mohamed, A.R.; Bhatia, S. Palm oil: Addressing issues and towards sustainable development. Renew. Sustain. Energy Rev. 2009, 13, 420–427. [Google Scholar] [CrossRef]
- Sumathi, S.; Chai, S.P.; Mohamed, A.R.Ã. Utilization of oil palm as a source of renewable energy in Malaysia. Renew. Sustain. Energy Rev. 2008, 12, 2404–2421. [Google Scholar] [CrossRef]
- Yai, H.; Yai, H. Biomass Residues from Palm Oil Mills in Thailand: An Overview on Quantity and Potential Usage. Biomass Bioenergy 1996, 1, 387–395. [Google Scholar]
- Sukiran, M.A. Pyrolysis of Empty Oil Palm Fruit Bunches Using the Quartz Fluidised-Fixed Bed Reactor. Univ. Malaya 2008, 8–35. [Google Scholar]
- Watkins, D.; Nuruddin, M.; Hosur, M.; Tcherbi-Narteh, A.; Jeelani, S. Extraction and characterization of lignin from different biomass resources. J. Mater. Res. Technol. 2015, 4, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Hornung, A.; Dasappa, S. Thermochemical conversion of biomass. In Transformation of Biomass: Theory to Practice; John Wiley & Sons: West Sussex, UK, 2014; p. 134. [Google Scholar]
- Morgan, T.J. An overview of the organic and inorganic phase composition of biomass. Fuel 2017, 94, 1–33. [Google Scholar]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [Green Version]
- Banks, S.W.; Bridgwater, A.V. Catalytic Fast Pyrolysis for Improved Liquid Quality. In Handbook of Biofuels Production; Luque, R., Lin, C.S.K., Wilson, K., Clark, J., Eds.; Elsevie: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Yahaya, H.; Hamdan, H.; Dolah, R.; Muhid, N.; Rashidi, K.A.; Rashidi, S.B. Catalytic fast-pyrolysis process design and equipment setup for converting palm oil empty fruit bunch biomass to bio-oil. J. Adv. Res. Fluid Mech. Therm. Sci. 2017, 32, 1–8. [Google Scholar]
- Czernik, S. Catalytic Pyrolysis of Biomass. In Advanced Biofuels and Bioproducts; Lee, J.W., Ed.; Springer Science+Business Media: New York, NY, USA, 2013; pp. 119–127. [Google Scholar]
- Wang, S.; Guo, Z.; Cai, Q.; Guo, L. Catalytic conversion of carboxylic acids in bio-oil for liquid hydrocarbons production. Biomass Bioenergy 2012, 45, 138–143. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, R.; Yin, R.; Mei, Y. Upgrading of bio-oil from biomass fast pyrolysis in China: A review. Renew. Sustain. Energy Rev. 2013, 24, 66–72. [Google Scholar] [CrossRef]
- Lu, Y.; Wei, X.-Y.; Liu, F.-J.; Zong, Z.-M.; Rong, L.-C.; Zhao, Y.-P.; Fan, X.; Wang, S.-Z.; Yue, X.-M.; Mukasa, R.; et al. Environmental Effects FTIR and Mass Spectral Analyses of an Upgraded Bio-oil FTIR and Mass Spectral Analyses of an Upgraded Bio-oil. Energy Sources Part A Recovery Util. Environ. Eff. 2009, 32, 370–375. [Google Scholar]
- Babich, I.V.; van der Hulst, M.; Lefferts, L.; Moulijn, J.A.; O’Connor, P.; Seshan, K. Catalytic pyrolysis of microalgae to high-quality liquid bio-fuels. Biomass Bioenergy 2011, 35, 3199–3207. [Google Scholar] [CrossRef]
- French, R.; Czernik, S. Catalytic pyrolysis of biomass for biofuels production. Fuel Process. Technol. 2010, 91, 25–32. [Google Scholar] [CrossRef]
- Qi, Z. Review of biomass pyrolysis oil properties and upgrading research. Energy Convers. Manag. 2007, 48, 87–92. [Google Scholar]
- Lim, X.Y.; Andrésen, M.J. Pyro-catalytic deoxgenated bio-oil from palm oil empty fruit bunch and fronds with boric oxide in a fixed-bed reactor. Fuel Process. Technol. 2011, 92, 1796–1804. [Google Scholar] [CrossRef]
- Hamdan, H.; Dolah, R.; Yahaya, H.; Basir, N.M.; Karnik, R. Conversion of Oil Palm Empty Fruit Bunch (EFB) Biomass to Bio-Oil and Jet-Fuel by Catalytic Fast-Pyrolysis Process. ASM Sci. J. 2021, 14, 1–11. [Google Scholar] [CrossRef]
- Basir, N.M.; Jamil, N.A.M.; Hamdan, H. Conversion of Jet Biofuel Range Hydrocarbons from Palm Oil over Zeolite Hybrid Catalyst. Nanomater. Nanotechnol. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Auta, M.; Ern, L.M.; Hameed, B.H. Fixed-bed Catalytic and Non-Catalytic Empty Fruit Bunch Biomass Pyrolysis. J. Anal. Appl. Pyrolysis 2014, 107, 67–72. [Google Scholar] [CrossRef]
- Thangalazhy-Gopakumar, S.; Lee, C.W.; Gan, S.; Ng, H.K.; Lee, L.Y. Comparison of Bio-Oil Properties from Non-Catalytic and In-Situ Catalytic Fast Pyrolysis of Palm Empty Fruit Bunch. Mater. Today-Proc. 2018, 5, 23456–23465. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, R.; Huang, H.; Xiao, G. Comparison of non-catalytic and catalytic fast pyrolysis of corncob in a fluidized bed reactor. Bioresour. Technol. 2009, 100, 1428–1434. [Google Scholar] [CrossRef]
- Triantafyllidis, K.S.; Lappas, A.A.; Stocker, M. (Eds.) The Role of Catalysis for The Sustainable Production of Bio-Fuels and Bio-Chemicals; Elsevier: Oxford, UK, 2013. [Google Scholar]
- Wyman, C.E.; Balan, V.; Dale, B.E.; Elander, R.T.; Falls, M.; Hames, B.; Holtzapple, M.T.; Ladisch, M.R.; Lee, Y.Y.; Mosier, N. Comparative data on effects of leading pretreatments and enzyme loadings and formulations on sugar yields from different switchgrass sources. Bioresour. Technol. 2011, 102, 11052–11062. [Google Scholar] [CrossRef]
- Kumar, R.S.; Sivakumar, S.; Joshuva, A.; Deenadayalan, G.; Vishnuvardhan, R. Bio-fuel Production from Martynia annua L. Seeds using Slow Pyrolysis Reactor and its Effects on Diesel Engine Performance, Combustion and Emission Characteristics. Energy 2021, 217, 119327. [Google Scholar] [CrossRef]
- Pozo, C.; Rego, F.; Yang, Y.; Puy, N.; Bartroli, J.; Fabregas, E.; Bridgwater, A.V. Converting Coffee Silverskin to Value-Added Products by a slow Pyrolysis-based Biorefinery Process. Fuel Process. Technol. 2021, 214, 106708. [Google Scholar] [CrossRef]
- Lan, P.; Xu, Q.; Zhou, M.; Lan, L.; Zhang, S.; Yan, Y. Catalytic Steam Reforming of Fast Pyrolysis Bio-Oil in Fixed Bed and Fluidized Bed Reactors. Chem. Eng. Technol. 2010, 12, 2021–2028. [Google Scholar] [CrossRef]
- Zin, R.M.; Lea-Langton, A.; Dupont, V.; Twigg, M.V. High hydrogen yield and purity from palm empty fruit bunch and pine pyrolysis oils. Int. J. Hydrog. Energy 2012, 37, 10627–10638. [Google Scholar] [CrossRef] [Green Version]
- Koriakin, A.; Moon, S.; Kim, D.-W.; Lee, C.-H. Liquefaction of oil palm empty fruit bunch using sub- and supercritical tetralin, n-dodecane, and their mixture. Fuel 2017, 208, 184–192. [Google Scholar] [CrossRef]
- Akhtar, J.; Amin, N.S. A review on operating parameters for optimum liquid oil yield in biomass pyrolysis. Renew. Sustain. Energy Rev. 2012, 16, 5101–5109. [Google Scholar] [CrossRef]
- Li, X.; Chen, G.; Liu, C.; Ma, W.; Yan, B.; Zhang, J. Hydrodeoxygenation of lignin-derived bio-oil using molecular sieves supported metal catalysts: A critical review. Renew. Sustain. Energy Rev. 2017, 71, 296–308. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Rasul, M.G.; Chowdhury, A.A.; Ashwath, N. Biofuels production through biomass pyrolysis—A technological review. Energies 2012, 5, 4952–5001. [Google Scholar] [CrossRef]
- Tang, Z.; Lu, Q.; Zhang, Y.; Zhu, X.; Guo, Q. One Step Bio-Oil Upgrading through Hydrotreatment, Esterification, and Cracking. Ind. Eng. Chem. Res. 2009, 48, 6923–6929. [Google Scholar] [CrossRef]
- Bridgwater, A.V.; Peacocke, G.V.C. Fast pyrolysis processes for biomass. Renew. Sustain. Energy Rev. 2000, 4, 1–73. [Google Scholar] [CrossRef]
- Mohamed, A.R.; Hamzah, Z.; Daud, M.Z.M.; Zakaria, Z. The effects of holding time and the sweeping nitrogen gas flowrates on the pyrolysis of EFB using a fixed bed reactor. Procedia Eng. 2013, 53, 185–191. [Google Scholar] [CrossRef] [Green Version]
- Xuan, T.; Lim, Y.; Jang, S.; Chung, H.; Lee, Y. Process Design and Economics for Bioethanol Production Process from Palm Empty Fruit Bunch (EFB). In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Zhang, H.; Xiao, R.; Wang, D.; He, G.; Shao, S.; Zhang, J.; Zhong, Z. Biomass fast pyrolysis in a fluidized bed reactor under N2, CO2, CO, CH4 and H2 atmospheres. Bioresour. Technol. 2011, 102, 4258–4264. [Google Scholar] [CrossRef]
- Production of Gasoline and Diesel from Biomass via Fast Pyrolysis, Hydrotreating and Hydrocracking: A Design Case. Available online: https://www.pnnl.gov/main/publications/external/technical_reports/PNNL-18284.pdf (accessed on 9 September 2021).
- Perkins, G.; Bhaskar, T.; Konarova, M. Process development status of fast pyrolysis technologies for the manufacture of renewable transport fuels from biomass. Renew. Sustain. Energy Rev. 2018, 90, 292–315. [Google Scholar] [CrossRef]
- Brassard, P.; Godbout, S.; Raghavan, V. Pyrolysis in auger reactors for biochar and bio-oil production: A review. Biosyst. Eng. 2017, 161, 80–92. [Google Scholar] [CrossRef]
- Gurentsov, E.; Priemchenko, K.; Grimm, H.; Orthner, H.; Wiggers, H.; Borchers, C.; Jander, H.; Eremin, A.; Schulz, C. Synthesis of Small Carbon Nanoparticles in a Microwave Plasma Flow Reactor. Z. Phys. Chem. 2013, 227, 357–370. [Google Scholar] [CrossRef]
- Salema, A.A.; Afzal, M.T.; Bennamoun, L. Pyrolysis of corn stalk biomass briquettes in a scaled-up microwave technology. Bioresour. Technol. 2017, 233, 353–362. [Google Scholar] [CrossRef]
- Soria, J.; Zeng, K.; Asensio, D.; Gauthier, D.; Flamant, G.; Mazza, G. Comprehensive CFD modelling of solar fast pyrolysis of beech wood pellets. Fuel Process. Technol. 2017, 158, 226–237. [Google Scholar] [CrossRef]
- Annual Report 2006 IEA Bioenergy. Available online: http://www.globalbioenergy.org/uploads/media/0707_IEA_-_Bioenergy_annual_report.pdf (accessed on 9 September 2021).
- Balat, M.; Balat, M.; Kirtay, E.; Balat, H. Main routes for the thermo-conversion of biomass into fuels and chemicals. Part 1: Pyrolysis systems. Energy Convers. Manag. 2009, 50, 3147–3157. [Google Scholar] [CrossRef]
- Arbeláez, J.I.M.; Janna, F.C.; Garcia-Pérez, M. Fast pyrolysis of biomass: A review of relevant aspects. Part I: Parametric study. Dyna 2015, 82, 239–248. [Google Scholar] [CrossRef]
- Babu, B.V. Biomass Pyrolysis: A state-of-the-art review. Biofuels Bioprod. Biorefining 2012, 6, 246–256. [Google Scholar] [CrossRef]
- Sellin, N.; Krohl, D.R.; Marangoni, C.; Souza, O. Oxidative fast pyrolysis of banana leaves in fluidized bed reactor. Renew. Energy 2016, 96, 56–64. [Google Scholar] [CrossRef]
- Chan, Y.; Dang, V.I.; Yusup, S.; Lim, M.T.; Zain, A.M.; Uemura, Y. Studies on Catalytic Pyrolysisof Empty Fruit Bunch (EFB) using Taguchi’s L9 Orthogonal Array. J. Energy Inst. 2014, 87, 227–234. [Google Scholar] [CrossRef]
- Ro, D.; Kim, Y.-M.; Lee, I.-G.; Jae, J.; Jung, S.-C.; Kimg, S.C.; Park, Y.-K. Bench scale catalytic fast pyrolysis of empty fruit bunches over low cost catalysts and HZSM-5 using a fixed bed reactor. J. Clean. Prod. 2018, 176, 298–303. [Google Scholar] [CrossRef]
- Demirbas, A. Progress and recent trends in biofuels. Prog. Energy Combust. Sci. 2007, 33, 1–18. [Google Scholar] [CrossRef]
- Ali, R.; Daut, I.; Taib, S. A review on existing and future energy sources for electrical power generation in Malaysia. Renew. Sustain. Energy Rev. 2012, 16, 4047–4055. [Google Scholar] [CrossRef]
- Czernik, S.; Bridgwater, A.V. Overview of applications of biomass fast pyrolysis oil. Energy Fuels 2004, 18, 590–598. [Google Scholar] [CrossRef]
- Furnaces and Boilers|Department of Energy. Available online: https://energy.gov/energysaver/furnaces-and-boilers (accessed on 26 August 2017).
- Oil-Fired Boilers and Furnaces|Department of Energy. Available online: https://energy.gov/energysaver/oil-fired-boilers-and-furnaces (accessed on 28 August 2017).
- Bridgwater, A.V.; Meier, D.; Radlein, D. An overview of fast pyrolysis of biomass. Org. Geochem. 1999, 30, 1479–1493. [Google Scholar] [CrossRef]
- Lehto, J.; Oasmaa, A.; Solantausta, Y.; Kytö, M.; Chiaramonti, D. Fuel oil quality and combustion of fast pyrolysis bio-oils. VTT Publ. 2013, 87, 79. [Google Scholar]
- Yoo, H.; Choi, H.S.; Lee, J.; Park, H.C.; Yang, W. The Fast Pyrolysis Characteristics of Palm Empty Fruit Bunch: The Yield and Homogeneity of Biocrudeoil Affected by Ash. Environ. Sci. Technol. 2014, 33, 482–489. [Google Scholar] [CrossRef]
- Oasmaa, A.; Czernik, S. Fuel Oil Quality of Biomass Pyrolysis Oils-State of the Art for the End Users. Energy Fuels 1999, 13, 914–921. [Google Scholar] [CrossRef]
- Kabir, G.; Hameed, B.H. Recent progress on catalytic pyrolysis of lignocellulosic biomass to high-grade bio-oil and bio-chemicals. Renew. Sustain. Energy Rev. 2017, 70, 945–967. [Google Scholar] [CrossRef]
- Di Blasi, C. Modeling chemical and physical processes of wood and biomass pyrolysis. Prog. Energy Combust. Sci. 2008, 34, 47–90. [Google Scholar] [CrossRef]
- Wang, S. High-Efficiency Separation of Bio-Oil. In Biomass Now–Sustainable Growth and Use; Matovic, M.D., Ed.; IntechOpen: London, UK, 2013. [Google Scholar]
- Ridgway Watt, P. Molecular Distillation. Vacuum 1956, 6, 113–160. [Google Scholar] [CrossRef]
- Wang, S.; Gua, Y.; Liu, Q.; Yao, Y.; Guo, Z.; Luo, Z.; Cen, K. Separation of bio-oil by molecular distillation. Fuel Process. Technol. 2009, 90, 738–745. [Google Scholar] [CrossRef]
- Guo, X.; Wang, S.; Guo, Z.; Liu, Q.; Luo, Z.; Cen, K. Pyrolysis characteristics of bio-oil fractions separated by molecular distillation. Appl. Energy 2010, 87, 2892–2898. [Google Scholar] [CrossRef]
- Vitasari, C.R.; Meindersma, G.W.; de Haan, A.B. Water extraction of pyrolysis oil: The first step for the recovery of renewable chemicals. Bioresour. Technol. 2011, 102, 7204–7210. [Google Scholar] [CrossRef]
- Yoosuk, B.; Boonpo, J.; Udomsap, P.; Sukkasi, S. Investigation of operating parameters of water extraction processes for improving bio-oil quality. Korean J. Chem. Eng. 2014, 31, 2229–2236. [Google Scholar] [CrossRef]
- Wang, H.; Elliott, D.C.; French, R.J.; Deutch, S.; Iisa, K. Biomass Conversion to Produce Hydrocarbon Liquid Fuel Via Hot-vapor Filtered Fast Pyrolysis and Catalytic Hydrotreating. J. Vis. Exp. 2016, 118, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Vásquez, M.C.; Silva, E.E.; Castillo, E.F. Hydrotreatment of vegetable oils: A review of the technologies and its developments for jet biofuel production. Biomass Bioenergy 2017, 105, 197–206. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, T.; Ma, L.; Chen, G. Upgrading of fast pyrolysis liquid fuel from biomass over Ru/γ-Al2O3 catalyst. Energy Convers. Manag. 2012, 55, 172–177. [Google Scholar]
- Xu, Y.; Wang, T.; Ma, L.; Zhang, Q.; Wang, L. Upgrading of liquid fuel from the vacuum pyrolysis of biomass over the Mo-Ni/γ-Al2O3 catalysts. Biomass Bioenergy 2009, 33, 1030–1036. [Google Scholar] [CrossRef]
- Guo, J.; Ruan, R.; Zhang, Y. Hydrotreating of phenolic compounds separated from bio-oil to alcohols. Ind. Eng. Chem. Res. 2012, 51, 6599–6604. [Google Scholar] [CrossRef]
- Piemonte, V.; Capocelli, M.; Orticello, G.; di Paola, L. Bio-oil production and upgrading: New challenges for membrane applications. Membr. Technol. Biorefining 2016, 32, 263–287. [Google Scholar]
- Technomanage. Hydrotreating. Available online: http://http://technomanage.com/Process%20Technology%20Database/Refinery/Hydrotreating/hydrotreating.htm (accessed on 10 October 2017).
- Gutierrez, A.; Turpeinen, E.M.; Viljava, T.R.; Krause, O. Hydrodeoxygenation of model compounds on sulfided CoMo/γ-Al2O3 and NiMo/γ-Al2O3 catalysts; Role of sulfur-containing groups in reaction networks. Catal. Today 2017, 285, 125–134. [Google Scholar] [CrossRef]
- Wang, J.; Chang, J.; Fan, J. Upgrading of Bio-oil by Catalytic Esterification and Determination of Acid Number for Evaluating Esterification Degree. Energy Fuels 2010, 12, 3251–3255. [Google Scholar] [CrossRef]
- Junming, X.; Jianchun, J.; Yunjuan, S.; Yanju, L. Bio-oil upgrading by means of ethyl ester production in reactive distillation to remove water and to improve storage and fuel characteristics. Biomass Bioenergy 2008, 32, 1056–1061. [Google Scholar] [CrossRef]
- Yu, W.; Tang, Y.; Mo, L.; Chen, P.; Lou, H.; Zheng, X. Bioresource Technology One-step hydrogenation—Esterification of furfural and acetic acid over bifunctional Pd catalysts for bio-oil upgrading. Bioresour. Technol. 2011, 102, 8241–8246. [Google Scholar] [CrossRef]
- Zhu, J.F.; Wang, J.C.; Li, Q.X. Transformation of bio-oil into BTX by bio-oil catalytic cracking. Chin. J. Chem. Phys. 2013, 26, 477–483. [Google Scholar] [CrossRef]
- Zhang, H.; Carlson, T.R.; Xiao, R.; Huber, G.W. Catalytic fast pyrolysis of wood and alcohol mixtures in a fluidized bed reactor. Green Chem. 2012, 14, 98–110. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Ellis, N. Upgrading Bio-oil through Emulsification with Biodiesel: Thermal Stability. Energy Fuels 2010, 23, 2699–2706. [Google Scholar] [CrossRef]
- Methane Methanol Price. Available online: https://www.methanex.com/our-business/pricing (accessed on 25 October 2017).
- Chen, T.; Wu, C.; Liu, R.; Fei, W.; Liu, S. Effect of hot vapor filtration on the characterization of bio-oil from rice husks with fast pyrolysis in a fluidized-bed reactor. Bioresour. Technol. 2011, 102, 6178–6185. [Google Scholar] [CrossRef] [PubMed]
- Park, L.K.E.; Ren, S.; Yiacoumi, S.; Ye, X.P.; Borole, A.P.; Tsouris, C. Separation of Switchgrass Bio-Oil by Water/Organic Solvent Addition and pH Adjustment. Energy Fuels 2016, 30, 2164–2173. [Google Scholar] [CrossRef]
- Refinery Project Hydrogenation Unit, Petroleum Refinery Equipment for Sale, Waste Oil Distillation Plant-Buy Refinery Project Hydrogenation Unit, Petroleum Refinery Equipment for Sale. Available online: https://www.alibaba.com/product-detail/Refinery-project-hydrogenation-unit-Petroleum-refinery_60684898533.html?spm=a2700.7724838.2017121.5.78d6a6a3Y1xSYt&s=p (accessed on 25 October 2017).
- Biomass Hydrodeoxygenation. 2016. Available online: http://large.stanford.edu/courses/2016/ph240/adams2/ (accessed on 24 October 2017).
- Saswattecha, K.; Kroeze, C.; Jawjit, W.; Hein, L. Options to reduce environmental impacts of palm oil production in Thailand. J. Clean. Prod. 2016, 137, 370–393. [Google Scholar] [CrossRef]
- Yogalakshmi, K.N.; Poornima Devi, T.; Sivashanmugam, P.; Kavitha, S.; Yukesh Kannah, R.; Sunita Varjani, S.; Adishkumar, A.G.; Rajesh, B.J. Lignocellulosic biomass-based pyrolysis: A comprehensive review. Chemosphere 2021, 286, 131824. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).