Pyrolysis of Solid Digestate from Sewage Sludge and Lignocellulosic Biomass: Kinetic and Thermodynamic Analysis, Characterization of Biochar
Abstract
:1. Introduction
Research Motivation and Paper Organization
2. Materials and Methods
2.1. Preparation and Characterization of Feedstocks and Products
2.1.1. Feedstocks Preparation
2.1.2. Characterization of Feedstocks and Biochars
2.2. Thermogravimetric Analysis (TGA) and Pyrolysis of Feedstocks
2.3. Kinetic and Thermodynamic Analysis
2.3.1. Kinetic Models
2.3.2. Thermodynamic Parameters
2.4. Cress Seed Germination Test
2.5. Adsorption Tests
3. Results and Discussion
3.1. Characterization of Feedstock Materials
3.2. Thermogravimetric Analysis
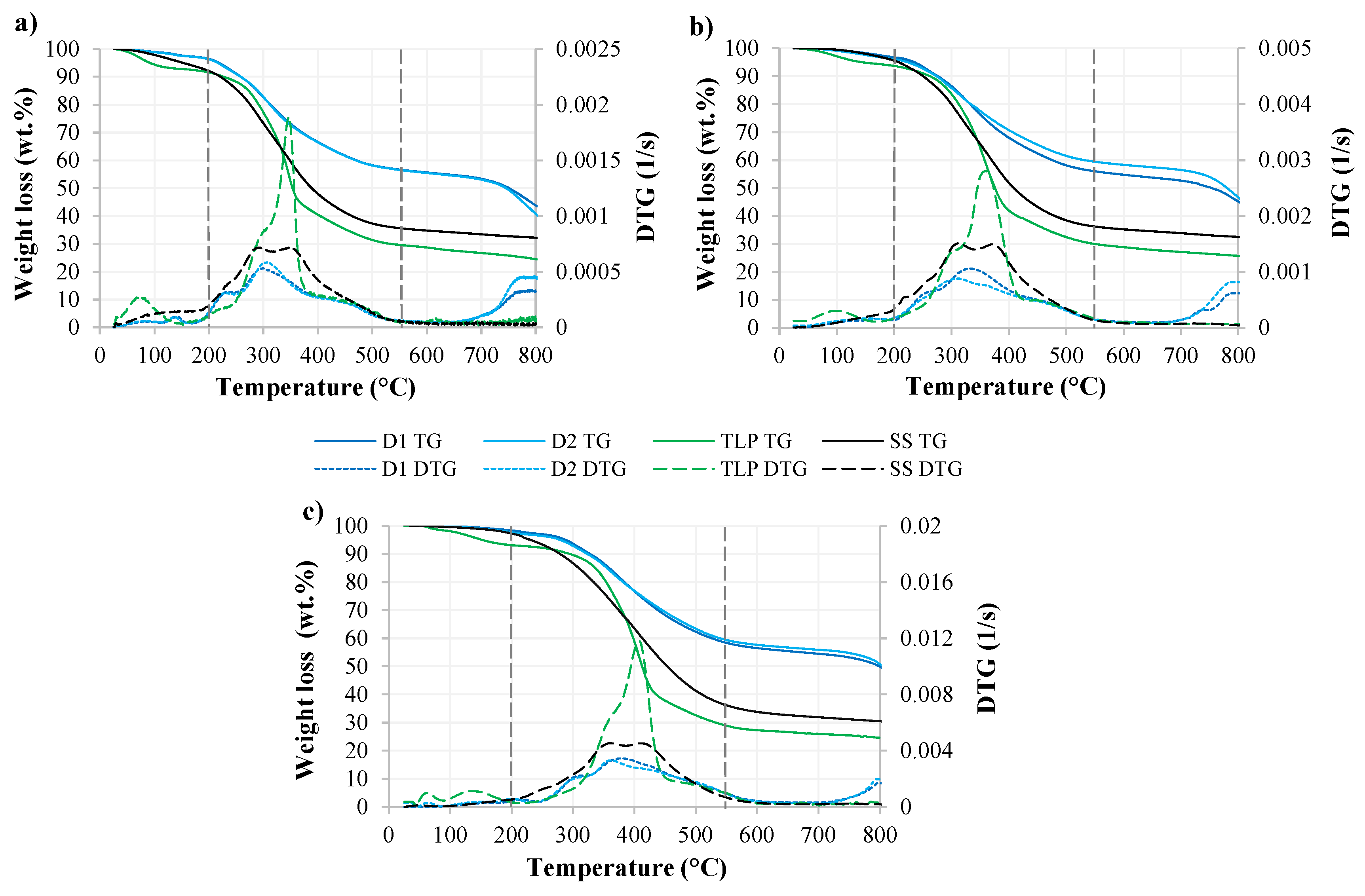
3.2.1. Analysis of TG and DTG Curves
3.2.2. The Influence of the Heating Rate
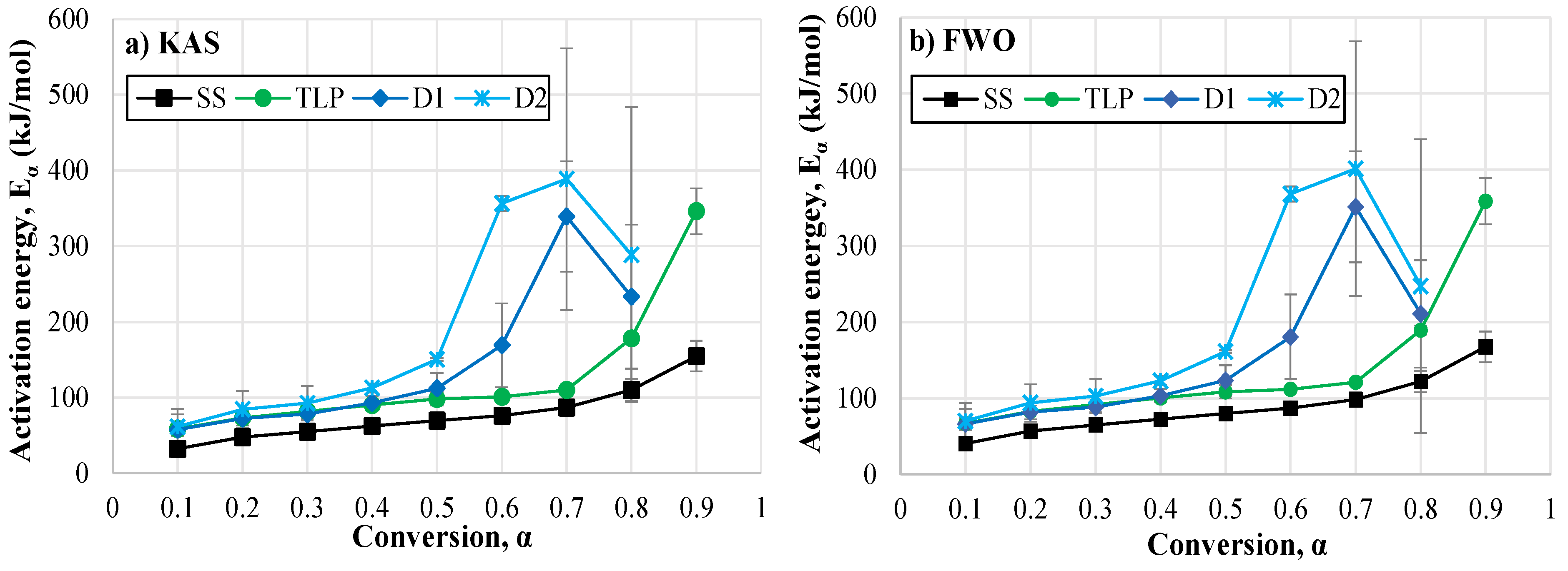
3.3. Kinetic Analysis
3.3.1. Activation Energy
3.3.2. The Pre-Exponential Factor
3.3.3. Kinetic Compensation Effect
3.4. Thermodynamic Analysis
3.4.1. Enthalpy ()
3.4.2. Gibbs Free Energy ()
3.4.3. Entropy ()
3.5. Characterization of Biochars
3.5.1. Chemical Characteristics of Biochars
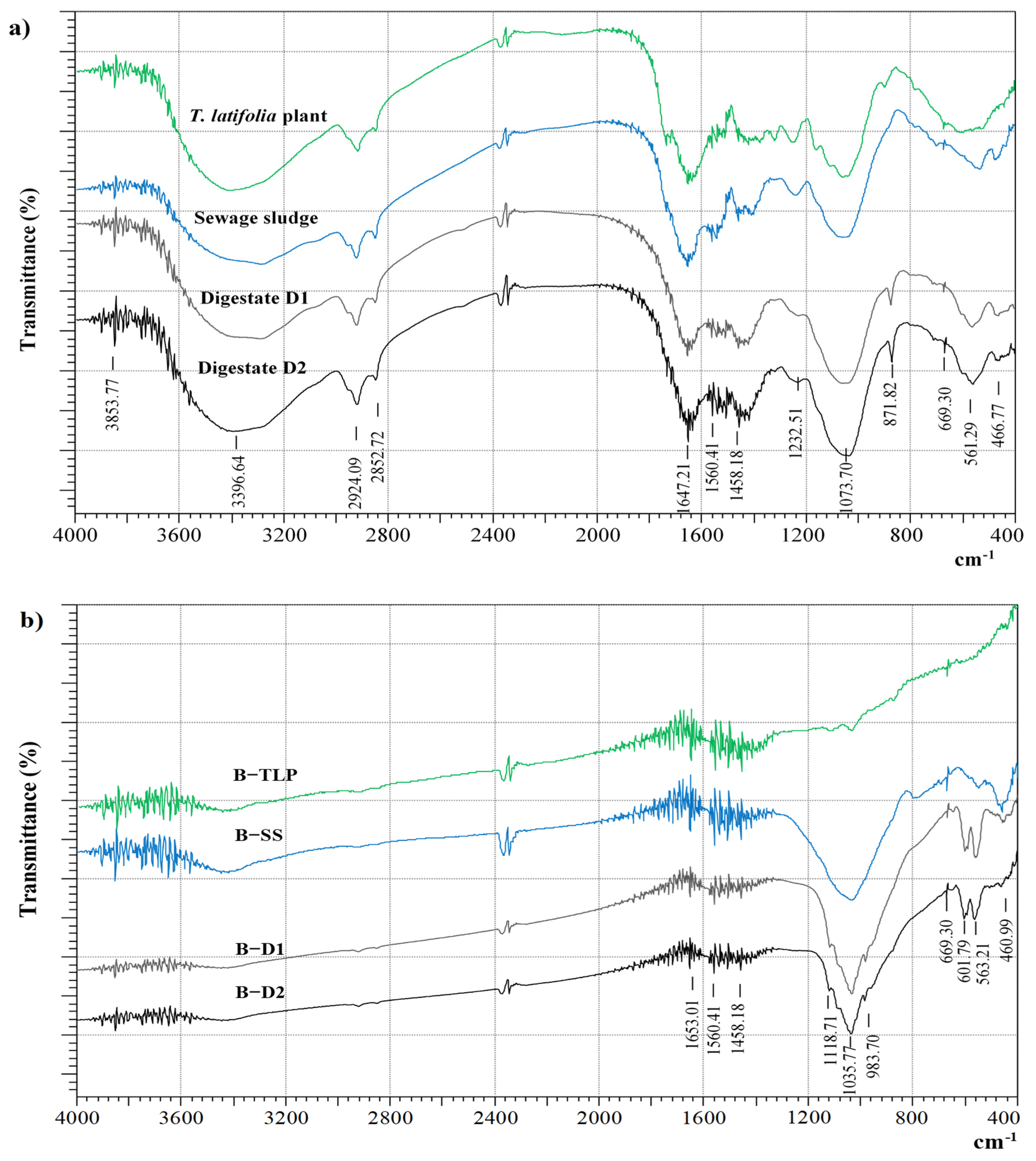
3.5.2. FTIR Analysis
3.5.3. SEM–EDS Analysis
3.5.4. XRD Analysis
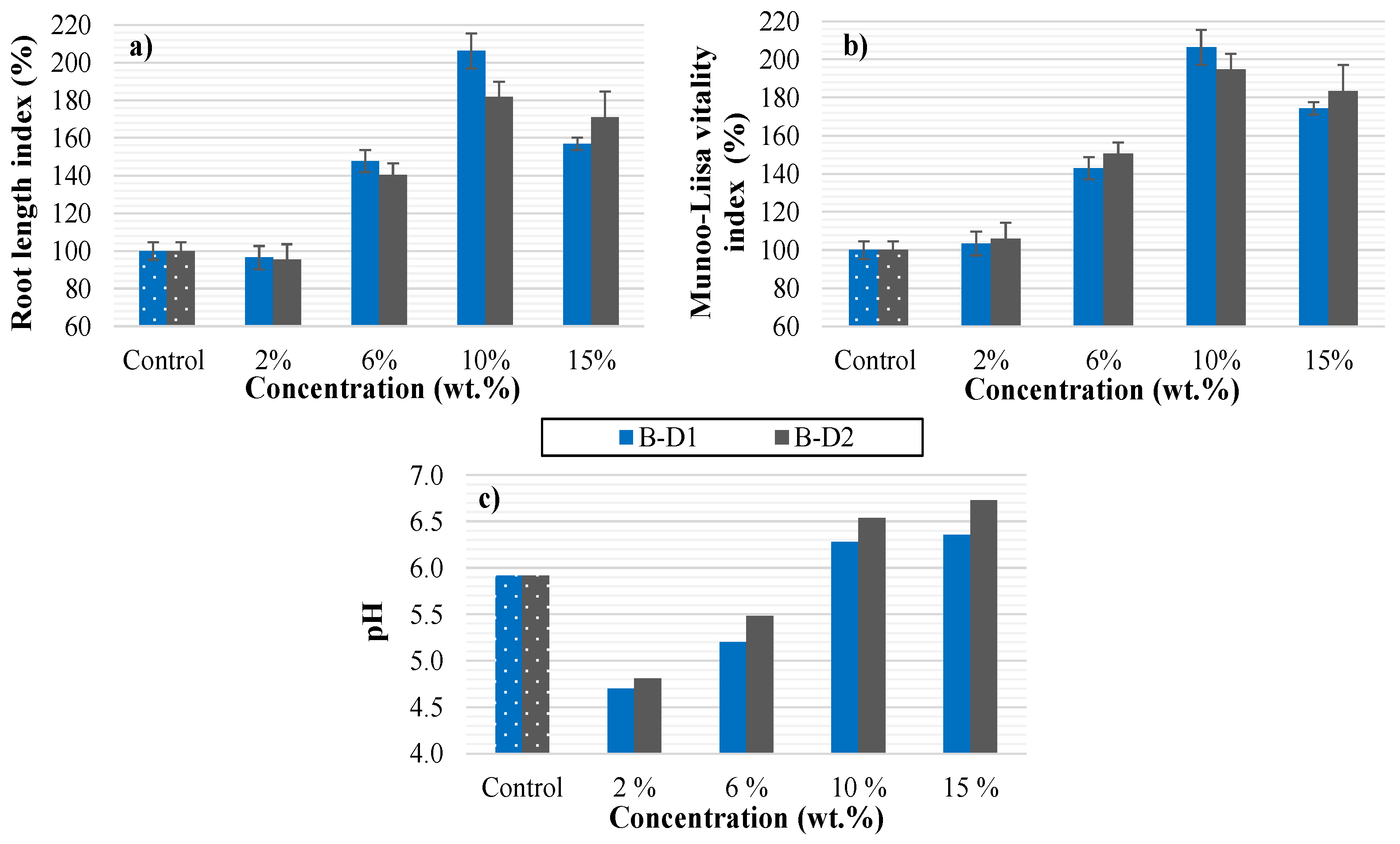
3.5.5. The Potential of Digestate-Derived Biochars for Use as a Soil Enhancer
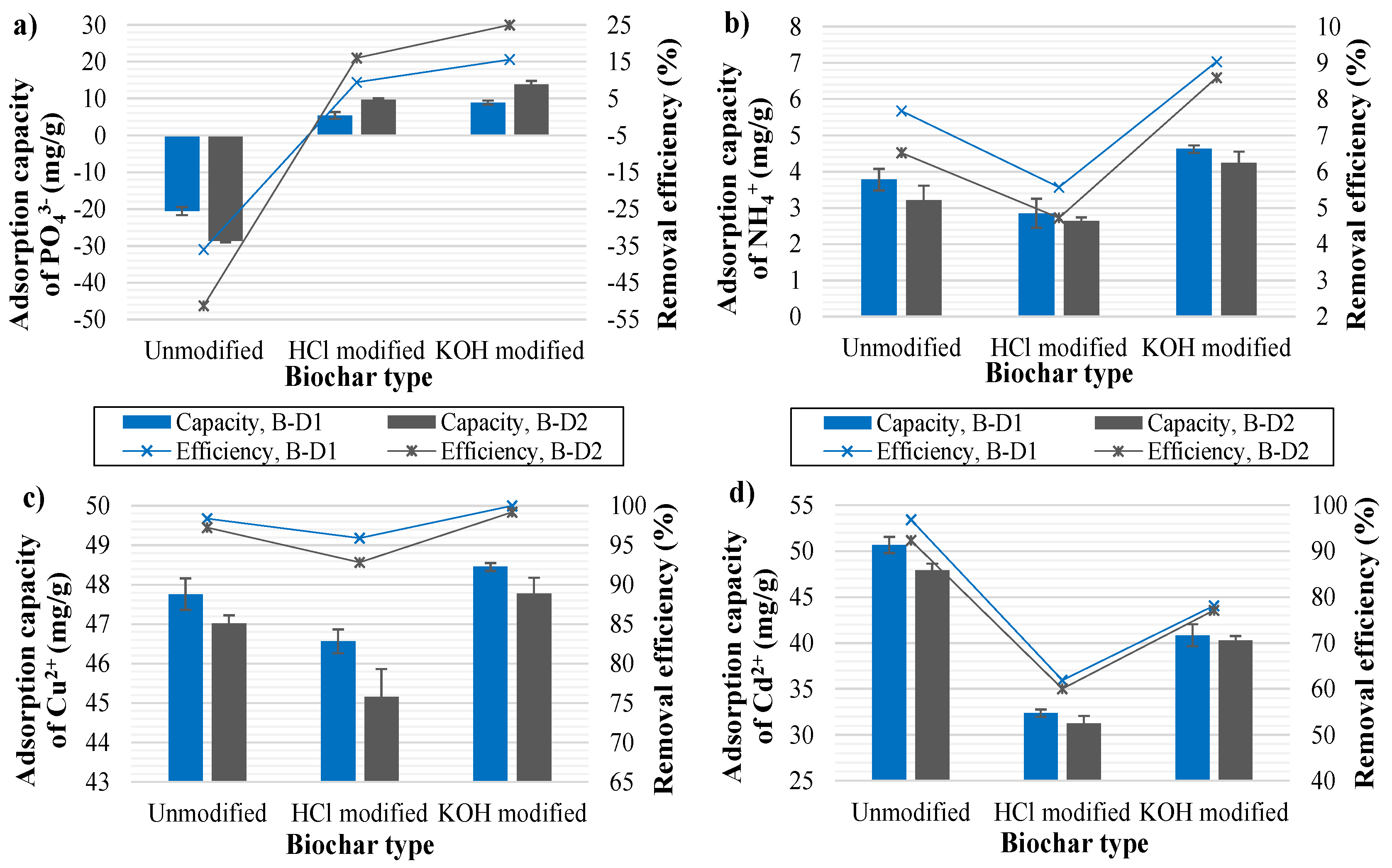
3.5.6. Biosorption Potential of Digestate-Derived Biochars
4. Conclusions
- Thermogravimetric analysis revealed that the digestate samples had lower weight loss than raw SS or TLP due to pre-treatment with AD and gave higher biochar yield. values were higher for digestate than for raw samples. The maximum values were obtained for digestate composed of a mixture of SS and TLP. The KAS and FWO models showed excellent matching for raw materials, while for digestates, lower correlations were observed, most likely because of heterogeneous constitution, which influenced the pyrolysis process. Variation of the thermodynamic parameters ( and ) indicated that the degradation of digestates is more complex than degradation of SS or TLP. TLP with the highest values exhibited the highest bioenergy potential.
- Chemical characterization of the biochars revealed high nutrient content and, thus, good prospects for their further utilization. Biochars performed very well with regard to biosorption of heavy metals (Cu and Cd), while biosorption of NH4+ and PO43− ions was less efficient. Modification of biochars with KOH significantly improved their biosorption ability for all ionic species, whereas HCl modification was found to be efficient only in the case of PO43− adsorption. Germination tests with cress seeds showed that digestate-derived biochars can be used as soil amenders at a concentration of up to 10 wt.%. SS digestate-derived biochar showed better performance than the biochar derived from a digestate mixture of SS and TLP, the advantage of the latter being its lower heavy metal content.
Limitations and Directions for Future Studies
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soria-Verdugo, A.; Goos, E.; Morato-Godino, A.; García-Hernando, N.; Riedel, U. Pyrolysis of biofuels of the future: Sewage sludge and microalgae—Thermogravimetric analysis and modelling of the pyrolysis under different temperature conditions. Energy Convers. Manag. 2017, 138, 261–272. [Google Scholar] [CrossRef] [Green Version]
- Zainol Abidin, M.A.; Mahyuddin, M.N.; Mohd Zainuri, M.A.A. Solar Photovoltaic Architecture and Agronomic Management in Agrivoltaic System: A Review. Sustainability 2021, 13, 7846. [Google Scholar] [CrossRef]
- Mehariya, S.; Patel, A.K.; Obulisamy, P.K.; Punniyakotti, E.; Wong, J.W.C. Co-digestion of food waste and sewage sludge for methane production: Current status and perspective. Bioresour. Technol. 2018, 265, 519–531. [Google Scholar] [CrossRef]
- Rasam, S.; Moshfegh Haghighi, A.; Azizi, K.; Soria-Verdugo, A.; Keshavarz Moraveji, M. Thermal behavior, thermodynamics and kinetics of co-pyrolysis of binary and ternary mixtures of biomass through thermogravimetric analysis. Fuel 2020, 280, 118665. [Google Scholar] [CrossRef]
- Sobek, S.; Werle, S. Kinetic modelling of waste wood devolatilization during pyrolysis based on thermogravimetric data and solar pyrolysis reactor performance. Fuel 2020, 261, 116459. [Google Scholar] [CrossRef]
- Cucchiella, F.; D’Adamo, I.; Gastaldi, M. Sustainable waste management: Waste to energy plant as an alternative to landfill. Energy Convers. Manag. 2017, 131, 18–31. [Google Scholar] [CrossRef]
- Khan, M.F.; Pervez, A.; Modibbo, U.M.; Chauhan, J.; Ali, I. Flexible Fuzzy Goal Programming Approach in Optimal Mix of Power Generation for Socio-Economic Sustainability: A Case Study. Sustainability 2021, 13, 8256. [Google Scholar] [CrossRef]
- D’Adamo, I.; Falcone, P.M.; Huisingh, D.; Morone, P. A circular economy model based on biomethane: What are the opportunities for the municipality of Rome and beyond? Renew. Energy 2021, 163, 1660–1672. [Google Scholar] [CrossRef]
- European Commission. A New Bioeconomy Strategy for a Sustainable Europe; European Comission: Brussels, Belgium, 2018.
- D’Adamo, I.; Falcone, P.M.; Morone, P. A New Socio-economic Indicator to Measure the Performance of Bioeconomy Sectors in Europe. Ecol. Econ. 2020, 176, 106724. [Google Scholar] [CrossRef]
- Cao, Y.; Pawłowski, A. Sewage sludge-to-energy approaches based on anaerobic digestion and pyrolysis: Brief overview and energy efficiency assessment. Renew. Sustain. Energy Rev. 2012, 16, 1657–1665. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Dai, X.; Mei, Q.; Ding, G. Thermogravimetry–Fourier transform infrared spectrometry–mass spectrometry technique to evaluate the effect of anaerobic digestion on gaseous products of sewage sludge sequential pyrolysis. J. Anal. Appl. Pyrolysis 2017, 126, 288–297. [Google Scholar] [CrossRef]
- Ahmed, M.; Andreottola, G.; Elagroudy, S.; Negm, M.S.; Fiori, L. Coupling hydrothermal carbonization and anaerobic digestion for sewage digestate management: Influence of hydrothermal treatment time on dewaterability and bio-methane production. J. Environ. Manag. 2021, 281, 111910. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.; Amutio, M.; Lopez, G.; Bilbao, J.; Olazar, M. Fast co-pyrolysis of sewage sludge and lignocellulosic biomass in a conical spouted bed reactor. Fuel 2015, 159, 810–818. [Google Scholar] [CrossRef]
- Feng, Q.; Lin, Y. Integrated processes of anaerobic digestion and pyrolysis for higher bioenergy recovery from lignocellulosic biomass: A brief review. Renew. Sustain. Energy Rev. 2017, 77, 1272–1287. [Google Scholar] [CrossRef]
- Hu, Z.-H.; Yu, H.-Q.; Yue, Z.-B.; Harada, H.; Li, Y.-Y. Kinetic analysis of anaerobic digestion of cattail by rumen microbes in a modified UASB reactor. Biochem. Eng. J. 2007, 37, 219–225. [Google Scholar] [CrossRef]
- Ciria, P.; Solano, M.; Soriano, P. Role of Macrophyte Typha latifolia in a Constructed Wetland for Wastewater Treatment and Assessment of Its Potential as a Biomass Fuel. Biosyst. Eng. BIOSYST ENG 2005, 92, 535–544. [Google Scholar] [CrossRef]
- Grosshans, R. Cattail (Typha spp.) Biomass Harvesting for Nutrient Capture and Sustainable Bioenergy for Integrated Watershed Management. Ph.D. Thesis, University of Manitoba, Winnipeg, MB, Canada, 2014. [Google Scholar]
- Hu, Z.-H.; Yue, Z.-B.; Yu, H.-Q.; Liu, S.-Y.; Harada, H.; Li, Y.-Y. Mechanisms of microwave irradiation pretreatment for enhancing anaerobic digestion of cattail by rumen microorganisms. Appl. Energy 2012, 93, 229–236. [Google Scholar] [CrossRef]
- Petrovič, A.; Simonič, M.; Čuček, L. Nutrient recovery from the digestate obtained by rumen fluid enhanced anaerobic co-digestion of sewage sludge and cattail: Precipitation by MgCl2 and ion exchange using zeolite. J. Environ. Manag. 2021, 290, 112593. [Google Scholar] [CrossRef]
- Jaruwat, D.; Udomsap, P.; Chollacoop, N.; Eiad-ua, A. Preparation of carbon supported catalyst from cattail leaves for biodiesel fuel upgrading application. Mater. Today Proc. 2019, 17, 1319–1325. [Google Scholar] [CrossRef]
- Zhang, B.; Joseph, G.; Wang, L.; Li, X.; Shahbazi, A. Thermophilic anaerobic digestion of cattail and hydrothermal carbonization of the digestate for co-production of biomethane and hydrochar. J. Environ. Sci. Health Part A 2020, 55, 230–238. [Google Scholar] [CrossRef]
- Arun, J.; Gopinath, K.P.; Sivaramakrishnan, R.; Madhav, N.V.; Abhishek, K.; Ramanan, V.G.K.; Pugazhendhi, A. Bioenergy perspectives of cattails biomass cultivated from municipal wastewater via hydrothermal liquefaction and hydro-deoxygenation. Fuel 2021, 284, 118963. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Mehmood, M.A.; Taqvi, S.T.H.; Elkamel, A.; Liu, C.-G.; Xu, J.; Rahimuddin, S.A.; Gull, M. Pyrolysis, kinetics analysis, thermodynamics parameters and reaction mechanism of Typha latifolia to evaluate its bioenergy potential. Bioresour. Technol. 2017, 245, 491–501. [Google Scholar] [CrossRef] [Green Version]
- Bartocci, P.; Tschentscher, R.; Stensrød, R.; Barbanera, M.; Fantozzi, F. Kinetic Analysis of Digestate Slow Pyrolysis with the Application of the Master-Plots Method and Independent Parallel Reactions Scheme. Molecules 2019, 24, 1657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedoić, R.; Jurić, F.; Ćosić, B.; Pukšec, T.; Čuček, L.; Duić, N. Beyond energy crops and subsidised electricity—A study on sustainable biogas production and utilisation in advanced energy markets. Energy 2020, 201, 117651. [Google Scholar] [CrossRef]
- D’Adamo, I.; Falcone, P.M.; Gastaldi, M.; Morone, P. RES-T trajectories and an integrated SWOT-AHP analysis for biomethane. Policy implications to support a green revolution in European transport. Energy Policy 2020, 138, 111220. [Google Scholar] [CrossRef]
- Hung, C.-Y.; Tsai, W.-T.; Chen, J.-W.; Lin, Y.-Q.; Chang, Y.-M. Characterization of biochar prepared from biogas digestate. Waste Manag. 2017, 66, 53–60. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, B. Investigation of thermodynamic parameters in the pyrolysis conversion of biomass and manure to biochars using thermogravimetric analysis. Bioresour. Technol. 2013, 146, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Li, Y.; Zhang, J.; Wu, S.; Cao, Y.; Liang, P.; Zhang, J.; Wong, M.H.; Wang, M.; Shan, S.; et al. Influence of pyrolysis temperature on properties and environmental safety of heavy metals in biochars derived from municipal sewage sludge. J. Hazard. Mater. 2016, 320, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Rangabhashiyam, S.; Balasubramanian, P. The potential of lignocellulosic biomass precursors for biochar production: Performance, mechanism and wastewater application—A review. Ind. Crop. Prod. 2019, 128, 405–423. [Google Scholar]
- Xue, Y.; Wang, C.; Hu, Z.; Zhou, Y.; Xiao, Y.; Wang, T. Pyrolysis of sewage sludge by electromagnetic induction: Biochar properties and application in adsorption removal of Pb(II), Cd(II) from aqueous solution. Waste Manag. 2019, 89, 48–56. [Google Scholar] [CrossRef]
- Wang, C.; Bi, H.; Lin, Q.; Jiang, X.; Jiang, C. Co-pyrolysis of sewage sludge and rice husk by TG–FTIR–MS: Pyrolysis behavior, kinetics, and condensable/non-condensable gases characteristics. Renew. Energy 2020, 160, 1048–1066. [Google Scholar] [CrossRef]
- Folgueras, M.B.; Alonso, M.; Díaz, R.M. Influence of sewage sludge treatment on pyrolysis and combustion of dry sludge. Energy 2013, 55, 426–435. [Google Scholar] [CrossRef]
- Gao, N.; Kamran, K.; Quan, C.; Williams, P.T. Thermochemical conversion of sewage sludge: A critical review. Prog. Energy Combust. Sci. 2020, 79, 100843. [Google Scholar] [CrossRef]
- Ruiz-Gómez, N.; Quispe, V.; Ábrego, J.; Atienza-Martínez, M.; Murillo, M.B.; Gea, G. Co-pyrolysis of sewage sludge and manure. Waste Manag. 2017, 59, 211–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortés, A.M.; Bridgwater, A.V. Kinetic study of the pyrolysis of miscanthus and its acid hydrolysis residue by thermogravimetric analysis. Fuel Process. Technol. 2015, 138, 184–193. [Google Scholar] [CrossRef] [Green Version]
- Mallick, D.; Poddar, M.K.; Mahanta, P.; Moholkar, V.S. Discernment of synergism in pyrolysis of biomass blends using thermogravimetric analysis. Bioresour. Technol. 2018, 261, 294–305. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, C.; Yan, F.; Shao, N.; Tang, Y.; Zhang, Z. Product characteristics and kinetics of sewage sludge pyrolysis driven by alkaline earth metals. Energy 2018, 153, 921–932. [Google Scholar] [CrossRef]
- Magdziarz, A.; Werle, S. Analysis of the combustion and pyrolysis of dried sewage sludge by TGA and MS. Waste Manag. 2014, 34, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Zaker, A.; Chen, Z.; Zaheer-Uddin, M.; Guo, J. Co-pyrolysis of sewage sludge and low-density polyethylene—A thermogravimetric study of thermo-kinetics and thermodynamic parameters. J. Environ. Chem. Eng. 2020, 9, 104554. [Google Scholar] [CrossRef]
- González Arias, J.; Gil, M.; Fernández, R.; Martinez, J.; Fernández, C.; Papaharalabos, G.; Gómez, X. Integrating anaerobic digestion and pyrolysis for treating digestates derived from sewage sludge and fat wastes. Environ. Sci. Pollut. Res. 2020, 27, 32603–32614. [Google Scholar] [CrossRef]
- Sanchez, M.E.; Otero, M.; Gómez, X.; Morán, A. Thermogravimetric kinetic analysis of the combustion of biowastes. Renew. Energy 2009, 34, 1622–1627. [Google Scholar] [CrossRef]
- Akor, C.I.; Osman, A.I.; Farrell, C.; McCallum, C.S.; John Doran, W.; Morgan, K.; Harrison, J.; Walsh, P.J.; Sheldrake, G.N. Thermokinetic study of residual solid digestate from anaerobic digestion. Chem. Eng. J. 2021, 406, 127039. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Liu, H.; Zhao, M.; Soomro, A.F.; Memon, M.Z.; Dupont, V. Thermogravimetric and kinetic analysis to discern synergy during the co-pyrolysis of microalgae and swine manure digestate. Biotechnol. Biofuels 2019, 12, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Wang, R.; Duan, X.; Wang, S.; Ren, N.; Ho, S.-H. Production, properties, and catalytic applications of sludge derived biochar for environmental remediation. Water Res. 2020, 187, 116390. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Cao, X.; Zhao, L.; Sun, T. Comparison of sewage sludge- and pig manure-derived biochars for hydrogen sulfide removal. Chemosphere 2014, 111, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.-Y.; Deng, J.-H.; Huang, G.-F.; Li, K.; Cai, K.-Z.; Liu, Y.; Huang, F. Relative distribution of Cd2+ adsorption mechanisms on biochars derived from rice straw and sewage sludge. Bioresour. Technol. 2019, 272, 114–122. [Google Scholar] [CrossRef]
- Melia, P.M.; Busquets, R.; Hooda, P.S.; Cundy, A.B.; Sohi, S.P. Driving forces and barriers in the removal of phosphorus from water using crop residue, wood and sewage sludge derived biochars. Sci. Total Environ. 2019, 675, 623–631. [Google Scholar] [CrossRef] [Green Version]
- Yousaf, B.; Liu, G.; Ubaid Ali, M.; Abbas, Q.; Liu, Y.; Ullah, H.; Imtiyaz Cheema, A. Decisive role of vacuum-assisted carbonization in valorization of lignin-enriched (Juglans regia-shell) biowaste. Bioresour. Technol. 2021, 323, 124541. [Google Scholar] [CrossRef]
- Fan, J.; Li, Y.; Yu, H.; Li, Y.; Yuan, Q.; Xiao, H.; Li, F.; Pan, B. Using sewage sludge with high ash content for biochar production and Cu(II) sorption. Sci. Total Environ. 2020, 713, 136663. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, Y.; Wang, H.; Lu, W.; Zhou, Z.; Zhang, Y.; Ren, L. Influence of pyrolysis temperature on characteristics and heavy metal adsorptive performance of biochar derived from municipal sewage sludge. Bioresour. Technol. 2014, 164, 47–54. [Google Scholar] [CrossRef]
- Yousaf, B.; Liu, G.; Abbas, Q.; Ali, M.U.; Wang, R.; Ahmed, R.; Wang, C.; Al-Wabel, M.I.; Usman, A.R.A. Operational control on environmental safety of potentially toxic elements during thermal conversion of metal-accumulator invasive ragweed to biochar. J. Clean. Prod. 2018, 195, 458–469. [Google Scholar] [CrossRef]
- Naqvi, S.R.; Hameed, Z.; Tariq, R.; Taqvi, S.A.; Ali, I.; Niazi, M.B.K.; Noor, T.; Hussain, A.; Iqbal, N.; Shahbaz, M. Synergistic effect on co-pyrolysis of rice husk and sewage sludge by thermal behavior, kinetics, thermodynamic parameters and artificial neural network. Waste Manag. 2019, 85, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Abbas, Q.; Ali, M.U.; Amina; Cheema, A.I.; Yousaf, B.; Rinklebe, J. Synergistic effects of low-/medium-vacuum carbonization on physico-chemical properties and stability characteristics of biochars. Chem. Eng. J. 2019, 373, 44–57. [Google Scholar] [CrossRef]
- Başer, B.; Yousaf, B.; Yetis, U.; Abbas, Q.; Kwon, E.E.; Wang, S.; Bolan, N.S.; Rinklebe, J. Formation of nitrogen functionalities in biochar materials and their role in the mitigation of hazardous emerging organic pollutants from wastewater. J. Hazard. Mater. 2021, 416, 126131. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Huang, H.; Lv, X.; Zhao, N.; Guo, G.; Zhang, D. Removal of phosphate from aqueous solution by dolomite-modified biochar derived from urban dewatered sewage sludge. Sci. Total Environ. 2019, 687, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Wongrod, S.; Simon, S.; Guibaud, G.; Lens, P.N.L.; Pechaud, Y.; Huguenot, D.; van Hullebusch, E.D. Lead sorption by biochar produced from digestates: Consequences of chemical modification and washing. J. Environ. Manag. 2018, 219, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ai, S.; Nzediegwu, C.; Kwak, J.-H.; Islam, M.S.; Li, Y.; Chang, S.X. Carboxyl and hydroxyl groups enhance ammonium adsorption capacity of iron (III) chloride and hydrochloric acid modified biochars. Bioresour. Technol. 2020, 309, 123390. [Google Scholar] [CrossRef] [PubMed]
- Saadat, S.; Raei, E.; Talebbeydokhti, N. Enhanced removal of phosphate from aqueous solutions using a modified sludge derived biochar: Comparative study of various modifying cations and RSM based optimization of pyrolysis parameters. J. Environ. Manag. 2018, 225, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Nuagah, M.B.; Boakye, P.; Oduro-Kwarteng, S.; Sokama-Neuyam, Y.A. Valorization of faecal and sewage sludge via pyrolysis for application as crop organic fertilizer. J. Anal. Appl. Pyrolysis 2020, 151, 104903. [Google Scholar] [CrossRef]
- Song, X.D.; Xue, X.Y.; Chen, D.Z.; He, P.J.; Dai, X.H. Application of biochar from sewage sludge to plant cultivation: Influence of pyrolysis temperature and biochar-to-soil ratio on yield and heavy metal accumulation. Chemosphere 2014, 109, 213–220. [Google Scholar] [CrossRef]
- Rehman, R.A.; Rizwan, M.; Qayyum, M.F.; Ali, S.; Zia-ur-Rehman, M.; Zafar-ul-Hye, M.; Hafeez, F.; Iqbal, M.F. Efficiency of various sewage sludges and their biochars in improving selected soil properties and growth of wheat (Triticum aestivum). J. Environ. Manag. 2018, 223, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.; Guwy, A.; Kalyuzhnyi, S.; Jenicek, P.; Van Lier, J. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SIST EN 14346:2007, Characterization of Waste—Calculation of Dry Matter by Determination of Dry Residue or Water Content; Slovenian Institute for Standardization (SIST): Ljubljana, Slovenia, 2007.
- SIST-TS CEN/TS 16023:2014, Characterization of Waste—Determination of Gross Calorific Value and Calculation of Net Calorific Value; Slovenian Institute for Standardization (SIST): Ljubljana, Slovenia, 2014.
- Channiwala, S.A.; Parikh, P.P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- EN 16170:2016, Sludge, Treated Biowaste and Soil—Determination of Elements Using Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES); European Committee for Standardization: Brussels, Belgium, 2016.
- Naqvi, S.R.; Tariq, R.; Hameed, Z.; Ali, I.; Taqvi, S.A.; Naqvi, M.; Niazi, M.B.K.; Noor, T.; Farooq, W. Pyrolysis of high-ash sewage sludge: Thermo-kinetic study using TGA and artificial neural networks. Fuel 2018, 233, 529–538. [Google Scholar] [CrossRef]
- Yao, Z.; Yu, S.; Su, W.; Wu, W.; Tang, J.; Qi, W. Kinetic studies on the pyrolysis of plastic waste using a combination of model-fitting and model-free methods. Waste Manag. Res. 2020, 38, 77–85. [Google Scholar] [CrossRef]
- Tahir, M.H.; Çakman, G.; Goldfarb, J.L.; Topcu, Y.; Naqvi, S.R.; Ceylan, S. Demonstrating the suitability of canola residue biomass to biofuel conversion via pyrolysis through reaction kinetics, thermodynamics and evolved gas analyses. Bioresour. Technol. 2019, 279, 67–73. [Google Scholar] [CrossRef]
- Kong, L.; Liu, J.; Han, Q.; Zhou, Q.; He, J. Integrating metabolomics and physiological analysis to investigate the toxicological mechanisms of sewage sludge-derived biochars to wheat. Ecotoxicol. Environ. Saf. 2019, 185, 109664. [Google Scholar] [CrossRef]
- SIST EN 16086-2:2012, Soil Improvers and Growing Media—Determination of Plant Response—Part 2: Petri Dish Test Using Cress; Slovenian Institute for Standardization (SIST): Ljubljana, Slovenia, 2012.
- Chemetova, C.; Fabião, A.; Gominho, J.; Ribeiro, H. Range analysis of Eucalyptus globulus bark low-temperature hydrothermal treatment to produce a new component for growing media industry. Waste Manag. 2018, 79, 1–7. [Google Scholar] [CrossRef]
- Maunuksela, L.; Herranen, M.; Torniainen, M. Quality assessment of biogas plant end products by plant bioassays. Int. J. Environ. Sci. Dev. 2012, 3, 305. [Google Scholar] [CrossRef]
- DIN 38 406-E5-1, Photometric Determination of Ammonium Nitrogen by Sodium Dichloroisocyanurate and Sodium Salicylate; DIN—German Institute for Standardization: Berlin, Germany, 1983.
- SIST EN ISO 6878:2004, Water Quality—Determination of Phosphorus—Ammonium Molybdate Spectrometric Method; ISO: Geneva, Switzerland, 2004.
- Tang, Y.; Alam, M.S.; Konhauser, K.O.; Alessi, D.S.; Xu, S.; Tian, W.; Liu, Y. Influence of pyrolysis temperature on production of digested sludge biochar and its application for ammonium removal from municipal wastewater. J. Clean. Prod. 2019, 209, 927–936. [Google Scholar] [CrossRef]
- Government of the Republic of Slovenia. Decree on the use of sludge from municipal sewage treatment plants in agriculture. In Official Gazette of the Republic of Slovenia, No. 62/08; The Official Gazette of the Republic of Slovenia Ltd., Public Company: Ljubljana, Slovenia, 2008. [Google Scholar]
- Ahmad, M.S.; Mehmood, M.A.; Al Ayed, O.S.; Ye, G.; Luo, H.; Ibrahim, M.; Rashid, U.; Arbi Nehdi, I.; Qadir, G. Kinetic analyses and pyrolytic behavior of Para grass (Urochloa mutica) for its bioenergy potential. Bioresour. Technol. 2017, 224, 708–713. [Google Scholar] [CrossRef]
- Syed-Hassan, S.S.A.; Wang, Y.; Hu, S.; Su, S.; Xiang, J. Thermochemical processing of sewage sludge to energy and fuel: Fundamentals, challenges and considerations. Renew. Sustain. Energy Rev. 2017, 80, 888–913. [Google Scholar] [CrossRef]
- Mehmood, M.A.; Ye, G.; Luo, H.; Liu, C.; Malik, S.; Afzal, I.; Xu, J.; Ahmad, M.S. Pyrolysis and kinetic analyses of Camel grass (Cymbopogon schoenanthus) for bioenergy. Bioresour. Technol. 2017, 228, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Shen, D.; Jin, W.; Hu, J.; Xiao, R.; Luo, K. An overview on fast pyrolysis of the main constituents in lignocellulosic biomass to valued-added chemicals: Structures, pathways and interactions. Renew. Sustain. Energy Rev. 2015, 51, 761–774. [Google Scholar] [CrossRef]
- Bedoić, R.; Ocelić Bulatović, V.; Čuček, L.; Ćosić, B.; Špehar, A.; Pukšec, T.; Duić, N. A kinetic study of roadside grass pyrolysis and digestate from anaerobic mono-digestion. Bioresour. Technol. 2019, 292, 121935. [Google Scholar] [CrossRef]
- Kaur, R.; Gera, P.; Jha, M.K.; Bhaskar, T. Pyrolysis kinetics and thermodynamic parameters of castor (Ricinus communis) residue using thermogravimetric analysis. Bioresour. Technol. 2018, 250, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Rasool, T.; Kumar, S. Kinetic and Thermodynamic Evaluation of Pyrolysis of Plant Biomass using TGA. Mater. Today Proc. 2020, 21, 2087–2095. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, F.; Yi, W.; Li, Z.; Shen, X.; Niu, W. Comparison study on pyrolysis characteristics and kinetics of corn stover and its digestate by TG-FTIR. BioResources 2017, 12, 8240–8254. [Google Scholar] [CrossRef]
- Gao, N.; Li, J.; Qi, B.; Li, A.; Duan, Y.; Wang, Z. Thermal analysis and products distribution of dried sewage sludge pyrolysis. J. Anal. Appl. Pyrolysis 2014, 105, 43–48. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Z.; Cheng, X.; Ma, C. Non-isothermal TGA study on the combustion reaction kinetics and mechanism of low-rank coal char. RSC Adv. 2018, 8, 22909–22916. [Google Scholar] [CrossRef] [Green Version]
- Naqvi, S.R.; Ali, I.; Nasir, S.; Ali Ammar Taqvi, S.; Atabani, A.E.; Chen, W.-H. Assessment of agro-industrial residues for bioenergy potential by investigating thermo-kinetic behavior in a slow pyrolysis process. Fuel 2020, 278, 118259. [Google Scholar] [CrossRef]
- Yin, Q.; Liu, M.; Ren, H. Biochar produced from the co-pyrolysis of sewage sludge and walnut shell for ammonium and phosphate adsorption from water. J. Environ. Manag. 2019, 249, 109410. [Google Scholar] [CrossRef]
- Huang, H.-j.; Yang, T.; Lai, F.-y.; Wu, G.-q. Co-pyrolysis of sewage sludge and sawdust/rice straw for the production of biochar. J. Anal. Appl. Pyrolysis 2017, 125, 61–68. [Google Scholar] [CrossRef]
- Xie, S.; Yu, G.; Li, C.; Li, J.; Wang, G.; Dai, S.; Wang, Y. Treatment of high-ash industrial sludge for producing improved char with low heavy metal toxicity. J. Anal. Appl. Pyrolysis 2020, 150, 104866. [Google Scholar] [CrossRef]
- Arauzo, P.J.; Atienza-Martínez, M.; Ábrego, J.; Olszewski, M.P.; Cao, Z.; Kruse, A. Combustion Characteristics of Hydrochar and Pyrochar Derived from Digested Sewage Sludge. Energies 2020, 13, 4164. [Google Scholar] [CrossRef]
- Liu, Q.; Fang, Z.; Liu, Y.; Liu, Y.; Xu, Y.; Ruan, X.; Zhang, X.; Cao, W. Phosphorus speciation and bioavailability of sewage sludge derived biochar amended with CaO. Waste Manag. 2019, 87, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Wang, M.; Liu, J.; Tang, S.; Chen, H.; Guo, T.; Yang, G.; Chen, Y. Removal of phosphate from aqueous solution by sewage sludge-based activated carbon loaded with pyrolusite. J. Water Reuse Desalination 2017, 8, 192–201. [Google Scholar] [CrossRef] [Green Version]
- Ren, N.; Tang, Y.; Li, M. Mineral additive enhanced carbon retention and stabilization in sewage sludge-derived biochar. Process Saf. Environ. Prot. 2018, 115, 70–78. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Poon, C.S. Using incinerated sewage sludge ash as a high-performance adsorbent for lead removal from aqueous solutions: Performances and mechanisms. Chemosphere 2019, 226, 587–596. [Google Scholar] [CrossRef]
- Xing, J.; Li, L.; Li, G.; Xu, G. Feasibility of sludge-based biochar for soil remediation: Characteristics and safety performance of heavy metals influenced by pyrolysis temperatures. Ecotoxicol. Environ. Saf. 2019, 180, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Yu, G.; Ma, J.; Wang, G.; Wang, Q.; You, F.; Li, J.; Wang, Y.; Li, C. Chemical speciation and distribution of potentially toxic elements in soilless cultivation of cucumber with sewage sludge biochar addition. Environ. Res. 2020, 191, 110188. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Dhanjal, D.S.; Datta, S.; Bhatia, D.; Dhiman, J.; Samuel, J.; Prasad, R.; Singh, J. A sustainable paradigm of sewage sludge biochar: Valorization, opportunities, challenges and future prospects. J. Clean. Prod. 2020, 269, 122259. [Google Scholar] [CrossRef]
- Cui, X.; Hao, H.; He, Z.; Stoffella, P.J.; Yang, X. Pyrolysis of wetland biomass waste: Potential for carbon sequestration and water remediation. J. Environ. Manag. 2016, 173, 95–104. [Google Scholar] [CrossRef]
- Almanassra, I.W.; McKay, G.; Kochkodan, V.; Ali Atieh, M.; Al-Ansari, T. A state of the art review on phosphate removal from water by biochars. Chem. Eng. J. 2021, 409, 128211. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, Z.; Deng, L. Adsorption Behaviors and Removal Efficiencies of Inorganic, Polymeric and Organic Phosphates from Aqueous Solution on Biochar Derived from Sewage Sludge of Chemically Enhanced Primary Treatment Process. Water 2018, 10, 869. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Song, G.; Gelardi, D.L.; Huang, L.; Khan, E.; Mašek, O.; Parikh, S.J.; Ok, Y.S. Evaluating biochar and its modifications for the removal of ammonium, nitrate, and phosphate in water. Water Res. 2020, 186, 116303. [Google Scholar] [CrossRef]
- Wongrod, S.; Simon, S.; van Hullebusch, E.D.; Lens, P.N.L.; Guibaud, G. Changes of sewage sludge digestate-derived biochar properties after chemical treatments and influence on As(III and V) and Cd(II) sorption. Int. Biodeterior. Biodegrad. 2018, 135, 96–102. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, D.; Gao, F.; Li, M.; Luo, X. Effects of Biochar-Derived Sewage Sludge on Heavy Metal Adsorption and Immobilization in Soils. Int. J. Environ. Res Public Health 2017, 14, 681. [Google Scholar] [CrossRef]
- Matheri, A.N.; Eloko, N.S.; Ntuli, F.; Ngila, J.C. Influence of pyrolyzed sludge use as an adsorbent in removal of selected trace metals from wastewater treatment. Case Stud. Chem. Environ. Eng. 2020, 2, 100018. [Google Scholar] [CrossRef]
- Chen, Y.; Li, M.; Li, Y.; Liu, Y.; Chen, Y.; Li, H.; Li, L.; Xu, F.; Jiang, H.; Chen, L. Hydroxyapatite modified sludge-based biochar for the adsorption of Cu2+ and Cd2+: Adsorption behavior and mechanisms. Bioresour. Technol. 2021, 321, 124413. [Google Scholar] [CrossRef] [PubMed]
| Feedstock | Biochar Mark | Pyrolysis Conditions | Tested Heating Rates |
|---|---|---|---|
| Sewage sludge digestate (D1) * | B-D1 | 25–800 °C, inert atmosphere (nitrogen flow of 100 mL/min) | 15, 30, and 100 °C/min |
| Digestate of sewage sludge mixture with T. latifolia plant (D2) * | B-D2 | ||
| Sewage sludge (SS) | B-SS | ||
| T. latifolia plant (TLP) | B-TLP |
| Parameter | Sewage Sludge (SS) | T. latifolia Plant (TLP) | Digestate D1 a | Digestate D2 a |
|---|---|---|---|---|
| Dry matter (wt.%) | 17.42 | 19.77 | 12.16 | 14.38 |
| Moisture content (wt.%) | 82.58 | 80.23 | 87.84 | 85.62 |
| Volatile matter (wt.%) b | 70.99 | 79.42 | 57.87 | 60.18 |
| Ash (wt.%) b | 18.48 | 7.31 | 35.66 | 31.08 |
| FC (wt.%) b | 10.52 | 13.27 | 6.47 | 8.74 |
| HHV (MJ/kg) | 19.91 | 17.02 | 12.75 | 12.64 |
| HHVtheoretical (MJ/kg) | 19.93 | 20.50 | 13.01 | 13.30 |
| C (wt.%) | 42.30 | 45.79 | 31.90 | 34.83 |
| H (wt.%) | 6.75 | 7.10 | 4.15 | 3.73 |
| N (wt.%) | 8.10 | 3.63 | 4.35 | 3.58 |
| S (wt.%) | 1.16 | 0.49 | 1.35 | 1.09 |
| O (wt.%) | 23.21 | 35.68 | 22.59 | 25.69 |
| H/C c | 1.91 | 1.86 | 1.56 | 1.29 |
| O/C c | 0.41 | 0.58 | 0.53 | 0.55 |
| N/C c | 0.16 | 0.07 | 0.12 | 0.09 |
| P (wt.%) | 2.61 | 0.57 | 1.01 | 0.86 |
| Ca (wt.%) | 2.04 | 1.45 | 5.35 | 4.87 |
| Mg (wt.%) | 0.82 | 0.32 | 0.34 | 0.26 |
| K (wt.%) | 0.82 | 3.44 | 1.15 | 1.18 |
| Si (wt.%) | 0.14 | 0.04 | 0.09 | 0.05 |
| Fe (wt.%) | 0.88 | 0.18 | 0.31 | 0.21 |
| Cd (mg/kg d.m.) | 1.03 | 1.05 | <1 | <1 |
| Crtotal (mg/kg d.m.) | 45.15 | 1.42 | 38.58 | 30.69 |
| Cu (mg/kg d.m.) | 173.61 | 6.24 | 165.59 | 153.10 |
| Ni (mg/kg d.m.) | 25.35 | 1.68 | 17.10 | 13.07 |
| Pb (mg/kg d.m.) | 26.66 | 2.10 | 15.85 | 12.04 |
| Zn (mg/kg d.m.) | 740.79 | 26.73 | 596.36 | 489.40 |
| Sample | Heating Rate (°C/min) | Weight Loss (wt.%) | Total Weight Loss (wt.%) | Final Residue (wt.%) | DTGmax (1/s) | |||
|---|---|---|---|---|---|---|---|---|
| Dehydration (Stage I) | Active Pyrolysis (Stage II) | Passive Pyrolysis (Stage III) | ||||||
| Digestate D1 | 15 | 2.70 | 40.44 | 13.33 | 56.47 | 43.53 | 299 | 5.31 × 10−4 |
| 30 | 3.12 | 41.47 | 10.60 | 55.18 | 44.82 | 332 | 1.06 × 10−3 | |
| 100 | 2.09 | 41.79 | 6.83 | 50.71 | 49.29 | 380 | 3.44 × 10−3 | |
| Digestate D2 | 15 | 2.63 | 40.49 | 16.72 | 59.85 | 40.15 | 306 | 5.84 × 10−4 |
| 30 | 3.51 | 37.19 | 13.78 | 54.48 | 45.52 | 312 | 8.79 × 10−4 | |
| 100 | 3.18 | 38.47 | 7.89 | 49.54 | 50.46 | 362 | 3.32 × 10−3 | |
| Sewage sludge (SS) | 15 | 7.05 | 56.79 | 3.96 | 67.80 | 32.20 | 292 | 7.17 × 10−4 |
| 30 | 4.23 | 59.34 | 3.94 | 67.51 | 32.49 | 311 | 1.51 × 10−3 | |
| 100 | 3.38 | 63.01 | 3.45 | 69.85 | 30.15 | 359 | 5.28 × 10−3 | |
| T. latifolia plant (TLP) | 15 | 7.22 | 54.96 | 13.37 | 75.55 | 24.45 | 347 | 1.89 × 10−3 |
| 30 | 5.60 | 55.85 | 12.91 | 74.36 | 25.64 | 359 | 2.80 × 10−3 | |
| 100 | 7.17 | 55.45 | 12.81 | 75.44 | 24.56 | 408 | 1.18 × 10−2 | |
| Feedstock | (kJ/mol) | (1/s) | (kJ/mol) | (kJ/mol) | (J/mol·K) | Kinetic Model | Ref. |
|---|---|---|---|---|---|---|---|
| Digestate D1 (sewage sludge) | 66–351 | 6.73 × 103–3.80 × 1030 | 61–347 | 160–168 | (−185)–(327) | FWO | This study |
| Digestate D2 (sewage sludge + T. latifolia plant) | 70–401 | 1.37 × 104–5.43 × 1034 | 65–397 | 161–170 | (−180)–(406) | FWO | This study |
| SS digestate | 90–227 | / | / | / | / | FWO | [42] |
| SS digestate | 49–198 | 5.49 × 101–7.92 × 1014 | / | / | / | nth-order reaction model | [34] |
| Sewage sludge co-digested with grease waste (ratio 95:5) | 132–226 | / | / | / | / | FWO | [42] |
| Swine manure digestate | 179–223 | 2.55 × 1016–1.45 × 1020 | 179–219 | 143–147 | (54)–(127) | FWO | [45] |
| Lignocellulosic biomass digestate | 75–175 | 1.83 × 10−2–9.74 × 109 | / | / | / | FWO | [44] |
| Lignocellulosic biomass digestate | 130–230 | 1.05 × 102–7.83 × 1015 | / | / | / | Starink model-free method | [25] |
| Roadside grass digestate | 30–175 | 6.74 × 10−3–1.59 × 1015 | / | / | / | KAS | [85] |
| Corn stover digestate | 99–331 | 1012–1022 | / | / | / | DAEM a | [88] |
| Sewage sludge | 41–167 | 2.12 × 101–4.85 × 1013 | 36–163 | 161–167 | (−233)–(4) | FWO | This study |
| 63–323 | 3.22 × 104–5.78 × 1026 | 70–318 | 85–90 | (−90)–(650) | FWO | [41] | |
| 46–232 | 1.02 × 109–3.97 × 1019 | 41–227 | 53–295 | (−151)–(63) | FWO | [69] | |
| 48–82 | 1.34 × 101–5.92 × 105 | 11–134 | / | / | Coats and Redfern | [89] | |
| 75–292 | / | / | / | / | FWO | Wang 2020 [33] | |
| 200–400 | 1015–1025 | / | / | / | DAEM a | [1] | |
| T. latifolia plant | 67–359 | 2.18 × 103–4.83 × 1028 | 62–354 | 174–183 | (−195)–(290) | FWO | This study |
| 135–204 | 7.6 × 109–7.9 × 1015 | 130–199 | 171–173 | (−70)–(45) | FWO | [24] | |
| Para grass | 152–242 | 3.06 × 1011–2.26 × 1019 | 113–237 | 169–173 | (−98)–(111) | FWO | [80] |
| Camel grass | 85–193 | 1.77 × 105–4.70 × 1014 | 79–188 | 174–178 | (−159)–(23) | FWO | [82] |
| Chicken manure | 149–288 | 1.00 × 106–1.00 × 1014 | 165–170 | 158–175 | (−8)–(12) | FWO | [29] |
| Castor residue | 102–216 | 3.06 × 108–6.26 × 1018 | 97–211 | 151–154 | (−97)–(101) | FWO | [86] |
| Canola residue | 129–391 | 6.5 × 109–3.4 × 1027 | 136–385 | 158–212 | (−51)–(284) | DAEM a | [71] |
| FWO Method | KAS Method | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| α | (1/s) | R2 | (kJ/mol) | (kJ/mol) | (J/mol·K) | (1/s) | R2 | (kJ/mol) | (kJ/mol) | (J/mol·K) |
| Digestate D1 | ||||||||||
| 0.1 | 6.73 × 103 | 0.99 | 61.46 | 167.53 | −185.36 | 9.14 × 102 | 0.98 | 52.63 | 168.21 | −201.97 |
| 0.2 | 2.19 × 105 | 0.99 | 77.03 | 166.52 | −156.39 | 2.62 × 104 | 0.99 | 67.51 | 167.11 | −174.06 |
| 0.3 | 9.87 × 105 | 1.00 | 83.80 | 166.14 | −143.89 | 1.06 × 105 | 0.99 | 73.77 | 166.72 | −162.42 |
| 0.4 | 2.67 × 107 | 0.99 | 98.76 | 165.40 | −116.46 | 2.67 × 106 | 0.99 | 88.29 | 165.91 | −135.63 |
| 0.5 | 2.09 × 109 | 0.98 | 118.67 | 164.56 | −80.21 | 1.90 × 108 | 0.98 | 107.70 | 165.01 | −100.14 |
| 0.6 | 5.10 × 1014 | 0.93 | 175.86 | 162.75 | 22.91 | 4.17 × 1013 | 0.92 | 164.27 | 163.07 | 2.10 |
| 0.7 | 3.80 × 1030 | 0.93 | 346.57 | 159.59 | 326.76 | 2.74 × 1029 | 0.93 | 334.23 | 159.76 | 304.89 |
| 0.8 | 3.38 × 1017 | 0.92 | 206.03 | 162.02 | 76.91 | 4.14 × 1019 | 0.88 | 228.43 | 161.54 | 116.90 |
| Digestate D2 | ||||||||||
| 0.1 | 1.37 × 104 | 0.92 | 65.47 | 169.53 | −179.58 | 1.95 × 103 | 0.90 | 56.74 | 170.17 | −195.76 |
| 0.2 | 2.47 × 106 | 0.95 | 89.12 | 168.14 | −136.36 | 3.09 × 105 | 0.94 | 79.61 | 168.65 | −153.66 |
| 0.3 | 1.66 × 107 | 0.96 | 97.86 | 167.71 | −120.55 | 1.87 × 106 | 0.95 | 87.83 | 168.20 | −138.71 |
| 0.4 | 1.37 × 109 | 1.00 | 118.25 | 166.83 | −83.85 | 1.43 × 108 | 1.00 | 107.78 | 167.26 | −102.64 |
| 0.5 | 4.92 × 1012 | 1.00 | 156.39 | 165.53 | −15.77 | 4.64 × 1011 | 1.00 | 145.35 | 165.88 | −35.42 |
| 0.6 | 5.21 × 1031 | 1.00 | 363.45 | 161.55 | 348.42 | 4.38 × 1030 | 1.00 | 351.68 | 161.71 | 327.85 |
| 0.7 | 5.43 × 1034 | 0.97 | 396.52 | 161.14 | 406.20 | 3.63 × 1033 | 0.97 | 383.64 | 161.30 | 383.71 |
| 0.8 | 4.49 × 1020 | 0.79 | 242.63 | 163.47 | 136.61 | 2.79 × 1024 | 0.82 | 283.96 | 162.73 | 209.22 |
| Sewage sludge (SS) | ||||||||||
| 0.1 | 2.12 × 101 | 0.99 | 35.80 | 167.48 | −233.13 | 2.83 × 100 | 0.98 | 27.43 | 168.57 | −249.89 |
| 0.2 | 9.99 × 102 | 1.00 | 52.28 | 165.88 | −201.12 | 1.15 × 102 | 1.00 | 42.95 | 166.72 | −219.12 |
| 0.3 | 6.06 × 103 | 1.00 | 60.14 | 165.27 | −186.13 | 6.31 × 102 | 0.99 | 50.29 | 166.05 | −204.94 |
| 0.4 | 3.43 × 104 | 1.00 | 67.76 | 164.75 | −171.71 | 3.34 × 103 | 0.99 | 57.53 | 165.46 | −191.09 |
| 0.5 | 1.90 × 105 | 1.00 | 75.32 | 164.28 | −157.51 | 1.71 × 104 | 0.99 | 64.68 | 164.95 | −177.52 |
| 0.6 | 8.76 × 105 | 0.99 | 82.13 | 163.90 | −144.77 | 7.30 × 104 | 0.99 | 71.09 | 164.54 | −165.44 |
| 0.7 | 1.17 × 107 | 0.99 | 93.72 | 163.31 | −123.22 | 9.07 × 105 | 0.99 | 82.28 | 163.89 | −144.49 |
| 0.8 | 2.24 × 109 | 0.99 | 117.38 | 162.30 | −79.53 | 1.57 × 108 | 0.99 | 105.39 | 162.79 | −101.61 |
| 0.9 | 4.85 × 1013 | 0.99 | 162.77 | 160.82 | 3.46 | 2.86 × 1012 | 0.99 | 149.86 | 161.19 | −20.07 |
| T. latifolia plant (TLP) | ||||||||||
| 0.1 | 2.18 × 103 | 0.94 | 61.54 | 182.65 | −195.40 | 4.00 × 102 | 0.92 | 53.47 | 183.32 | −209.49 |
| 0.2 | 5.89 × 104 | 1.00 | 77.43 | 181.55 | −167.99 | 8.05 × 103 | 1.00 | 67.81 | 182.19 | −184.54 |
| 0.3 | 3.72 × 105 | 1.00 | 86.39 | 181.02 | −152.68 | 4.71 × 104 | 1.00 | 76.34 | 181.62 | −169.86 |
| 0.4 | 2.36 × 106 | 1.00 | 95.44 | 180.54 | −137.30 | 2.83 × 105 | 1.00 | 85.06 | 181.10 | −154.94 |
| 0.5 | 1.24 × 107 | 1.00 | 103.57 | 180.13 | −123.53 | 1.42 × 106 | 0.99 | 92.94 | 180.66 | −141.53 |
| 0.6 | 2.31 × 107 | 1.00 | 106.65 | 179.99 | −118.33 | 2.56 × 106 | 1.00 | 95.84 | 180.51 | −136.62 |
| 0.7 | 1.48 × 108 | 1.00 | 115.82 | 179.58 | −102.88 | 1.60 × 107 | 1.00 | 104.83 | 180.08 | −121.40 |
| 0.8 | 1.37 × 1014 | 1.00 | 184.27 | 177.27 | 11.29 | 1.39 × 1013 | 1.00 | 172.82 | 177.59 | −7.70 |
| 0.9 | 4.83 × 1028 | 0.99 | 353.61 | 173.98 | 289.81 | 3.93 × 1027 | 0.99 | 340.87 | 174.17 | 268.96 |
| Parameter | Biochar | |||
|---|---|---|---|---|
| B-SS | B-TLP | B-D1 | B-D2 | |
| Biochar yield (wt.%) | 32.49 | 25.64 | 44.82 | 45.52 |
| HHV (MJ/kg) | 11.84 | 12.92 | 11.61 | 12.15 |
| Ash (wt.%) a | 56.22 | 41.06 | 70.20 | 64.78 |
| C (wt.%) | 37.10 | 53.42 | 24.33 | 29.03 |
| H (wt.%) | 0.58 | 1.47 | 0.18 | 0.31 |
| N (wt.%) | 5.28 | 3.33 | 1.39 | 1.48 |
| S (wt.%) | 0.05 | 0.21 | 0.92 | 0.59 |
| O (wt.%) | 0.77 | 0.51 | 2.98 | 3.81 |
| H/C b | 0.19 | 0.33 | 0.09 | 0.13 |
| O/C b | 0.02 | 0.01 | 0.09 | 0.10 |
| N/C b | 0.12 | 0.05 | 0.05 | 0.04 |
| P (wt.%) | 7.94 | 1.67 | 2.11 | 1.72 |
| Ca (wt.%) | 6.60 | 11.57 | 8.60 | 12.37 |
| Mg (wt.%) | 2.34 | 1.78 | 0.74 | 0.58 |
| K (wt.%) | 2.51 | 1.78 | 2.04 | 3.35 |
| Si (wt.%) | 0.13 | 0.04 | 0.09 | 0.05 |
| Fe (wt.%) | 1.49 | 0.28 | 0.54 | 0.52 |
| Cd (mg/kg d.m.) | <1 | <1 | <1 | <1 |
| Crtotal (mg/kg d.m.) | 60.45 | 6.40 | 62.31 | 47.58 |
| Cu (mg/kg d.m.) | 300.77 | 11.00 | 369.27 | 283.25 |
| Ni (mg/kg d.m.) | 33.78 | 3.00 | 29.77 | 26.64 |
| Pb (mg/kg d.m.) | 51.88 | 1.40 | 25.09 | 20.73 |
| Zn (mg/kg d.m.) | 2352.42 | 56.90 | 1179.38 | 939.61 |
| pH | 9.36 | 10.89 | 11.05 | 11.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrovič, A.; Vohl, S.; Cenčič Predikaka, T.; Bedoić, R.; Simonič, M.; Ban, I.; Čuček, L. Pyrolysis of Solid Digestate from Sewage Sludge and Lignocellulosic Biomass: Kinetic and Thermodynamic Analysis, Characterization of Biochar. Sustainability 2021, 13, 9642. https://doi.org/10.3390/su13179642
Petrovič A, Vohl S, Cenčič Predikaka T, Bedoić R, Simonič M, Ban I, Čuček L. Pyrolysis of Solid Digestate from Sewage Sludge and Lignocellulosic Biomass: Kinetic and Thermodynamic Analysis, Characterization of Biochar. Sustainability. 2021; 13(17):9642. https://doi.org/10.3390/su13179642
Chicago/Turabian StylePetrovič, Aleksandra, Sabina Vohl, Tjaša Cenčič Predikaka, Robert Bedoić, Marjana Simonič, Irena Ban, and Lidija Čuček. 2021. "Pyrolysis of Solid Digestate from Sewage Sludge and Lignocellulosic Biomass: Kinetic and Thermodynamic Analysis, Characterization of Biochar" Sustainability 13, no. 17: 9642. https://doi.org/10.3390/su13179642
APA StylePetrovič, A., Vohl, S., Cenčič Predikaka, T., Bedoić, R., Simonič, M., Ban, I., & Čuček, L. (2021). Pyrolysis of Solid Digestate from Sewage Sludge and Lignocellulosic Biomass: Kinetic and Thermodynamic Analysis, Characterization of Biochar. Sustainability, 13(17), 9642. https://doi.org/10.3390/su13179642








