Wood and Black Liquor-Based N-Doped Activated Carbon for Energy Application
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
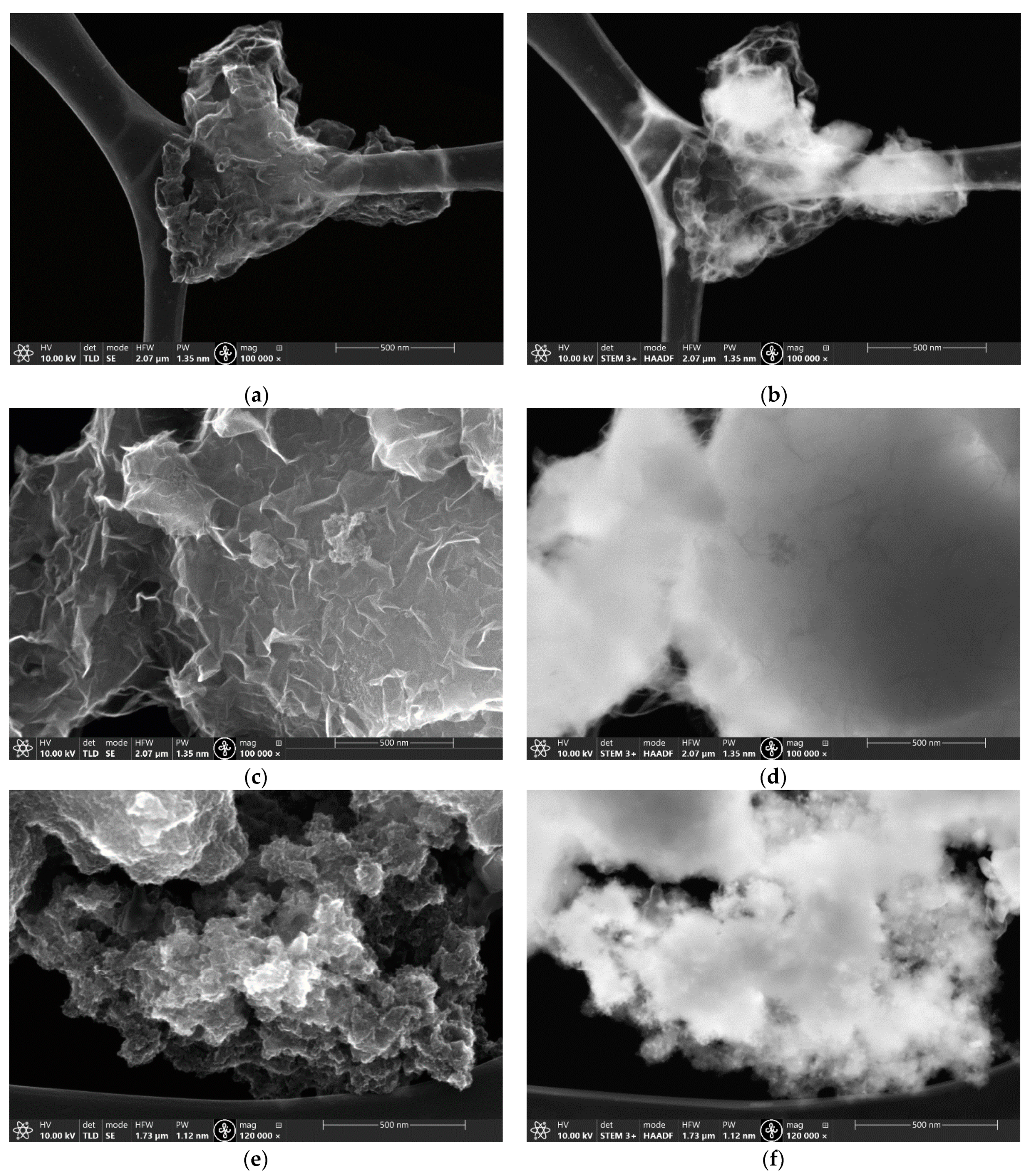
3.1. Characterization of the Materials
3.2. Electrochemical Characterization
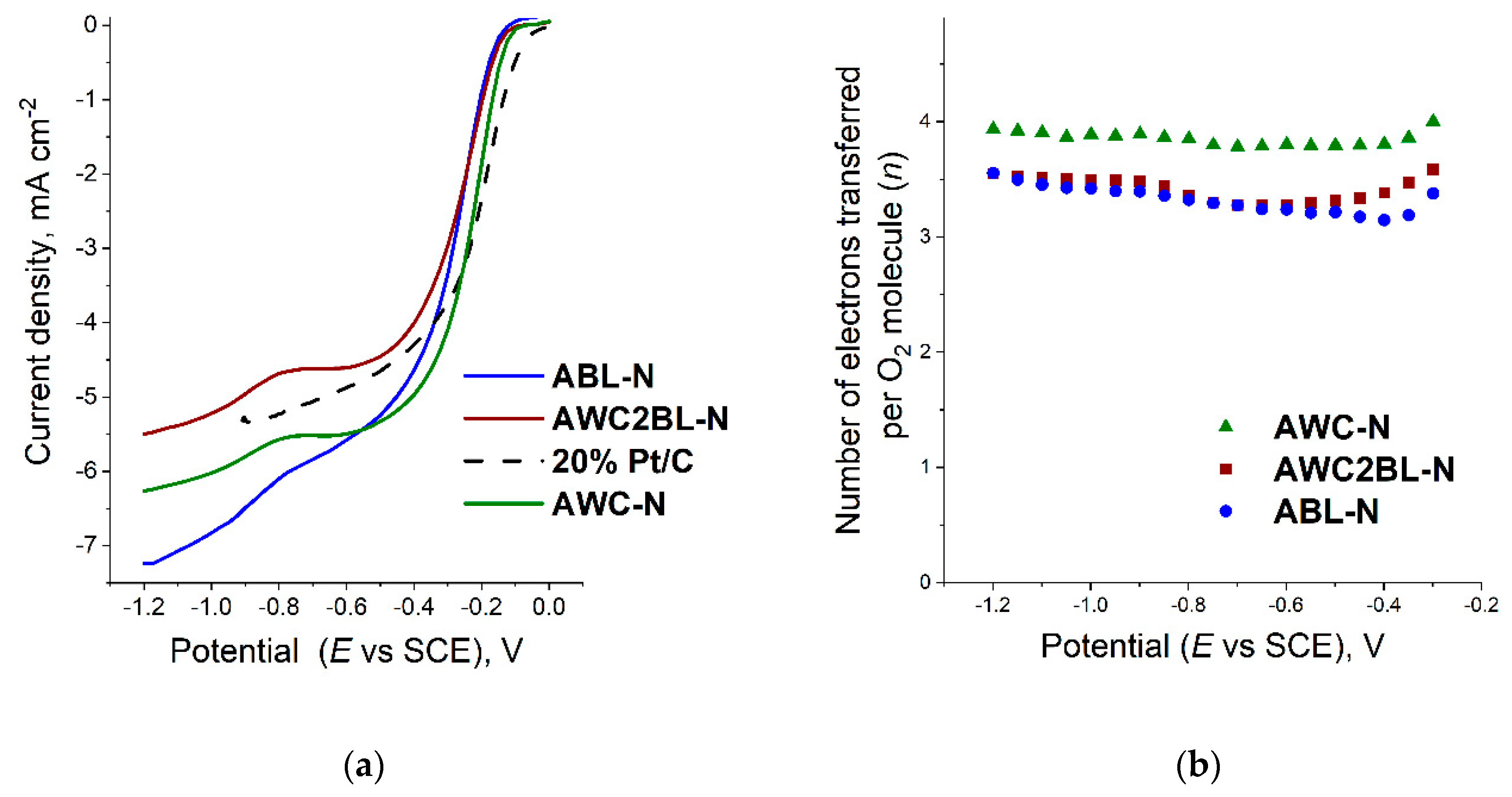
3.2.1. Oxygen Reduction Reaction (ORR) on Catalysts in Alkaline Media
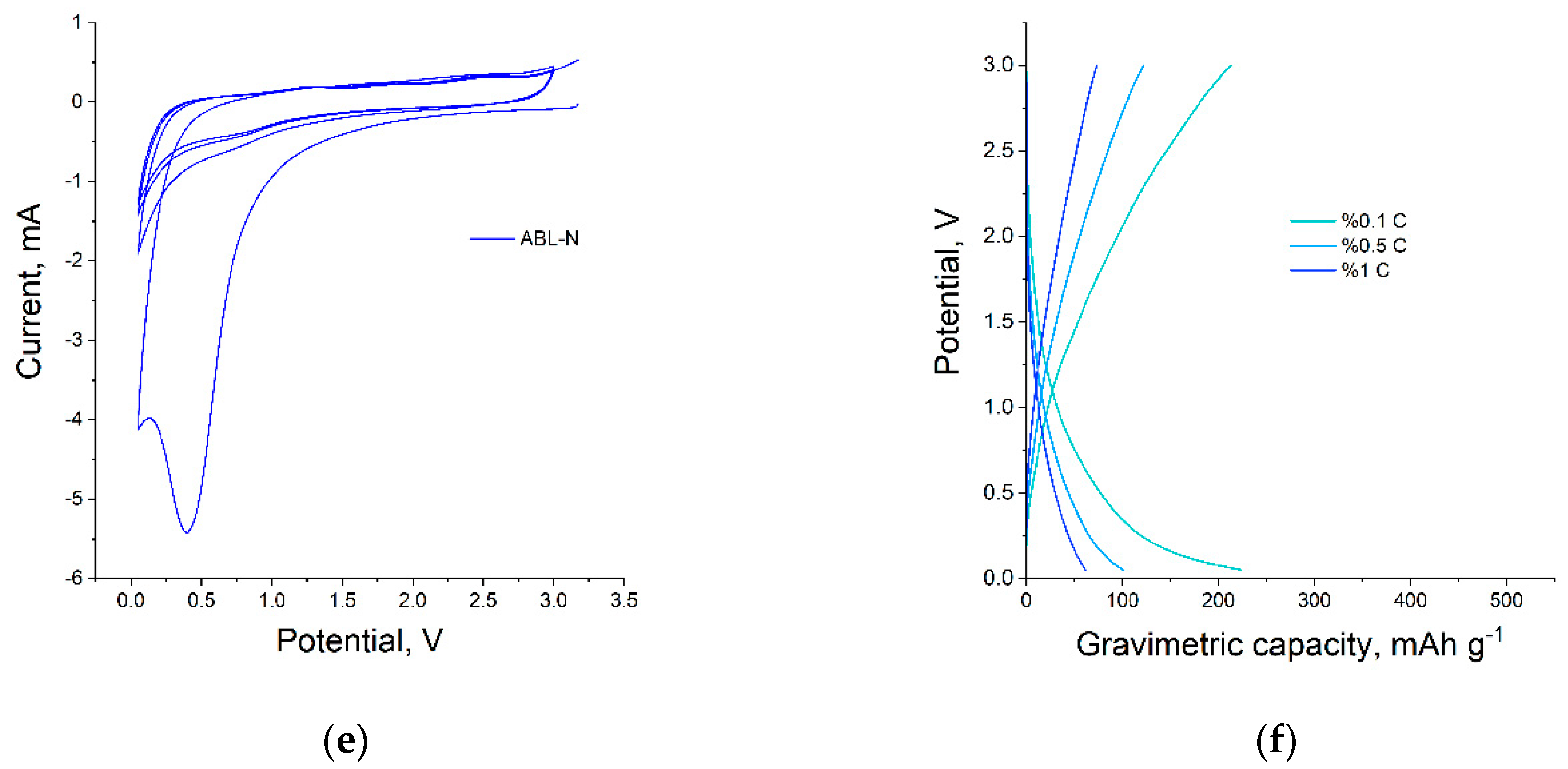
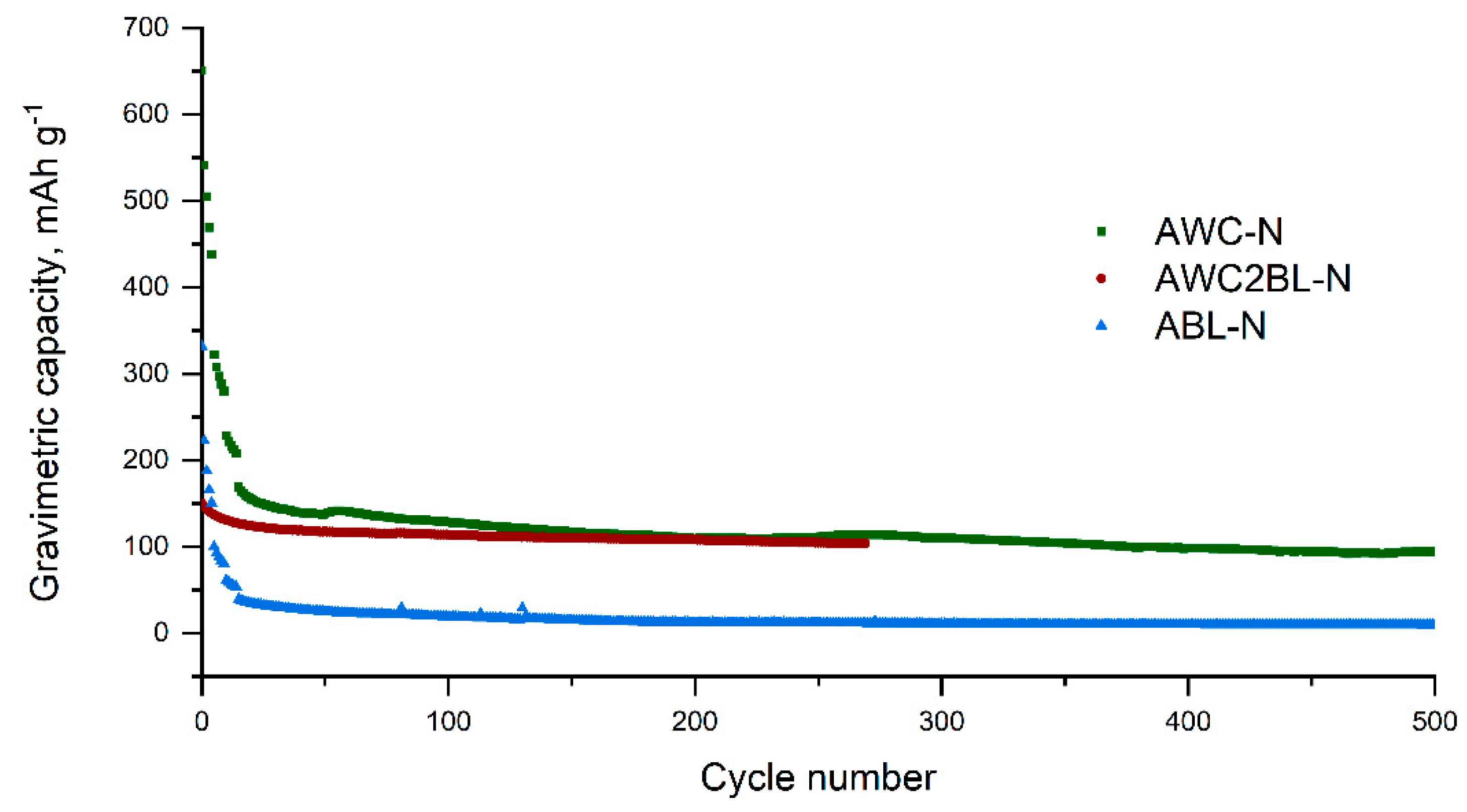
3.2.2. Anode Material in Li Ion Batteries
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, R.; Sahoo, S.; Joanni, E.; Singh, R.K.; Tan, W.K.; Kar, K.K.; Matsuda, A. Recent progress in the synthesis of graphene and derived materials for next generation electrodes of high performance lithium ion batteries. Prog. Energy Combust. Sci. 2019, 75, 100786. [Google Scholar] [CrossRef]
- Vaalma, C.; Buchholz, D.; Weil, M.; Passerini, S. A cost and resource analysis of sodium-ion batteries. Nat. Rev. Mater. 2018, 3, 1–11. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, C.Y. Cost-effective iron-based aqueous redox flow batteries for large-scale energy storage application: A review. J. Power Sources 2021, 493, 229445. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Kluska, J.; Ochnio, M.; Kardaś, D.; Heda, L. The influence of temperature on the physicochemical properties of products of pyrolysis of leather-tannery waste. Waste Manag. 2019, 88, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.X.; Moghaddam, T.B.; Chen, M.Z.; Wu, S.P.; Zhang, Y.; Zhang, X.R.; Adhikari, S.; Zhang, X. Effects of pyrolysis parameters on physicochemical properties of biochar and bio-oil and application in asphalt. Sci. Total Environ. 2021, 780, 146448. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sahoo, S.; Joanni, E.; Singh, R.K.; Maegawa, K.; Tan, W.K.; Kawamura, G.; Kar, K.K.; Matsuda, A. Heteroatom doped graphene engineering for energy storage and conversion. Mater. Today 2020, 39, 47–65. [Google Scholar] [CrossRef]
- Niu, J.; Shao, R.; Liang, J.J.; Dou, M.L.; Li, L.Z.; Huang, Y.Q.; Wang, F. Biomass-derived mesopore-dominant porous carbons with large specific surface area and high defect density as high performance electrode materials for Li-ion batteries and supercapacitors. Nano Energy 2017, 36, 322–330. [Google Scholar] [CrossRef]
- Volperts, A.; Plavniece, A.; Dobele, G.; Zhurinsh, A.; Kruusenberg, I.; Kaare, K.; Locs, J.; Tamasauskaite-Tamasiunaite, L.; Norkus, E. Biomass based activated carbons for fuel cells. Renew. Energy 2019, 141, 40–45. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.Y.; Liu, J.H. Biomass-derived porous carbon materials for advanced lithium sulfur batteries. J. Energy Chem. 2019, 34, 171–185. [Google Scholar] [CrossRef] [Green Version]
- Abioye, A.M.; Ani, F.N. Recent development in the production of activated carbon electrodes from agricultural waste biomass for supercapacitors: A review. Renew. Sustain. Energy Rev. 2015, 52, 1282–1293. [Google Scholar] [CrossRef]
- Wang, M.K.; Cheng, S.; Yao, M.H.; Zhu, Y.Y.; Wu, P.; Luo, H.W.; Yang, L.F.; Tang, L.J.; Liu, M.L. Synthesis of biomass-derived 3D porous graphene-like via direct solid-state transformation and its potential utilization in lithium-ion battery. Ionics (Kiel) 2018, 24, 1879–1886. [Google Scholar] [CrossRef]
- Balčiūnaitė, A.; Budrytė, E.; Vaičiūnienė, J.; Niaura, G.; Kruusenberg, I.; Kaare, K.; Volperts, A.; Dobele, G.; Zurins, A.; Tamašauskaitė-Tamašiūnaitė, L.; et al. One-Pot Synthesis of Nitrogen-Doped Carbon Supported Mn-Co Nanoparticles for Hydrazine Oxidation. ECS Trans. 2020, 97, 593–603. [Google Scholar] [CrossRef]
- Bandosz, T.J.; Ren, T. Porous carbon modified with sulfur in energy related applications. Carbon N. Y. 2017, 118, 561–577. [Google Scholar] [CrossRef]
- Xiong, D.B.; Li, X.F.; Fan, L.L.; Bai, Z.M. Three-dimensional heteroatom-doped nanocarbon for metal-free oxygen reduction electrocatalysis: A review. Catalysts 2018, 8, 301. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, D.D.; Hu, L.B.; Wang, G.; Dai, B.; Yu, F.; Zhang, L.L. A review of biomass-derived graphene and graphene-like carbons for electrochemical energy storage and conversion. New Carbon Mater. 2021, 36, 350–372. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, H.; Luo, X.D.; Sun, C.Y. SiO2-decorated graphite felt electrode by silicic acid etching for iron-chromium redox flow battery. Electrochim. Acta 2020, 336, 135646. [Google Scholar] [CrossRef]
- Dobele, G.; Dizhbite, T.; Gil, M.V.; Volperts, A.; Centeno, T.A. Production of nanoporous carbons from wood processing wastes and their use in supercapacitors and CO2 capture. Biomass Bioenergy 2012, 46, 145–154. [Google Scholar] [CrossRef] [Green Version]
- Volperts, A.; Dobele, G.; Zhurinsh, A.; Vervikishko, D.; Shkolnikov, E.; Ozolinsh, J. Wood-based activated carbons for supercapacitor electrodes with a sulfuric acid electrolyte. Xinxing Tan Cailiao/New Carbon Mater. 2017, 32, 319–326. [Google Scholar] [CrossRef]
- Bansal, R.C.; Goyal, M. Activated Carbon Adsorption; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Abbas, Q.; Raza, R.; Shakir, I.; Olabi, A.G. Heteroatom doped high porosity carbon nanomaterials as electrodes for energy storage in electrochemical capacitors: A review. J. Sci. Adv. Mater. Devices 2019, 4, 341–352. [Google Scholar] [CrossRef]
- Flowers, P.; Neth, E.J.; Robinson, W.R.; Theopold, K.; Langley, R.E. Chemistry: Atoms First, 2nd ed.; OpenStax: Houston, TX, USA, 2019. [Google Scholar]
- Rauls, E.; Gerstmann, U.; Frauenheim, T.; Overhof, H. The different behavior of nitrogen and phosphorus as n-type dopants in SiC. Phys. B Condens. Matter 2003, 340–342, 184–189. [Google Scholar] [CrossRef]
- Bajwa, D.S.; Pourhashem, G.; Ullah, A.H.; Bajwa, S.G. A concise review of current lignin production, applications, products and their environment impact. Ind. Crop. Prod. 2019, 139, 111526. [Google Scholar] [CrossRef]
- Mandeep; Gupta, G.K.; Liu, H.; Shukla, P. Pulp and paper industry–based pollutants, their health hazards and environmental risks. Curr. Opin. Environ. Sci. Health 2019, 12, 48–56. [Google Scholar]
- Haile, A.; Gelebo, G.G.; Tesfaye, T.; Mengie, W.; Mebrate, A.A.; Abuhay, A.; Limeneh, D.Y. Pulp and paper mill wastes: Utilizations and prospects for high value-added biomaterials. Bioresour. Bioprocess. 2021, 8, 35. [Google Scholar] [CrossRef]
- Viel, M.; Collet, F.; Lanos, C. Effect of compaction on multi-physical properties of hemp-black liquor composites. J. Mater. Res. Technol. 2020, 9, 2487–24940. [Google Scholar] [CrossRef]
- Eugenio, M.E.; Ibarra, D.; Martín-Sampedro, R.; Espinosa, E.; Bascón, I.; Rodríguez, A. Alternative Raw Materials for Pulp and Paper Production in the Concept of a Lignocellulosic Biorefinery. In Cellulose; Pascual, A.R., Martin, M.E.E., Eds.; BoD—Books on Demand: London, UK, 2019; p. 78. [Google Scholar]
- Collard, F.-X.; Blin, J. A review on pyrolysis of biomass constituents: Mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew. Sustain. Energy Rev. 2014, 38, 594–608. [Google Scholar] [CrossRef]
- Cheremisinoff, N.P.; Rosenfeld, P. Sources of air emissions from pulp and paper mills. In Handbook of Pollution Prevention and Cleaner Production; Elsevier: Amsterdam, Holland, The Netherlands, 2010; pp. 179–259. [Google Scholar]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Van, K.L.; Thu, T.L.T.; Thu, T.N.H.; Hoang, V.H. Activated Carbon by KOH and NaOH Activation: Preparation and Electrochemical Performance in K2SO4 and Na2SO4 Electrolytes. Russ. J. Electrochem. 2019, 55, 900–907. [Google Scholar]
- Ndifreke, W.E.; Aydinlik, N.P. KOH modified Thevetia peruviana shell activated carbon for sorption of dimethoate from aqueous solution. J. Environ. Sci. Health Part B 2018, 54, 1–13. [Google Scholar] [CrossRef]
- Gunasekaran, S.S.; Elumalali, S.K.; Kumaresan, T.K.; Meganathan, R.; Ashok, A.; Pawar, V.; Vediappan, K.; Ramasamy, G.; Karazhanov, S.Z.; Raman, K.; et al. Partially graphitic nanoporous activated carbon prepared from biomass for supercapacitor application. Mater. Lett. 2018, 218, 165–168. [Google Scholar] [CrossRef]
- Dobele, D.; Vervikishko, D.; Volperts, A.; Bogdanovich, N.; Shkolnikov, E. Characterization of the pore structure of nanoporous activated carbons produced from wood waste. Holzforschung 2013, 67, 587–594. [Google Scholar] [CrossRef]
- Liu, Y.; Li, K.S.; Ge, B.C.; Pu, L.T.; Liu, Z.Q. Influence of Micropore and Mesoporous in Activated Carbon Air-cathode Catalysts on Oxygen Reduction Reaction in Microbial Fuel Cells. Electrochim. Acta 2016, 214, 110–118. [Google Scholar] [CrossRef]
- Xing, Z.; Ju, Z.C.; Zhao, Y.L.; Wan, J.L.; Zhu, Y.B.; Qiang, Y.H.; Qian, Y.T. One-pot hydrothermal synthesis of Nitrogen-doped graphene as high-performance anode materials for lithium ion batteries. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Shruthi, T.K.; Kumar, M.S.; Arjunan, M.; Pratap, A.; Chandrasekaran, N. Graphene oxide aided structural tailoring of 3-D N-doped amorphous carbon network for enhanced energy storage. RSC Adv. 2015, 5, 93423–93432. [Google Scholar] [CrossRef]
- Zhang, X.L.; Yu, D.L.; Zhang, Y.Q.; Guo, W.H.; Ma, X.X.; He, X.Q. Nitrogen- and sulfur-doped carbon nanoplatelets via thermal annealing of alkaline lignin with urea as efficient electrocatalysts for oxygen reduction reaction. RSC Adv. 2020, 6, 104183–104192. [Google Scholar] [CrossRef]
- Zhang, J.M.; He, J.; Zheng, H.Y.; Li, R.; Gou, X.L. N,S dual-doped carbon nanosheet networks with hierarchical porosity derived from biomass of Allium cepa as efficient catalysts for oxygen reduction and Zn–air batteries. J. Mater. Sci. 2020, 55, 7464–7476. [Google Scholar] [CrossRef]
- Wu, X.X.; Tian, Z.W.; Hu, L.Q.; Huang, S.; Cai, J.J. Macroalgae-derived nitrogen-doped hierarchical porous carbons with high performance for H2 storage and supercapacitors. RSC Adv. 2017, 7, 32795–32805. [Google Scholar] [CrossRef] [Green Version]
- Lai, L.F.; Potts, J.R.; Zhan, D.; Wang, L.; Poh, C.K.; Tang, C.H.; Gong, H.; Shen, Z.X.; Lin, J.Y.; Ruoff, R.S. Exploration of the active center structure of nitrogen-doped graphene-based catalysts for oxygen reduction reaction. Energy Environ. Sci. 2012, 5, 7936–7942. [Google Scholar] [CrossRef]
- Guo, D.H.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalyst. Science 2016, 351, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Si, W.Y.; He, J.J.; Sun, L.; Zhang, C.F.; Wang, N.; Yang, Z.; Li, X.D.; Wang, X.; Deng, W.Q.; et al. Selectively nitrogen-doped carbon materials as superior metal-free catalysts for oxygen reduction. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Alsmeyer, C.D.; McCreery, L.R. Raman spectroscopy of carbon materials: Structural basis of observed spectra. Chem. Mater. 2002, 2, 557–563. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095. [Google Scholar] [CrossRef] [Green Version]
- Gadipelli, S.; Guo, Z.X. Graphene-based materials: Synthesis and gas sorption, storage and separation. Prog. Mater. Sci. 2015, 69, 1–60. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Tian, T.; Chen, Y.; Niu, Y.; Tang, J.; Qin, L.C. Synthesis of graphene from dry ice in flames and its application in supercapacitors. Chem. Phys. Lett. 2014, 591, 78–81. [Google Scholar] [CrossRef] [Green Version]
- Qiu, T.; Yang, J.G.; Bai, X.J.; Wang, Y.L. The preparation of synthetic graphite materials with hierarchical pores from lignite by one-step impregnation and their characterization as dye absorbents. RSC Adv. 2019, 9, 12737–12746. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Zhu, Y.M. Structural characteristics of coal vitrinite during pyrolysis. Energy Fuels 2014, 28, 3645–3654. [Google Scholar] [CrossRef]
- Arrebola, J.C.; Caballero, A.; Hernán, L.; Morales, J. Graphitized Carbons of Variable Morphology and Crystallinity: A Comparative Study of Their Performance in Lithium Cells. J. Electrochem. Soc. 2009, 156, A986. [Google Scholar] [CrossRef]
- Aso, H.; Matsuoka, K.; Sharma, A.; Tomita, A. Structural analysis of PVC and PFA carbons prepared at 500–1000 °C based on elemental composition, XRD, and HRTEM. Carbon 2004, 42, 2963–2973. [Google Scholar] [CrossRef]
- Arie, A.A.; Kristianto, H.; Susanti, R.F.; Devianto, H.; Halim, M.; Lee, J.K. Structural and preliminary electrochemical characteristics of palm oil based carbon nanospheres as anode materials in lithium ion batteries. Carbon Lett. 2016, 18, 80–83. [Google Scholar] [CrossRef] [Green Version]
- Yin, P.F.; Deng, Y.; Zhang, L.; Li, N.; Feng, X.; Wang, J.; Zhang, Y. Facile synthesis and microwave absorption investigation of activated carbon@Fe3O4 composites in the low frequency band. RSC Adv. 2018, 8, 23048–23057. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-J.; Pyun, S.-I.; Lee, S.-K.; Kang, S.-J.L. Fundamentals of Rotating Disc and Ring-Disc Electrode Techniques and their Applications to Study of the Oxygen Reduction Mechanism at Pt/C Electrode for Fuel Cells. Isr. J. Chem. 2008, 48, 215–228. [Google Scholar] [CrossRef]
- Kaare, K.; Yu, E.; Käämbre, T.; Volperts, A.; Dobele, G.; Zhurinsh, A.; Niaura, G.; TamasauskaiteTamasiunaite, L.; Norkus, E.; Kruusenberg, I. Biomass-derived Graphene-like Catalyst Material for Oxygen Reduction Reaction. ChemNanoMat 2021, 7, 307–313. [Google Scholar] [CrossRef]
- Lide, D.R. Handbook of Chemistry and Physics, 81st ed.; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Davis, R.E.; Horvath, G.L.; Tobias, C.W. The solubility and diffusion coefficient of oxygen in potassium hydroxide solutions. Electrochim. Acta 1967, 12, 287–297. [Google Scholar] [CrossRef]
- MCMB Mesocarbon Microbeads Graphite Powder Anode Raw Materials For Lithium Battery. Available online: https://www.batterymaking.com/product/mcmb-mesocarbon-microbeads-graphite-powder-anode-raw-materials-for-lithium-battery_p158.html (accessed on 10 August 2021).
- Hansen, S.; Quiroga-González, E.; Carstensen, J.; Föll, H. Size-dependent cyclic voltammetry study of silicon microwire anodes for lithium ion batteries. Electrochim. Acta 2016, 217, 283–291. [Google Scholar] [CrossRef]
- Chang, X.H.; Li, W.; Yang, J.F.; Xu, L.; Zheng, J.; Li, X.G. Direct plasma deposition of amorphous Si/C nanocomposites as high performance anodes for lithium ion batteries. J. Mater. Chem. A 2015, 3, 3522–3528. [Google Scholar] [CrossRef]
- Wang, W.; Sun, Y.; Liu, B.; Wang, S.G.; Cao, M.H. Porous carbon nanofiber webs derived from bacterial cellulose as an anode for high performance lithium ion batteries. Carbon 2015, 91, 56–65. [Google Scholar] [CrossRef]
- Tang, K.; White, R.J.; Mu, X.K.; Titirici, M.M.; Aken, P.V.A.; Maier, J. Hollow carbon nanospheres with a high rate capability for lithium-based batteries. ChemSusChem 2012, 5, 400–403. [Google Scholar] [CrossRef]
- Zhou, H.T.; Wang, X.H.; Chen, D. Li-Metal-Free Prelithiation of Si-Based Negative Electrodes for Full Li-Ion Batteries. ChemSusChem 2015, 8, 2737–2744. [Google Scholar] [CrossRef]
- Yu, K.F.; Li, J.; Qi, H.; Liang, C. High-capacity activated carbon anode material for lithium-ion batteries prepared from rice husk by a facile method. Diam. Relat. Mater. 2018, 86, 139–145. [Google Scholar] [CrossRef]
- Cetinkaya, T.; Ozcan, S.; Uysal, M.; Guler, M.O.; Akbulut, H. Free-standing flexible graphene oxide paper electrode for rechargeable Li–O2 batteries. J. Power Sources 2014, 267, 140–147. [Google Scholar] [CrossRef]
- Dao, T.D.; Hong, J.E.; Ryu, K.S.; Jeong, H.M. Super-tough functionalized graphene paper as a high-capacity anode for lithium ion batteries. Chem. Eng. J. 2014, 250, 257–266. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Yeh, F.-H.; Yu, I.-S. A Commercial Carbonaceous Anode with a-Si Layers by Plasma Enhanced Chemical Vapor Deposition for Lithium Ion Batteries. J. Compos. Sci. 2020, 4, 72. [Google Scholar] [CrossRef]
- Plavniece, A.; Dobele, G.; Volperts, A.; Zhurinsh, A.; Kruusenberg, I. Wood-based nitrogen doped activated carbon for fuel cells. IOP Conf. Ser. Mater. Sci. Eng. 2019, 503, 012001. [Google Scholar] [CrossRef]



| Material | Yield, % * | Ash, % | N, % | C, % | H, % | O, % | S, % |
|---|---|---|---|---|---|---|---|
| AWC | 38.5 | 0.01 | 0.72 | 96.18 | 0.69 | 2.41 | 0.13 |
| AWC-N | 34.7 | 0.01 | 4.06 | 93.99 | 0.71 | 1.24 | 0.12 |
| AWC2BL | 11.5 | 3.04 | 0.82 | 87.66 | 2.96 | 5.24 | 3.32 |
| AWC2BL-N | 10.4 | 3.15 | 4.21 | 92.40 | 1.66 | 1.21 | 0.52 |
| ABL | 6.2 | 6.74 | 0.49 | 70.07 | 2.32 | n.a. | 15.94 |
| ABL-N | 5.6 | 6.94 | 4.75 | 86.29 | 2.30 | n.a. | 3.07 |
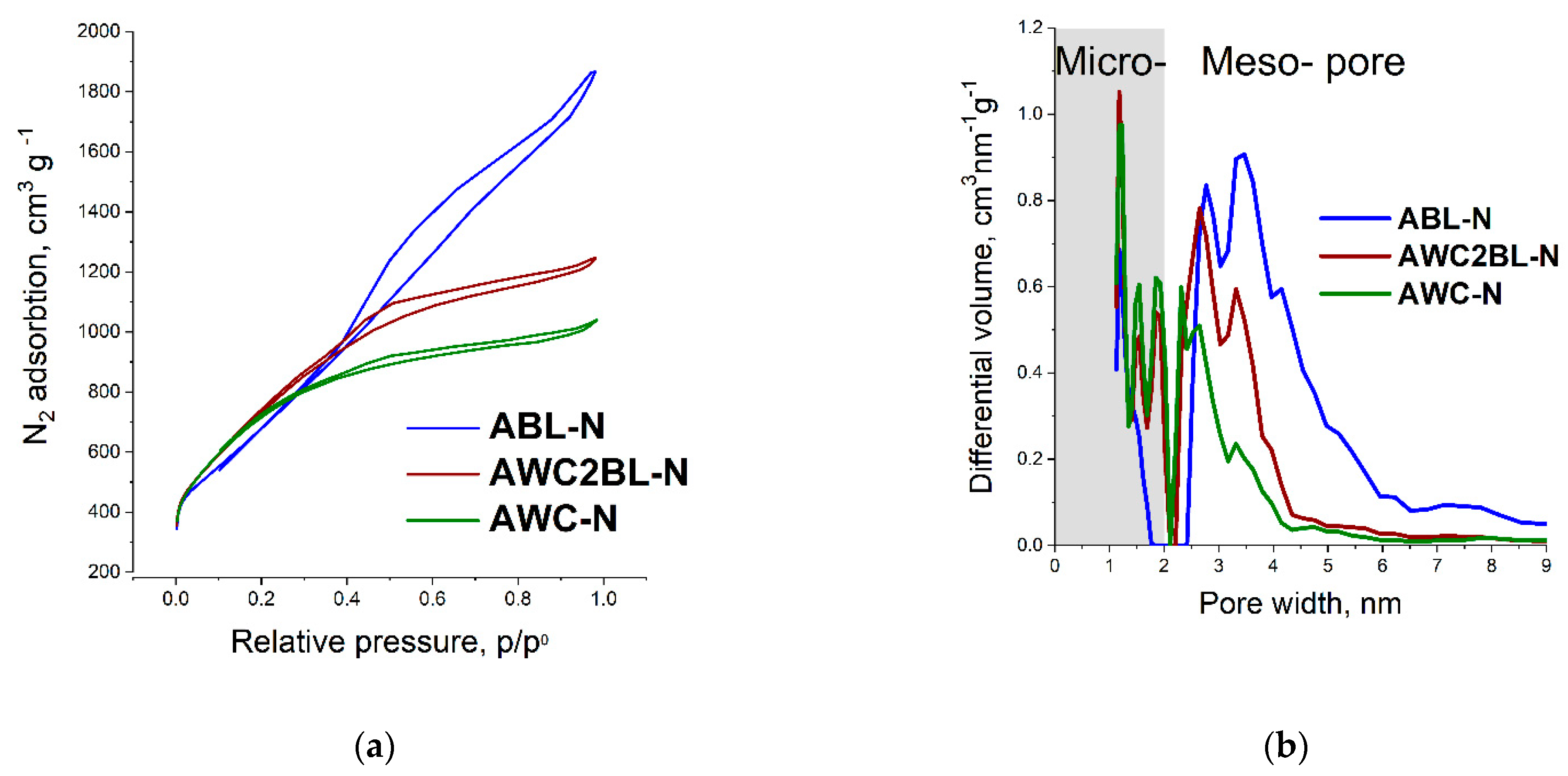
| Material | Specific Surface Area (BET), m2 g−1 | Total Pore Volume, cm3 g−1 | Micropore Volume, cm3 g−1 | Mesopore Volume, cm3 g−1 | Average Pore Width, (nm) |
|---|---|---|---|---|---|
| AWC | 2733 | 1.52 | 0.86 | 0.66 | 2.23 |
| AWC-N | 2631 | 1.60 | 0.82 | 0.79 | 2.44 |
| AWC2BL | 2754 | 1.91 | 0.87 | 1.04 | 2.77 |
| AWC2BL-N | 2690 | 1.92 | 0.84 | 1.08 | 2.87 |
| ABL | 2104 | 2.35 | 0.68 | 1.67 | 4.42 |
| ABL-N | 2481 | 2.88 | 0.82 | 2.06 | 4.66 |
| Electrode Material | Working Potential Range, V | Electrolyte | Gravimetric Capacity, mAh g−1 | Capacity Retention | Ref. |
|---|---|---|---|---|---|
| Graphene oxide paper | 0.01–3.0 | - | 702 at 50 mAg−1 | 73% after 55 cycles | [66] |
| Functionalized graphene paper | 0.01–3.0 | 1.15 M LiPF6 in EC:EMC:DMC (3:2:5) | 450 at 300 mAg−1 | - | [67] |
| Si/C composite | 0.01–1.2 | 1.0 M LiPF6 in EC: PC: DMC (1:1:1) | 455 at 0.1C | 78% after 40 cycles | [68] |
| AWC-N | 0.05–3.0 | LiPF6 in EC: DMC (1:1) | 543 at 0.5 C | 26% after 50 cycles | |
| AWC2BL-N | 0.05–3.0 | LiPF6 in EC: DMC (1:1) | 149 at 0.5 C | 78% after 50 cycles | |
| ABL-N | 0.05–3.0 | LiPF6 in EC: DMC (1:1) | 224 at 0.5 C | 12% after 50 cycles |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plavniece, A.; Volperts, A.; Dobele, G.; Zhurinsh, A.; Kaare, K.; Kruusenberg, I.; Kaprans, K.; Knoks, A.; Kleperis, J. Wood and Black Liquor-Based N-Doped Activated Carbon for Energy Application. Sustainability 2021, 13, 9237. https://doi.org/10.3390/su13169237
Plavniece A, Volperts A, Dobele G, Zhurinsh A, Kaare K, Kruusenberg I, Kaprans K, Knoks A, Kleperis J. Wood and Black Liquor-Based N-Doped Activated Carbon for Energy Application. Sustainability. 2021; 13(16):9237. https://doi.org/10.3390/su13169237
Chicago/Turabian StylePlavniece, Ance, Aleksandrs Volperts, Galina Dobele, Aivars Zhurinsh, Kätlin Kaare, Ivar Kruusenberg, Kaspars Kaprans, Ainars Knoks, and Janis Kleperis. 2021. "Wood and Black Liquor-Based N-Doped Activated Carbon for Energy Application" Sustainability 13, no. 16: 9237. https://doi.org/10.3390/su13169237
APA StylePlavniece, A., Volperts, A., Dobele, G., Zhurinsh, A., Kaare, K., Kruusenberg, I., Kaprans, K., Knoks, A., & Kleperis, J. (2021). Wood and Black Liquor-Based N-Doped Activated Carbon for Energy Application. Sustainability, 13(16), 9237. https://doi.org/10.3390/su13169237