Coagulation-Flocculation of Aquaculture Wastewater Using Green Coagulant from Garcinia kola Seeds: Parametric Studies, Kinetic Modelling and Cost Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Aquaculture Wastewater (AW) Collection
2.2. Preparation of the GKC
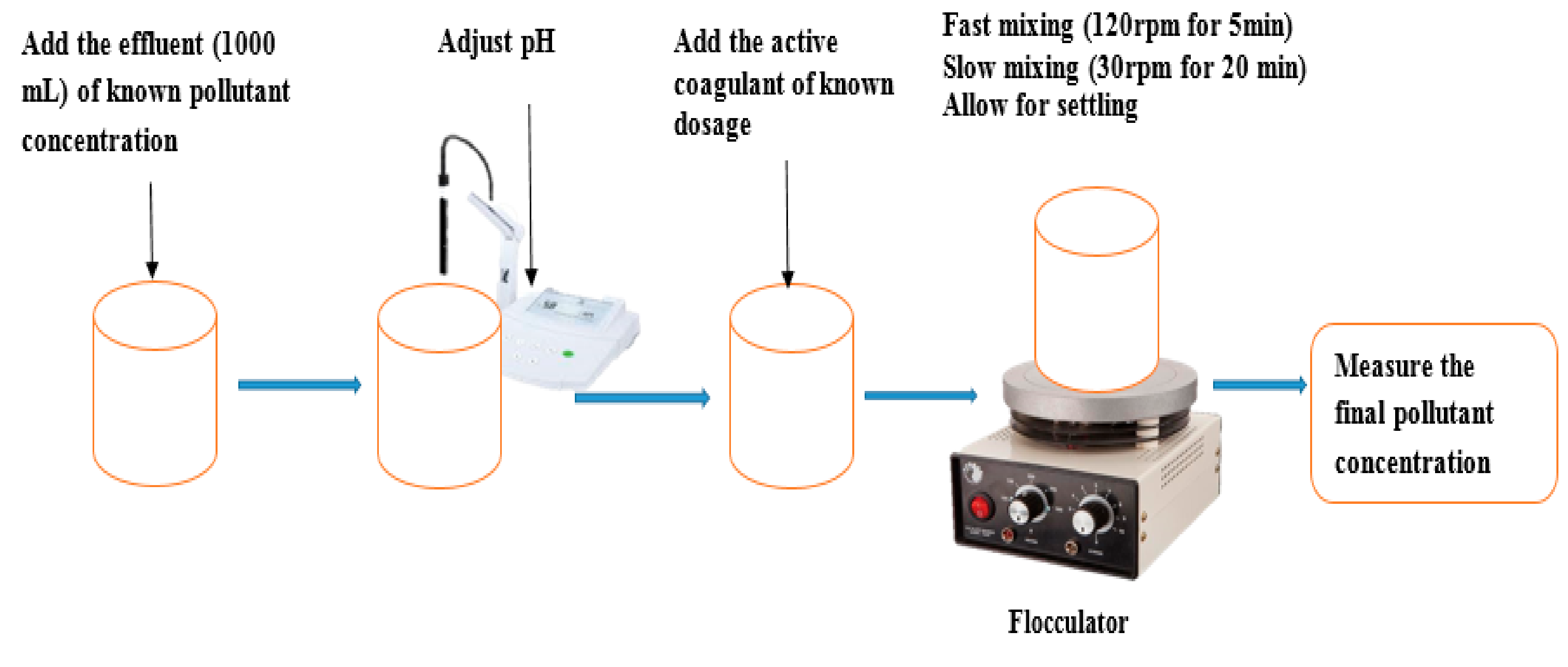
2.3. Coagulation-Flocculation Procedure
2.4. Coagulation-Flocculation Kinetics Study
2.5. Cost Estimation
3. Results
3.1. Physio-Chemical Characteristics of the Garcinia kola Seeds
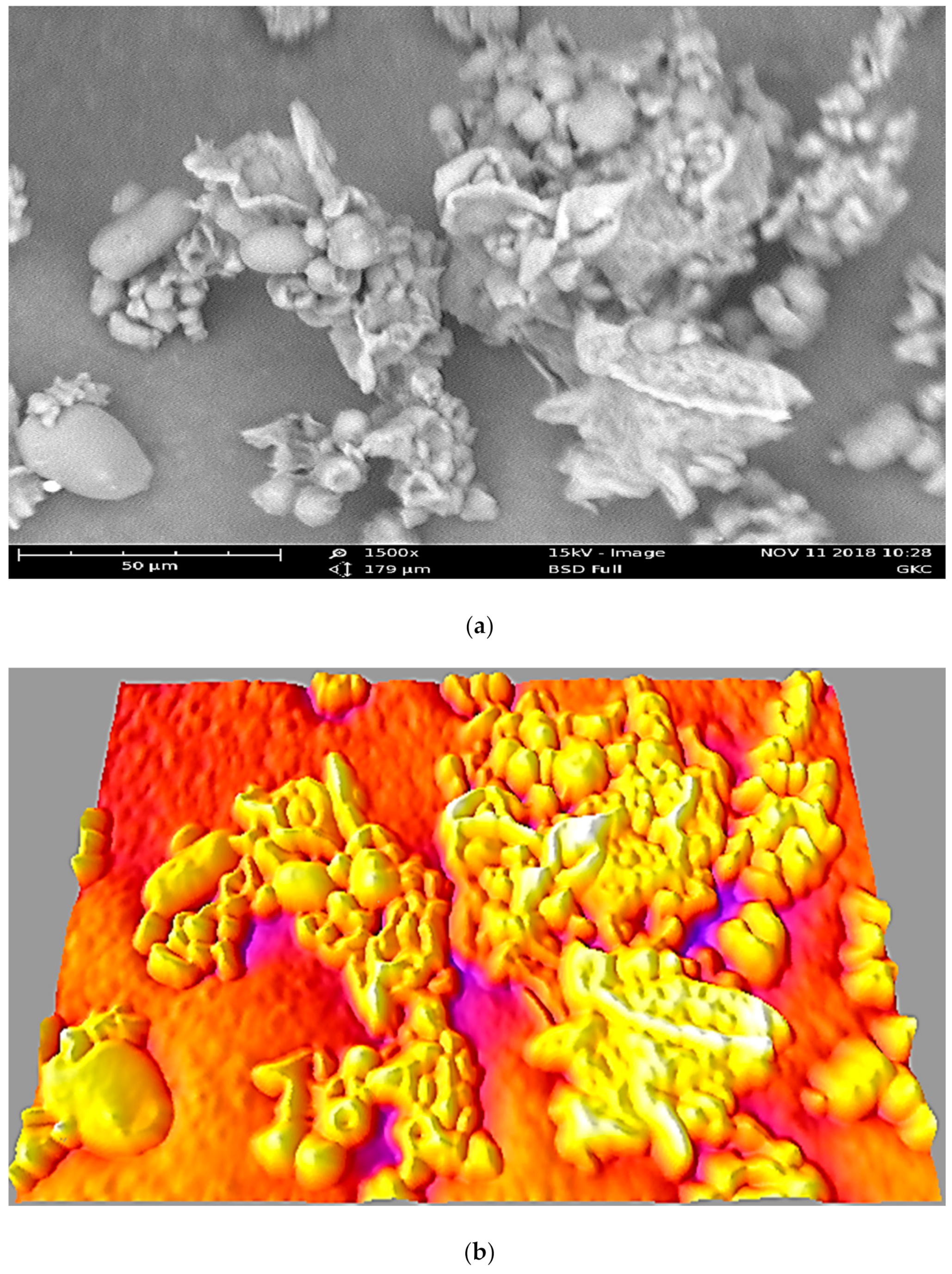
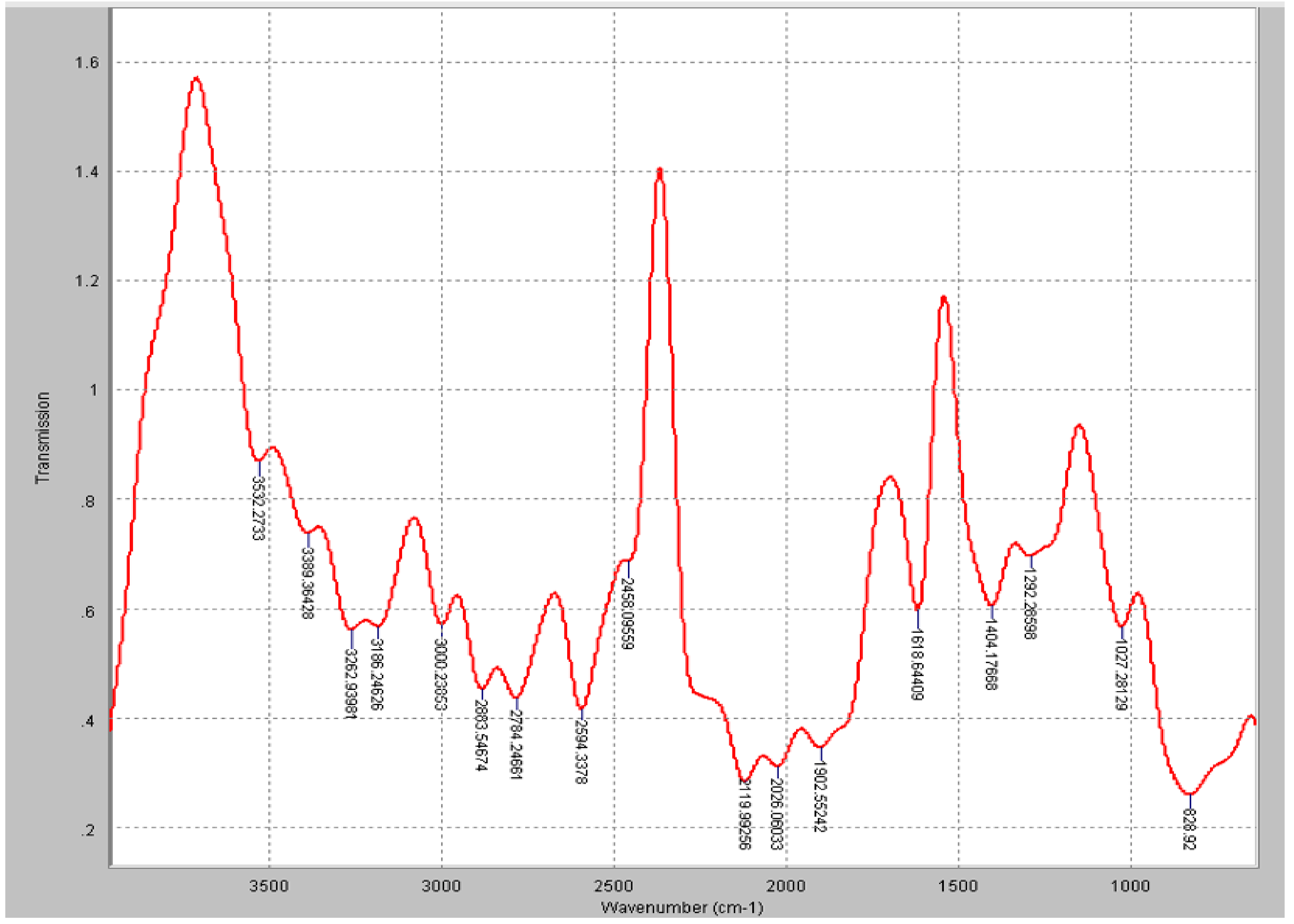
3.2. SEM and FTIR Characterisation of the GKC
3.3. Effect of Process Factors
3.3.1. Effect of GKC Dose
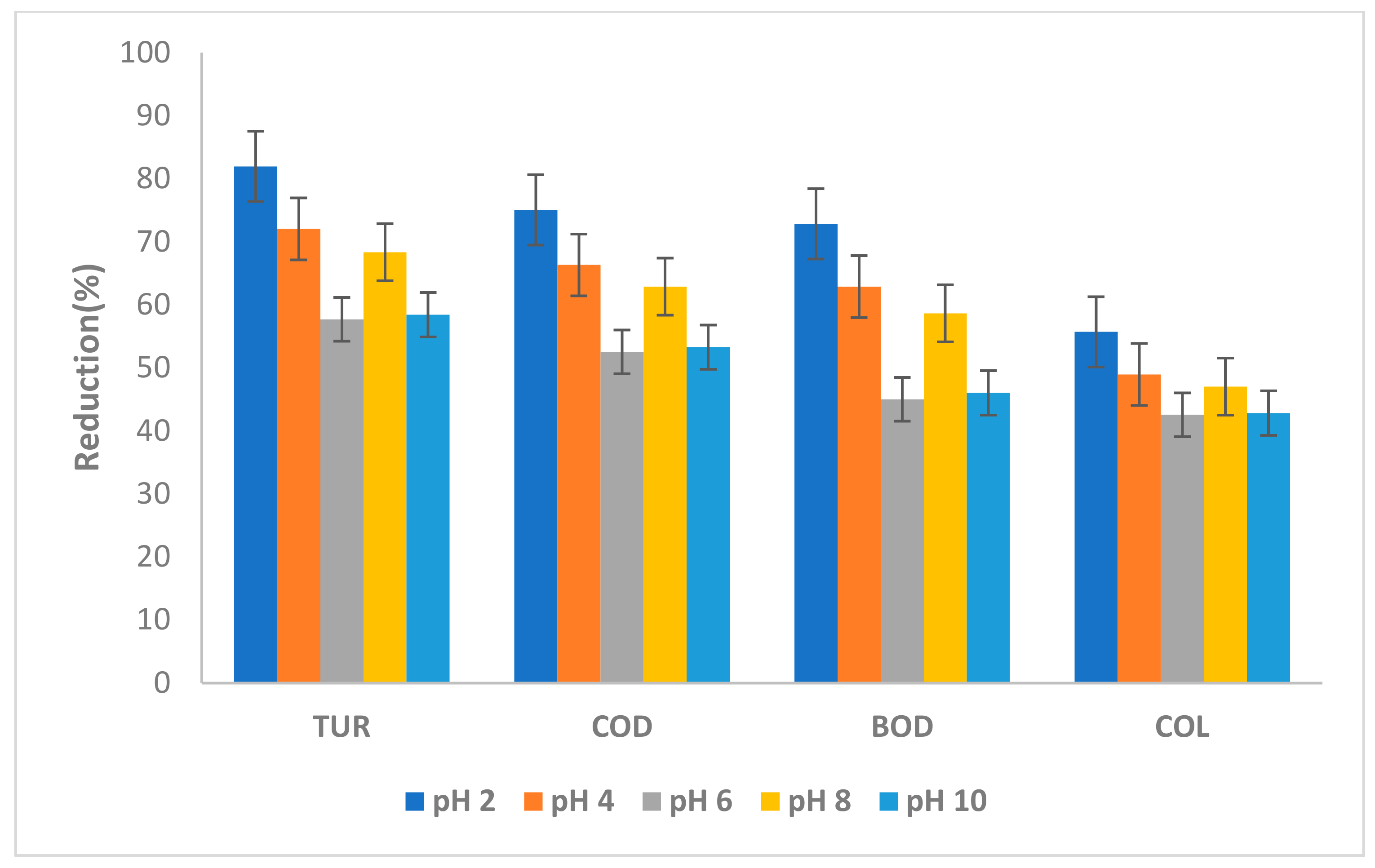
3.3.2. Effect of AW pH
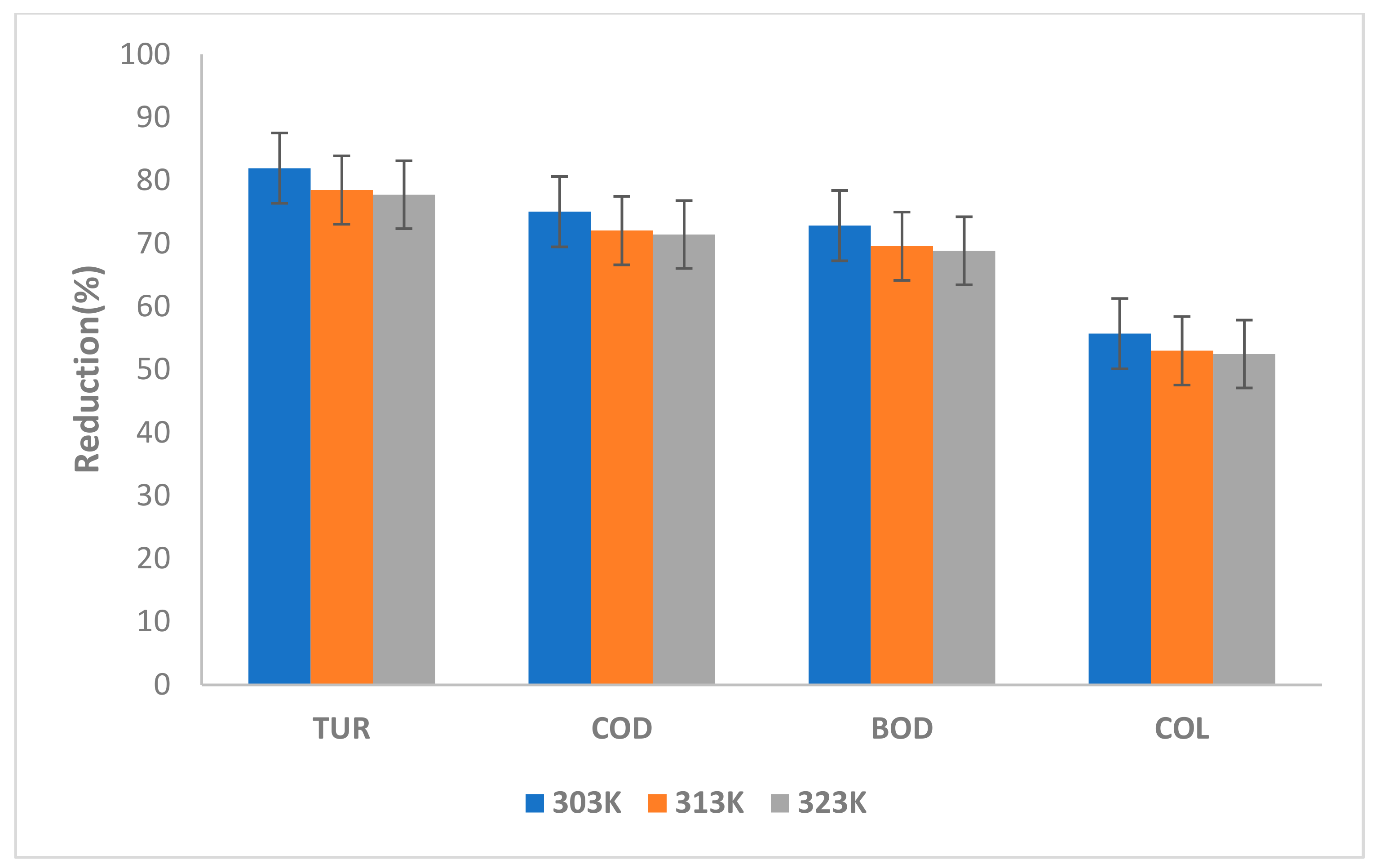
3.3.3. Effect of Temperature on TURB, COD, BOD and COL Reduction
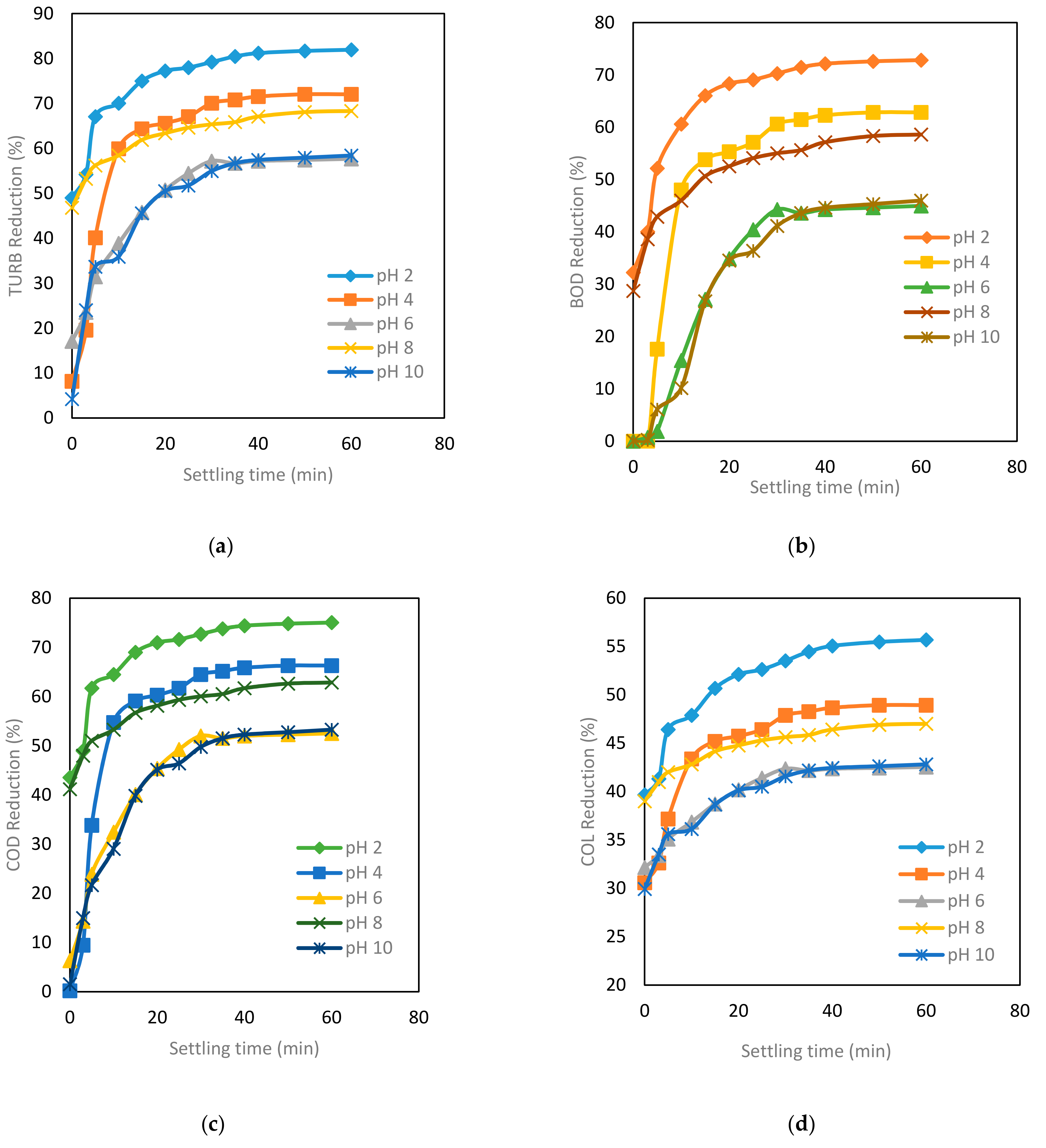
3.3.4. Effect of Settling Time on TURB, COD, BOD and COL Reduction
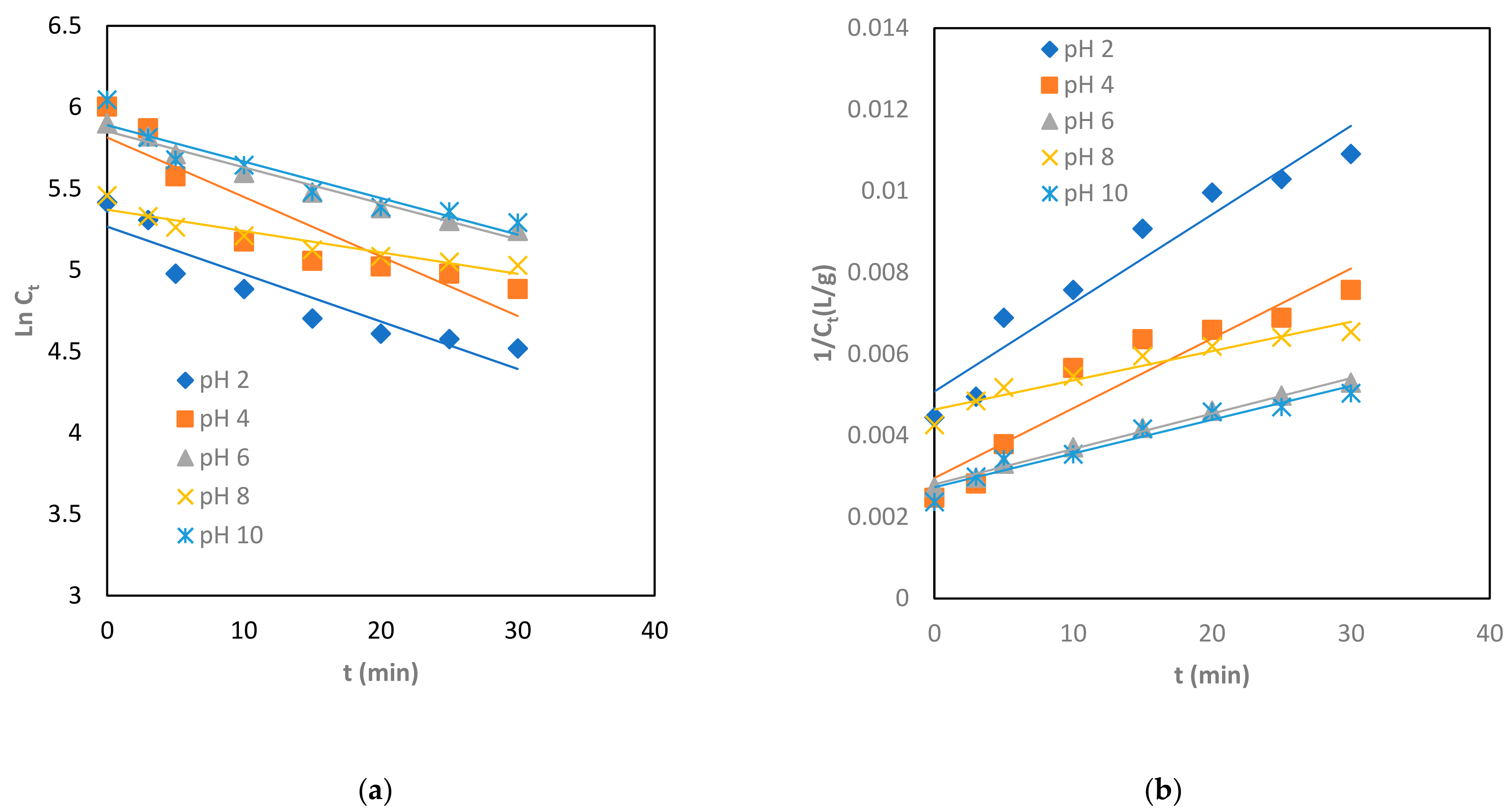
3.4. Brownian Coagulation-Flocculation Kinetics on the Process
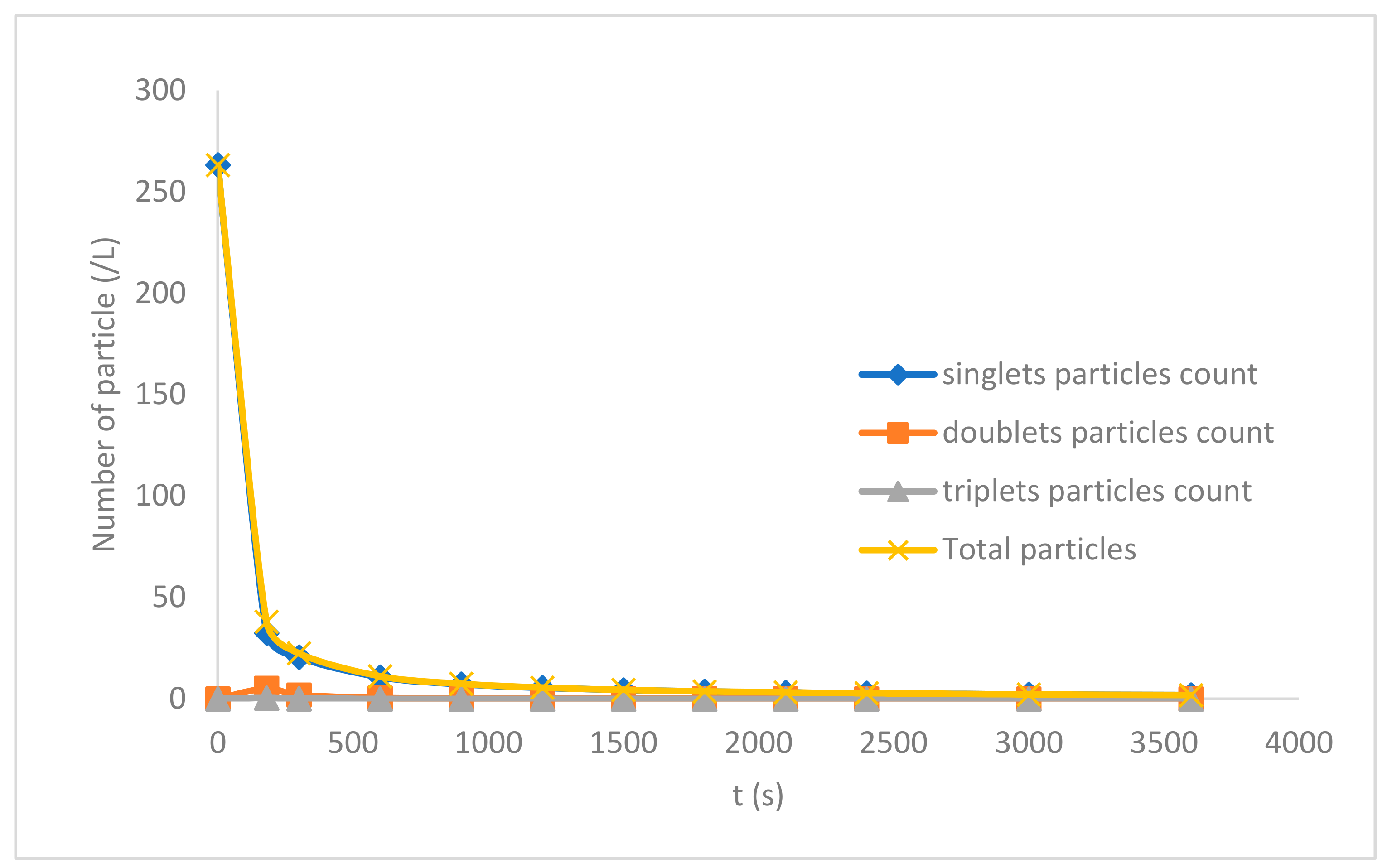
3.5. Particle Distribution Behaviour of the Process
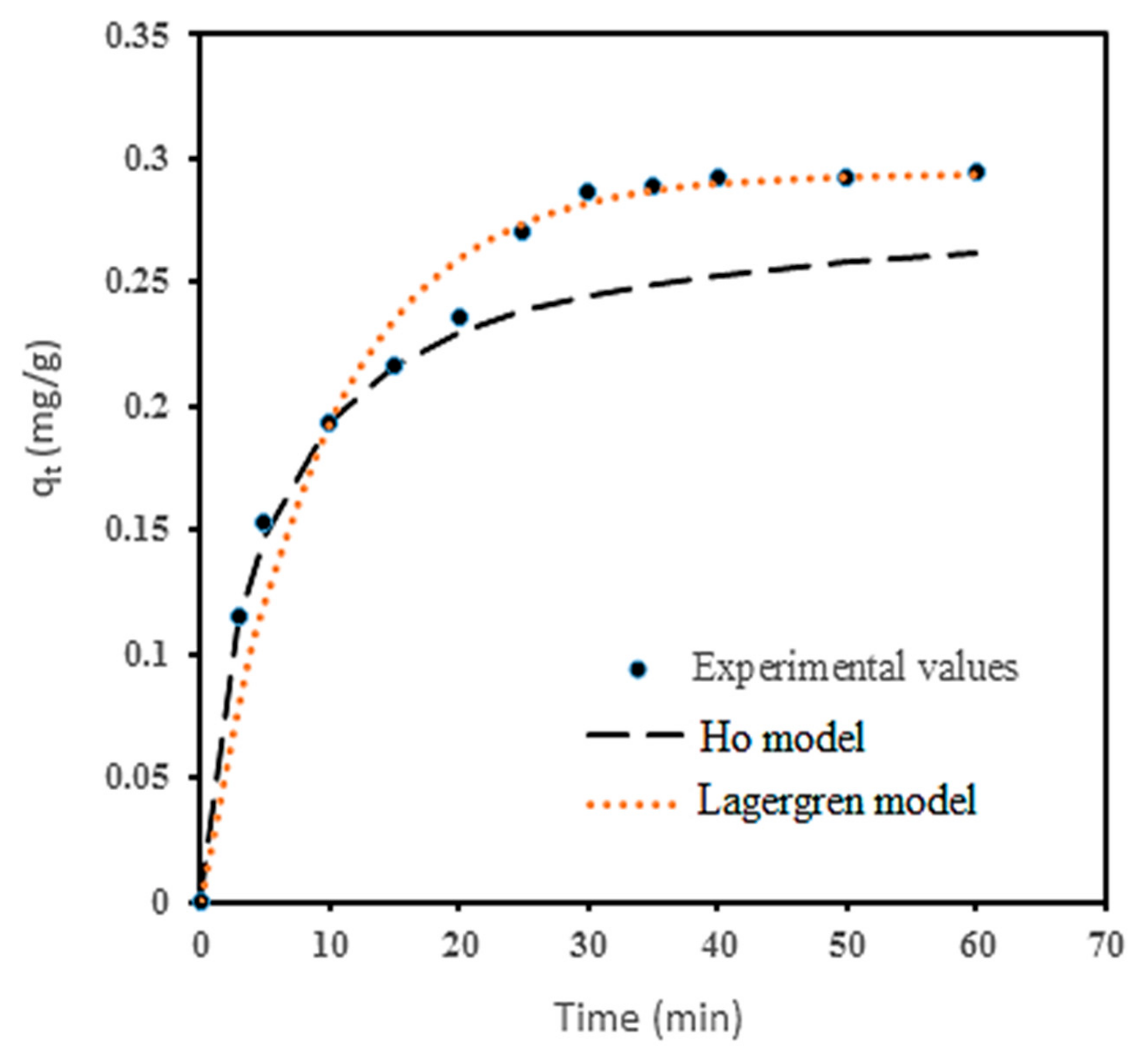
3.6. Coagulation-Adsorption Kinetics Studies
3.7. Cost Analysis on the Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharrer, M.J.; Rishel, K.; Summerfelt, S. Evaluation of geotextile filtration applying coagulant and flocculant amendments for aquaculture biosolids dewatering and phosphorus removal. Aquacult. Eng. 2009, 40, 1–10. [Google Scholar] [CrossRef]
- Ebeling, J.M.; Timmons, M.B. Recirculating Aquaculture; Cayuga Aqua Ventures: Freeville, NY, USA, 2010. [Google Scholar]
- Ermukdakul, T.; Chawaloesphonsiya, N.; Boonchayaanant, B.; Pungrasmi, W.; Painmanakul, P. Different Approaches for the Separation of Suspended Solids in Aquaculture System. J. Water Environ. Technol. 2013, 11, 59–70. [Google Scholar] [CrossRef]
- Walker, S. Scoping Science Assessment of the Impacts of Freshwater Aquaculture on the Canadian Environment; National Water Research Institute, Environment Canada: Burlington, ON, Canada; Saskatoon, SK, Canada, 2003. [Google Scholar]
- Ighalo, J.O.; Igwegbe, C.A.; Aniagor, C.O.; Oba, S.N. A Review of Methods for the Removal of Penicillins from Water. J. Water Process. Eng. 2021, 39, 101886. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Adeniyi, A.G.; Marques, G. Internet of Things for Water Quality Monitoring and Assessment: A Comprehensive Review. In Artificial Intelligence for Sustainable Development: Theory, Practice and Future Applications; Hassanien, A.E., Bhatnagar, R., Darwish, A., Eds.; Springer Nature: Basingstoke, UK, 2021; Volume 912, pp. 245–259. [Google Scholar]
- Ighalo, J.O.; Adeniyi, A.G.; Eletta, A.A.O.; Ojetimi, N.I.; Ajala, O.J. Evaluation of Luffa Cylindrica Fibers in A Biomass Packed Bed for The Treatment of Fish Pond Effluent Before Environmental Release. Sustain. Water Resour. Manag. 2020, 6. [Google Scholar] [CrossRef]
- Sahu, O.; Chaudhari, P. Review on chemical treatment of industrial waste water. J. Appl. Sci. Environ. Manag. 2013, 17, 241–257. [Google Scholar] [CrossRef]
- Kumar, V.; Othman, N.; Asharuddin, S. Applications of natural coagulants to treat wastewater—A review. In Proceedings of the ISCEE 2016 MATEC Web of Conferences, Melaka, Malaysia, 5–6 December 2016. [Google Scholar]
- Adinolfi, M.; Corsaro, M.M.; Lanzetta, R.; Parrilli, M.; Folkard, G.; Grant, W.; Sutherland, J. Composition of the coagulant polysaccharide fraction from Strychnos potatorum seeds. Carbohydr. Res. 1994, 263, 103–110. [Google Scholar] [CrossRef]
- Igwegbe, C.A.; Onukwuli, O.D.; Ighalo, J.O.; Menkiti, M.C. Bio-coagulation-flocculation (BCF) of municipal solid waste leachate using picralima nitida extract: RSM and ANN modelling. Curr. Res. Green Sustain. Chem. 2021, 4, 100078. [Google Scholar] [CrossRef]
- Teh, C.Y.; Wu, T.Y. The potential use of natural coagulants and flocculants in the treatment of urban waters. In Proceedings of the Conference on Process Integration, Modelling and Optimisation for Energy Saving and Pollution Reduction (PRES 2014), Crete, Greece, 20–23 October 2014; pp. 1603–1608. [Google Scholar]
- Olajide, O.J.; Adeniyi, P.A. Studies on effects of aqueous Garcinia kola extract on the lateral geniculate body and rostral colliculus of adult Wistar rats. Med Pract. Rev. 2011, 2, 23–28. [Google Scholar]
- Iwu, M.W.; Duncan, A.R.; Okunji, C.O. New antimicrobials of plant origin. Perspectives on New Crops and New Uses; ASHS Press: Alexandria, VA, USA, 1999; pp. 457–462. [Google Scholar]
- Igwegbe, C.A. Evaluation of Bio- and Electro- Coagulants’ Activities on Fish Pond Wastewater and Solid Waste Leachate. Ph.D. Thesis, Department of Chemical Engineering, Faculty of Engineering, Nnamdi Azikiwe University, Awka, Nigeria, 2019. [Google Scholar]
- Ghebremichael, K.A.; Gunaratna, K.; Henriksson, H.; Brumer, H.; Dalhammar, G. A simple purification and activity assay of the coagulant protein from Moringa oleifera seed. Water Res. 2005, 39, 2338–2344. [Google Scholar] [CrossRef]
- Menkiti, M.C.; Ezemagu, I.G. Sludge characterization and treatment of produced water (PW) using Tympanotonus fuscatus coagulant (TFC). Petroleum 2015, 1, 51–62. [Google Scholar] [CrossRef]
- Collins, T.J. ImageJ for microscopy. BioTechniques 2007, 43, S25–S30. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Adeniyi, A.G.; Igwegbe, C.A. 3D Reconstruction and Morphological Analysis of Electrostimulated Hyperthermophile Biofilms of Thermotoga neapolitana. Biotechnol. Lett. 2021, 43, 1303–1309. [Google Scholar] [CrossRef]
- Nwabanne, J.T.; Obi, C.C. Coagulation-Flocculation Performance of Snail Shell Biomass in Abattoir Wastewater Treatment. J. Chem. Technol. Metall. 2019, 54, 1177–1188. [Google Scholar]
- Mageshkumar, M.; Karthikeyan, R. Modelling the kinetics of coagulation process for tannery industry effluent treatment using Moringa oleifera seeds protein. Desalination Water Treat. 2016, 57, 14954–14964. [Google Scholar] [CrossRef]
- Smoluchowski, M. Versuch einer mathematischen Theorie der Koagulationskinetik kolloider Lösungen. Z. Phys. Chem. 1917, 92, 129–168. [Google Scholar] [CrossRef]
- Igwegbe, C.A.; Onukwuli, O.D.; Ighalo, J.O.; Umembamalu, C.J.; Adeniyi, A.G. Comparative analysis on the electrochemical reduction of colour, COD and turbidity from municipal solid waste leachate using aluminium, iron and hybrid electrodes. Sustain. Water Resour. Manag. 2021, 7, 1–18. [Google Scholar] [CrossRef]
- Menkiti, M. Studies on Coagulation and Flocculation of Coal Washery Effluent: Turbid Metric Approach. Master’s Thesis, Nnamdi Azikiwe University, Awka, Nigeria, 2007. [Google Scholar]
- Ugonabo, V.I.; Emembolu, L.N.; Igwegbe, C.A. Bio-Coag-Flocculation of Refined Petroleum Wastewater using Plant Extract: A Turbidimeric Approach. Int. J. Emerg. Eng. Res. Technol. 2016, 4, 19–26. [Google Scholar]
- Emembolu, L.N.; Igwegbe, C.A.; Ugonabo, V.I. Effect of Natural Biomass Treatment on Vegetable Oil Industry Effluent via Coag-Flocculation. Saudi J. Eng. Technol. 2016, 1, 172–179. [Google Scholar]
- Igwegbe, C.A.; Onukwuli, O.D.; Ighalo, J.O.; Umembamalu, C.J. Electrocoagulation-flocculation of aquaculture effluent using hybrid iron and aluminium electrodes: A comparative study. Chem. Eng. J. Adv. 2021, 6, 100107. [Google Scholar] [CrossRef]
- Ugonabo, V.I.; Emembolu, L.N.; Igwegbe, C.A.; Olaitan, S.A. Optimal evaluation of coag-flocculation factors for refined petroleum wastewater using plant extract. In International Conference 2016 Proceedings; Faculty of Engineering, Nnamdi Azikiwe University: Awka, Nigeria, 2016; pp. 548–557. [Google Scholar]
- Menkiti, M.C.; Ejimofor, M.I. Experimental and artificial neural network application on the optimization of paint effluent (PE) coagulation using novel Achatinoidea shell extract (ASE). J. Water Process. Eng. 2016, 10, 172–187. [Google Scholar] [CrossRef]
- Okey-Onyesolu, C.; Onukwuli, O.; Ejimofor, M.; Okoye, C. Kinetics and mechanistic analysis of particles decontamination from abattoir wastewater (ABW) using novel Fish Bone Chito-protein (FBC). Heliyon 2020, 6, e04468. [Google Scholar] [CrossRef]
- Ahmadi, S.; Mohammadi, L.; Rahdar, A.; Rahdar, S.; Dehghani, R.; Igwegbe, C.A.; Kyzas, G.Z. Acid Dye Removal from Aqueous Solution by Using Neodymium (III) Oxide Nanoadsorbents. Nanomaterials 2020, 10, 556–582. [Google Scholar] [CrossRef]
- Bouaouine, O.; Bourven, I.; Khalil, F.; Baudu, M. Identification of functional groups of Opuntia ficus-indica involved in coagulation process after its active part extraction. Environ. Sci. Pollut. Res. 2018, 25, 11111–11119. [Google Scholar] [CrossRef] [PubMed]
- Igwegbe, C.A.; Onukwuli, O.D. Removal of Total Dissolved Solids (TDS) from Aquaculture Wastewater by Coagulation-flocculation Process using Sesamum indicum extract: Effect of Operating Parameters and Coagulation-Flocculation kinetics. Pharm. Chem. J. 2019, 6, 32–45. [Google Scholar]
- Coruh, H.A. Use of Calcium Alginate as a Coagulant in Water treatment. Master’s Thesis, Middle East Technical University, Ankara, Turkey, 2005. [Google Scholar]
- AOAC. Official Method of Analysis. Method 935.14, 942.05 and 992.24, 18th ed.; AOAC: Washington, DC, USA, 2005. [Google Scholar]
- AOAC. Official Methods of Analysis, 14th ed.; Association of Official Analytical Chemists International Publisher: Arlington, VA, USA, 1984. [Google Scholar]
- AOAC. Total, Soluble, and Insoluble Dietary Fiber in Foods: Enzymatic Gravimetric Method, MES-TRIS Buffer, 17th ed.; Association of Official Analytical Chemists International Publisher: Arlington, VA, USA, 2000. [Google Scholar]
- AOAC. Determination of Protein Content in Food, Method 945.18-B.; AOAC International Publisher: Gaithersburg, MD, USA, 2005. [Google Scholar]
- AOAC. Oil in Cereal Adjuncts: Petroleum Ether Extraction Method, 17th ed.; Association of Official Analytical Chemists International Publisher: Arlington, VA, USA, 2000. [Google Scholar]
- AACC. AACC Method 44-15A. Approved Methods of the American Association for Clinical Chemistry (AACC), 10th ed.; AACC: Washington, DC, USA, 2000. [Google Scholar]
- Li, G.; Huang, Y.; Lin, J.; Yu, C.; Liu, Z.; Fang, Y.; Xue, Y.; Tang, C. Effective capture and reversible storage of iodine using foam-like adsorbents consisting of porous boron nitride microfibers. Chem. Eng. J. 2020, 382, 122833. [Google Scholar] [CrossRef]
- Sharma, B.; Dhuldhoya, N.; Merchant, U. Flocculants—An ecofriendly approach. J. Polym. Environ. 2006, 14, 195–202. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Adeniyi, A.G. A Mini-Review of the Morphological Properties of Biosorbents Derived from Plant Leaves. SN Appl. Sci. 2020, 2, 509. [Google Scholar] [CrossRef]
- Zhang, Z.; Xia, S.; Zhao, J.; Zhang, J. Characterization and flocculation mechanism of high efficiency microbial flocculant TJ-F1 from Proteus mirabilis. Colloids Surf. B. Biointerfaces 2010, 75, 247–251. [Google Scholar] [CrossRef]
- Assaad, E.; Azzouz, A.; Nistor, D.; Ursu, A.; Sajin, T.; Miron, D.; Monette, F.; Niquette, P.; Hausler, R. Metal removal through synergic coagulation–flocculation using an optimized chitosan–montmorillonite system. Appl. Clay Sci. 2007, 37, 258–274. [Google Scholar] [CrossRef]
- Zhang, D.; Crini, G.; Lichtfouse, E.; Rhimi, B.; Wang, C. Removal of Mercury Ions from Aqueous Solutions by Crosslinked Chitosan-based Adsorbents: A Mini Review. Chem. Rec. 2020, 20, 1220–1234. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Rahdar, A.; Igwegbe, C.A.; Mortazavi-Derazkola, S.; Banach, A.M.; Rahdar, S.; Singh, A.K.; Rodriguez-Couto, S.; Kyzas, G.Z. Praseodymium-doped cadmium tungstate (CdWO4) nanoparticles for dye degradation with sonocatalytic process. Polyhedron 2020, 190, 114792. [Google Scholar] [CrossRef]
- Igwegbe, C.A.; Onukwuli, O.D.; Onyechi, K.K.; Ahmadi, S. Equilibrium and Kinetics Analysis on Vat Yellow 4 Uptake from Aqueous Environment by Modified Rubber Seed Shells: Nonlinear modelling. J. Mater. Environ. Sci. 2020, 11, 1424–1444. [Google Scholar]
- Nharingo, T.; Zivurawa, M.; Guyo, U. Exploring the use of cactus Opuntia ficus indica in the biocoagulation–flocculation of Pb (II) ions from wastewaters. Int. J. Environ. Sci. Technol. 2015, 12, 3791–3802. [Google Scholar] [CrossRef]
- Ejimofor, M.I.; Menkiti, M.C.; Ezemagu, I.G. Comparative Studies on Removal of Turbid-Metric Particles (Tdsp) Using Animal Based Chito-Protein And Aluminium Sulfate On Paint Wastewater (Pww). Sigma J. Eng. Nat. Sci./Mühendislik Fen Bilimleri Dergisi 2020, 38, 1143–1159. [Google Scholar]
- Bhandari, V.M.; Ranade, V.V. Advanced physico-chemical methods of treatment for industrial wastewaters. Ind. Wastewater Treat. Recycl. Reuse 2014, 81–140. [Google Scholar] [CrossRef]
- Loganathan, P.; Gradzielski, M.; Bustamante, H.; Vigneswaran, S. Progress, challenges, and opportunities in enhancing NOM flocculation using chemically modified chitosan: A review towards future development. Environ. Sci. Water Res. Technol. 2020, 6, 45–61. [Google Scholar] [CrossRef]
- Fedala, N.; Lounici, H.; Drouiche, N.; Mameri, N.; Drouiche, M. physical parameters affecting coagulation of turbid water with Opuntia ficus-indica cactus. Ecol. Eng. 2015, 77, 33–36. [Google Scholar] [CrossRef]
- Cruz, D.; Pimentel, M.; Russo, A.; Cabral, W. Charge neutralization mechanism efficiency in water with high color turbidity ratio using aluminium sulfate and flocculation index. Water 2020, 12, 572. [Google Scholar] [CrossRef]
- Obiora-Okafo, I.; Onukwuli, O.; Eli-Chukwu, N. Evaluation of bio-coagulants for colour removal from dye synthetic wastewater: Characterization, adsorption kinetics, and modelling approach. Water SA 2020, 46, 300–312. [Google Scholar]
- Teh, C.Y.; Wu, T.Y.; Juan, J.C. Potential use of rice starch in coagulation–flocculation process of agro-industrial wastewater: Treatment performance and flocs characterization. Ecol. Eng. 2014, 71, 509–519. [Google Scholar] [CrossRef]
- Shak, K.P.Y.; Wu, T.Y. Coagulation–flocculation treatment of high-strength agro-industrial wastewater using natural Cassia obtusifolia seed gum: Treatment efficiencies and flocs characterization. Chem. Eng. J. 2014, 256, 293–305. [Google Scholar] [CrossRef]
- Zuki, N.M.; Ismail, N.; Omar, F.M. Evaluation of zeta potential and particle size measurements of multiple coagulants in semiconductor wastewater. In Proceedings of the 6th International Conference on Environment (ICENV2018), Penang, Malaysia, 11–13 December 2018; p. 020036. [Google Scholar]
- Zhou, Z.; Yang, Y.; Li, X.; Wang, W.; Wu, Y.; Wang, C.; Luo, J. Coagulation performance and flocs characteristics of recycling pre-sonicated condensate sludge for low-turbidity surface water treatment. Sep. Purif. Technol. 2014, 123, 1–8. [Google Scholar] [CrossRef]
- Phalakornkule, C.; Mangmeemak, J.; Intrachod, K.; Nuntakumjorn, B. Pretreatment of palm oil mill effluent by electrocoagulation and coagulation. Sci. Asia 2010, 36, 142–149. [Google Scholar] [CrossRef]
- Marriott, N.G.; Robertson, G. Essentials of Food Sanitation; Springer Science & Business Media: Berlin, Germany, 1997. [Google Scholar]
- Bhatia, S.; Othman, Z.; Ahmad, A.L. Pretreatment of palm oil mill effluent (POME) using Moringa oleifera seeds as natural coagulant. J. Hazard. Mater. 2007, 145, 120–126. [Google Scholar] [CrossRef]
- Ugonabo, I.V.; Onukwuli, O.; Ezechukwu, C. Deturbidization of Pharmaceutical Industry Wastewater Using Natural Coagulant: Response Surface Methodology Applied. Int. J. Progress. Sci. Technol. 2020, 22, 258–267. [Google Scholar]
- Okolo, B.I.; Nnaji, P.C.; Menkiti, M.C.; Ugonabo, V.I.; Onukwuli, O.D. Application of single angle turbidimetry on coag-flocculation effect of Detarium microcarpum seed in brewery effluent. Mater. Sci. Appl. 2014, 5. [Google Scholar] [CrossRef][Green Version]
- Hunter, R. Introduction to Modern Colloid Science; Oxford University Press: Oxford, UK, 1993. [Google Scholar]
- De Oliveira Reis, G. Study of the Mechanism of Acid Coagulation of Hevea Latex and of the Rheological Properties of Resulting Gels. Ph.D. Thesis, Université de Montpellier, Montpellier, France, 2015. [Google Scholar]
- Ifeanyi, U.V.; Chukwudi, M.M.; Okechukwu, O.D. Effect of coag-flocculation kinetics on telfairia occidentalis seed coagulant (TOC) in pharmaceutical wastewater. Int. J. Multidiscip. Sci. Eng. 2012, 3, 22–33. [Google Scholar]
- Park, S.; Kruis, F.; Lee, K.; Fissan, H. Evolution of particle size distributions due to turbulent and Brownian coagulation. Aerosol. Sci. Technol. 2002, 36, 419–432. [Google Scholar] [CrossRef]
- Balarak, D.; Zafariyan, M.; Igwegbe, C.A.; Onyechi, K.K.; Ighalo, J.O. Adsorption of Acid Blue 92 Dye from Aqueous Solutions by Single-Walled Carbon Nanotubes: Isothermal, Kinetic, and Thermodynamic Studies. Environ. Process. 2021, 8, 869–888. [Google Scholar] [CrossRef]
- Iwuozor, K.O.; Ighalo, J.O.; Ogunfowora, L.A.; Adeniyi, A.G.; Igwegbe, C.A. An Empirical Literature Analysis of Adsorbent Performance for Methylene Blue Uptake from Aqueous Media. J. Environ. Chem. Eng. 2021, 9, 105658. [Google Scholar] [CrossRef]
- Beltrán-Heredia, J.; Sánchez-Martín, J.; Gómez-Muñoz, C. Performance and characterization of a new tannin-based coagulant. Appl. Water Sci. 2012, 2, 199–208. [Google Scholar] [CrossRef]
- Miller, S.M.; Fugate, E.J.; Craver, V.O.; Smith, J.A.; Zimmerman, J.B. Toward understanding the efficacy and mechanism of Opuntia spp. as a natural coagulant for potential application in water treatment. Environ. Sci. Technol. 2008, 42, 4274–4279. [Google Scholar] [CrossRef]
- Lagergren, S.; Svenska, B.K. On the theory of so-called adsorption of dissolved substances. R. Swed. Acad. Sci. Doc. Band 1898, 24, 1–13. [Google Scholar]
- Oba, S.N.; Ighalo, J.O.; Aniagor, C.O.; Igwegbe, C.A. Removal of ibuprofen from aqueous media by adsorption: A comprehensive review. Sci. Total Environ. 2021, 780, 146608. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Aniagor, C.O.; Igwegbe, C.A.; Ighalo, J.O.; Oba, S.N. Adsorption of doxycycline from aqueous media: A review. J. Mol. Liq. 2021, 334, 116124. [Google Scholar] [CrossRef]
- Mohamed, L.A.; Aniagor, C.O.; Hashem, A. Isotherms and kinetic modelling of mycoremediation of hexavalent chromium contaminated wastewater. Clean. Eng. Technol. 2021, 4, 100192. [Google Scholar] [CrossRef]
- Igwegbe, C.A.; Ighalo, J.O.; Onyechi, K.K.; Onukwuli, O.D. Adsorption of Congo red and malachite green using H3PO4 and NaCl-modified activated carbon from rubber (Hevea brasiliensis) seed shells. Sustain. Water Resour. Manag. 2021, 7, 63. [Google Scholar] [CrossRef]
- Ahmadi, S.; Igwegbe, C.A. Kinetic Studies on penicillin G removal from aqueous environments by cupric oxide nanoparticles. Arch. Hyg. Sci. 2021, 10, 86–96. [Google Scholar]
- Igwegbe, C.A.; Ighalo, J.O.; Ghosh, S.; Ahmadi, S.; Ugonabo, V.I. Pistachio (Pistacia vera) Waste as Adsorbent for Wastewater Treatment: A Review. Biomass Convers. Biorefinery 2021, 1–18. [Google Scholar] [CrossRef]
- Hevira, L.; Ighalo, J.O.; Aziz, H.; Zein, R. Terminalia catappa shell as low-cost biosorbent for the removal of methylene blue from aqueous solutions. J. Ind. Eng. Chem. 2021, 97, 188–199. [Google Scholar] [CrossRef]


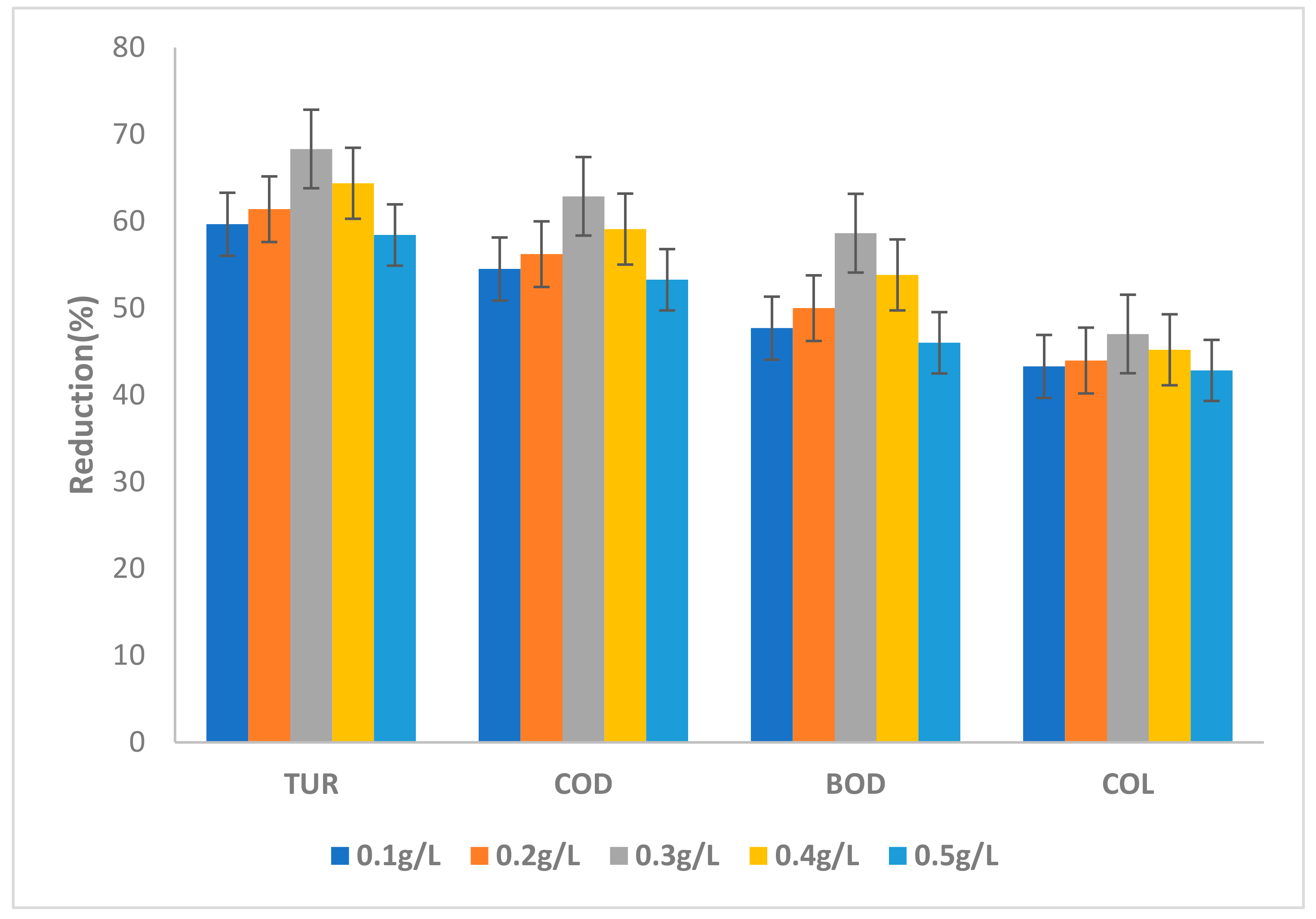
| Parameter | Value |
|---|---|
| Absorbance at 275 nm | 0.842 |
| Ammoniacal nitrogen | 0.829 mg/L |
| Appearance | Yellowish green |
| Biochemical oxygen demand (BOD5) | 317 mg/L |
| Biodegradability index (BI) | 0.42 |
| Calcium | 5.40 mg/L |
| Chemical oxygen demand (COD) | 758 mg/L |
| Chloride | 2.44 mg/L |
| Dissolved oxygen | 9.6 mg/L |
| Conductivity | 1963 µS/cm |
| Iron | 0.425 mg/L |
| Nitrogen | 0.829 mg/L |
| pH | 7.9 |
| Potassium | 5.45 mg/L |
| Temperature | 303.2 K |
| Total dissolved solids | 650 mg/L |
| Total phosphorus | 0.91 mg/L |
| Total solids | 695 mg/L |
| Total suspended solids | 45 mg/L |
| TURB | 404 NTU |
| Parameters | Unit | Values % | Procedure |
|---|---|---|---|
| Ash content | % | 4.147 | AOAC method 942.05 [35] |
| Bulk density | g/mL | 0.22 | AOAC method PA 105 [36] |
| Carbohydrate | % | 68.33 | FAO [11] |
| Fibre | % | 3.940 | AOAC method 978.10 [37] |
| Protein | % | 11.27 | AOAC method 945.18-B [38] |
| Fat | % | 3.030 | AOAC method 920.39 [39] |
| Moisture | % | 9.280 | AACC method 44-15A [40] |
| Yield of coagulant | % | 80.46 | Menkiti and Ezemagu [17] |
| Factor | pH 2 | pH 4 | pH 6 | pH 8 | pH 10 |
|---|---|---|---|---|---|
| R2 | 0.8679 | 0.8321 | 0.973 | 0.8991 | 0.8933 |
| KC (L/mg min) | 0.0291 | 0.0365 | 0.0221 | 0.0131 | 0.0224 |
| C0 (mg/L) | 193.78 | 334.82 | 348.14 | 214.99 | 361.41 |
| rp (mg/min) | −0.0291Ct2 | −0.0365Ct2 | −0.0221Ct2 | −0.0131Ct2 | −0.0224Ct2 |
| Factor | pH 2 | pH 4 | pH 6 | pH 8 | pH 10 |
|---|---|---|---|---|---|
| R2 | 0.9292 | 0.8975 | 0.9931 | 0.9313 | 0.9457 |
| KC (L/mg min) | 0.0002 | 0.0002 | 0.0001 | 0.0001 | 0.0001 |
| C0 (mg/L) | 196.08 | 333.33 | 357.14 | 217.39 | 217.39 |
| rp (mg/min) | −0.0002Ct2 | −0.0002Ct2 | −0.00009Ct2 | −0.00007Ct2 | −0.00007Ct2 |
| (min) | 51 | 30 | 62.222 | 131.43 | 131.43 |
| (min) | 102 | 60 | 124.44 | 262.86 | 262.86 |
| ΒF (L/ mg min) | 0.0004 | 0.0004 | 0.0002 | 0.0002 | 0.0001 |
| KC (L/min) | |||||
| (L/mg) | |||||
| D1 | 0.0082 | 0.0082 | 0.0037 | 0.0029 | 0.0003 |
| Time (s) | Singlet Particles Count | Doublet Particles Count | Triplet Particles Count | Total Particles |
|---|---|---|---|---|
| 0 | 263.16 | 0 | 0 | 263.16 |
| 180 | 32.468 | 5.1981 | 0.4332 | 38.099 |
| 300 | 20.492 | 1.8754 | 0.1146 | 22.482 |
| 600 | 10.661 | 0.4691 | 0.0168 | 11.147 |
| 900 | 7.2046 | 0.2085 | 0.0053 | 7.4184 |
| 1200 | 5.4407 | 0.1173 | 0.0023 | 5.5603 |
| 1500 | 4.3706 | 0.0751 | 0.0012 | 4.4469 |
| 1800 | 3.6523 | 0.0521 | 0.0007 | 3.7051 |
| 2100 | 3.1368 | 0.0383 | 0.0004 | 3.1755 |
| 2400 | 2.7488 | 0.0293 | 0.0003 | 2.7784 |
| 3000 | 2.2036 | 0.0188 | 0.0002 | 2.2225 |
| 3600 | 1.8389 | 0.0130 | 0.0001 | 1.8520 |
| Model | Parameter | Value | Unit |
|---|---|---|---|
| Lagergren | qe | 0.2938 | mg/g |
| K1 | 0.1075 | min−1 | |
| h0 | 0.0316 | mg/g/min | |
| R2 | 0.9987 | - | |
| X2 | 0.0905 | - | |
| MPSD | 5.8045 | - | |
| ARE | 0.2793 | - | |
| SAE | 0.0905 | - | |
| Δqe(%) | 5.5196 | - | |
| SSE | 0.0082 | - | |
| Ho | qe | 0.2818 | mg/g |
| K2 | 0.7774 | g/mg/min | |
| h0 | 0.0617 | mg/g/min | |
| R2 | 0.9856 | - | |
| X2 | 0.2968 | - | |
| MPSD | 19.040 | - | |
| ARE | 0.9161 | - | |
| SAE | 0.2968 | - | |
| Δqe(%) | 9.9967 | - | |
| SSE | 0.0881 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Igwegbe, C.A.; Ighalo, J.O.; Onukwuli, O.D.; Obiora-Okafo, I.A.; Anastopoulos, I. Coagulation-Flocculation of Aquaculture Wastewater Using Green Coagulant from Garcinia kola Seeds: Parametric Studies, Kinetic Modelling and Cost Analysis. Sustainability 2021, 13, 9177. https://doi.org/10.3390/su13169177
Igwegbe CA, Ighalo JO, Onukwuli OD, Obiora-Okafo IA, Anastopoulos I. Coagulation-Flocculation of Aquaculture Wastewater Using Green Coagulant from Garcinia kola Seeds: Parametric Studies, Kinetic Modelling and Cost Analysis. Sustainability. 2021; 13(16):9177. https://doi.org/10.3390/su13169177
Chicago/Turabian StyleIgwegbe, Chinenye Adaobi, Joshua O. Ighalo, Okechukwu Dominic Onukwuli, Ifeoma Amaoge Obiora-Okafo, and Ioannis Anastopoulos. 2021. "Coagulation-Flocculation of Aquaculture Wastewater Using Green Coagulant from Garcinia kola Seeds: Parametric Studies, Kinetic Modelling and Cost Analysis" Sustainability 13, no. 16: 9177. https://doi.org/10.3390/su13169177
APA StyleIgwegbe, C. A., Ighalo, J. O., Onukwuli, O. D., Obiora-Okafo, I. A., & Anastopoulos, I. (2021). Coagulation-Flocculation of Aquaculture Wastewater Using Green Coagulant from Garcinia kola Seeds: Parametric Studies, Kinetic Modelling and Cost Analysis. Sustainability, 13(16), 9177. https://doi.org/10.3390/su13169177









