Breeding Alfalfa (Medicago sativa L.) in Mixture with Grasses
Abstract
1. Introduction
2. Materials and Methods
3. Results
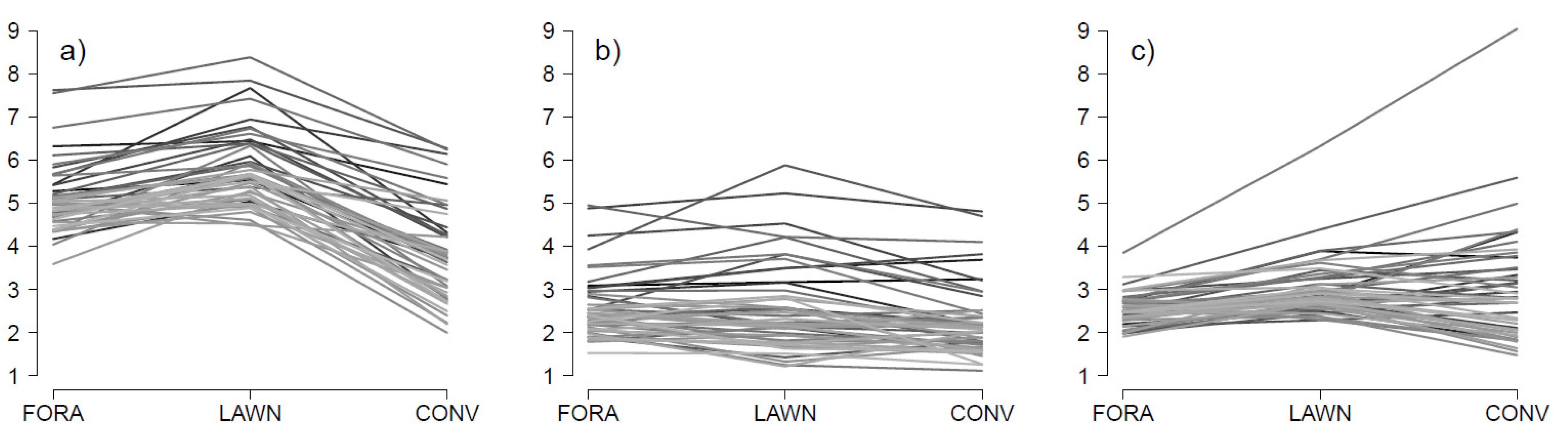
3.1. Variance Explained by Accession, Cultivation System and Their Interaction
3.2. Correlations among Cultivation Systems
4. Discussion
4.1. Will Different Cultivation Systems Lead to Different Selection Decisions?
4.2. What Plants to Select in What System for More Resilient Organic Systems?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nyfeler, D.; Huguenin-Elie, O.; Suter, M.; Frossard, E.; Lüscher, A. Grass-legume mixtures can yield more nitrogen than legume pure stands due to mutual stimulation of nitrogen uptake from symbiotic and non-symbiotic sources. Agric. Ecosyst. Environ. 2011, 140, 155–163. [Google Scholar] [CrossRef]
- Fustec, J.; Lesuffleur, F.; Mahieu, S.; Cliquet, J.-B. Nitrogen rhizodeposition of legumes. A review. Agron. Sustain. Dev. 2010, 30, 57–66. [Google Scholar] [CrossRef]
- Zanetti, S.; Hartwig, U.A.; van Kessel, C.; Lüscher, A.; Hebeisen, T.; Frehner, M.; Fischer, B.U.; Hendrey, G.R.; Blum, H.; Nösberger, J. Does nitrogen nutrition restrict the CO2 response of fertile grassland lacking legumes? Oecologia 1997, 112, 17–25. [Google Scholar] [CrossRef]
- Finn, J.A.; Kirwan, L.; Connolly, J.; Sebastià, M.T.; Helgadottir, A.; Baadshaug, O.H.; Bélanger, G.; Black, A.; Brophy, C.; Collins, R.P.; et al. Ecosystem function enhanced by combining four functional types of plant species in intensively managed grassland mixtures: A 3-year continental-scale field experiment. J. Appl. Ecol. 2013, 50, 365–375. [Google Scholar] [CrossRef]
- Lüscher, A.; Mueller-Harvey, I.; Soussana, J.F.; Rees, R.M.; Peyraud, J.L. Potential of legume-based grassland–livestock systems in Europe: A review. Grass Forage Sci. 2014, 69, 206–228. [Google Scholar] [CrossRef] [PubMed]
- Sampoux, J.P.; Baudouin, P.; Bayle, B.; Béguier, V.; Bourdon, P.; Chosson, J.F.; Deneufbourg, F.; Galbrun, C.; Ghesquière, M.; Noël, D.; et al. Breeding perennial grasses for forage usage: An experimental assessment of trait changes in diploid perennial ryegrass (Lolium perenne L.) cultivars released in the last four decades. Field Crop. Res. 2011, 123, 117–129. [Google Scholar] [CrossRef]
- Hill, J. Breeding components for mixture performance. Euphytica 1996, 92, 135–138. [Google Scholar] [CrossRef]
- Annicchiarico, P. Breeding white clover for increased ability to compete with associated grasses. J. Agric. Sci. 2003, 140, 255–266. [Google Scholar] [CrossRef]
- Litrico, I. Strategy avenues for breeding plants for multispecies grasslands. In Grassland Science in Europe; Huguenin-Elie, O., Studer, B., Kölliker, R., Reheul, D., Probo, M., Barre, P., Feuerstein, U., Roldán-Ruiz, I., Mariotte, P., Hopkins, A., Eds.; European Grassland Federation EGF; Wageningen Academic Publishers: Wageningen, The Netherlands, 2019; Volume 24, pp. 27–35. [Google Scholar]
- Litrico, I.; Violle, C. Diversity in Plant Breeding: A New Conceptual Framework. Trends Plant Sci. 2015, 20, 604–613. [Google Scholar] [CrossRef]
- Sampoux, J.P.; Giraud, H.; Litrico, I. Which recurrent selection scheme to improve mixtures of crop species? Theoretical expectations. G3 Genes Genomes Genet. 2020, 10, 89–107. [Google Scholar] [CrossRef]
- Annicchiarico, P.; Collins, R.P.; De Ron, A.M.; Firmat, C.; Litrico, I.; Hauggaard-Nielsen, H. Do we need specific breeding for legume-based mixtures. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press Ltd.: London, UK; Elsevier Science Ltd.: London, UK, 2019; Volume 157, pp. 141–215. [Google Scholar]
- Charles, J.; Lehmann, J. Intérêt des mélanges de graminées et de légumineuses pour la production fourragère en Suisse. Fourrages 1989, 119, 311–320. [Google Scholar]
- Rowe, D.E.; Brink, G.E. Heritabilities and genetic correlations of white clover clones grown in three environments. Crop Sci. 1993, 33, 1149–1152. [Google Scholar] [CrossRef]
- Caradus, J.R.; Mackay, A.C.; Van Den Bosch, J.; Woodfield, D.R. Comparative evaluation of white clover cultivars in spaced plant and small mixed species plot trials. N. Z. J. Agric. Res. 1989, 32, 433–436. [Google Scholar] [CrossRef]
- Dijkstra, J.; De Vos, A.L.F. The evaluation of selections of white clover (Trifolium repens L.) in monoculture and in mixture with grass. Euphytica 1972, 21, 432–449. [Google Scholar] [CrossRef]
- Zannone, L.; Rotili, P.; Paoletti, R.; Scotti, C. Experimental studies of grass-legume associations. Agronomie 1986, 6, 931–940. [Google Scholar] [CrossRef]
- Rotili, P.; Zannone, L.; Jacquars, P. Effects of association on the evaluation of lucerne populations. Ann. Amelior. Plant. 1976, 28, 139–145. [Google Scholar]
- Maamouri, A.; Louarn, G.; Béguier, V.; Julier, B. Performance of lucerne genotypes for biomass production and nitrogen content differs in monoculture and in mixture with grasses and is partly predicted from traits recorded on isolated plants. Crop. Pasture Sci. 2017, 68, 942–951. [Google Scholar] [CrossRef]
- Klein, T.; Holzkämper, A.; Calanca, P.; Fuhrer, J. Adaptation options under climate change for multifunctional agriculture: A simulation study for western Switzerland. Reg. Environ. Chang. 2014, 14, 167–184. [Google Scholar] [CrossRef]
- Suter, D.; Rosenberg, E.; Frick, R. Standardmischungen für den Futterbau. Revision 2021–2024; Arbeitsgemeinschaft zur Förderung des Futterbaus (AGFF) & Agroscope: Zurich, Switzerland, 2021. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Falconer, D.S.; Mackay, T.F. Introduction to Quantitative Genetics; Longman Group Ltd.: Harlow, UK, 1996. [Google Scholar]
- Fyfe, J.L.; Rogers, H.H. Effects of varying variety and spacing on yields and composition of mixtures of lucerne and tall fescue. J. Agric. Sci. 1965, 64, 351–359. [Google Scholar] [CrossRef]
- Rumbaugh, M.D.; Pendery, B.M. Stability of forage yield of alfalfa clones grown with five associate species. Can. J. Plant Sci. 1991, 71, 453–459. [Google Scholar] [CrossRef][Green Version]
- Zannone, L.; Assemat, L.; Rotili, P.; Jacquard, P. An experimental study of intraspecific competition within several forage crops. Agronomie 1983, 3, 451–459. [Google Scholar] [CrossRef]
- Samac, D.A.; Rhodes, L.H.; Lamp, W.O. Compendium of Alfalfa Diseases and Pests; Am Phytopath Society: St. Paul, MN, USA, 2016. [Google Scholar] [CrossRef]
- Fasoula, D.A.; Fasoula, V.A. Competitive Ability and Plant Breeding. Plant Breed. Rev. 1997, 14, 89–138. [Google Scholar]
- Chamblee, D.S.; Collins, M. Relationships with other species in a mixture. In Alfalfa and Alfalfa Improvement; Hanson, A.A., Barnes, D.K., Hill, R.R.J., Eds.; American Society of Agronomy (ASA): Madison, WI, USA, 1988; Volume 29, pp. 439–461. [Google Scholar]
- Maamouri, A.; Louarn, G.; Gastal, F.; Béguier, V.; Julier, B. Effects of lucerne genotype on morphology, biomass production and nitrogen content of lucerne and tall fescue in mixed pastures. Crop. Pasture Sci. 2015, 66, 192–204. [Google Scholar] [CrossRef]


| Variance Components | ||||||
|---|---|---|---|---|---|---|
| Trait 1 | ||||||
| Vigor traits | ||||||
| vigor_2017_1 | 0.83 | (0.46) | 0.09 | (0.05) | 1.79 | (1.00) |
| vigor_2018_1 | 0.59 | (0.18) | 0.06 | (0.02) | 3.24 | (1.00) |
| vigor_2018_2 | 0.72 | (0.28) | 0.01 | (0.00) | 2.59 | (1.00) |
| vigor_2018_3 | 0.62 | (0.25) | 0.04 | (0.02) | 2.49 | (1.00) |
| vigor_2018_4 | 0.69 | (0.30) | 0.06 | (0.03) | 2.28 | (1.00) |
| vigor_2019_1 | 0.63 | (0.24) | 0.07 | (0.03) | 2.60 | (1.00) |
| Disease occurrence traits | ||||||
| sclerotinia_2018_1a | 0.005 | (0.00) | 0.089 | (0.04) | 2.23 | (1.00) |
| sclerotinia_2018_1b | 0.018 | (0.01) | 0.092 | (0.03) | 2.86 | (1.00) |
| sclerotinia_2019_1 | 0.000 | (0.00) | 0.006 | (0.02) | 0.32 | (1.00) |
| peronospora_2018_1 | 0.605 | (0.24) | 0.000 | (0.00) | 2.51 | (1.00) |
| Morphological traits | ||||||
| habit_2018_1 | 0.39 | (0.42) | 0.28 | (0.30) | 0.95 | (1.00) |
| habit_2018_2 | 0.29 | (0.31) | 0.09 | (0.10) | 0.95 | (1.00) |
| stem-diameter_2018_1 | 0.45 | (0.19) | 0.02 | (0.01) | 2.33 | (1.00) |
| leaf-stem-ratio_2018_1 | 0.11 | (0.10) | 0.00 | (0.00) | 1.19 | (1.00) |
| leaf-stem-ratio_2018_2 | 0.16 | (0.11) | 0.01 | (0.01) | 1.50 | (1.00) |
| rp | |||
|---|---|---|---|
| Trait 1 | FORA-LAWN | FORA-CONV | LAWN-CONV |
| Vigor traits | |||
| vigor_2017_1 | 0.90 ** (0.83 **) | 0.73 ** (0.44) | 0.74 ** (0.47) |
| vigor_2018_1 | 0.76 ** (−0.09) | 0.74 ** (0.01) | 0.75 ** (0.55 *) |
| vigor_2018_2 | 0.83 ** (0.23) | 0.85 ** (0.52*) | 0.86 ** (0.56 *) |
| vigor_2018_3 | 0.82 ** (0.29) | 0.78 ** (0.06) | 0.80 ** (0.74 **) |
| vigor_2018_4 | 0.84 ** (0.06) | 0.79 ** (−0.33) | 0.79 ** (−0.05) |
| vigor_2019_1 | 0.85 ** (0.24) | 0.74 ** (0.05) | 0.71 ** (−0.23) |
| Mean vigor | 0.83 ** (0.26) | 0.77 ** (0.13) | 0.78 ** (0.34) |
| Disease occurrence traits | |||
| sclerotinia_2018_1a | 0.16 | −0.05 | 0.06 |
| sclerotinia_2018_1b | 0.30 * | −0.02 | 0.17 |
| sclerotinia_2019_1a | −0.02 | −0.07 | −0.20 |
| peronospora_2018_1 | 0.86 ** | 0.82 ** | 0.86 ** |
| Morphological traits | |||
| habit_2018_1 | 0.73 ** | 0.57 ** | 0.86 ** |
| habit_2018_2 | 0.77 ** | 0.79 ** | 0.78 ** |
| stem-diameter_2018_1 | 0.77 ** | 0.72 ** | 0.78 ** |
| leaf-stem-ratio_2018_1 | 0.53 ** | 0.80 ** | 0.49 ** |
| leaf-stem-ratio_2018_2 | 0.71 ** | 0.62 ** | 0.44 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grieder, C.; Kempf, K.; Schubiger, F.X. Breeding Alfalfa (Medicago sativa L.) in Mixture with Grasses. Sustainability 2021, 13, 8929. https://doi.org/10.3390/su13168929
Grieder C, Kempf K, Schubiger FX. Breeding Alfalfa (Medicago sativa L.) in Mixture with Grasses. Sustainability. 2021; 13(16):8929. https://doi.org/10.3390/su13168929
Chicago/Turabian StyleGrieder, Christoph, Katharina Kempf, and Franz Xaver Schubiger. 2021. "Breeding Alfalfa (Medicago sativa L.) in Mixture with Grasses" Sustainability 13, no. 16: 8929. https://doi.org/10.3390/su13168929
APA StyleGrieder, C., Kempf, K., & Schubiger, F. X. (2021). Breeding Alfalfa (Medicago sativa L.) in Mixture with Grasses. Sustainability, 13(16), 8929. https://doi.org/10.3390/su13168929






