Abstract
Various treatments are carried out in order to extend both the shelf life and storage life of fresh fruit and vegetables after harvest and among them non-toxic for humans, environmentally and economically friendly alternative treatments are gained more importance. In the current study, methyl jasmonate (MeJA), cytokinin, and lavender oil which are eco-friendly and safe for human health were applied on apricot fruit. The treated fruit were stored at 0 °C and 90–95% relative humidity for 25 days and catalase (CAT), superoxide dismutase (SOD), and ascorbate peroxidase (APX) enzyme activities and lipid peroxidation of apricots after treatments were studied. According to the findings obtained from the study, it was observed that 5 ppm cytokinin and 1000 ppm lavender oil treatments of apricot fruit gave better APX and CAT enzyme activity, respectively. In addition, better SOD enzyme activity in fruit was obtained with MeJA + lavender oil treatments. As a result, it can be emphasized that the product quality of apricot fruit is preserved as both the eco-friendly application of MeJA, cytokinin, and lavender oil separately from each other and the treatment of combinations between these compounds activate the enzymatic antioxidant defense systems of apricot fruit after harvest.
1. Introduction
Fruit and vegetables are a huge portion of the food supply chain and are depended on globally for good health and nutrition. The supply of good food, however, greatly depends on good pre and postharvest handling practices. Substantial research has been carried out to preserve the quality of fresh horticultural produce. Eco-friendly technology for pre and postharvest produce quality presents the scope of emerging eco-friendly technologies to maintain the postharvest quality of fresh produce in terms of safety and nutrition [1,2,3,4,5,6].
Apricot (Prunus armeniaca L.) is one of the most popular fruit species preferred in the world due to its high nutritional and antioxidant properties and taste and aroma characteristics [7,8]. Turkey dominates world apricot production for a long time because of favorable climate and soil conditions for apricot growing [9,10]. World fresh apricot production was around 4.08 million tons and Turkey contributes 846 thousand tons of this production [11]. As of 2019, apricot production is carried out on an area of 561 thousand hectares in the world, and 23.35% of this production area is located in Turkey.
It is well known that fresh fruit can be available on the markets in certain periods that depend on the harvest periods of different fruit species. The consumers demand fresh fruit out of season thus the fruit has been storing for a longer period of time to meet these consumer demands. Post-harvest storage of apricot fruit is limited due to the fact that the flesh of apricots is perishable and easily spoiled, has a medium respiration rate, and is susceptible to decay and spoilage. In order to present a quality product to the consumer, the fruit must be kept under suitable conditions after harvest. Apricots can be stored for a very short time such as 3–5 days under normal room conditions. However, at appropriate temperature (0 °C) and humidity (90–95%) levels, this period can be extended to approximately 2–4 weeks [12,13]. In addition to fruit, apricot kernels are also a valuable food source, and apricot blossoms are edible, too [14,15].
In recent years, there has been an increasing interest in the use of natural compounds in extending the post-harvest shelf life of agricultural products and maintaining product quality [16,17,18]. Jasmonic acids (JA), which are showed to be effective in maintaining the quality of many food products, are compounds that are activated by the effect of lipoxygenase (LOX3) enzyme found in chloroplasts obtained from linoleic acid in the plant [19]. Jasmonic acids become active against biotic and abiotic stress conditions in the plant, and they stimulate the formation of flavonoids, alkaloids, polypeptides, terpenoids, and phytoalexins in the plant by promoting proteinase enzyme synthesis [20,21].
Methyl jasmonate (MeJA), on the other hand, is the methyl ester of JA, and it has been determined to be effective in increasing aromatic components and anthocyanins in the plant, providing chlorophyll degradation, reducing blackening and chilling damage, preventing fungal growth, and increasing plant resistance against pathogens. The effectiveness of MeJA against chilling damage during storage was determined in horticulture crops [22,23,24].
It is known that endogenous hormones, including cytokinins, have an important effect on the prevention of post-harvest aging. Cytokinins can prevent the degradation of proteins due to their effects on tRNA in the cell [25,26]. It has been reported by different researchers that postharvest cytokinin treatments gave positive results in broccoli [26,27], and cauliflower [28].
Scientific studies show that the effects of the substances used as edible coatings on the product are different from each other. Essential oils, which have an antimicrobial effect, are used extensively to prevent microbial deterioration. One of the largest groups of natural components is essential oils. Essential oils increase the shelf life of unprocessed or processed foods by reducing the rate of microbial growth [29]. Some of these substances extend the life of the products by contributing to the plant’s defense system against infectious organisms [29,30,31].
Essential oils are found in different proportions in edible and medicinal plants. Essential oils and their components are frequently used as flavoring agents in foods and are well known to have broad-spectrum antimicrobial effects [32,33]. Among them, it has been observed that the oils of clove, thyme, rosemary, mountain thyme, sage, and vanilla plants are very active against microorganisms. They generally show an inhibitory effect against Gram-positive rather than Gram-negative bacteria [34,35].
There is no study in the literature on the effects of postharvest MeJA, cytokinin, lavender oil (Lavandula angustifolia Mill.), MeJA + lavender, and cytokinin + lavender treatments on antioxidative enzymes (CAT, SOD and APX) and lipid peroxidation in apricot fruit. The aim of the study is to investigate the effects of these treatments on CAT, SOD, and APX enzyme activities and lipid peroxidation in apricots stored at 0 °C and 90–95% relative humidity for 25 days.
2. Materials and Methods
2.1. Plant Material
In the study, the ‘Bebeco’ apricot cultivar collected from Van Yüzüncü Yıl University Application and Research Experimental orchard was used as fruit material (when three-quarters of the fruit surface, and half of the fruit pulp had a yellow color). After being harvested, the fruit was precooled to +4 °C for 12 h. Nitrozyme, an organic cytokinin, commercially available 100% lavender oil and MeJA (PubChem CID: 5281929, 95% Sigma Aldrich, cat no.392707, St. Louis, MO, USA) were used. Then, fruit with the same maturity stage were divided into 6 groups by applying control, 0.2 mM MeJA, 1000 ppm lavender oil, 5 ppm cytokinin, 0.2 mM MeJA + 1000 ppm lavender, and 5 ppm cytokinin + 1000 ppm lavender. While the control group was dipped in distilled water, the others were dipped for 5 min at 20 °C, preparing treatment solutions before, respectively. After the treatments, apricot fruit were stored in foam plates in 3 repetitions (each package per 400 g), covered with stretch film, and stored at 0 °C with 90–95% relative humidity in the cold storage room. Analyses were carried out at 5-day intervals throughout the trial. The research was carried out in the Cold Storage Facilities of Van Yüzüncü Yıl University, Faculty of Agriculture, Department of Horticulture.
2.2. Material Preparation for Antioxidative Enzyme Assays
One gram of frozen fruit pulp sample was homogenized (Ika Ultra-Turrax T20 Basic, Staufen, Germany) with a mixture of 5 mL cold 0.1 M Na-phosphate, 0.5 mM NaEDTA and 1 mM ascorbic acid (pH: 7.5) for one minute, and then was centrifuged at 18,000× g for 5 min at 4 °C. Ascorbate peroxidase (APX) activity was determined immediately on the homogenate prepared in this way. For the determination of CAT and SOD activities, 1 g frozen fruit pulp sample was homogenized with 5 mL cold 0.1 M Na-phosphate, 0.5 mM Na-EDTA mixture (pH: 7.5) for one minute, and then was centrifuged at 18,000× g for 5 min at 4 °C [36].
2.3. Ascorbate Peroxidase (APX) Activity
APX activity was measured at a wavelength of 290 nm based on the reduction of H2O2 connected to ascorbic acid. A mixture of 50 mM phosphate buffer (KH2PO4), 0.5 mM ascorbic acid, 0.1 mM EDTA, 1.5 mM H2O2 was used as the reaction solution (pH: 7.0). Next, 0.1 mL of fruit pulp extract was mixed with 3 mL of reaction solution. Readings were taken at 0 and 60 s at 290 nm wavelength in the spectrophotometer (Thermo Scientific Genesys 10S Model UV-VIS spectrophotometer, Waltham, MA, USA). The reaction was initiated by the addition of 0.1 mL of enzyme extract. Evaluation was made by considering the change in absorbance within 1 min [36].
2.4. Catalase (CAT) Activity
CAT was determined by monitoring the disappearance of H2O2 at a wavelength of 240 nm. A mixture of 0.05 M phosphate buffer (KH2PO4) and 1.5 mM H2O2 was used as the reaction solution (pH: 7.0). A 2.5 mL aliquot of reaction solution was mixed with 0.2 mL of fruit pulp extract. Readings were taken at 0 and 60 s at 240 nm wavelength in the spectrophotometer (Thermo Scientific Genesys 10S Model UV-VIS spectrophotometer USA). The reaction was initiated by the addition of 0.1 mL of enzyme extract. Evaluation was made taking into account the change in absorbance within 1 min [36].
2.5. Superoxide Dismutase (SOD) Activity
It was determined by the inhibition of nitroblue tetrazolium (NBT) at a wavelength of 560 nm. A mixture of 50 mM Na-phosphate buffer (Na2HPO4 × H2O2), 0.1 mM Na-EDTA, 33 µM NBT, 5 µM riboflavin, 13 mM methionine was used as the reaction solution (pH: 7.0). A 2.5 mL aliquot of the reaction solution was mixed with 0.2 mL of fruit pulp extract. The reaction was achieved by keeping it under 75 µmol m−2 s−1 (40 W) light for 10 min at 25 °C. The control solution was kept in the dark for the same time without enzyme. The control and reaction solution were read at 560 nm wavelength in the spectrophotometer (Thermo Scientific Genesys 10S Model UV-VIS spectrophotometer USA). SOD activity was determined as the activity that reduces 50% of NBT as a unit [36].
2.6. Lipid Peroxidation
Lipid peroxidation in fruit is expressed as malondialdehyde (MDA) content. A 0.5 g sample of fruit flesh was homogenized with 10 mL of 0.1% trichloroacetic acid (TCA) for one minute and then centrifuged at 15,000× g for 5 min at 4 °C. A 1 mL aliquot of the clear part of the centrifuged sample was taken and 0.5% thiobarbituric acid (TBA) dissolved in 4 mL of 20% TCA was added to it. After the mixture was kept at 95 °C for 30 min, it was rapidly cooled in an ice bath and centrifuged at 10,000× g for 10 min, its absorbance was determined at 532 and 600 nm wavelengths (Thermo Scientific Genesys 10S Model UV-VIS spectrophotometer USA) in the clear part and MDA content was determined [37].
2.7. Statistical Analysis
Descriptive statistics for the searched features; expressed as mean and standard error. The study was carried out as factorial experiments with three factors including storage periods, and treatments based on a randomized design with 3 replicates. N = 90 for APX, CAT, SOD, and MDA (three replicates × five storage periods × six treatments for each replicate), and the test of normality was performed according to Kolmogorov–Smirnov. One way ANOVA for completely randomized design was conducted for analyzing the effects of storage periods and treatments. Duncan’s test was used to determine the difference between the averages of treatments and storage periods following the analysis of variance. The statistical significance level was taken as 5% in the calculations and the “SPSS version 20.0” statistical package program was used for the calculations.
3. Results
During the storage period, an increase in ascorbic peroxidase (APX) enzyme activity was observed in general considering all treatments compared to the beginning of storage, but higher enzyme activity was observed in the treated fruit samples (Table 1). The highest enzyme activity was determined on the 15th day of storage on 5 ppm cytokinin applied fruit as 0.655 mmol/g−1. The difference between storage periods was found to be statistically significant. When the difference between the treatments was examined, it was observed that fruit samples treated with 5 ppm cytokinin were the most effective treatments to obtain the highest APX level.

Table 1.
Changes in APX (mmol g−1), CAT (mmol g−1), SOD (unit g−1) enzyme activities, and MDA (nmol g−1) levels during the storage period of ‘Bebeco’ apricot cultivar (fresh weight base). Data are expressed as mean ± standard error.
It was observed that there was an increase in catalase (CAT) enzyme activity in all treatments from the beginning of the storage period. However, it was determined that the fruit samples subjected to the treatments contained higher CAT enzyme activity during storage. The difference between storage times was found to be statistically significant. Compared to the control group fruit, it was observed that there was a statistically significant increase in the CAT enzyme activity of the fruit subjected to the treatments. When the changes in superoxide dismutase (SOD) enzyme activity during the storage period are examined; it was determined that the highest SOD activity occurred after 10 days of storage. In addition, it was observed that there was a statistically significant increase on the 15th day of storage. The difference between treatments was found to be statistically significant (Table 1).
Malondialdehyde (MDA) levels increase in harvested products during the storage period, especially due to aging. As a matter of fact, in the present study, it was determined that MDA content increased in all fruit groups during the storage period. However, compared to the control group fruit, it was determined that MDA accumulation was relatively inhibited in the treated fruit groups. The difference between storage times was found to be statistically significant. In addition, compared to the control group, a statistically significant decrease was observed in the MDA level of the fruit subjected to the treatments (Table 2).

Table 2.
Changes in MDA (nmol g−1) levels during the storage period of ‘Bebeco’ apricot cultivar (fresh weight base). Data are expressed as mean ± standard error.
Changes in APX, CAT, SOD enzyme activities, and MDA levels as a percentage during the storage period of the ‘Bebeco’ apricot cultivar (See Figure 1).
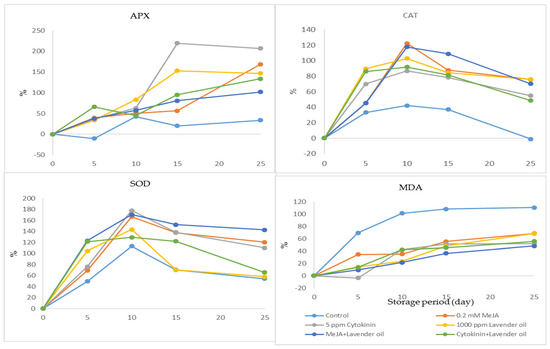
Figure 1.
Changes in APX, CAT, SOD enzyme activities, and MDA levels as a percentage during the storage period of the ‘Bebeco’ apricot cultivar.
4. Discussion
Antioxidative enzymes, which are associated with both aging and defense mechanisms, play an important role in suppressing oxidative stress. Enzymes such as APX, CAT, and SOD are the main enzymes that can protect cells from oxidative damage by scavenging free oxygen radicals [38]. The accumulation of free radicals causes oxidative damage and accelerates the development of various aging-related disorders. With the increase of free radicals, the SOD enzyme catalyzes the decomposition of the superoxide radical into molecular O2 and H2O2. On the other hand, H2O2 enters into chain reactions where it is detoxified by CAT and POD [39]. The CAT enzyme reduces H2O2 to water and oxygen. APX, on the other hand, acts as an electron donor to ascorbic acid and prevents the formation of H2O2 [40].
Liu et al. [41], in their study on cucumber, suggested that methyl jasmonate (MeJA) reduces chilling damage and increases CAT enzyme activity by reducing H2O2 accumulation. Many researchers have stated that the chilling damage prevented by MeJA applications is related to the increase in CAT enzyme activity [42,43,44,45]. According to Zhu and Tian [24], they suggested that MeJA applications activate CAT and POD activities, causing H2O2 to deteriorate and that MeJA increases endurance in tomatoes by regulating the production of reactive oxygen species, and also causes an increase in CAT enzyme activity.
Menga et al. [46] in their study on mushrooms reported that SOD and CAT enzymes have an important role in protecting mushroom quality and protection against oxidative stress since they provide membrane integrity by destroying free oxygen radicals. Again, in the same study, higher SOD and CAT activity were detected in mushrooms treated with MeJA during storage compared to the control. In addition, it has been suggested that accumulation of free radicals leads to lipid peroxidation, leading to disruption of membrane integrity, thus causing MDA accumulation in the cell [47]. It has been reported that MeJA applied to kidney beans after harvest significantly reduces MDA levels [48]. In the current study, it was observed that the effects of MeJA and MeJA + lavender applications on antioxidative enzymes and MDA were similar to the above studies.
Cytokinins are phytohormones that can promote cell division and play a role in plant growth regulation, development, and differentiation [49,50,51]. Information on the role of cytokinin applications in regulating ripening and senescence in harvested fruit is limited in the literature. However, postharvest, the treatment of a synthetic cytokinin, BAP (6-Benzylaminopurine), alone or in combination with other chemicals is known to be effective in controlling decay in crops [52,53,54]. It has also been reported to delay cell wall degradation and softening in fruit [55]. BAP applied to litchi fruit after harvest caused higher CAT, SOD, and APX enzyme activity and lower MDA content in fruit compared to control fruit [54]. In the current study, similar results were obtained with the above-mentioned study, with higher CAT, SOD, and APX enzyme activity and lower MDA content in fruit treated with 5 ppm cytokinin and 5 ppm cytokinin + 1000 ppm lavender oil compared to the control.
Several studies have been conducted on volatile compounds, such as methyl jasmonate, salicylic acid, and tea oil, to maintain product quality in fruit and vegetables [56,57,58,59,60]. In addition, researchers reported that essential oils are environmentally friendly applications and perform safer antibacterial and antifungal activities for human health [61,62,63,64]. One of the essential oils, lavender oil (Lavandula angustifolia Mill.) mainly contains linalool and linalyl acetate, with moderate levels of lavandulyl acetate, terpinen-4-ol, and lavandulol [65]. There are studies in the literature that MeJA applied in different crops after harvest reduces chilling damage and protects product quality by preventing the accumulation of reactive oxygen species by increasing the activity of enzymes such as CAT, SOD, and APX, which are antioxidative enzymes. However, studies on the effect of lavender oil alone or in combination with MeJA on antioxidative enzymes are not yet available in the literature. In the current study, it was determined that the application of MeJA + lavender resulted in higher SOD and CAT enzyme activity in apricot fruit, as well as lower MDA content, compared to fruit treated with MeJA alone.
5. Conclusions
As a result, synthetic fungicides are mainly used to control post-harvest diseases of fruit and vegetables around the world today. However, the development of fungicide-resistant pathogens and the growing consumer trend around the world to reduce harmful chemicals in the food chain has promptly led to the discovery of alternative biocontrol methods with high efficiency, low residue rates, non-toxic, environmentally, and economically friendly. In the current study, it was determined that the treatments of MeJA, cytokinin, and lavender oil, which are environmentally friendly and safe for human health, have positive effects on antioxidative enzymes (CAT, SOD, and APX) and lipid peroxidation in apricot fruit during the storage period. According to the findings obtained from the study, it was observed that 5 ppm cytokinin applied for APX enzyme activity and 1000 ppm lavender oil applied for CAT enzyme activity gave better results. In addition, better results were obtained in fruit treated with MeJA + lavender oil in terms of SOD enzyme activity and MDA content. As a matter of fact, the high antioxidative enzyme activities in post-harvest products are accepted as an indication that product quality is preserved during storage and chilling damage is inhibited. Therefore, it can be said that the product quality of apricot fruit is preserved as both the application of MeJA, cytokinin, and lavender oil separately from each other and the application of combinations between these compounds activate the enzymatic antioxidant defense systems after harvest.
Author Contributions
S.C., N.Y., F.I. and O.T. were responsible for analysis. S.C. coordinated and organized all research activities. H.I.S., S.E., E.R. and T.N. contributed to writing and editing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The cooperation and research were supported by the program no. 6.2.10 ref.: 51834/2017-MZE-17253, sub-program “National Program of Conservation and Utilization of Plant Genetic Resources and Agrobiodiversity” which is funded by the Ministry of Agriculture of the Czech Republic.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All-new research data were presented in this contribution.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Ercisli, S.; Esitken, A.; Cangi, R.; Sahin, F. Adventitious root formation of kiwifruit in relation to sampling date, IBA and Agrobacterium rubi inoculation. Plant Growth Regul. 2003, 41, 133–137. [Google Scholar] [CrossRef]
- Campbell, O.E.; Merwin, I.A.; Padilla-Zakour, O.I. Characterization and the effect of maturity at harvest on the phenolic and carotenoid content of northeast USA apricot (Prunus armeniaca) varieties. J. Agric. Food Chem. 2013, 61, 12700–12710. [Google Scholar] [CrossRef] [PubMed]
- Dogan, H.; Ercisli, S.; Jurikova, T.; Temim, E.; Leto, A.; Hadziabulic, A.; Tosun, M.; Narmanlioglu, H.K.; Zia-Ul-Haq, M. Physicochemical and antioxidant characteristics of fruits of cape gooseberry (Physalis peruviana L.) from Turkey. Oxid. Commun. 2014, 37, 1005–1014. [Google Scholar]
- Hu, W.; Yang, H.; Tie, W.; Yan, Y.; Ding, Z.; Liu, Y.; Wu, C.; Wang, J.; Reiter, R.J.; Tan, D.X.; et al. Natural variation in banana varieties highlights the role of melatonin in postharvest ripening and quality. J. Agric. Food Chem. 2017, 65, 9987–9994. [Google Scholar] [CrossRef]
- Abd El-Naby, S.K.M.A.; Mohamed, A.A.A.; El-Naggar, Y.I.M. Effect of melatonin, GA3 and NAA on vegetative growth, yield and quality of ‘Canino’ apricot fruits. Acta Sci. Pol. Hortorum Cultus 2019, 18, 167–174. [Google Scholar] [CrossRef]
- Engin, S.P.; Mert, C. The effects of harvesting time on the physicochemical components of aronia berry. Turk. J. Agric. For. 2020, 44, 361–370. [Google Scholar] [CrossRef]
- Karatas, N.; Sengul, M. Some important physicochemical and bioactive characteristics of the main apricot cultivars from Turkey. Turk. J. Agric. For. 2020, 44, 651–661. [Google Scholar] [CrossRef]
- Medina-Santamarina, J.; Zapata, P.J.; Valverde, J.M.; Valero, D.; Serrano, M.; Guillén, F. Melatonin treatment of apricot trees leads to maintenance of fruit quality attributes during storage at chilling and non-chilling temperatures. Agronomy 2021, 11, 917. [Google Scholar] [CrossRef]
- Ercisli, S. Apricot culture in Turkey. Sci. Res. Essays 2009, 4, 715–719. [Google Scholar]
- Gecer, M.K.; Kan, T.; Gundogdu, M.; Ercisli, S.; Ilhan, G.; Sagbas, H.I. Physicochemical characteristics of wild and cultivated apricots (Prunus armeniaca L.) from Aras valley in Turkey. Genet. Resour. Crop Evol. 2020, 67, 935–945. [Google Scholar] [CrossRef]
- FAOSTAT. 2019. Available online: http://www.fao.org/faostat/en/#compare (accessed on 28 January 2021).
- Egea, M.I.; Sánchez-Bel, P.; Martínez-Madrid, M.C.; Flores, F.B.; Romojaro, F. The effect of beta ionization on the antioxidant potential of “Búlida” apricot and its relationship with quality. Postharvest Biol. Technol. 2007, 46, 63–70. [Google Scholar] [CrossRef]
- Cavusoglu, S.; Islek, F.; Yilmaz, N.; Tekin, O. Kayısıda (Prunus armeniaca L.) metil jasmonate, sitokinin ve lavanta yağı uygulamalarının hasat sonrası fizyolojisi üzerine etkileri. Yüzüncü Yıl Univ. Tarm Bilimleri Derg. 2020, 30, 136–146. [Google Scholar]
- Rampáčková, E.; Göttingerová, M.; Gála, P.; Kiss, T.; Ercişli, S.; Nečas, T. Evaluation of Protein and Antioxidant Content in Apricot Kernels as a Sustainable Additional Source of Nutrition. Sustainability 2021, 13, 4742. [Google Scholar] [CrossRef]
- Göttingerová, M.; Kumšta, M.; Nečas, T. Health-benefitting Biologically Active Substances in Edible Apricot Flowers. HortScience 2020, 55, 1372–1377. [Google Scholar] [CrossRef]
- Hossein, M.; Mahmood, G.; Davood, B. Effect of different coatings on post-harvest quality and bioactive compounds of pomegranate (Punica granatum L.) fruits. J. Food Sci. Technol. 2015, 52, 4507–4514. [Google Scholar]
- Nourozi, F.; Sayyari, M. Enrichment of Aloe vera gel with basil seed mucilage preserve bioactive compounds and postharvest quality of apricot fruits. Sci. Hortic. 2020, 262, 109041. [Google Scholar] [CrossRef]
- Sortino, G.; Saletta, F.; Puccio, S.; Scuderi, D.; Allegra, A.; Inglese, P.; Farina, V. Extending the shelf life of white peach fruit with 1-Methylcyclopropene and Aloe arborescens edible coating. Agriculture 2020, 10, 151. [Google Scholar] [CrossRef]
- Vick, B.A.; Zimmerman, D.C. Biosynthesis of jasmonic acid by several plant species. Plant Physiol. 1984, 75, 458–461. [Google Scholar] [CrossRef]
- Gundlach, H.; Muller, M.J.; Kutchan, T.M.; Zenk, M.H. Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc. Natl. Acad. Sci. USA 1992, 89, 2389–2393. [Google Scholar] [CrossRef]
- Mueller, M.J.; Brodschelm, W.; Spannagl, E.; Zenk, M.H. Signaling in the elicitation process is mediated through the octadecanoid pathway leading to jasmonic acid. Proc. Natl. Acad. Sci. USA 1993, 90, 7490–7494. [Google Scholar] [CrossRef] [PubMed]
- Meir, S.; Philosoph-Hadas, S.; Lurie, S.; Droby, S.; Akerman, M.; Zauberman, G.; Shapiro, B.; Cohen, E.; Fuchs, Y. Reduction of chilling injury in stored avocado, grapefruit, and bell pepper by methyl jasmonate. Can. J. Bot. 1996, 74, 870–874. [Google Scholar] [CrossRef]
- Pérez, A.G.; Sanz, C.; Olías, R.; Olías, J.M. Effect of methyl jasmonate on in vitro strawberry ripening. J. Agric. Food Chem. 1997, 45, 3733–3737. [Google Scholar] [CrossRef]
- Zhu, Z.; Tian, S.P. Resistant responses of tomato fruit treated with exogenous methyl jasmonate to Botrytis cinerea infection. Sci. Hortic. 2012, 142, 38–43. [Google Scholar] [CrossRef]
- Kays, S.J. Postharvest Physiology of Perishable Plant Products; Van Nostrand Reinhold: New York, NY, USA, 1991; p. 507. [Google Scholar]
- Clarke, S.F.; Jameson, P.E.; Downs, C. The influence of 6-benzylaminopurine on post-harvest senescence of floral tissues of broccoli (Brassica oleracea var Italica). Plant Growth Regul. 1994, 14, 21–27. [Google Scholar] [CrossRef]
- Fuller, G.; Kuhnle, J.A.; Corse, J.W.; Mackey, B.E. Use of natural cytokinin’s to extend the storage life of broccoli (Brassica oleracea, Itaica group). J. Am. Soc. Hort. Sci. 1977, 102, 480–484. [Google Scholar]
- Cavusoglu, S.; Halloran, N. The effect of pre-harvest cytokinin application total phenolic content and polyphenoloxidase on post-harvest physiology of cauliflower (Brassica oleracea L. Botrytis). In Proceedings of the XXVIII International Horticultural Congress, Lisboa, Portugal, 22–26 August 2010; p. 114. [Google Scholar]
- Mastromatteo, M.; Conte, A.; Del Nobile, M.A. Combined used of modified atmosphere packaging and natural compounds for food preservation. Food Eng. Rev. 2010, 2, 28–38. [Google Scholar] [CrossRef]
- Deans, S.G.; Ritchie, G. Antimicrobial properties of plant essential oils. Int. J. Food Microbiol. 1987, 5, 165–180. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, Y.J.; Hong, K.H.; Kwon, Y.K.; Sim, K.C.; Lee, J.Y.; Cho, H.Y.; Kim, I.S.; Han, S.B.; Lee, C.W.; et al. Isolation of antimicrobial substances from natural products and their preservative effects. Food Sci. Biotechnol. 2001, 10, 59–71. [Google Scholar]
- Alzoreky, N.S.; Nakahara, K. Antimicrobial activity of extracts from some edible plants commonly consumed in Asia. Int. Food Microbial. 2002, 80, 223–230. [Google Scholar] [CrossRef]
- Packiyasothy, E.V.; Kyle, S. Antimicrobial properties of some herb essential oil. Food Aust. 2002, 54, 384–387. [Google Scholar]
- Zaika, L.L. Spice and herbs: Their antimicrobial activity and its determinaton. J. Food Saf. 1988, 9, 97–118. [Google Scholar] [CrossRef]
- Meng, D.; Zhang, Y.; Yang, R.; Wang, J.; Zhang, X.; Sheng, J.; Wang, J.; Fan, Z. Arginase participates in the methyl jasmonate-regulated quality maintenance of postharvest Agaricus bisporus fruit bodies. Postharvest Biol. Technol. 2017, 132, 7–14. [Google Scholar] [CrossRef]
- Jebara, S.; Jebara, M.; Limam, F.; Aouani, M.E. Changes in ascorbate peroxidase, catalase, guaiacol peroxidase and superoxide dismutase activities in common bean (Phaseolus vulgaris) nodules under salt stress. J. Plant Physiol. 2005, 162, 929–936. [Google Scholar] [CrossRef]
- Guneri Bagci, E. Determination of Physiological and Biochemical Parameters Symptomatic for Oxidative Stress in Chickpea (Cicer arietinum L.) Cultivars under Droughtvars under Drought. Ph.D. Thesis, Ankara University, Ankara, Turkey, 2010. [Google Scholar]
- Sun, J.; You, X.; Li, L.; Peng, H.; Su, W.; Li, C.; He, Q.; Liao, F. Effects of a phospholipase D inhibitor on postharvest enzymatic browning and oxidative stress of litchi fruit. Postharvest Biol. Technol. 2011, 62, 288–294. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Zhou, Q.; Ma, C.; Cheng, S.; Wei, B.; Liu, X.; Ji, S. Changes in antioxidative metabolism accompanying pitting development in stored blueberry fruit. Postharvest Biol. Technol. 2014, 88, 88–95. [Google Scholar] [CrossRef]
- Liu, Y.; Yanga, X.; Zhua, S.; Wang, Y. Postharvest application of MeJA and NO reduced chilling injury in cucumber (Cucumis sativus) through inhibition of H2O2 accumulation. Postharvest Biol. Technol. 2016, 119, 77–83. [Google Scholar] [CrossRef]
- Cao, S.; Zheng, Y.; Wang, K.; Rui, H.; Tang, S. Effect of methyl jasmonate on cell wall modification of loquat fruit in relation to chilling injury after harvest. Food Chem. 2009, 118, 641–647. [Google Scholar] [CrossRef]
- Esim, N.; Atici, O. Nitric oxide improves chilling tolerance of maize by affecting apoplastic antioxidative enzymes in leaves. Plant Growth Regul. 2014, 72, 29–38. [Google Scholar] [CrossRef]
- Wu, B.; Guo, Q.; Li, Q.; Ha, Y.; Li, X.; Chen, W. Impact of postharvest nitric oxide treatment on antioxidant enzymes and related genes in banana fruit in response to chilling tolerance. Postharvest Biol. Technol. 2014, 92, 157–163. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Meenune, M. Effect of methyl jasmonate on physiological and biochemical quality changes of longkong fruit under low temperature storage. Fruits 2015, 70, 69–75. [Google Scholar] [CrossRef]
- Mangena, T.; Muyima, N.Y.O. Comparative evaluation of the antimicrobial activities of essential oils of Artemisia afra, Pteronia incana and Rosemarinus officinalis on selected bacteria and yeast strains. Lett. Appl. Microbiol. 1999, 28, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.X.; Duan, L.S.; Tian, X.L.; Wang, B.M.; Eneji, A.E.; Li, Z.H. Coronatinealleviatessalinity stress in cotton by improving the antioxidative defense system and radical-scavenging activity. J. Plant Physiol. 2008, 165, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Wang, Q.; Lv, J.; Gao, L.; Zuo, J.; Shi, J. Amelioration of postharvest chilling injury in cowpea (Vigna sinensis) by methyl jasmonate (MeJA) treatments. Sci. Hortic. 2016, 203, 95–101. [Google Scholar] [CrossRef]
- Gan, S.; Amasino, M.R. Cytokinins in plant senescence: From spray and pray to clone and play. Bioassays 1996, 18, 557–565. [Google Scholar] [CrossRef]
- Hwang, L.; Sheen, J.; Muller, B. Cytokinin signaling networks. Ann. Rev. Plant Biol. 2012, 63, 353–380. [Google Scholar] [CrossRef]
- Markovich, O.; Steiner, E.; Kouril, S.; Tarkowski, P.; Aharoni, A.; Elbaum, R. Silicon promotes cytokinin biosynthesis and delays senescence in Arabidopsis and Sorghum. Plant. Cell Environ. 2017, 40, 1189–1196. [Google Scholar] [CrossRef]
- Yu, T.; Wang, L.P.; Yin, Y.; Feng, F.Q.; Zheng, X.D. Suppression of postharvest blue mould of apple fruit by Cryptococcus laurentii and N-6-benzyladenine. J. Sci. Food Agric. 2008, 88, 1266–1271. [Google Scholar] [CrossRef]
- Zheng, X.D.; Yu, T.; Chen, R.L.; Huang, B.; Wu, V.C.H. Inhibiting Penicillium expansum infection on pear fruit by Cryptococcus laurentii and cytokinin. Postharvest Biol. Technol. 2007, 45, 221–227. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, X.; Zhang, Z.; Jiang, G.; Feng, L.; Duan, X.; Jiang, Y. 6-Benzylaminopurine improves the quality of harvested litchi fruit. Postharvest Biol. Technol. 2018, 143, 137–142. [Google Scholar] [CrossRef]
- Massolo, J.F.; Lemoine, M.L.; Chaves, A.R.; Concellon, A.; Vicente, A.R. Benzylaminopurine (BAP) treatments delay cell wall degradation and softening, improving quality maintenance of refrigerated summer squash. Postharvest Biol. Technol. 2014, 93, 122–129. [Google Scholar] [CrossRef]
- Chanjirakul, K.; Wang, S.Y.; Wang, C.Y.; Siriphanich, J. Effect of natural volatile compounds on antioxidant capacity and antioxidant enzymes in raspberries. Postharvest Biol. Technol. 2006, 40, 106–115. [Google Scholar] [CrossRef]
- Ghasemnezhad, M.; Javaherdashti, M. Effect of methyl jasmonate treatment on antioxidant capacity, internal quality and postharvest life of raspberry fruit. Casp. J. Environ. Sci. 2008, 1, 73–78. [Google Scholar]
- Giménez, M.J.; Valverde, J.M.; Valero, D.; Zapata, P.J.; Castillo, S.; Serrano, M. Postharvest methyl salicylate treatments delay ripening and maintain quality attributes and antioxidant compounds of ‘Early Lory’ sweet cherry. Postharvest Biol. Technol. 2016, 117, 102–109. [Google Scholar] [CrossRef]
- Muengkaew, R.; Chaiprasart, P.; Warrington, I. Changing of physiochemical properties and color development of mango fruit sprayed methyl Jasmonate. Sci. Hortic. 2016, 198, 70–77. [Google Scholar] [CrossRef]
- Awad, A.H.R.; Parmar, A.; Ali, M.R.; El-Mogy, M.M.; Abdelgawad, K.F. Extending the Shelf-Life of Fresh-Cut Green Bean Pods by Ethanol, Ascorbic Acid, and Essential Oils. Foods 2021, 10, 1103. [Google Scholar] [CrossRef]
- Javed, S.; Shoaib, A.; Mahmood, Z.; Mushtaq, S.; Iftikhar, S. Analysis of phytochemical constituents of Eucalyptus citriodora L. responsible for antifungal activity against post-harvest fungi. Nat. Prod. Res. 2012, 26, 1732–1736. [Google Scholar] [CrossRef]
- Petretto, G.L.; Chessa, M.; Piana, A.; Masia, M.D.; Foddai, M.; Mangano, G.; Culeddu, N.; Afifi Fatma, U.; Pintore, G. Chemical and biological study on the essential oil of Artemisia caerulescens L. ssp. Densiflora (Viv.). Nat. Prod. Res. 2013, 27, 1709–1715. [Google Scholar] [CrossRef]
- Bouabidi, W.; Hanana, M.; Gargouri, S.; Amri, I.; Fezzani, T.; Ksontini, M.; Jamoussi, B.; Hamrouni, L. Chemical composition, phytotoxic and antifungal properties of Ruta chalepensis L. essential oils. Nat. Prod. Res. 2015, 29, 864–868. [Google Scholar] [CrossRef]
- Moghaddam, M.; Taheri, P.; Pirbalouti, A.G.; Mehdizadeh, L. Chemical composition and antifungal activity of essential oil from the seed of Echinophora platyloba DC. against phytopathogens fungi by two different screening methods. LWT-Food Sci. Technol. 2015, 61, 536–542. [Google Scholar] [CrossRef]
- Dupuy, N.; Gaydou, V.; Kister, J. Quantitative analysis of lavender (Lavandula angustifolia) essential oil using multiblock data from infrared spectroscopy. Am. J. Analyt. Chem. 2014, 5, 633–645. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).