Risk Assessment of Heavy Metals in Basmati Rice: Implications for Public Health
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Soil
2.3. Roots, Shoots, Grains
2.4. Digestion of Soil, Grains, Roots and Shoots Samples
2.5. Metal Analyses
2.6. Quality Control
2.7. Statistical Analysis
2.8. Bioaccumulation Factor
2.9. Translocation Factor (TF)
2.10. Pollution Load Index (PLI)
2.11. Enrichment Factor
2.12. Daily Intake of Metals
2.13. Health Risk Index
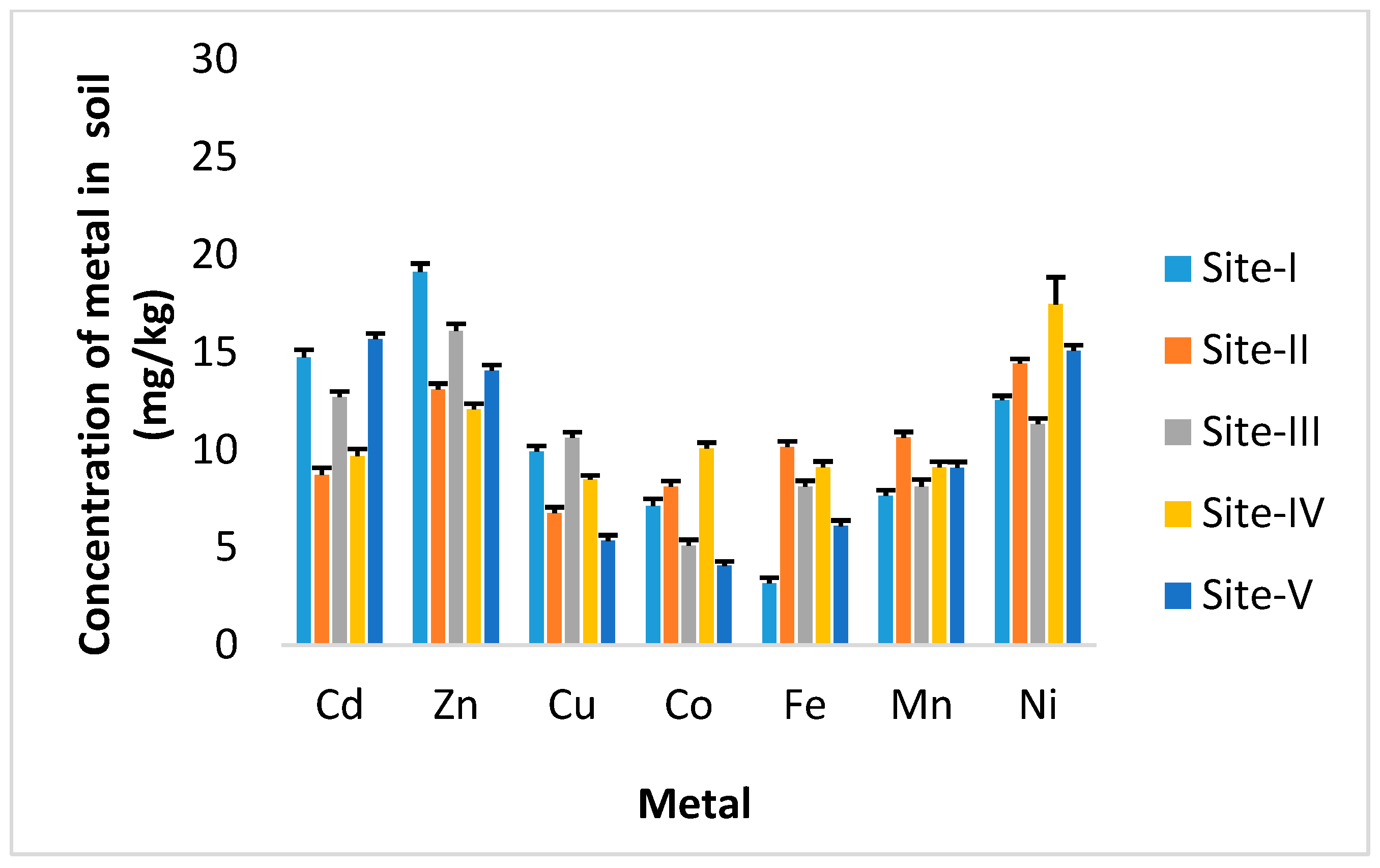
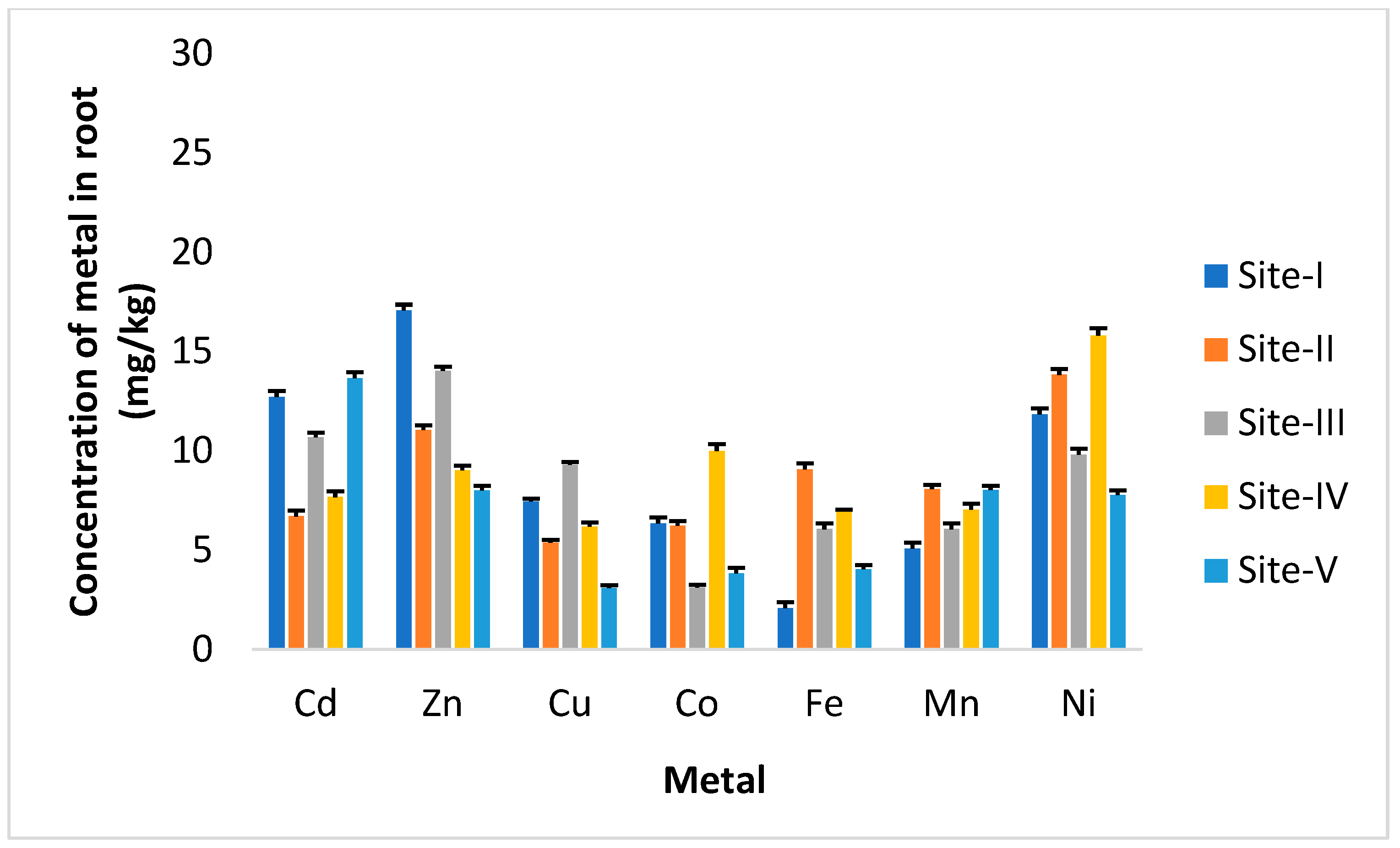
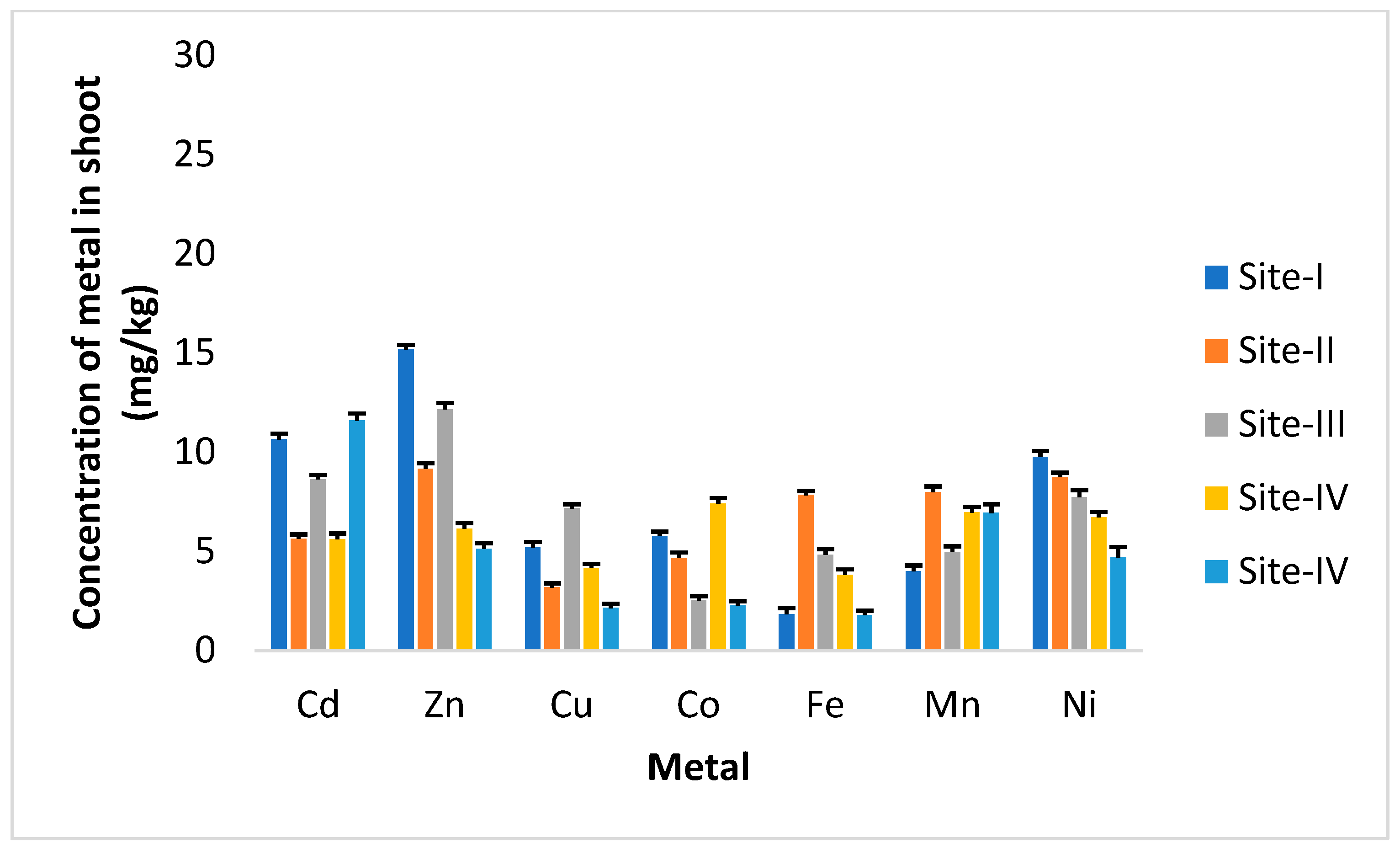
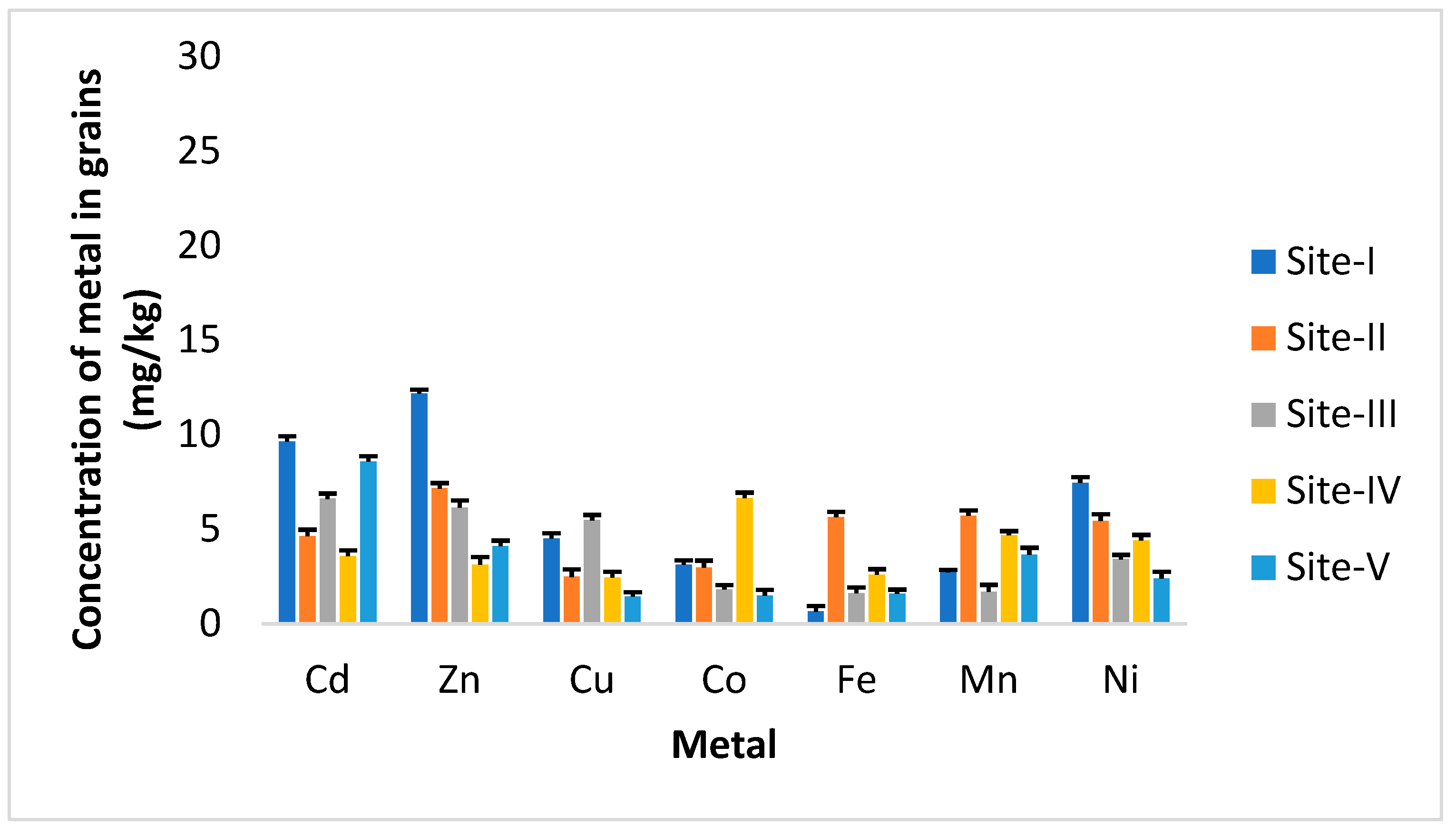
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fresco, L. Rice is life. J. Food Compos. Anal. 2005, 18, 249–253. [Google Scholar] [CrossRef]
- Kennedy, G.; Burlingame, B.; Nguyen, V.N. Nutrition contribution of rice and impact of biotechnology and biodiversity in rice consuming countries. In Proceedings of the 20th Session of the International Rice Commission, Bangkok, Thailand, 23–26 July 2002. [Google Scholar]
- Kennedy, G.; Burlingame, B. Analysis of food composition data on rice from a plant genetic resources perspective. Food Chem. 2003, 80, 589–596. [Google Scholar] [CrossRef]
- Agriculture Statistics of Pakistan 2010–2011; Government of Pakistan, Pakistan Bureau of Statistics: Islamabad, Pakistan, 2011.
- Saleem, M.H.; Ali, S.; Rehman, M.; Hasanuzzaman, M.; Rizwan, M.; Irshad, S.; Shafiq, F.; Iqbal, M.; Alharbi, B.M.; Alnusaire, T.S.; et al. Jute: A Potential Candidate for Phytoremediation of Metals—A Review. Plants 2020, 9, 258. [Google Scholar] [CrossRef]
- Saleem, M.H.; Ali, S.; Hussain, S.; Kamran, M.; Chattha, M.S.; Ahmad, S.; Aqeel, M.; Rizwan, M.; Aljarba, N.H.; Alkahtani, S.; et al. Flax (Linum usitatissimum L.): A Potential Candidate for Phytoremediation? Biological and Economical Points of View. Plants 2020, 9, 496. [Google Scholar] [CrossRef]
- Saleem, M.H.; Ali, S.; Rehman, M.; Rana, M.S.; Rizwan, M.; Kamran, M.; Imran, M.; Riaz, M.; Soliman, M.H.; Elkelish, A.; et al. Influence of phosphorus on copper phytoextraction via modulating cellular organelles in two jute (Corchorus capsularis L.) varieties grown in a copper mining soil of Hubei Province, China. Chemosphere 2020, 248, 126032. [Google Scholar] [CrossRef]
- Zaheer, I.E.; Ali, S.; Saleem, M.H.; Arslan Ashraf, M.; Ali, Q.; Abbas, Z.; Rizwan, M.; El-Sheikh, M.A.; Alyemeni, M.N.; Wijaya, L. Zinc-lysine Supplementation Mitigates Oxidative Stress in Rapeseed (Brassica napus L.) by Preventing Phytotoxicity of Chromium, When Irrigated with Tannery Wastewater. Plants 2020, 9, 1145. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.H.; Kamran, M.; Zhou, Y.; Parveen, A.; Rehman, M.; Ahmar, S.; Malik, Z.; Mustafa, A.; Anjum, R.M.A.; Wang, B.; et al. Appraising growth, oxidative stress and copper phytoextraction potential of flax (Linum usitatissimum L.) grown in soil differentially spiked with copper. J. Environ. Manag. 2020, 257, 109994. [Google Scholar] [CrossRef]
- Saleem, M.H.; Fahad, S.; Khan, S.U.; Din, M.; Ullah, A.; EL Sabagh, A.; Hossain, A.; Llanes, A.; Liu, L. Copper-induced oxidative stress, initiation of antioxidants and phytoremediation potential of flax (Linum usitatissimum L.) seedlings grown under the mixing of two different soils of China. Environ. Sci. Pollut. Res. 2019, 27, 5211–5221. [Google Scholar] [CrossRef]
- Zaheer, I.E.; Ali, S.; Saleem, M.H.; Imran, M.; Alnusairi, G.S.H.; Alharbi, B.M.; Riaz, M.; Abbas, Z.; Rizwan, M.; Soliman, M.H. Role of iron–lysine on morpho-physiological traits and combating chromium toxicity in rapeseed (Brassica napus L.) plants irrigated with different levels of tannery wastewater. Plant Physiol. Biochem. 2020, 155, 70–84. [Google Scholar] [CrossRef]
- Javed, M.T.; Saleem, M.H.; Aslam, S.; Rehman, M.; Iqbal, N.; Begum, R.; Ali, S.; Alsahli, A.A.; Alyemeni, M.N.; Wijaya, L. Elucidating silicon-mediated distinct morpho-physio-biochemical attributes and organic acid exudation patterns of cadmium stressed Ajwain (Trachyspermum ammi L.). Plant Physiol. Biochem. 2020, 157, 23–37. [Google Scholar] [CrossRef]
- Afzal, J.; Saleem, M.H.; Batool, F.; Elyamine, A.M.; Rana, M.S.; Shaheen, A.; El-Esawi, M.A.; Tariq Javed, M.; Ali, Q.; Arslan Ashraf, M.; et al. Role of Ferrous Sulfate (FeSO4) in Resistance to Cadmium Stress in Two Rice (Oryza sativa L.) Genotypes. Biomolecules 2020, 10, 1693. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, I.E.; Ali, S.; Saleem, M.H.; Ali, M.; Riaz, M.; Javed, S.; Sehar, A.; Abbas, Z.; Rizwan, M.; El-Sheikh, M.A.; et al. Interactive role of zinc and iron lysine on Spinacia oleracea L. growth, photosynthesis and antioxidant capacity irrigated with tannery wastewater. Physiol. Mol. Biol. Plants 2020, 26, 2435–2452. [Google Scholar] [CrossRef]
- Rehman, M.; Liu, L.; Wang, Q.; Saleem, M.H.; Bashir, S.; Ullah, S.; Peng, D. Copper environmental toxicology, recent advances, and future outlook: A review. Environ. Sci. Pollut. Res. 2019, 26, 18003–18016. [Google Scholar] [CrossRef]
- Saleem, M.H.; Ali, S.; Seleiman, M.F.; Rizwan, M.; Rehman, M.; Akram, N.A.; Liu, L.; Alotaibi, M.; Al-Ashkar, I.; Mubushar, M. Assessing the Correlations between Different Traits in Copper-Sensitive and Copper-Resistant Varieties of Jute (Corchorus capsularis L.). Plants 2019, 8, 545. [Google Scholar] [CrossRef]
- Saleem, M.H.; Fahad, S.; Rehman, M.; Saud, S.; Jamal, Y.; Khan, S.; Liu, L. Morpho-physiological traits, biochemical response and phytoextraction potential of short-term copper stress on kenaf (Hibiscus cannabinus L.) seedlings. PeerJ 2020, 8, e8321. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.; Danish, M.; Saleem, M.H.; Malik, Z.; Parveen, A.; Abbasi, G.H.; Jamil, M.; Ali, S.; Afzal, S.; Riaz, M.; et al. Application of abscisic acid and 6-benzylaminopurine modulated morpho-physiological and antioxidative defense responses of tomato (Solanum lycopersicum L.) by minimizing cobalt uptake. Chemosphere 2021, 263, 128169. [Google Scholar] [CrossRef]
- Saleem, M.H.; Ali, S.; Kamran, M.; Iqbal, N.; Azeem, M.; Javed, M.T.; Ali, Q.; Haider, M.Z.; Irshad, S.; Rizwan, M.; et al. Ethylenediaminetetraacetic Acid (EDTA) Mitigates the Toxic Effect of Excessive Copper Concentrations on Growth, Gaseous Exchange and Chloroplast Ultrastructure of Corchorus capsularis L. and Improves Copper Accumulation Capabilities. Plants 2020, 9, 756. [Google Scholar] [CrossRef] [PubMed]
- Hashem, I.A.; Abbas, A.Y.; El-Hamed, A.E.-N.H.A.; Salem, H.M.; El-Hosseiny, O.E.; Abdel-Salam, M.A.; Saleem, M.H.; Zhou, W.; Hu, R. Potential of rice straw biochar, sulfur and ryegrass (Lolium perenne L.) in remediating soil contaminated with nickel through irrigation with untreated wastewater. PeerJ 2020, 8, e9267. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.I.; Safdar, H.; Ahmad, K.; Wajid, K.; Bashir, H.; Ugulu, I.; Dogan, Y. Copper bioaccumulation and translocation in forages grown in soil irrigated with sewage water. Pak. J. Bot. 2020, 52, 111–119. [Google Scholar] [CrossRef]
- Dogan, Y.; Ugulu, I.; Durkan, N.; Unver, M.C.; Mert, H.H. Determination of some ecological characteristics and economical importance of Vitex agnus-castus. Eurasia. J. Biosci. 2011, 5, 10–18. [Google Scholar] [CrossRef]
- Yorek, N.; Ugulu, I.; Sahin, M.; Dogan, Y. A qualitative investigation of students’ understanding about ecosystem and its components. Nat. Montenegrina 2010, 9, 931–981. [Google Scholar]
- Khan, Z.I.; Ahmad, K.; Safdar, H.; Ugulu, I.; Wajid, K.; Bashir, H.; Dogan, Y. Manganese bioaccumulation and translocation of in forages grown in soil irrigated with city effluent: An evaluation on health risk. Res. J. Pharmaceut. Biol. Chem. Sci. 2018, 9, 759–770. [Google Scholar]
- Khan, Z.I.; Ahmad, K.; Rehman, S.; Ashfaq, A.; Mehmood, N; Ugulu, I.; Dogan, Y. Effect of sewage water irrigation on accumulation of metals in soil and wheat in Punjab, Pakistan. Pak. J. Anal. Environ. Chem. 2019, 20, 60–66. [Google Scholar]
- Ugulu, I. Development and validation of an instrument for assessing attitudes of high school students about recycling. Environ. Educ. Res. 2015, 21, 916–942. [Google Scholar]
- Ugulu, I.; Khan, Z.I.; Sheik, Z.; Ahmad, K.; Bashir, H.; Ashfaq, A. Effect of wastewater irrigation as an alternative irrigation resource on heavy metal accumulation in ginger (Zingiber officinale Rosc.) and human health risk from consumption. Arab. J. Geosci. 2021, 14, 702. [Google Scholar] [CrossRef]
- Ugulu, I.; Unver, M.C.; Dogan, Y. Potentially toxic metal accumulation and human health risk from consuming wild Urtica urens sold on the open markets of Izmir. Euro-Mediterr. J. Environ. Integr. 2019, 4, 36. [Google Scholar] [CrossRef]
- Ugulu, I.; Khan, Z.I.; Rehman, S.; Ahmad, K.; Munir, M.; Bashir, H.; Nawaz, K. Trace Metal Accumulation in Trigonella foenum-graecum Irrigated with Wastewater and Human Health Risk of Metal Access Through the Consumption. Bull. Environ. Contam. Toxicol. 2019, 103, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Qureshi, T.M.; Ugulu, I.; Riaz, M.N.; An, Q.U.; Khan, Z.I.; Ahmad, K.; Ashfaq, A.; Bashir, H.; Dogan, Y. Mineral, vitamin and phenolic contents and sugar profiles of some prominent date palm (Phoenix dactylifera) varieties of Pakistan. Pak. J. Bot. 2019, 51, 171–178. [Google Scholar]
- Ugulu, I.; Akhter, P.; Khan, Z.I.; Akhtar, M.; Ahmad, K. Trace metal accumulation in pepper (Capsicum annuum L.) grown using organic fertilizers and health risk assessment from consumption. Food Res. Int. 2021, 140, 109992. [Google Scholar]
- Khan, Z.I.; Ugulu, I.; Zafar, A.; Mehmood, N.; Bashir, H.; Ahmad, K.; Sana, M. Biomonitoring of heavy metals accumulation in wild plants growing at soon valley, Khushab, Pakistan. Pak. J. Bot. 2021, 53, 247–252. [Google Scholar]
- Wang, M.-J. Land application of sewage sludge in China. Sci. Total Environ. 1997, 197, 149–160. [Google Scholar] [CrossRef]
- Ugulu, I.; Khan, Z.I.; Aslam, Z.; Ahmad, K.; Bashir, H.; Munir, M. Potentially toxic metal accumulation in grains of wheat variety Galaxy-2013 irrigated with sugar industry wastewater and human health risk assessment. Euro-Mediterr. J. Environ. Integr. 2021, 6, 38. [Google Scholar] [CrossRef]
- Khan, Z.I.; Ugulu, I.; Sahira, S.; Mehmood, N.; Ahmad, K.; Bashir, H.; Dogan, Y. Human health risk assessment through the comparative analysis of diverse irrigation regimes for Luffa (Luffa cylindrica (L.) Roem.). J. Water Sanit. Hyg. Dev. 2020, 10, 249–261. [Google Scholar] [CrossRef]
- Vukadinović, V.; Bertić, B. Agrochemistry and Plant Nutrition Practicum. Ph.D. Thesis, University J.J. Osijek, Osijek, Croatia, 1988. [Google Scholar]
- Steel, R.; Torrie, J.H. Principle and Procedures of Statistics, A Biometrical Approach, 2nd ed.; McGraw Hill: New York, NY, USA, 1980. [Google Scholar]
- Cui, Y.-J.; Zhu, Y.-G.; Zhai, R.-H.; Chen, D.-Y.; Huang, Y.-Z.; Qiu, Y.; Liang, J.-Z. Transfer of metals from soil to vegetables in an area near a smelter in Nanning, China. Environ. Int. 2004, 30, 785–791. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, J.Z.; Ouyang, Z.Y.; Soderlund, L.; Liu, G.H. Impacts of sewage irrigation on heavy metals distribution and contamination. Environ. Int. 2005, 31, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Tinker, P.B.; MacPherson, A.; West, T.S. Levels, distribution and chemical forms of trace elements in food plants. Philos. Trans. R. Soc. B Biol. Sci. 1981, 294, 41–55. [Google Scholar]
- Chary, N.S.; Kamala, C.; Raj, D.S.S. Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. Ecotoxicol. Environ. Saf. 2008, 69, 513–524. [Google Scholar] [CrossRef]
- Ge, K.Y. The Status of Nutrient and Meal of Chinese in the 1990s; People’s Health & Hygiene Press: Beijing, China, 1992; pp. 415–434. [Google Scholar]
- Wang, X.; Sato, T.; Xing, B.; Tao, S. Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci. Total Environ. 2005, 350, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Preliminary Remediation Goals, Region 9; USEPA. United States Environmental Protection Agency: Washington, DC, USA, 2002.
- Angula, E. The Tomlinson pollution index applied to heavy metal, mussel-watch data: A useful index to assess coastal pollution. Sci. Total Environ. 1996, 187, 19–56. [Google Scholar] [CrossRef]
- Harikumar, P.S.; Nasir, U.P.; Rahman, M.P.M. Distribution of heavy metals in the core sediments of a tropical wetland system. Int. J. Environ. Sci. Technol. 2009, 6, 225–232. [Google Scholar] [CrossRef]
- Jaishree, A.; Khan, T.I. Assessment of heavy metals’ risk on human health via dietary intake of cereals and vegetables from effluent irrigated land Jaipur district, Rajasthan. Int. J. Innov. Res. Sci. Eng. Technol. 2015, 4, 5142–5148. [Google Scholar]
- European Union. Commission Regulation (EC) No. 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in foodstuffs. Off. J. Eur. Union 2006, 364, 5–24. [Google Scholar]
- Exposure Factors Handbook; VolumeII-Food Ingestion Factors. EPA/600//P-95/002Fa; USEPA. United States Environmental Protection Agency: Washington, DC, USA, 1997.
- Othman, A.A.; Ali, R.; Othman, A.M.; Ali, J.; Habila, M.A. Assessment of toxic metals in wheat crops grown in selected soils, irrigated by different water sources. Arab. J. Chem. 2016, 9, 1555–1562. [Google Scholar] [CrossRef]
- Lorestani, B.; Cheraghi, M.; Yousefi, N. Accumulation of Pb, Fe, Mn, Cu and Zn in plants and choice of hyper accumulator plants in the industrial town of Vian, Iran. Arch. Biol. Sci. 2011, 63, 739–745. [Google Scholar] [CrossRef]
- Juen, L.L.; Aris, A.Z.; Ying, L.W.; Haris, H. Bioconcentration and translocation efficiency of metals in paddy (Oryza sativa): A case study from Alor Setar, Kedah, Malaysia. Sains Malays. 2014, 43, 521–528. [Google Scholar]
- FAO/WHO. Codex Alimentarius Commission, Food Additives and Contaminants. Joint FAO/WHO Food Standards Programme, ALINORM 01/12A: 1-289, Food and Agriculture. Available online: http://www.fao.org (accessed on 27 July 2001).
- Eticha, T.; Hymete, A. Determination of some heavy metals in barley locally grown for brewing and its malt in Ethiopia. J. Bioanal. Biomed. 2015, 7, 171–173. [Google Scholar]
- Singh, A.; Sharma, R.K.; Agarwal, M.; Marshal, F.M. Health risk assessment of heavy metals via dietary intake of foodstuffs from wastewater irrigated site of dry tropical area of India. Food Chem. Toxicol. 2010, 48, 611–619. [Google Scholar] [CrossRef]
- Dutch Standards. Circular on Target Values and Intervention Values for Soil Remediation; Ministry of Housing, Spatial Planning and the Environment: Bilthoven, The Netherlands, 2000.
- Dosumu, O.O.; Abdus-Salam, N.; Oguntoye, S.; Afdekale, F.A. Trace metals bioaccumulation by some Nigerian vegetables. Centrepoint 2005, 13, 23–32. [Google Scholar]
- Singh, R.; Singh, D.P.; Kumar, N.; Bhargavai, S.K.; Barman, S.C. Accumulation of translocation of heavy metals in soil and plants from fly ash contaminated area. J. Environ. Biol. 2010, 31, 421–430. [Google Scholar]
- Wajid, K.; Ahmad, K.; Khan, Z.I.; Nadeem, M.; Bashir, H.; Chen, F.; Ugulu, I. Effect of organic manure and mineral fertilizers on bioaccumulation and translocation of trace metals in maize. Bull. Environ. Contam. Toxicol. 2020, 104, 649–657. [Google Scholar]
- Badawy, K.R.; Abd El-Gawad, A.M.; Osman, H.E. Health risks assessment of heavy metals and microbial contamination in water, soil and agricultural foodstuff from wastewater irrigation at Sahl El-Hessania area, Egyptian. J. Appl. Sci. Res. 2013, 9, 3091–3107. [Google Scholar]
- Singh, J.; Upadhyay, S.K.; Pathak, R.K.; Gupta, V. Accumulation of heavy metals in soil and paddy crop (Oryza sativa), irrigated with water of Ramgarh Lake, Gorakhpur, UP, India. Toxicol. Environ. Chem. 2010, 93, 462–472. [Google Scholar] [CrossRef]
- Brunetti, G.; Farrag, K.; Soler-Rovira, P.; Ferrara, M.; Nigro, F.; Senesi, N. Heavy metals accumulation and distribution in durum wheat and barley grown in contaminated soils under Mediterranean field conditions. J. Plant Interact. 2012, 7, 160–174. [Google Scholar] [CrossRef][Green Version]
- Khan, Z.I.; Arshad, N.; Ahmad, K.; Nadeem, M.; Ashfaq, A.; Wajid, K.; Bashir, H.; Munir, M.; Huma, B.; Memoona, H.; et al. Toxicological potential of cobalt in forage for ruminants grown in polluted soil: A health risk assessment from trace metal pollution for livestock. Environ. Sci. Pollut. Res. 2019, 26, 15381–15389. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Malik, R.N. Human health risk assessment of heavy metals via consumption of contaminated vegetables collected from different irrigation sources in Lahore, Pakistan. Arab. J. Chem. 2014, 7, 91–99. [Google Scholar] [CrossRef]
- Ogunkunle, C.O.; Varun, M.; Jimoh, M.A.; Olorunmaiye, K.S.; Fatoba, P.O. Evaluating the trace metal pollution of an urban paddy soil and bioaccumulation in rice (Oryza sativa L.) with the associated dietary risks to local population: A case study of Ilorin, north-central Nigeria. Environ. Earth Sci. 2016, 75, 1383. [Google Scholar] [CrossRef]
- Balkhair, K.; Ashraf, M.A. Field accumulation risks of heavy metals in soil and vegetable crop irrigated with sewage water in western region of Saudi Arabia. Saudi J. Biol. Sci. 2016, 23, S32–S44. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.I.; Nisar, A.; Ugulu, I.; Ahmad, K.; Wajid, K.; Bashir, H.; Dogan, Y. Determination of cadmium concentrations of vegetables grown in soil irrigated with wastewater: Evaluation of health risk to the public. Egypt. J. Bot. 2019, 59, 753–762. [Google Scholar]
- Satpathy, D.; Reddy, M.V.; Dhal, S.P. Risk assessment of heavy metals contamination in paddy soil, plants, and grains (Oryza sativa L.) at the East Coast of India. Biomed. Res. Int. 2014, 2014, 545473. [Google Scholar] [CrossRef]
- Bibi, Z.; Khan, Z.I.; Ahmad, K.; Ashraf, M.; Hussain, A.; Akram, N.A. Vegetables as a potential source of metals and metalloids for human nutrition, A case study of (Momordica charantia) grown in soil irrigated with domestic sewage water in Sargodha, Pakistan. Pak. J. Zool. 2014, 46, 633–664. [Google Scholar]
- Fan, Y.; Zhu, T.; Li, M.; He, J.; Huang, R. Heavy Metal Contamination in Soil and Brown Rice and Human Health Risk Assessment near Three Mining Areas in Central China. J. Healthc. Eng. 2017, 2017, 4124302. [Google Scholar] [CrossRef] [PubMed]

| Metal | Cd | Co | Zn | Fe | Cu | Mn | Ni |
|---|---|---|---|---|---|---|---|
| Wave length (nm) | 228.8 | 250.0 | 213.9 | 248.3 | 324.8 | 279.5 | 232.0 |
| Lamp current low (mA) | 8 | 9.5 | 8 | 12 | 6 | 12 | 12 |
| Slit width (nm) | 0.7 | 0.2 | 0.7 | 0.2 | 0.7 | 0.2 | 0.2 |
| Air flow rate (L/min) | 15 | 15 | 15 | 15 | 15 | 15 | 15 |
| Burner height (mm) | 7 | 10 | 7 | 9 | 7 | 9 | 7 |
| Source of Variation | Degree of Freedom | Mean Square | ||||||
|---|---|---|---|---|---|---|---|---|
| Cd | Zn | Cu | Co | Fe | Mn | Ni | ||
| Paddy Soil | ||||||||
| Sites | 4 | 0.206 | 0.004 | 0.004 | 0.002 | 0.002 | 0.003 | 0.002 |
| Error | 10 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| Root | ||||||||
| Sites | 4 | 0.158 *** | 0.073 *** | 0.001 *** | 0.001 *** | 0.124 *** | 0.002 *** | 0.001 *** |
| Error | 10 | 0.002 | 0.001 | 0.001 | 0.001 | 0.002 | 0.001 | 0.001 |
| Shoot | ||||||||
| Sites | 4 | 0.003 *** | 0.002 *** | 0.003 *** | 0.002 *** | 0.002 *** | 0.002 *** | 0.001 *** |
| Error | 10 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| Grain | ||||||||
| Sites | 4 | 0.004 *** | 0.002 *** | 0.001 *** | 0.002 *** | 0.002 *** | 0.003 *** | 0.002 *** |
| Error | 10 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| Site | Metal | ||||||
|---|---|---|---|---|---|---|---|
| Cd | Zn | Cu | Co | Fe | Mn | Ni | |
| Pollution load index | |||||||
| Site-I | 0.0928 | 0.3235 | 0.4217 | 0.4767 | 0.3222 | 0.3285 | 0.7139 |
| Site-II | 0.9732 | 0.2779 | 0.5389 | 0.3441 | 0.1287 | 0.2631 | 0.0433 |
| Site-III | 0.1924 | 0.3681 | 0.2989 | 0.4845 | 0.2338 | 0.3491 | 0.2624 |
| Site-IV | 0.7707 | 0.2318 | 0.2938 | 0.6279 | 0.3390 | 0.3059 | 0.9296 |
| Site-V | 0.6510 | 0.2763 | 0.4114 | 0.5504 | 0.1103 | 0.2414 | 0.3761 |
| Bioaccumulation factor | |||||||
| Site-I | 0.8129 | 0.6891 | 0.4147 | 0.4682 | 0.6611 | 0.7237 | 0.6001 |
| Site-II | 0.9873 | 0.4753 | 0.3212 | 0.6309 | 0.4245 | 0.6559 | 0.6105 |
| Site-III | 0.4453 | 0.7264 | 0.4952 | 0.5292 | 0.6834 | 0.6170 | 0.6482 |
| Site-IV | 0.2574 | 0.4688 | 0.5172 | 1.1664 | 0.6779 | 0.4231 | 0.3587 |
| Site-V | 0.2376 | 0.5559 | 0.3380 | 0.2930 | 0.3279 | 0.4462 | 0.2603 |
| Enrichment factor | |||||||
| Site-I | 6.0563 | 0.3045 | 0.1731 | 2.0131 | 0.0884 | 0.0677 | 0.5437 |
| Site-II | 7.355 | 0.2100 | 0.1348 | 2.7130 | 0.0568 | 0.0613 | 0.5531 |
| Site-III | 3.3177 | 0.3210 | 0.2077 | 2.2754 | 0.0914 | 0.0577 | 0.5873 |
| Site-IV | 1.9173 | 0.2072 | 0.2161 | 5.0155 | 0.0907 | 0.0396 | 0.3250 |
| Site-V | 1.7705 | 0.2457 | 0.1418 | 1.2601 | 0.0439 | 0.0417 | 0.2358 |
| Translocation factor | |||||||
| Site-I | 0.9019 | 0.8824 | 0.9423 | 0.8721 | 0.9392 | 0.9157 | 0.8964 |
| Site-II | 0.9255 | 0.9616 | 0.6756 | 0.9068 | 0.7082 | 0.8914 | 0.9222 |
| Site-III | 0.8790 | 0.9781 | 0.5891 | 0.8909 | 0.8615 | 0.8528 | 0.8752 |
| Site-IV | 0.5572 | 0.8514 | 0.8640 | 0.8173 | 0.8491 | 0.7281 | 0.8753 |
| Site-V | 0.6312 | 0.7864 | 0.5625 | 0.8361 | 0.5740 | 0.7486 | 0.5931 |
| Site | Metal | ||||||
|---|---|---|---|---|---|---|---|
| Cd | Zn | Cu | Co | Fe | Mn | Ni | |
| Daily intake of metal | |||||||
| Site-I | 0.0564 | 0.0566 | 0.0084 | 0.0225 | 0.0697 | 0.0639 | 0.0536 |
| Site-II | 0.0505 | 0.0336 | 0.0083 | 0.0339 | 0.0179 | 0.0465 | 0.0641 |
| Site-III | 0.0274 | 0.0671 | 0.0071 | 0.0223 | 0.0523 | 0.0579 | 0.0764 |
| Site-IV | 0.0215 | 0.0276 | 0.0073 | 0.0625 | 0.0752 | 0.0348 | 0.0361 |
| Site-V | 0.0156 | 0.0390 | 0.0067 | 0.0106 | 0.0118 | 0.0289 | 0.0187 |
| Health risk index | |||||||
| Site-I | 0.0378 | 0.0013 | 0.0010 | 0.5235 | 0.0012 | 0.0014 | 0.0059 |
| Site-II | 0.0339 | 0.0008 | 0.0009 | 0.7885 | 0.0003 | 0.0001 | 0.0072 |
| Site-III | 0.0184 | 0.0015 | 0.0008 | 0.5187 | 0.0009 | 0.0012 | 0.0084 |
| Site-IV | 0.0145 | 0.0006 | 0.0007 | 0.4529 | 0.0013 | 0.0007 | 0.0040 |
| Site-V | 0.0105 | 0.0009 | 0.0007 | 0.2469 | 0.0002 | 0.0006 | 0.0021 |
| RFD * mg/kg/day) | 1 × 10−3 | 3 × 10−1 | 4 × 10−2 | 43 × 10−2 | 1 × 10−1 | 14 × 10−1 | 2 × 10−2 |
| Metal | Soil-Root | Root-Shoot | Shoot-Grain |
|---|---|---|---|
| Cd | 0.169 | 0.664 | 0.893 * |
| Zn | 0.983 ** | 0.953 ** | 0.899 * |
| Cu | 0.548 | 0.480 | 0.820 |
| Co | 0.815 | 0.986 ** | 0.919 * |
| Fe | 0.988 ** | 0.991 ** | 1.00 ** |
| Mn | 0.981 | 0.949 | 0.978 |
| Ni | 0.879 | 0.982 | 0.988 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tariq, F.; Wang, X.; Saleem, M.H.; Khan, Z.I.; Ahmad, K.; Saleem Malik, I.; Munir, M.; Mahpara, S.; Mehmood, N.; Ahmad, T.; et al. Risk Assessment of Heavy Metals in Basmati Rice: Implications for Public Health. Sustainability 2021, 13, 8513. https://doi.org/10.3390/su13158513
Tariq F, Wang X, Saleem MH, Khan ZI, Ahmad K, Saleem Malik I, Munir M, Mahpara S, Mehmood N, Ahmad T, et al. Risk Assessment of Heavy Metals in Basmati Rice: Implications for Public Health. Sustainability. 2021; 13(15):8513. https://doi.org/10.3390/su13158513
Chicago/Turabian StyleTariq, Farah, Xiukang Wang, Muhammad Hamzah Saleem, Zafar Iqbal Khan, Kafeel Ahmad, Ifra Saleem Malik, Mudasra Munir, Shehzadi Mahpara, Naunain Mehmood, Tasneem Ahmad, and et al. 2021. "Risk Assessment of Heavy Metals in Basmati Rice: Implications for Public Health" Sustainability 13, no. 15: 8513. https://doi.org/10.3390/su13158513
APA StyleTariq, F., Wang, X., Saleem, M. H., Khan, Z. I., Ahmad, K., Saleem Malik, I., Munir, M., Mahpara, S., Mehmood, N., Ahmad, T., Memona, H., Ugulu, I., Fiaz, S., & Ali, S. (2021). Risk Assessment of Heavy Metals in Basmati Rice: Implications for Public Health. Sustainability, 13(15), 8513. https://doi.org/10.3390/su13158513








