Seafood Processing Chitin Waste for Electricity Generation in a Microbial Fuel Cell Using Halotolerant Catalyst Oceanisphaera arctica YHY1
Abstract
1. Introduction
2. Materials and Methods
2.1. Chitin Preparation, Isolation, and Identification of Chitin Degrading Microbes
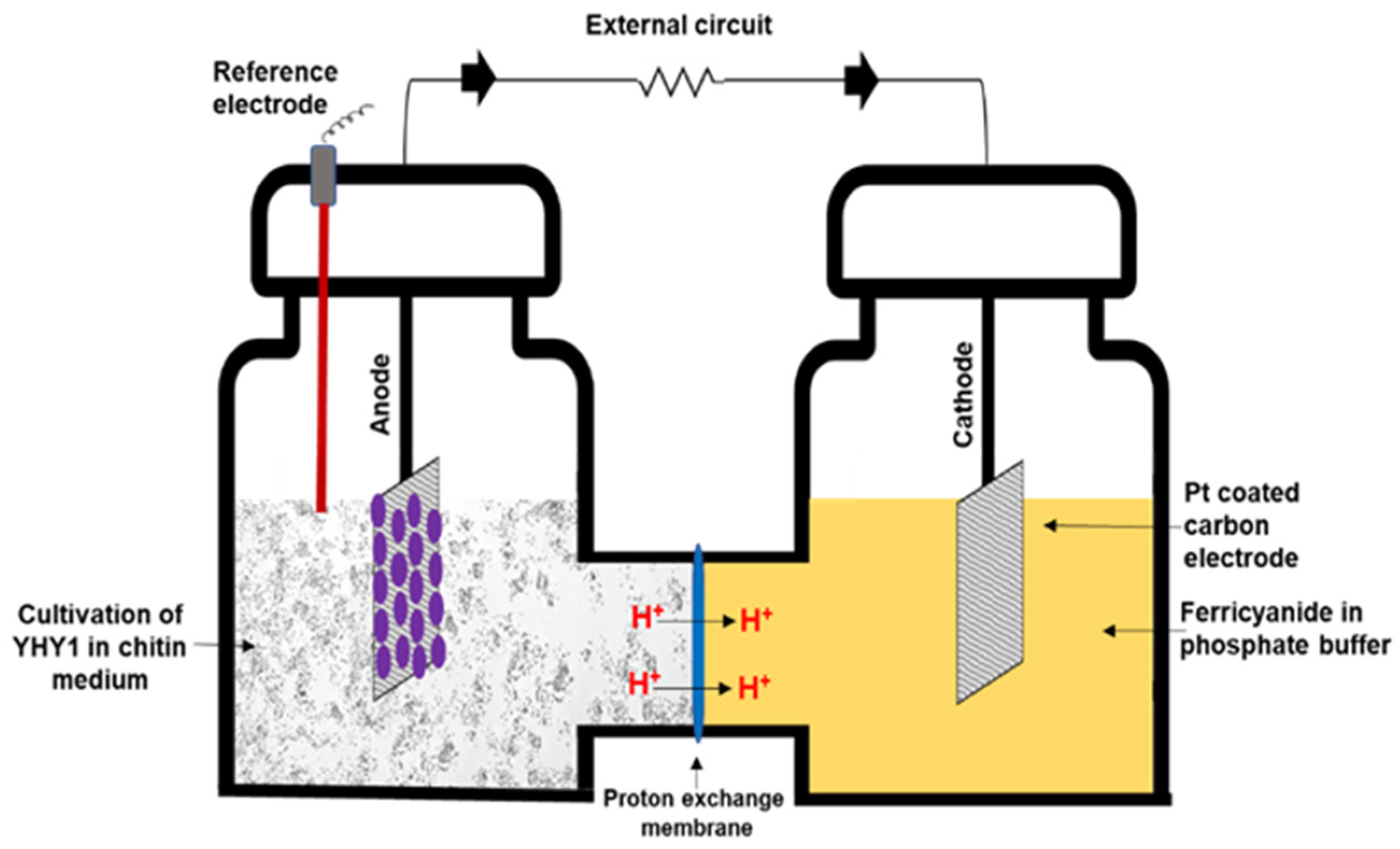
2.2. MFC Setup and Electrochemical Analysis
2.3. Analysis of Degradation Products
2.4. SEM, XRD and FTIR Analysis
3. Results
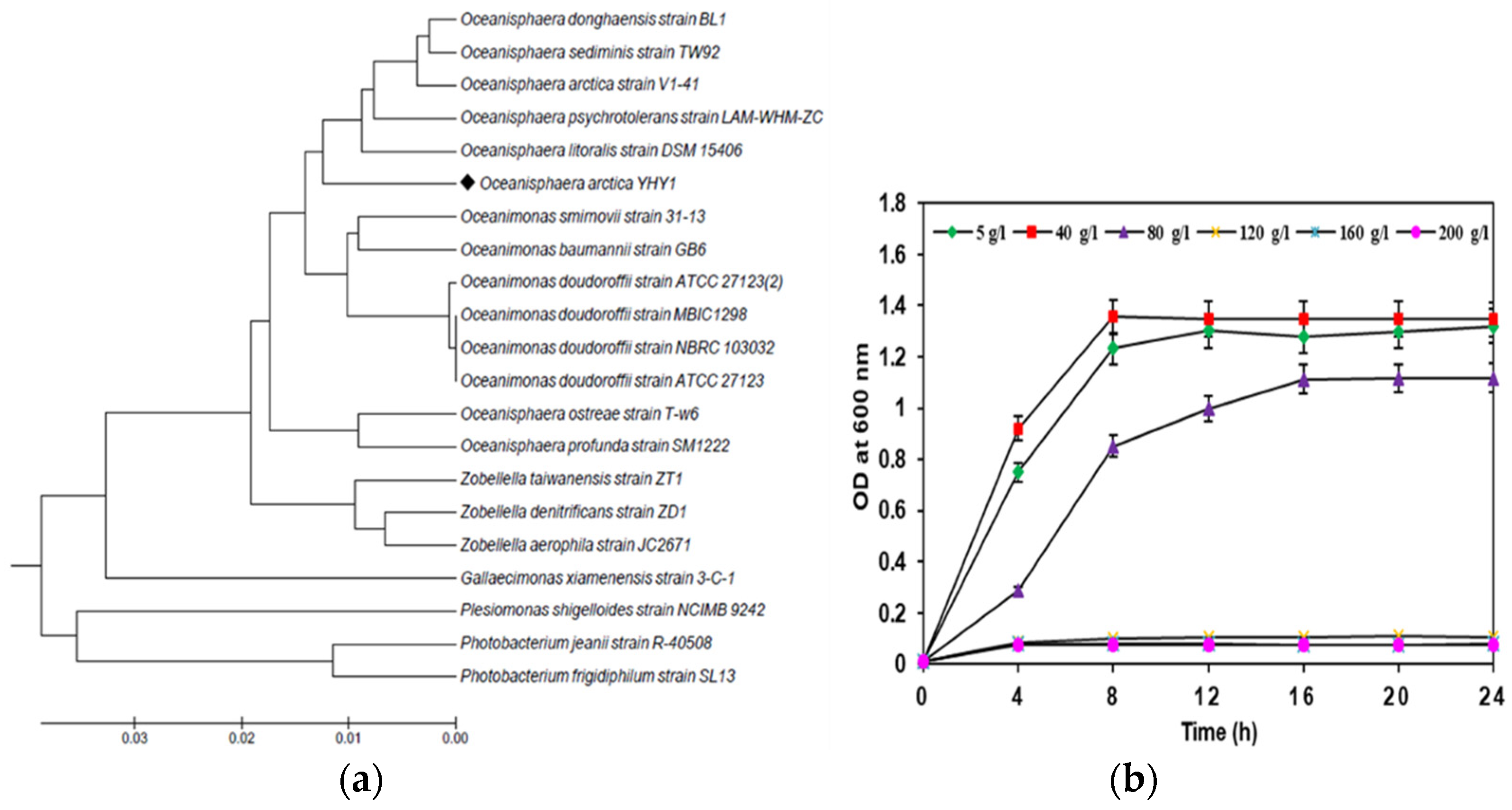
3.1. Identification, and Salt-Tolerance in the Chitinolytic Bacterium
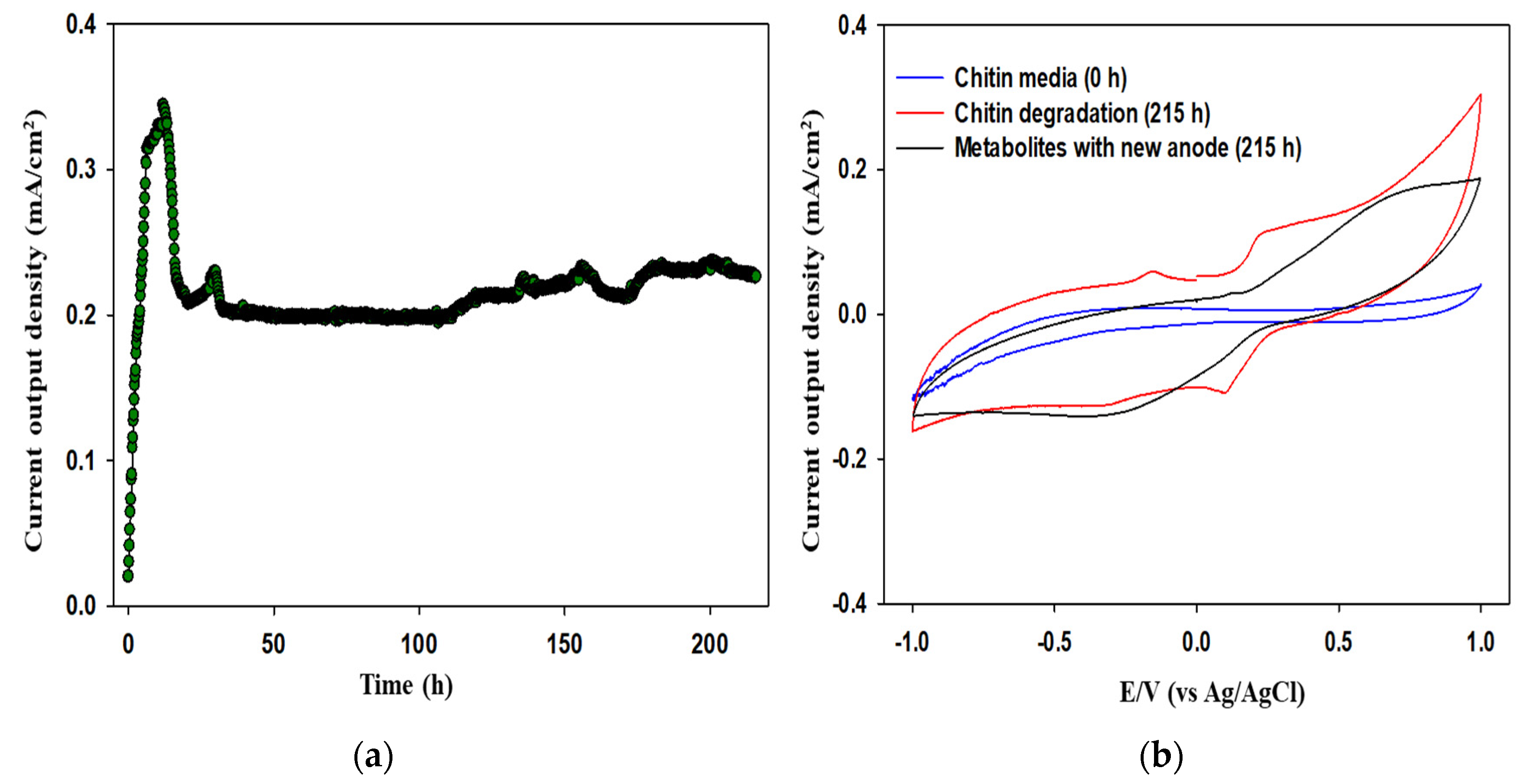
3.2. Electrochemical Assessment of MFCs Fueled with Chitin Waste
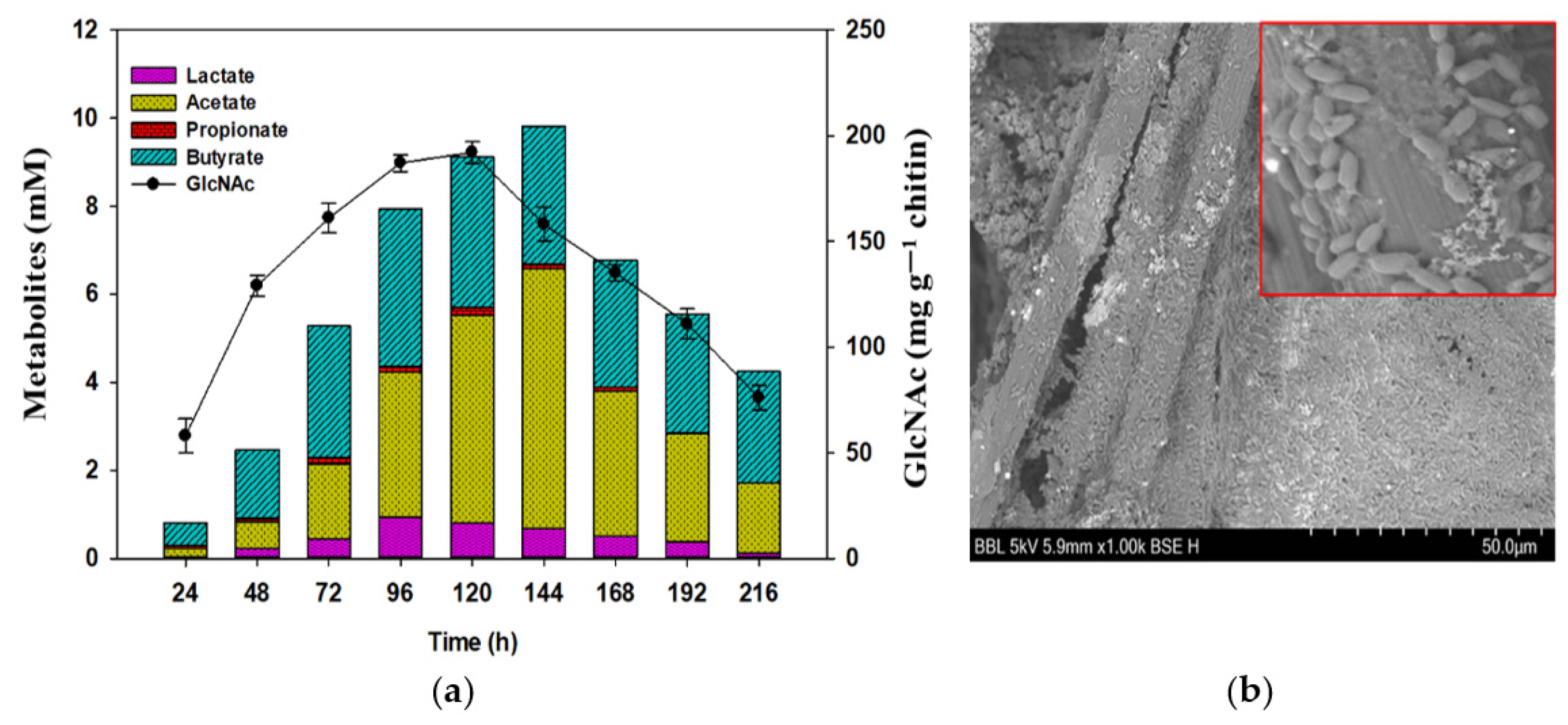
3.3. Chitin Degradation By-Products and Other Metabolites
3.4. Investigating Structural Changes in Chitin Polymer in MFCs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nirmal, N.P.; Santivarangkna, C.; Rajput, M.S.; Benjakul, S. Trends in shrimp processing waste utilization: An industrial prospective. Trends Food Sci. Technol. 2020, 103, 20–35. [Google Scholar] [CrossRef]
- Senphan, T.; Benjakul, S.; Kishimura, H. Characteristics and antioxidative activity of carotenoprotein from shells of Pacific white shrimp extracted using hepatopancreas proteases. Food Biosci. 2014, 5, 54–63. [Google Scholar] [CrossRef]
- Djellouli, M.; López-Caballero, M.E.; Arancibia, M.Y.; Karam, N.; Martínez-Alvarez, O. Antioxidant and antimicrobial enhancement by reaction of protein hydrolysates derived from shrimp by-products with glucosamine. Waste Biomass Valorization 2020, 11, 2491–2505. [Google Scholar] [CrossRef]
- Tan, Y.N.; Lee, P.P.; Chen, W.N. Microbial extraction of chitin from seafood waste using sugars derived from fruit waste-stream. AMB Express 2020, 10, 17. [Google Scholar] [CrossRef]
- Ching, Y.C.; Redzwan, G. Biological treatment of fish processing saline wastewater for reuse as liquid fertilizer. Sustainability 2017, 9, 1062. [Google Scholar] [CrossRef]
- Gurav, R.; Tang, J.; Jadhav, J. Novel chitinase producer Bacillus pumilus RST25 isolated from the shellfish processing industry revealed antifungal potential against phyto-pathogens. Int. Biodeterior. Biodegrad. 2017, 125, 228–234. [Google Scholar] [CrossRef]
- Gurav, R.; Bhatia, S.K.; Choi, T.R.; Jung, H.R.; Yang, S.Y.; Song, H.S.; Park, Y.L.; Han, Y.H.; Park, J.Y.; Kim, Y.G.; et al. Chitin biomass powered microbial fuel cell for electricity production using halophilic Bacillus circulans BBL03 isolated from sea salt harvesting area. Bioelectrochemistry 2019, 130, 107329. [Google Scholar] [CrossRef]
- Rezaei, F.; Richard, T.L.; Logan, B.E. Analysis of chitin particle size on maximum power generation, power longevity, and Coulombic efficiency in solid–substrate microbial fuel cells. J. Power Sources 2009, 192, 304–309. [Google Scholar] [CrossRef]
- Gurav, R.; Bhatia, S.K.; Choi, T.R.; Kim, H.J.; Song, H.S.; Park, S.L.; Lee, S.M.; Lee, H.S.; Kim, S.H.; Yoon, J.J.; et al. Utilization of different lignocellulosic hydrolysates as carbon source for electricity generation using novel Shewanella marisflavi BBL25. J. Clean. Prod. 2020, 277, 124084. [Google Scholar] [CrossRef]
- Gurav, R.; Bhatia, S.K.; Moon, Y.M.; Choi, T.R.; Jung, H.R.; Yang, S.Y.; Song, H.S.; Jeon, J.M.; Yoon, J.J.; Kim, Y.G.; et al. One-pot exploitation of chitin biomass for simultaneous production of electricity, n-acetylglucosamine and polyhydroxyalkanoates in microbial fuel cell using novel marine bacterium Arenibacter palladensis YHY2. J. Clean. Prod. 2019, 209, 324–332. [Google Scholar] [CrossRef]
- Li, S.-W.; Zeng, R.J.; Sheng, G.-P. An excellent anaerobic respiration mode for chitin degradation by Shewanella oneidensis MR-1 in microbial fuel cells. Biochem. Eng. J. 2017, 118, 20–24. [Google Scholar] [CrossRef]
- Li, S.W.; He, H.; Zeng, R.J.; Sheng, G.P. Chitin degradation and electricity generation by Aeromonas hydrophila in microbial fuel cells. Chemosphere 2017, 168, 293–299. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Gurav, R.; Choi, T.R.; Jung, H.R.; Yang, S.Y.; Moon, Y.M.; Song, H.S.; Jeon, J.M.; Choi, K.Y.; Yang, Y.H. Bioconversion of plant biomass hydrolysate into bioplastic (polyhydroxyalkanoates) using Ralstonia eutropha 5119. Bioresour. Technol. 2019, 271, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Focher, B.; Beltrame, P.L.; Naggi, A.; Torri, G. Alkaline N-deacetylation of chitin enhanced by flash treatments. Reaction kinetics and structure modifications. Carbohydr. Polym. 1990, 12, 405–418. [Google Scholar] [CrossRef]
- Faria, A.; Gonçalves, L.; Peixoto, J.M.; Peixoto, L.; Brito, A.G.; Martins, G. Resources recovery in the dairy industry: Bioelectricity production using a continuous microbial fuel cell. J. Clean. Prod. 2017, 140, 971–976. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Fu, G.; Zhang, Z. Simultaneous electricity generation and nitrogen and carbon removal in single-chamber microbial fuel cell for high-salinity wastewater treatment. J. Clean. Prod. 2020, 276, 123203. [Google Scholar] [CrossRef]
- Abbas, S.Z.; Rafatullah, M.; Ismail, N.; Shakoori, F.R. Electrochemistry and microbiology of microbial fuel cells treating marine sediments polluted with heavy metals. RSC Adv. 2018, 8, 18800–18813. [Google Scholar] [CrossRef]
- Carmona-Martinez, A.A.; Harnisch, F.; Fitzgerald, L.A.; Biffinger, J.C.; Ringeisen, B.R.; Schröder, U. Cyclic voltammetric analysis of the electron transfer of Shewanella oneidensis MR-1 and nanofilament and cytochrome knock-out mutants. Bioelectrochemistry 2011, 81, 74–80. [Google Scholar] [CrossRef]
- Richter, H.; Nevin, K.P.; Jia, H.; Lowy, D.A.; Lovley, D.R.; Tender, L.M. Cyclic voltammetry of biofilms of wild type and mutant Geobacter sulfurreducens on fuel cell anodes indicates possible roles of OmcB, OmcZ, type IV pili, and protons in extracellular electron transfer. Energy Environ. Sci. 2009, 2, 506–516. [Google Scholar] [CrossRef]
- Qian, X.; Reguera, G.; Mester, T.; Lovley, D.R. Evidence that OmcB and OmpB of Geobacter sulfurreducens are outer membrane surface proteins. FEMS Microbiol. Lett. 2007, 277, 21–27. [Google Scholar] [CrossRef]
- Stephen, C.S. Abundance of the multiheme c-type cytochrome omcB increases in outer biofilm layers of electrode-grown Geobacter sulfurreducens. PLoS ONE 2014, 9, 104336. [Google Scholar] [CrossRef]
- Steidl, R.J.; Lampa-Pastirk, S.; Reguera, G. Mechanistic stratification in electroactive biofilms of Geobacter sulfurreducens mediated by pilus nanowires. Nat. Commun. 2016, 7, 12217. [Google Scholar] [CrossRef] [PubMed]
- Gurav, R.; Bhatia, S.K.; Choi, T.R.; Choi, Y.K.; Kim, H.J.; Song, H.S.; Lee, S.M.; Park, S.L.; Lee, H.S.; Koh, J.; et al. Application of macroalgal biomass derived biochar and bioelectrochemical system with Shewanella for the adsorptive removal and biodegradation of toxic azo dye. Chemosphere 2021, 264, 128539. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Kim, K.Y. Antagonistic potential of Bacillus pumilus L1 against root-knot nematode, Meloidogyne arenaria. J. Phytopathol. 2016, 164, 29–39. [Google Scholar] [CrossRef]
- Mao, L.; Verwoerd, W.S. Theoretical exploration of optimal metabolic flux distributions for extracellular electron transfer by Shewanella oneidensis MR-1. Biotechnol. Biofuels 2014, 7, 118. [Google Scholar] [PubMed]
- Nakagawa, G.; Kouzuma, A.; Hirose, A.; Kasai, T.; Yoshida, G.; Watanabe, K. Metabolic characteristics of a glucose-utilizing Shewanella oneidensis strain grown under electrode-respiring conditions. PLoS ONE 2015, 10, 0138813. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Wei, G.; Mo, X.; Zhou, N.; Chen, K.; Ouyang, P. Enzymatic hydrolysis of chitin pretreated by bacterial fermentation to obtain pure N-acetyl-d-glucosamine. Green Chem. 2018, 20, 2320–2327. [Google Scholar] [CrossRef]
- Kumari, S.; Rath, P.; Kumar, A.S.; Tiwari, T.N. Extraction and characterization of chitin and chitosan from fishery waste by chemical method. Environ. Technol. Innov. 2015, 3, 77–85. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurav, R.; Bhatia, S.K.; Choi, T.-R.; Kim, H.-J.; Lee, H.-J.; Cho, J.-Y.; Ham, S.; Suh, M.-J.; Kim, S.-H.; Kim, S.-K.; et al. Seafood Processing Chitin Waste for Electricity Generation in a Microbial Fuel Cell Using Halotolerant Catalyst Oceanisphaera arctica YHY1. Sustainability 2021, 13, 8508. https://doi.org/10.3390/su13158508
Gurav R, Bhatia SK, Choi T-R, Kim H-J, Lee H-J, Cho J-Y, Ham S, Suh M-J, Kim S-H, Kim S-K, et al. Seafood Processing Chitin Waste for Electricity Generation in a Microbial Fuel Cell Using Halotolerant Catalyst Oceanisphaera arctica YHY1. Sustainability. 2021; 13(15):8508. https://doi.org/10.3390/su13158508
Chicago/Turabian StyleGurav, Ranjit, Shashi Kant Bhatia, Tae-Rim Choi, Hyun-Joong Kim, Hong-Ju Lee, Jang-Yeon Cho, Sion Ham, Min-Ju Suh, Sang-Hyun Kim, Sun-Ki Kim, and et al. 2021. "Seafood Processing Chitin Waste for Electricity Generation in a Microbial Fuel Cell Using Halotolerant Catalyst Oceanisphaera arctica YHY1" Sustainability 13, no. 15: 8508. https://doi.org/10.3390/su13158508
APA StyleGurav, R., Bhatia, S. K., Choi, T.-R., Kim, H.-J., Lee, H.-J., Cho, J.-Y., Ham, S., Suh, M.-J., Kim, S.-H., Kim, S.-K., Yoo, D.-W., & Yang, Y.-H. (2021). Seafood Processing Chitin Waste for Electricity Generation in a Microbial Fuel Cell Using Halotolerant Catalyst Oceanisphaera arctica YHY1. Sustainability, 13(15), 8508. https://doi.org/10.3390/su13158508






