Enhancement of Biogas Production via Co-Digestion of Wastewater Treatment Sewage Sludge and Brewery Spent Grain: Physicochemical Characterization and Microbial Community
Abstract
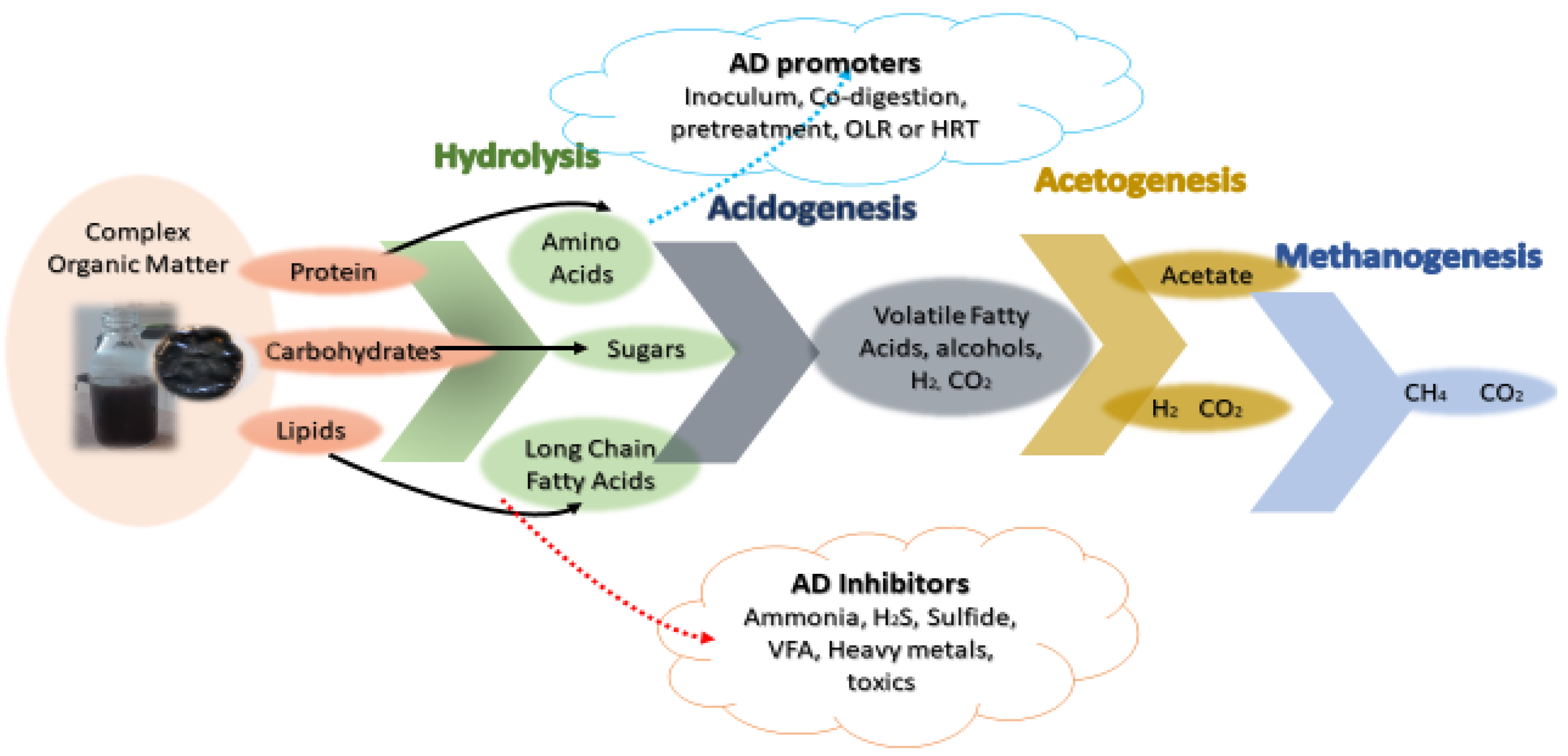
:1. Introduction
2. Materials and Method
2.1. Substrate Sampling and Source
2.2. Inoculum Preparation
2.3. Analysis Techniques
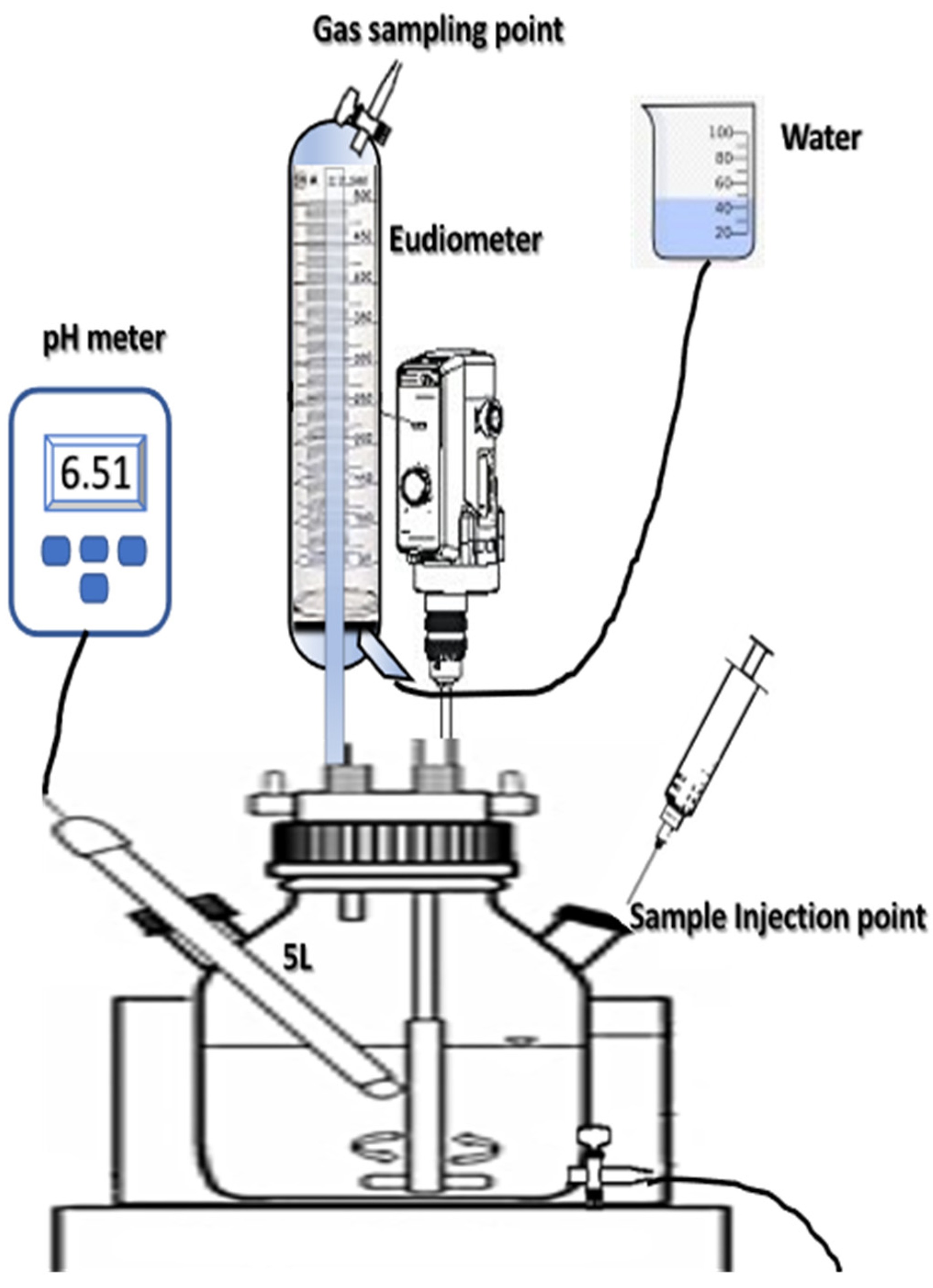
2.4. Batch Co-Digestion Experiment (Biochemical Methane Potential Apparatus)
Characteristics of Sewage Sludge and Spent Grain
2.5. Semi-Continuous Experimental Set-Up
3. Results and Discussion
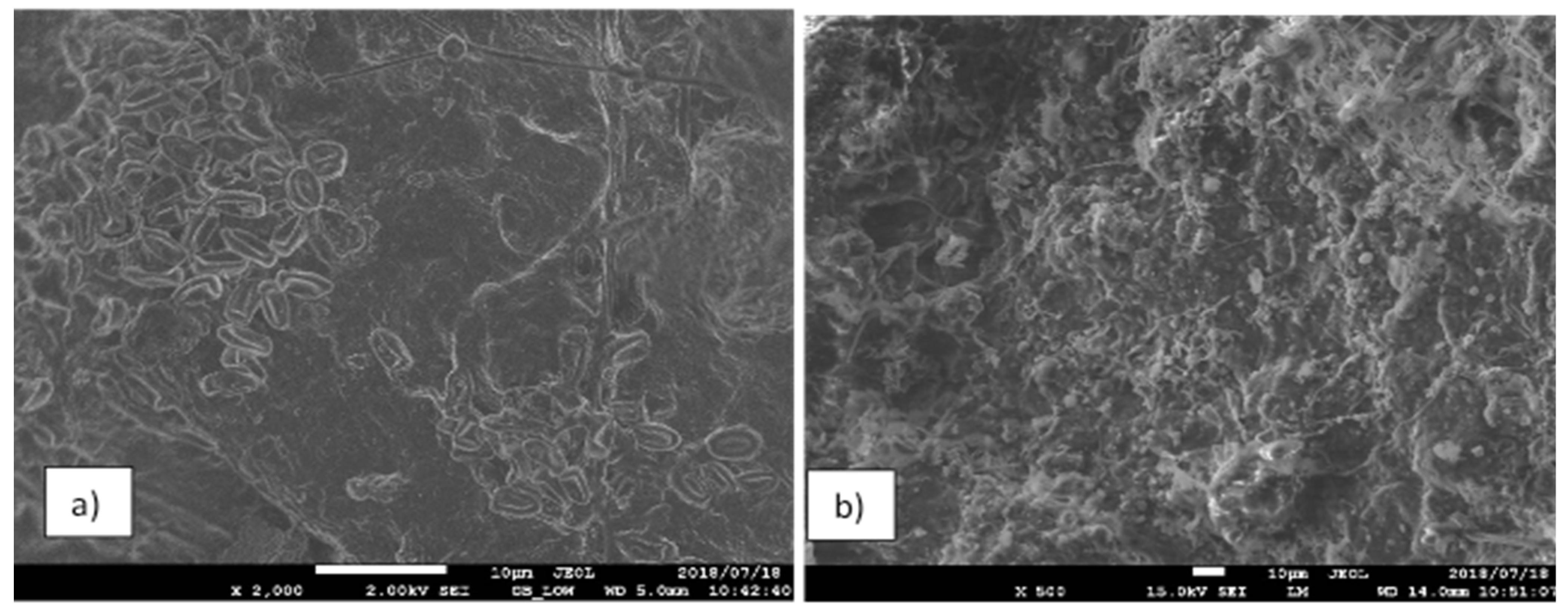
3.1. SEM Imaging
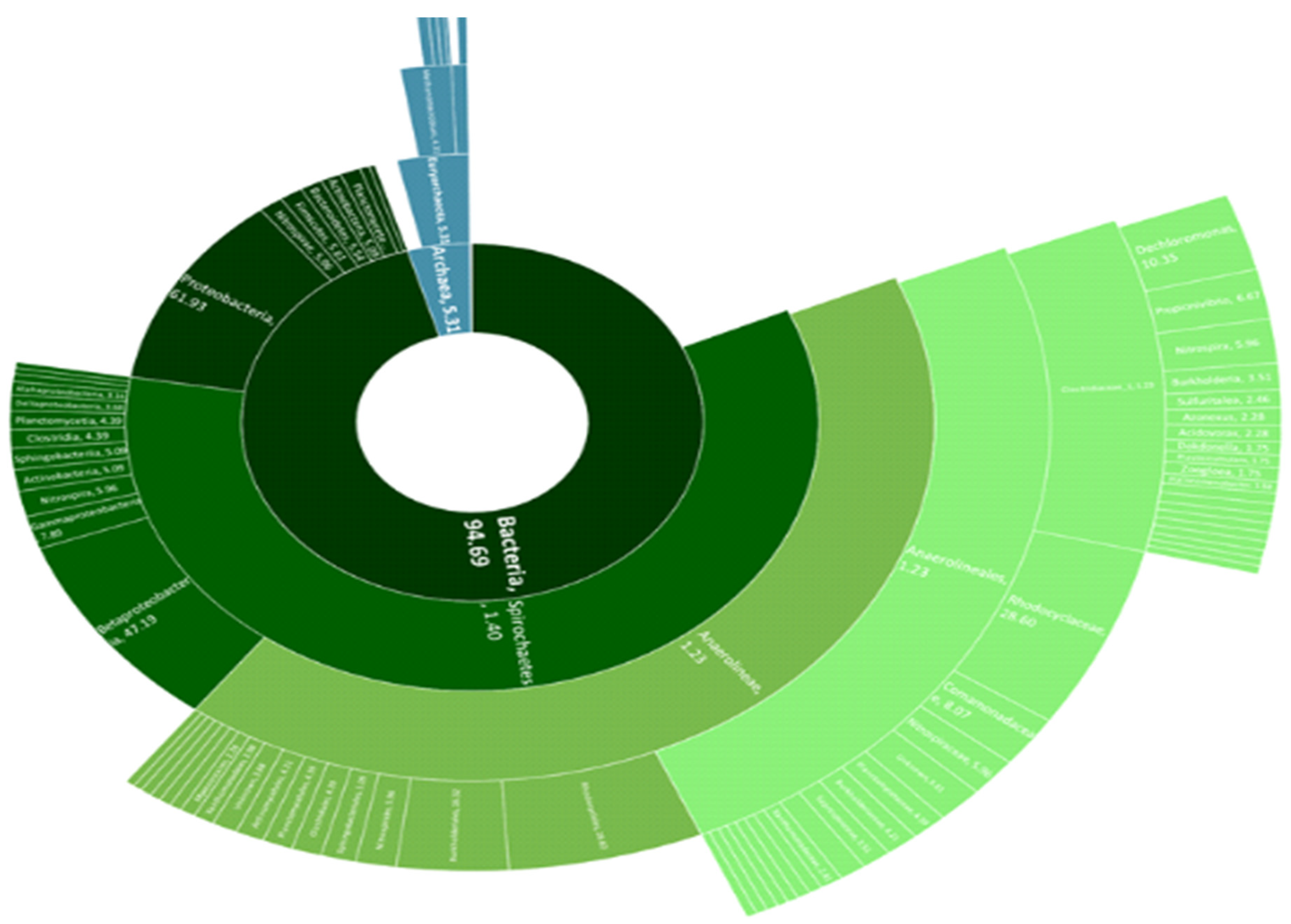
3.2. Taxonomic Profile of the Biogas Microbial Community
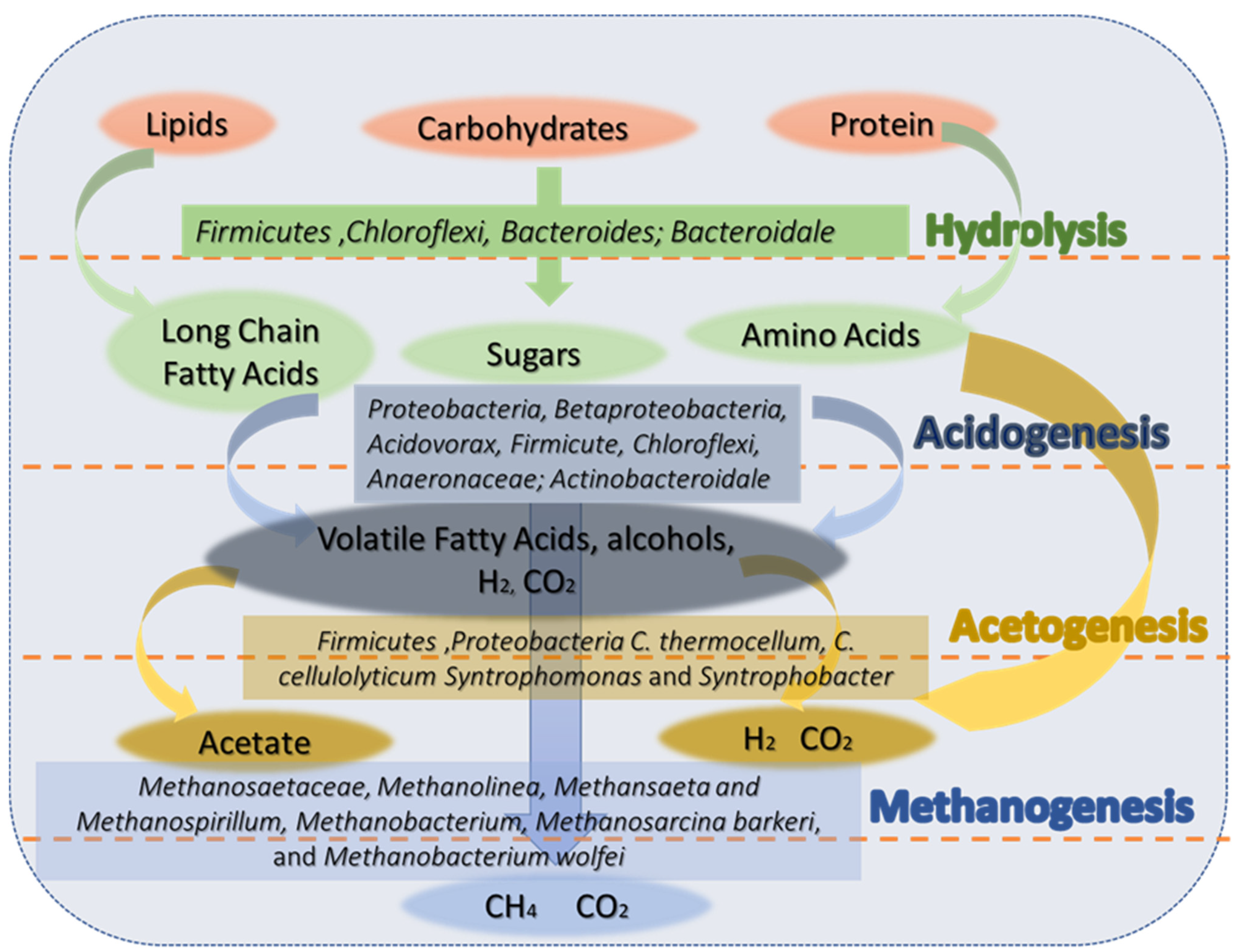
3.3. Metabolic Pathway Analysis
3.4. Anaerobic Co-Digestion Experiments
3.4.1. Methane Production: Batch Studies
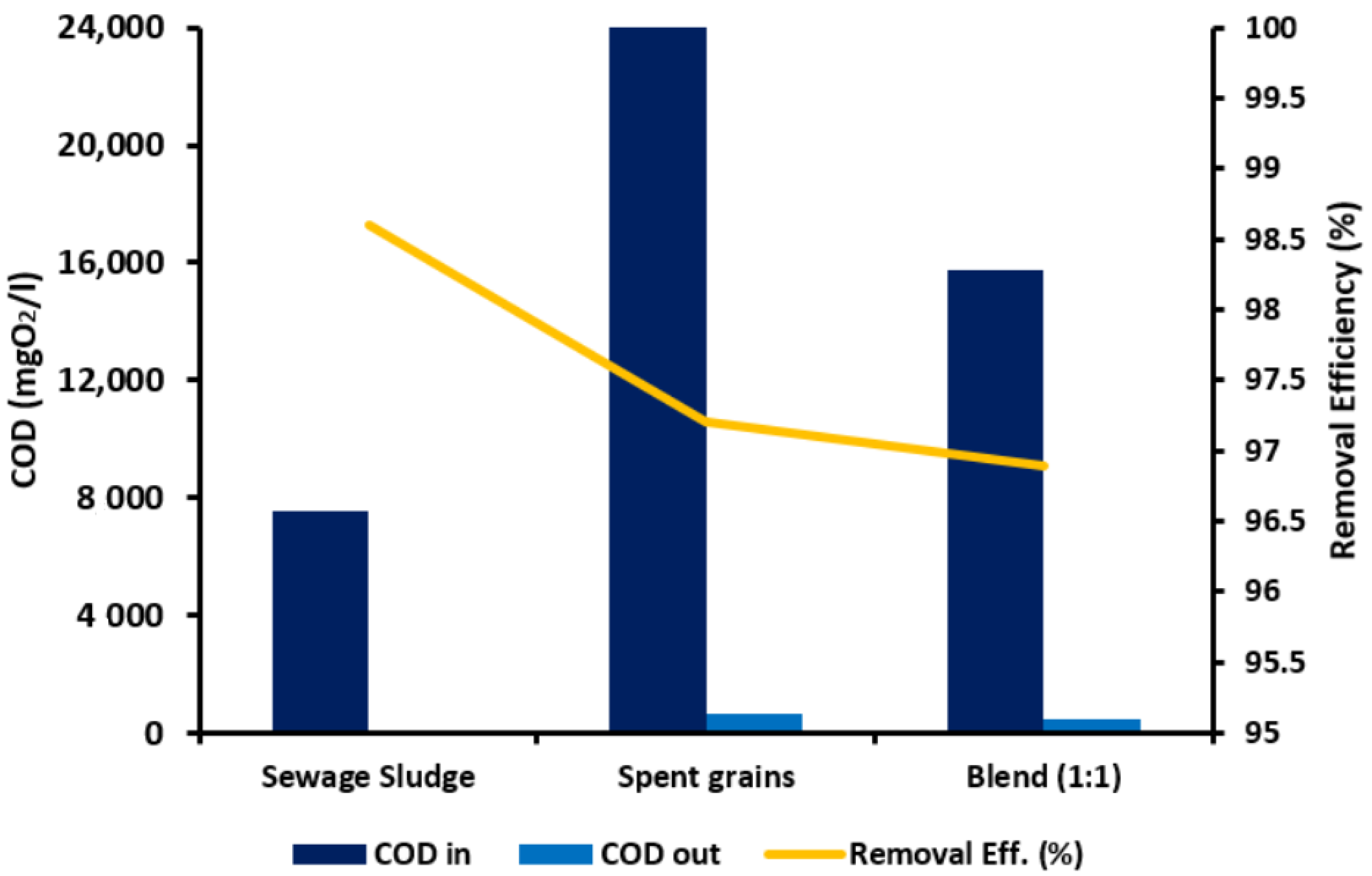
3.4.2. COD Removal Efficiency in AD
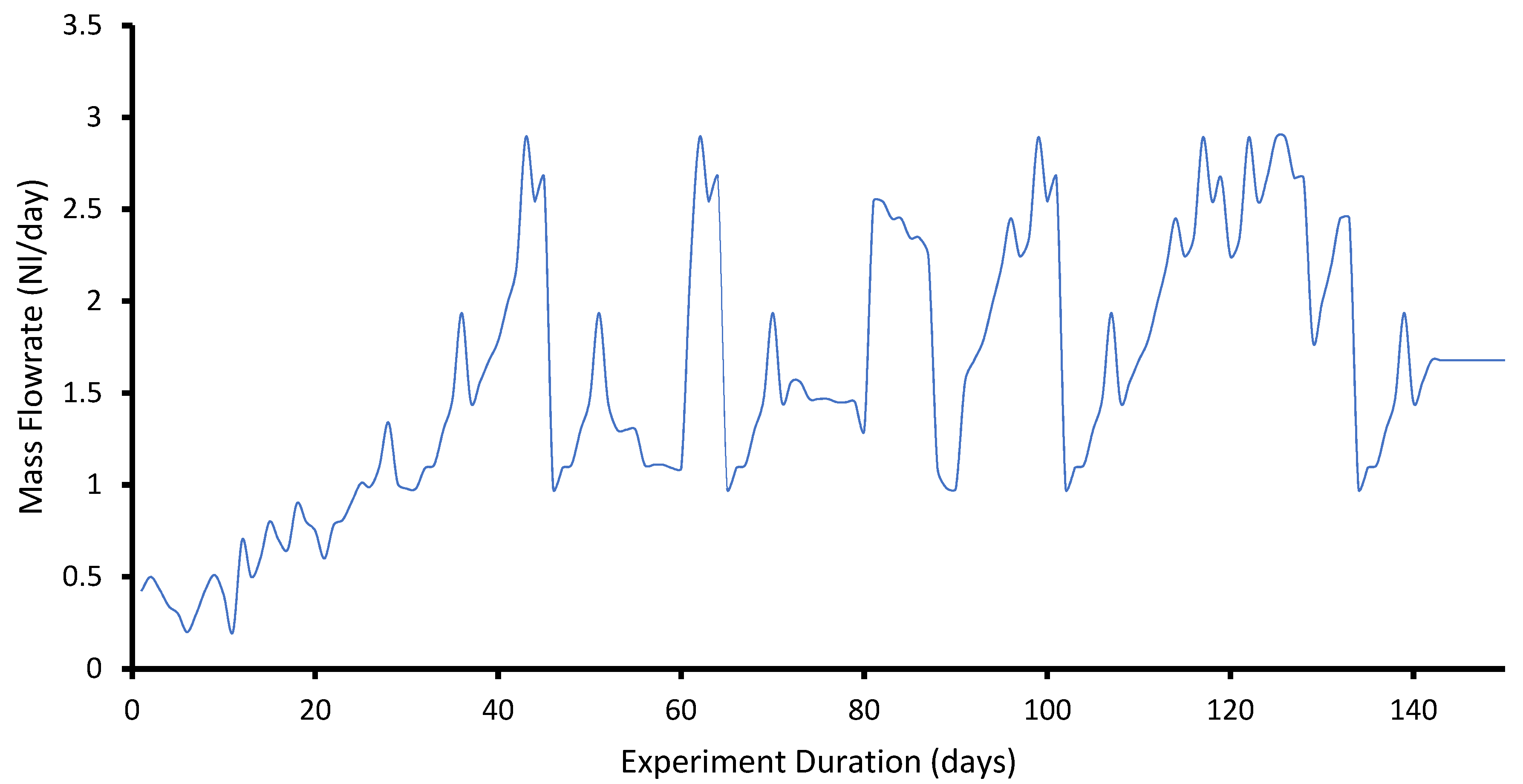
3.5. Biogas Production: Semi-Continuous Studies
4. Conclusions
- The microbial analysis indicated that Proteobacteria is the most abundant phylum, followed by the Bacteroidetes, Firmicutes, and Actinobacteria. The Bacteroidetes consist of fermentative bacteria, which are capable of hydrolysing and fermenting organic substances and acids into CO2 and H2. During the steady-state period, a significant shift occurred, with Proteobacteria becoming the most abundant phylum and hydrogenotrophic methanogens dominating over aceticlastic methanogens. The archaea community of hydrogenotrophic methanogen genus enrichment includes methanogens such as Methanobacterium, thermoautotrophicum, Methanosarcina barkeri, and Methanobacterium wolfei.
- The results confirm that the conducted BMP tests can definitely be used to assess the biogas and methane production from the co-digestion process. It was found that the cumulative biogas production of the mixture of sewage sludge and spent grain increased with increasing proportions of the spent grains. However, a negative effect on kinetics was observed in the presence of BSG and a major decline was observed for shortened HRT of 18 d, which seems to indicate the need to extend HRT. Importantly, the application of BSG, the substrate that is rich in organic compounds, significantly enhanced methane production. Regardless of the HRT, a stable process performance was maintained in co-digestion runs. However, a significant reduction in the biogas yield, from 10.23 to 2.02 NL/day, was also observed in a semi-continuous setup when the OLR reached a level of 2.90 gVS/L.day.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitchell, S.A.; de Wit, M.P.; Blignaut, J.N.; Crookes, D. Waste Water Treatment Plants: The Financing Mechanisms Associated with Achieving Green Drop Rating; Water Research Commission: Pretoria, South Africa, 2014. [Google Scholar]
- Van der Merwe-Botha, M.; Juncker, K.; Visser, A.; Boyd, R. Guiding Principles in the Design and Operation of a Wastewater Sludge Digestion Plant with Biogas and Power Generation: Report to the Water Research Commission; Water Research Commission: Pretoria, South Africa, 2016. [Google Scholar]
- Ntombela, C.; Funke, N.; Meissner, R.; Steyn, M.; Masangane, W. A Critical Look at South Africa’s Green Drop Programme. Water SA 2016, 42, 703–710. [Google Scholar] [CrossRef] [Green Version]
- Masindi, V.; Chatzisymeon, E.; Kortidis, I.; Foteinis, S. Assessing the sustainability of acid mine drainage (AMD) treatment in South Africa. Sci. Total Environ. 2018. [Google Scholar] [CrossRef] [Green Version]
- WRC. Permissible Utilisation and Disposal of Sewage Sludge, 1st ed.; Water Research Commission: Pretoria, South Africa, 1997; p. 83. [Google Scholar]
- Herbig, F.J.W. Talking dirty—Effluent and sewage irreverence in South Africa: A conservation crime perspective. Cogent Soc. Sci. 2019, 5, 1–18. [Google Scholar] [CrossRef]
- Hansen, K. Overview of Wastewater Treatment in South Africa; AWARD Technical Report Series; Association for Water and Rural Development (AWARD): Hoedspruit Limpopo, South Africa, 2015; pp. 25–63. [Google Scholar]
- Wirth, R.; Kovács, E.; Maróti, G.; Bagi, Z.; Rákhely, G.; Kovács, K.L. Characterization of a biogas-producing microbial community by short-read next generation DNA sequencing. Biotechnol. Biofuels 2012, 5, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Department of Energy. State of Renewable Energy in South Africa; Department of Energy: Pretoria, South Africa, 2017. [Google Scholar]
- Department of Energy. The South African Energy Sector Report 2019; Department of Energy: Pretoria, South Africa, 2019. [Google Scholar]
- National Planning Commission. National Development Plan (2030); National Planning Commission: Pretoria, South Africa, 2010; p. 70. ISBN 978-0-621-41180-5. [Google Scholar]
- Franks, L.; Euston-Brown, M.; Ferry, A.; Giljova, S. Municipal Wastewater Treatment Works—Biogas to Energy; GIZ-SALGA Municipal Renewable Energy Case Study Series; SALGA: Johannesburg, South Africa, 2013; pp. 1–6. [Google Scholar]
- Oke, M.A.; Suffian, M.; Annuar, M.; Simarani, K. Mixed Feedstock Approach to Lignocellulosic Ethanol Production—Prospects and Limitations. BioEnergy Res. 2016. [Google Scholar] [CrossRef] [Green Version]
- Cavinato, C. Anaerobic Digestion Fundamentals I; Summer School on Biogas Technology for Sustainable Second-Generation Biofuel Production; University of Jyvaskyla: Venezia, UK, 2011; pp. 1–47. [Google Scholar]
- Guo, J.; Peng, Y.; Ni, B.J.; Han, X.; Fan, L.; Yuan, Z.; Liu, C.; Li, H.; Zhang, Y.; Si, D.; et al. The Microbiology of Anaerobic Digesters. Environ. Technol. 2015, 14, 1–11. [Google Scholar] [CrossRef]
- Doloman, A.; Soboh, Y.; Walters, A.J.; Sims, R.C.; Miller, C.D. Qualitative Analysis of Microbial Dynamics during Anaerobic Digestion of Microalgal Biomass in a UASB Reactor. Int. J. Microbiol. 2017, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin Vincent, N.; Wei, Y.; Zhang, J.; Yu, D.; Tong, J. Characterization and Dynamic Shift of Microbial Communities during Start-Up, Overloading and Steady-State in an Anaerobic Membrane Bioreactor. Int. J. Environ. Res. Public Health 2018, 15, 1399. [Google Scholar] [CrossRef] [Green Version]
- Alemahdi, N.; Che Man, H.; Abd Rahman, N.; Nasirian, N.; Yang, Y. Enhanced mesophilic bio-hydrogen production of raw rice straw and activated sewage sludge by co-digestion. Int. J. Hydrogen Energy 2015, 40, 16033–16044. [Google Scholar] [CrossRef] [Green Version]
- Campanaro, S.; Treu, L.; Kougias, P.G.; De Francisci, D.; Valle, G.; Angelidaki, I. Metagenomic analysis and functional characterization of the biogas microbiome using high throughput shotgun sequencing and a novel binning strategy. Biotechnol. Biofuels 2016, 9, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Martínez, E.J.; Gil, M.V.; Fernandez, C.; Rosas, J.G.; Gómez, X. Anaerobic Codigestion of Sludge: Addition of Butcher’ s Fat Waste as a Co-substrate for Increasing Biogas Production. PLoS ONE 2016, 11, 1–13. [Google Scholar] [CrossRef]
- Zahan, Z.; Othman, M.Z.; Rajendram, W. Anaerobic Codigestion of Municipal Wastewater Treatment Plant Sludge with Food Waste: A Case Study. Biomed. Res. Int. 2016, 201. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, J.V.; Alves, M.M.; Costa, J.C. Biochemical methane potential of brewery by-products. Clean Technol. Environ. Policy 2018, 20, 435–440. [Google Scholar] [CrossRef]
- Jeung, J.H.; Chung, W.J.; Chang, S.W. Evaluation of anaerobic co-digestion to enhance the efficiency of livestock manure anaerobic digestion. Sustainability 2019, 11, 7170. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Kamble, S.; Kazmi, A.; Starklhors, M. Environmental and economic performance evaluation of municipal wastewater treatment plants in India: A life cycle approach. Water Sci. Technol. 2019, 79, 1102. [Google Scholar] [CrossRef] [Green Version]
- Dido, M.S.; Rieker, C.; Bursche, J.; Lopez Velarde Santos, M.; Hamhaber, J. Anaerobic Digestion of spent grains: Potential use in small-scale Biogas Digesters in Jos, Nigeria. Koln Bibl. Tech. Hochschule Koln 2014, 46, 19–25. [Google Scholar]
- Lebiocka, M.; Montusiewicz, A.; Cydzik-Kwiatkowska, A. Effect of bioaugmentation on biogas yields and kinetics in anaerobic digestion of sewage sludge. Int. J. Environ. Res. Public Health 2018, 15, 1717. [Google Scholar] [CrossRef] [Green Version]
- Szaja, A.; Montusiewicz, A.; Lebiocka, M.; Bis, M. The effect of brewery spent grain application on biogas yields and kinetics in co-digestion with sewage sludge. PeerJ 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Fernandez, C.; Barreiro-Vescovo, S.; de Godos, I.; Fernandez, M.; Zouhayr, A.; Ballesteros, M. Biochemical methane potential of microalgae biomass using different microbial inocula. Biotechnol. Biofuels 2018, 11, 1–11. [Google Scholar] [CrossRef]
- Gonzalez-Gil, L.; Carballa, M.; Lema, J.M. Cometabolic Enzymatic Transformation of Organic Micropollutants under Methanogenic Conditions. Environ. Sci. Technol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Pellera, F.M.; Gidarakos, E. Effect of substrate to inoculum ratio and inoculum type on the biochemical methane potential of solid agroindustrial waste. J. Environ. Chem. Eng. 2016, 4. [Google Scholar] [CrossRef]
- Achinas, S.; Euverink, G.J.W. Effect of combined inoculation on biogas production from hardly degradable material. Energies 2019, 12, 217. [Google Scholar] [CrossRef] [Green Version]
- Eaton, A.D.; Clesceri, L.S.; Franson, M.A.H.; American Public Health Association; Rice, E.W.; Greenberg, A.E. Standard Methods for the Examination of Water & Wastewater. Am. Public Health Assoc. 2005, 21, 256–269. [Google Scholar]
- Nielfa, A.; Cano, R.; Pérez, A.; Fdez-Polanco, M. Co-digestion of municipal sewage sludge and solid waste: Modelling of carbohydrate, lipid and protein content influence. Waste Manag. Res. 2015, 33, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Dioha, I.J.; Ikeme, C.; Nafi, T.; Soba, N.I.; Mbs, Y. Effect of Carbon to Nitrogen Ratio on Biogas Production. Int. Res. J. Nat. Sci. 2013, 1, 1–10. [Google Scholar]
- Wang, Q.; Liang, Y.; Zhao, P.; Li, Q.X.; Guo, S.; Chen, C. Potential and optimization of two-phase anaerobic digestion of oil refinery waste activated sludge and microbial community study. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mudzanani, K.; Ejekwu, O.; Ayeni, A.O.; Daramola, M.O. Dissolution kinetics of corncob in lithium perchlorate solvent system. In Proceedings of the 7th European Biomass Conference and Exhibition (EUBCE 2019), Lisbon, Portugal, 27–30 May 2019. [Google Scholar]
- Masebinu, S.O. Anaerobic Digestion Process Stabilisation and In-Situ Upgrading of a Biogas System. Ph.D. Thesis, University of Johannesburg, Johannesburg, South Africa, 2018. [Google Scholar]
- Han, M.; Zhang, J.; Chu, W.; Zhou, G.; Chen, J. Surface-modified sewage sludge-derived carbonaceous catalyst as a persulfate activator for phenol degradation. Int. J. Environ. Res. Public Health 2020, 17, 3286. [Google Scholar] [CrossRef]
- Yan, A.; Li, J.; Liu, L.; Ma, T.; Liu, J.; Ni, Y. Centrifugal dewatering of blended sludge from drinking water treatment plant and wastewater treatment plant. J. Mater. Cycles Waste Manag. 2018, 20, 421–430. [Google Scholar] [CrossRef]
- Cuetos, M.J.; Martinez, E.J.; Moreno, R.; Gonzalez, R.; Otero, M.; Gomez, X. Enhancing anaerobic digestion of poultry blood using activated carbon Enhancing anaerobic digestion of poultry blood. J. Adv. Res. 2017, 8, 297–307. [Google Scholar] [CrossRef]
- Siddique, M.N.I.; Wahid, Z.B.A. Enhanced Methane Yield by Codigestion of Sewage Sludge with Microalgae and Catering Waste Leachate. Water Environ. Res. 2018, 90, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Derilus, D.; Forestil, A.; Fortuné, J.; Polyanska, O.; Louime, C.; Gervais, G.; Massey, S.E. Functional Metagenomics Characterization of an Anaerobic Saltwater Bioreactor. J. Renew. Energy 2019, 2019, 4527628. [Google Scholar] [CrossRef]
- Treu, L.; Kougias, P.G.; Campanaro, S.; Bassani, I.; Angelidaki, I. Deeper insight into the structure of the anaerobic digestion microbial community; The biogas microbiome database is expanded with 157 new genomes. Bioresour. Technol. 2016, 216, 260–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Zhang, L.; Li, A. Enhanced anaerobic digestion of food waste by trace metal elements supplementation and reduced metals dosage by green chelating agent [S, S]—EDDS via improving metals bioavailability. Water Res. 2017, 84, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Kumar, M.; Kapoor, R.; Kumar, S.S.; Singh, L.; Vijay, V.; Vijay, V.K.; Kumar, V.; Thakur, I.S. Enhanced biogas production from municipal solid waste via co-digestion with sewage sludge and metabolic pathway analysis. Bioresour. Technol. 2020, 296, 90–123. [Google Scholar] [CrossRef] [PubMed]
- Oladejo, J.; Shi, K.; Luo, X.; Yang, G.; Wu, T. A review of sludge-to-energy recovery methods. Energies 2019, 12, 60. [Google Scholar] [CrossRef] [Green Version]
- Jingura, R.M.; Kamusoko, R. Methods for determination of biomethane potential of feedstocks: A review. Biofuel Res. J. 2017, 4. [Google Scholar] [CrossRef]
- De Mandal, S.; Chatterjee, R.; Kumar, N.S. Dominant bacterial phyla in caves and their predicted functional roles in C and N cycle. BMC Microbiol. 2017, 17, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, L.; Qin, K.; Ding, J.; Xue, M.; Yang, C.; Jiang, J.; Zhao, Q. Optimization of the co-digestion of sewage sludge, maize straw and cow manure: Microbial responses and effect of fractional organic characteristics. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wang, M.; Hu, X.; Song, Y. Effect of Total Solids Content on the Biogas Production and Phosphorus Release from Excess Sludge. Adv. Mater. Res. 2014, 1010, 1006–1009. [Google Scholar] [CrossRef]
- Zhang, Y.; Culhaoglu, T.; Pollet, B.; Melin, C.; Denoue, D.; Barriere, Y.; Baumberger, S.; Mechin, V. Impact of lignin structure and cell wall reticulation on maize cell wall degradability. J. Agric. Food Chem. 2011, 59, 10129–10135. [Google Scholar] [CrossRef]
- Ruan, D.; Zhou, Z.; Pang, H.; Yao, J.; Chen, G.; Qiu, Z. Enhancing methane production of anaerobic sludge digestion by microaeration: Enzyme activity stimulation, semi-continuous reactor validation and microbial community analysis. Bioresour. Technol. 2019, 289, 121643. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Zhao, N.; Song, J.; Wang, X. Interactive Effects of Chemical Composition of Food Waste during Anaerobic Co-Digestion under Thermophilic Temperature. Sustainability 2019, 11, 2933. [Google Scholar] [CrossRef] [Green Version]
- Goswami, R.; Chattopadhyay, P.; Shome, A.; Banerjee, S.N.; Chakraborty, A.K.; Mathew, A.K.; Chaudhury, S. An overview of physico-chemical mechanisms of biogas production by microbial communities: A step towards sustainable waste management. 3 Biotech 2016. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Park, C.; Kim, T.-H.; Lee, M.; Kim, S.; Kim, S.-W.; Lee, J. Effects of various pretreatments for enhanced anaerobic digestion with waste activated sludge. J. Biosci. Bioeng. 2003, 95, 271–275. [Google Scholar] [CrossRef]
- Yeneneh, A.M.; Sen, T.K.; Ang, H.M.; Kayaalp, A. The role of sludge pretreatment on the enhancement of anaerobic digester performance in wastewater treatment plants. In Physical, Chemical and Biological Treatment Processes for Water and Wastewater; Nova Publishers: Hauppauge, NY, USA, 2015; pp. 251–282. [Google Scholar]
- Zhang, J.; Zhao, W.; Zhang, H.; Wang, Z.; Fan, C.; Zang, L. Recent achievements in enhancing anaerobic digestion with carbon- based functional materials. Bioresour. Technol. 2018, 266, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Bong, C.P.C.; Lim, L.Y.; Lee, C.T.; Klemeš, J.J.; Ho, C.S.; Ho, W.S. The characterisation and treatment of food waste for improvement of biogas production during anaerobic digestion—A review. J. Clean. Prod. 2018, 172. [Google Scholar] [CrossRef]
- Dhinakaran, S.; Navaneethagopalakrishnan, A. Feasibility studies on performance improvement for tannery effluent through chemical oxidation- biological treatment. J. Chem. Pharm. Sci. 2015, 2015, 439–442. [Google Scholar]
- Ahring, B.K.; Sandberg, M.; Angelidaki, I. Volatile fatty acids as indicators of process imbalance in anaerobic digestors. Appl. Microbiol. Biotechnol. 1995, 43, 559–565. [Google Scholar] [CrossRef]
- Tian, X.; Trzcinski, A. Effects of Physico-Chemical Post-Treatments on the Semi-Continuous Anaerobic Digestion of Sewage Sludge. Environments 2017, 4, 49. [Google Scholar] [CrossRef] [Green Version]
- Sakeus, K. Anaerobic Digestion of Dairy Manure Wastewater, Food and Fruit Waste, A Sustainable Source of Bio-Energy and Waste Management. Master’s Thesis, Stellenbosch University, Stellenbosch, South Africa, 2016. [Google Scholar]


| Parameters | Inoculum | Sewage Sludge | Brewery Spent Grains | Blend (1:1) |
|---|---|---|---|---|
| pH | 7.20 | 6.90 | 6.10 | 6.64 |
| Total solids (w/w%) | 1.34 | 3.60 | 16.72 | 10.16 |
| Volatile solids (w/w%) | 1.58 | 98.70 | 97.50 | 98.10 |
| Carbon content, % | 37.14 | 35.23 | 87.30 | 61.27 |
| Nitrogen content, % | 3.23 | 8.60 | 2.50 | 5.55 |
| C/N ratio | 11.50 | 4.10 | 34.92 | 19.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mudzanani, K.; van Heerden, E.; Mbhele, R.; Daramola, M.O. Enhancement of Biogas Production via Co-Digestion of Wastewater Treatment Sewage Sludge and Brewery Spent Grain: Physicochemical Characterization and Microbial Community. Sustainability 2021, 13, 8225. https://doi.org/10.3390/su13158225
Mudzanani K, van Heerden E, Mbhele R, Daramola MO. Enhancement of Biogas Production via Co-Digestion of Wastewater Treatment Sewage Sludge and Brewery Spent Grain: Physicochemical Characterization and Microbial Community. Sustainability. 2021; 13(15):8225. https://doi.org/10.3390/su13158225
Chicago/Turabian StyleMudzanani, Khuthadzo, Esta van Heerden, Ryneth Mbhele, and Michael O. Daramola. 2021. "Enhancement of Biogas Production via Co-Digestion of Wastewater Treatment Sewage Sludge and Brewery Spent Grain: Physicochemical Characterization and Microbial Community" Sustainability 13, no. 15: 8225. https://doi.org/10.3390/su13158225
APA StyleMudzanani, K., van Heerden, E., Mbhele, R., & Daramola, M. O. (2021). Enhancement of Biogas Production via Co-Digestion of Wastewater Treatment Sewage Sludge and Brewery Spent Grain: Physicochemical Characterization and Microbial Community. Sustainability, 13(15), 8225. https://doi.org/10.3390/su13158225







