Soil Arthropods in the Douro Demarcated Region Vineyards: General Characteristics and Ecosystem Services Provided
Abstract
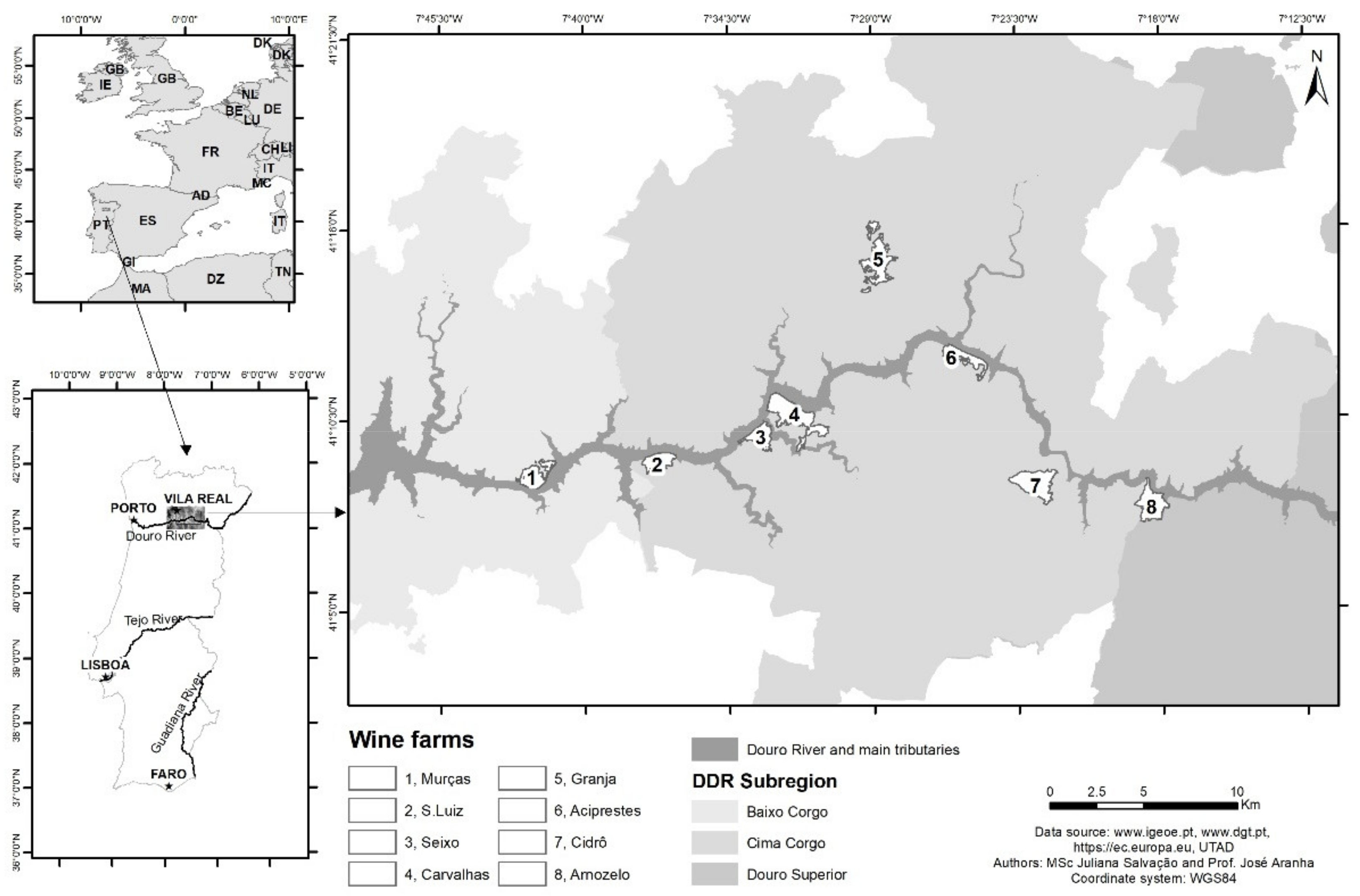
1. Introduction
2. Methods
3. Results
3.1. Abundance and Functional Roles of Soil-Surface Arthropods
3.2. Abundance and Functional Roles of Soil-Living Arthropods
4. Review of the Systematic Groups and Their Functionality
4.1. Chelicerata Arachnida
4.1.1. Dromopoda
Opiliones (Harvestmen)
Pseudoscorpiones (False Scorpions)
Scorpiones (Scorpions)
Solifugae (Camel Spiders)
4.1.2. Micrura
Acari (Mites)
Araneae (Spiders)
Palpigradi (Microwhip Scorpions)
4.2. Crustacea—Malacostraca
4.2.1. Amphipoda (Terrestrial Amphipods)
4.2.2. Isopoda (Terrestrial Isopods)
4.3. Hexapoda Entognatha
4.3.1. Collembola (Springtails)
4.3.2. Diplura (Diplurans)
4.3.3. Protura (Proturans)
4.4. Hexapoda Insecta
4.4.1. Coleoptera (Beetles)
Carabidae (Ground Beetles)
Chrysomelidae (Leaf Beetles)
Malachiidae (Soft-Winged Flower Beetles)
Corylophidae (Minute Fungus Beetles)
Latridiidae (Minute Brown Scavenger Beetles)
Phalacridae (Shining Flower Beetles)
Brentidae (Straight-Snouted Weevils)
Curculionidae (Snout Beetles)
Aderidae (Antlike Leaf Beetles)
Anthicidae (Antlike Flower Beetles)
Meloidae (Blister Beetles)
Tenebrionidae (Darkling Beetles)
Buprestidae (Wood-Boring Long-Horned Beetles)
Elateridae (Click Beetles)
Geotrupidae (Scarab Beetles)
Scarabaeidae (Scarab Beetles)
Scydmaenidae (Antlike Stone Beetle)
Staphylinidae (Rove Beetles)
4.4.2. Dictyoptera (Isoptera) (Termites)
4.4.3. Diptera (Flies)
4.4.4. Embioptera (Webspinners)
4.4.5. Hemiptera
Dictyopharidae (Dictyopharid planthoppers)
Cydnidae (Burrower Bugs)
Lygaeidae (Seed Bugs)
Pentatomidae (Stink Bugs)
Rhopalidae (Scentless Plant Bugs)
Scutelleridae (Shield-Backed Bug)
4.4.6. Hymenoptera
Dryinidae
Formicidae (Ants)
Mutillidae (Velvet Ants)
4.4.7. Orthoptera
Acrididae (Grasshoppers)
Gryllidae (True Crickets)
Gryllotalpidae (Mole Crickets)
4.4.8. Lepidoptera (Moths)
4.4.9. Neuroptera (Lacewings)
4.4.10. Thysanoptera (Thrips)
4.5. Myriapoda Chilopoda (Centipedes)
4.6. Myriapoda Diplopoda (Millipedes)
4.7. Myriapoda Pauropoda
4.8. Myriapoda Symphyla
5. Ecosystem Services Provided by Soil Arthropods in the Vineyard
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission. The Factory of Life. Why Soil Biodiversity Is so Important; Office for Official Publications of the European Union: Luxembourg, 2010; p. 22. [Google Scholar]
- Roger-Estrade, J.; Anger, C.; Bertrand, M.; Richard, G. Tillage and soil ecology: Partners for sustainable agriculture. Soil Till. Res. 2010, 111, 33–40. [Google Scholar] [CrossRef]
- Barrios, E. Soil biota, ecosystem services and land productivity. Ecol. Econ. 2007, 64, 269–285. [Google Scholar] [CrossRef]
- Stockdale, E.A.; Watson, C.A. Managing Soil Biota to Deliver Ecosystem Services; Natural England Commissioned Reports No 100; Newcastle University: Newcastle, UK, 2012; p. 141. [Google Scholar]
- Culliney, T.W. Role of Arthropods in Maintaining Soil Fertility. Agriculture 2013, 3, 629–659. [Google Scholar] [CrossRef]
- Menta, C.; Remelli, S. Soil Health and Arthropods: From Complex System to Worthwhile Investigation. Insects 2020, 11, 54. [Google Scholar] [CrossRef]
- Eisenbeis, G. Biology of Soil Invertebrates. In Intestinal Microorganisms of Termites and Other Invertebrates; König, H., Varma, A., Eds.; Soil Biology; Springer: Berlin, Germany, 2006; Volume 6, pp. 3–53. [Google Scholar]
- Wurst, S.; De Deyn, G.B.; Orwin, K. Soil Biodiversity and Functions. In Soil Ecology and Ecosystem Services; Wall, D.H., Bardgett, R.D., Behan-Pelletier, V., Herrick, J.E., Jones, T.H., Ritz, K., Six, J., Strong, D.R., van der Putten, W.H., Eds.; Oxford University Press: Oxford, UK, 2013; pp. 28–44. [Google Scholar]
- European Commission. European Atlas of Soil Biodiversity; Jeffery, S., Gardi, C., Jones, A., Montanarella, L., Marmo, L., Miko, L., Ritz, K., Peres, G., Römbke, J., van der Putten, W.H., Eds.; Publications Office of the European Union: Luxembourg, 2010; p. 127. [Google Scholar]
- Parisi, V.; Menta, C.; Gardi, C.; Jacomini, C.; Mozzanica, E. Microarthropod communities as a tool to assess soil quality and biodiversity: A new approach in Italy. Agric. Ecosyst. Environ. 2005, 105, 323–333. [Google Scholar] [CrossRef]
- Galli, L.; Capurro, M.; Menta, C.; Rellini, I. Is the QBS-ar index a good tool to detect the soil quality in Mediterranean areas? A cork tree Quercus suber L. (Fagaceae) wood as a case of study. Ital. J. Zool. 2014, 81, 126–135. [Google Scholar] [CrossRef]
- Çakır, M. The impact of wood ants (Formica rufa) mound on soil biological quality (QBS-ar) in a semi-arid pine forest. Pedobiologia 2019, 77, 150593. [Google Scholar] [CrossRef]
- Vignozzi, N.; Agnelli, A.E.; Brandi, G.; Gagnarli, E.; Goggioli, D.; Lagomarsino, A.; Pellegrini, S.; Simoncini, S.; Simoni, S.; Valboa, G.; et al. Soil ecosystem functions in a high-density olive orchard managed by different soil conservation practices. Appl. Soil Ecol. 2019, 134, 64–76. [Google Scholar] [CrossRef]
- Gonçalves, F.; Nunes, C.; Carlos, C.; López, Á.; Oliveira, I.; Teixeira, B.; Crespí, A.; Pinto, R.; Costa, C.A.; Torres, L. Do soil management practices affect activity density, diversity, and stability of soil arthropods in vineyards? Agric. Ecosyst. Environ. 2020, 294, 106863. [Google Scholar] [CrossRef]
- Galli, L. An user friendly tool to assess the effects on agricultural soils of different practices: The QBS-ar index. Mod. Concept Dev. Agron. 2020, 6, 680–682. [Google Scholar]
- Sánchez-Bayo, F.; Wyckhuys, F.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Sofo, A.; Mininni, A.N.; Ricciuti, P. Soil Macrofauna: A key factor for increasing soil fertility and promoting sustainable soil use in fruit orchard agrosystems. Agronomy 2020, 10, 456. [Google Scholar] [CrossRef]
- Trivellone, V.; Paltrinieri, L.P.; Jermini, M.; Moretti, M. Management pressure drives leafhopper communities in vineyards in Southern Switzerland. Insect Conserv. Divers. 2012, 5, 75–85. [Google Scholar] [CrossRef]
- Karimi, B.; Cahurel, J.-Y.; Gontier, L.; Charlier, L.; Chovelons, M.; Mahé, H.; Ranjard, L. A meta analysis of the ecotoxicological impact of viticultural practices on soil biodiversity. Environ. Chem. Lett. 2020, 18, 1947–1966. [Google Scholar] [CrossRef]
- Winter, S.; Bauer, T.; Strauss, P.; Kratschmer, S.; Paredes, D.; Popescu, D.; Landa, B.; Guzmán, G.; Gómez, J.A.; Guernion, M.; et al. Effects of vegetation management intensity on biodiversity and ecosystem services in vineyards: A meta-analysis. J. Appl. Ecol. 2018, 55, 2484–2495. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, C.I.; Altieri, M.A.; Ponti, L. Enhancing plant diversity for improved insect pest management in northern California organic vineyards. Acta Hortic. 2008, 785, 263–278. [Google Scholar] [CrossRef]
- Vaudour, E.; Costantini, E.A.C.; Jones, G.V.; Mocali, S. An overview of the recent approaches to terroir functional modelling, foot printing and zoning. Soil 2015, 1, 287–312. [Google Scholar] [CrossRef]
- Costantini, E.A.C.; Agnelli, A.E.; Fabiani, A.; Gagnarli, E.; Mocali, S.; Priori, S.; Valboa, G. Short-term recovery of soil physical, chemical, micro- and mesobiological functions in a new vineyard under organic farming. Soil 2015, 1, 443–457. [Google Scholar] [CrossRef]
- Viers, J.H.; Williams, J.N.; Nicholas, K.A.; Barbosa, O.; Kotzé, I.; Spence, L.; Webb, L.B.; Merenlender, A.; Reynolds, M. Vinecology: Pairing wine with nature. Conserv. Lett. 2013, 6, 287–299. [Google Scholar] [CrossRef]
- Instituto do Vinho do Porto e Douro. Área de Vinha e Sua Composição. 2021. Available online: https://www.ivdp.pt/ (accessed on 3 May 2021).
- Andresen, T.; De Aguiar, F.B.; Curado, M.J. The Alto Douro Wine Region greenway. Landsc. Urban Plan. 2004, 68, 289–303. [Google Scholar] [CrossRef]
- Andresen, T.; Rebelo, J. Assessment of the State of Conservation of the Property Alto Douro Wine Region—Evolutive and Living Cultural Landscape—Assessment Report; CCDRN/EMD, CIBIO UP-UTAD: Porto, Portugal, 2013; p. 118. [Google Scholar]
- Biagioli, G.; Vezzosi, R. 4. The Agrarian/Rural Organisation of Space, Production and Productivity: Its Characters. In European Guidelines for Wine Cultural Landscape Preservation and Enhancement—With Special Regard to Endangered Areas and Vineyards, Italy; Biagioli, G., Prats, M., Bender, J., Eds.; ViTour: Jacksonville, FL, USA, 2012; pp. 20–27. [Google Scholar]
- Havlicek, E. Soil biodiversity and bioindication: From complex thinking to simple acting. Eur. J. Soil Biol. 2012, 49, 80–84. [Google Scholar] [CrossRef]
- Villani, M.G.; Allee, L.L.; Díaz, A.; Robbins, P.S. Adaptive strategies of edaphic strategies. Annu. Rev. Entomol. 1999, 44, 233–256. [Google Scholar] [CrossRef]
- Gonçalves, F.; Carlos, C.; Aranha, J.; Torres, L. Does habitat heterogeneity affect the diversity of epigaeic arthropods in vineyards? Agric. For. Entomol. 2018, 20, 366–379. [Google Scholar] [CrossRef]
- Fauna Europaea 2020. Available online: https://fauna-eu.org (accessed on 28 December 2020).
- Machado, G.; Pinto-da-Rocha, R.; Giribet, G. What Are Harvestmen? In Harvestmen: The Biology of Opiliones; Pinto-da-Rocha, R., Machado, G., Giribet, G., Eds.; Harvard University Press: Cambridge, MA, USA, 2007; pp. 1–14. [Google Scholar]
- Bragagnolo, C.; Nogueira, A.A.; Pinto-da-Rocha, R.; Pardini, R. Harvestmen in an Atlantic forest fragmented landscape: Evaluating assemblage response to habitat quality and quantity. Biol. Conserv. 2007, 139, 389–400. [Google Scholar] [CrossRef]
- Gerlach, J.; Samways, M.; Pryke, J. Terrestrial invertebrates as bioindicators: An overview of available taxonomic groups. J. Insect Conserv. 2013, 17, 831–850. [Google Scholar] [CrossRef]
- Pryke, J.S.; Samways, M.J. Recovery of invertebrate diversity in a rehabilitated city landscape mosaic in the heart of a biodiversity hotspot. Landsc. Urban Plan. 2009, 93, 54–62. [Google Scholar] [CrossRef]
- Johnson, E.A.; Catley, K.M. Life in the Leaf Litter; Center for Biodiversity and Conservation, American Museum of Natural History: New York, NY, USA, 2002; p. 28. [Google Scholar]
- Scorpion. Encyclopædia Britannica, Inc. Available online: https://www.britannica.com/animal/scorpion (accessed on 4 January 2021).
- Hruskova-Martisova, M.; Pekár, S.; Cardoso, P. Natural history of the Iberian solifuge Gluvia dorsalis (Solifuges: Daesiidae). J. Arachnol. 2010, 38, 466–474. [Google Scholar] [CrossRef]
- The Arachnid Order Solifugae. Available online: http://www.solifugae.info/ (accessed on 4 January 2021).
- González-Moliné, A.L.; Melic, A.; Barrientos, J.A. Taxonomía, distribución geográfica e historia natural del endemismo ibérico Gluvia dorsalis (Latreille, 1817) (Solifugae: Daesiidae). Bol. Soc. Entomol. Aragonesa 2008, 42, 385–395. [Google Scholar]
- Dhooria, M.S. Fundamentals of Applied Acarology; Springer: Singapore, 2016; p. 470. [Google Scholar]
- Coleman, D.C.; Crossley, D.A., Jr.; Hendrix, P.F. Fundamentals of Soil Ecology, 2nd ed.; Academic Press: Cambridge, MA, USA, 2004. [Google Scholar]
- O’Neill, K.P.; Godwin, H.W.; Jimenez-Esquilin, A.E.; Battigelli, J.P. Reducing the dimensionality of soil micro invertebrate community datasets using indicator species analysis: Implications for ecosystem monitoring and soil management. Soil Biol. Biochem. 2010, 42, 145–154. [Google Scholar] [CrossRef]
- Jung, M.P.; Kim, S.T.; Kim, H.; Lee, J.H. Species diversity and community structure of ground-dwelling spiders in unpolluted and moderately heavy metal-polluted habitats. Water Air Soil Pollut. 2008, 195, 15–22. [Google Scholar] [CrossRef]
- Hoy, M.A. Soil Mites (Acari: Oribatida and Others). In Encyclopedia of Entomology; Capinera, J.L., Ed.; Springer: Dordrecht, Germany, 2008. [Google Scholar]
- Wise, D.H.; Snyder, W.E.; Tuntibunpakul, P.; Halaj, J. Spiders in decomposition food webs of agroecosystems: Theory and evidence. J. Arachnol. 1999, 27, 363–370. [Google Scholar]
- Marc, P.; Canard, A.; Ysnel, F. Spiders (Araneae) useful for pest limitation and bioindication. Agric. Ecosyst. Environ. 1999, 74, 229–273. [Google Scholar] [CrossRef]
- Culin, J.; Levi, H.W.; Levi, L.R. Spider. Available online: https://www.britannica.com/animal/spider-arachnid (accessed on 18 January 2021).
- Collingwood, C.; Prince, A. A guide to ants of Continental Portugal (Hymenoptera: Formicidae). Bol. Soc. Port. Entomol. 1998, 5, 1–49. [Google Scholar]
- Pekár, S.; Coddington, J.A.; Blackledge, T.A. Evolution of stenophagy in spiders (Araneae): Evidence based on the comparative analysis of spider diets. Evolution 2012, 66, 776–806. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, F.S. Ant-mimicking spiders: Strategies for living with social insects. Psyche J. Entomol. 2013, 2013, 839181. [Google Scholar] [CrossRef]
- Nelson, X.J.; Jackson, R.R. How spiders practice aggressive and Batesian mimicry. Curr. Zool. 2012, 58, 620–629. [Google Scholar] [CrossRef][Green Version]
- Hölldobler, B.; Wilson, E.O. The Ants; Belknap Press of Harvard University: Cambridge, MA, USA, 1990; p. 732. [Google Scholar]
- Pekár, S. Spiders (Araneae) in the pesticide world: An ecotoxicological review. Pest Manag. Sci. 2012, 68, 1438–1446. [Google Scholar] [CrossRef]
- Pfingstmann, A.; Paredes, D.; Buchholz, J.; Querner, P.; Bauer, T.; Strauss, P.; Kratschmer, S.; Winter, S.; Zaller, J. Contrasting effects of tillage and landscape structure on spiders and springtails in vineyards. Sustainability 2019, 11, 2095. [Google Scholar] [CrossRef]
- Fiera, C.; Ulrich, W.; Popescu, D.; Bunea, C.J.; Manu, M.; Nae, J.; Stan, M.; Markó, B.; Urák, I.; Giurginca, A.; et al. Effects of vineyard inter-row management on the diversity and abundance of plants and surface-dwelling invertebrates in Central Romania. J. Insect Conserv. 2020, 24, 175–185. [Google Scholar] [CrossRef]
- Jiménez-García, L.; García-Martínez, Y.G.; Marco-Mancebón, V.; Pérez-Moreno, I.; Jiménez-García, D. Biodiversity analysis of natural arthropods enemies in vineyard agroecosystems in La Rioja, Spain. J. Asia Pac. Entomol. 2019, 22, 308–315. [Google Scholar] [CrossRef]
- Branco, V.V.; Morano, E.; Cardoso, P. An update to the Iberian spider checklist (Araneae). Zootaxa 2019, 4614, 201–225. [Google Scholar] [CrossRef]
- Cardoso, P.; Pekár, S.; Jocqué, R.; Coddington, J.A. Global patterns of guild composition and functional diversity of spiders. PLoS ONE 2011, 6, e21710. [Google Scholar] [CrossRef] [PubMed]
- Pekár, S.; Toft, S. Can ant-eating Zodarion spiders (Araneae: Zodariidae) develop on a diet optimal for euryphagous arthropod predators? Physiol. Entomol. 2009, 34, 195–201. [Google Scholar] [CrossRef]
- Cushing, P.E. Myrmecomorphy and myrmecophily in spiders: A review. Fla. Entomol. 1997, 80, 165–193. [Google Scholar] [CrossRef]
- Pekár, S.; Smerda, J.; Hrušková, M.; Sedo, O.; Muster, C.; Cardoso, P.; Zdráhal, Z.; Korenko, S.; Bureš, P.; Líznarová, E.; et al. Prey-race drives differentiation of biotypes in ant-eating spiders. J. Anim. Ecol. 2012, 81, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Řezáč, M.; Pekár, S.; Lubin, Y. How oniscophagous spiders overcome woodlouse armour. J. Zool. 2008, 275, 64–71. [Google Scholar] [CrossRef]
- Smrž, J.; Kováč, Ĺ.; Mikeš, J.; Lukešová, A. Microwhip scorpions (Palpigradi) feed on heterotrophic cyanobacteria in Slovak caves—A curiosity among Arachnida. PLoS ONE 2013, 8, e75989. [Google Scholar] [CrossRef]
- Friend, J.A.; Richardson, A.M.M. Biology of Terrestrial Amphipods. Ann. Rev. Entomol. 1986, 31, 25–48. [Google Scholar] [CrossRef]
- Duncan, K.W. Terrestrial Talitridae (Crustacea: Amphipoda). In Fauna of New Zealand; Manaaki Whenua Press: Lincoln, New Zealand, 1994; Volume 31, p. 128. [Google Scholar]
- Fialkowski, W.; Rainbow, P.S.; Fialkowska, E.; Smith, B.D. Biomonitoring of trace metals along the Baltic Coast of Poland using the sandhopper Talitrus saltator (Montagu) (Crustacea: Amphipoda). Ophelia 2000, 52, 183–192. [Google Scholar] [CrossRef]
- Bagyaraj, D.J.; Nethravathi, C.J.; Nitin, K.S. Soil Biodiversity and Arthropods: Role in Soil Fertility. In Economic and Ecological Significance of Arthropods in Diversified Ecosystems; Chakravarthy, A.K., Sridhara, S., Eds.; Springer: Singapore, 2016. [Google Scholar]
- Souty-Grosset, C.; Faberi, A. Effect of agricultural practices on terrestrial isopods: A review. ZooKeys 2018, 801, 63–96. [Google Scholar] [CrossRef]
- Solomou, A.D.; Sfugaris, A.I.; Sfenthourakis, S. Terrestrial isopods as bioindicators for environmental monitoring in olive groves and natural ecosystems. J. Nat. Hist. 2019, 53, 1721–1735. [Google Scholar] [CrossRef]
- Drobne, D.; Blazic, M.; Van Gestel, C.A.; Leser, V.; Zidar, P.; Jemec, A.; Trebse, P. Toxicity of imidacloprid to the terrestrial isopod Porcellio scaber (Isopoda, Crustacea). Chemosphere 2008, 71, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Van Gestel, C.A.M.; Loureiro, S.; Zidar, P. Terrestrial isopods as model organisms in soil ecotoxicology: A review. ZooKeys 2018, 801, 127–162. [Google Scholar] [CrossRef]
- Blanusa, M.; Mrkovich-Milic, R.; Durbesic, P. Lead and cadmium in soil and Isopoda Woodlice in Croatia. Ecotox. Environ. Saf. 2002, 52, 198–202. [Google Scholar] [CrossRef]
- Paoletti, M.G.; Hassall, M. Woodlice (Isopoda: Oniscidea): Their potential for assessing sustainability and use as bioindicators. Agric. Ecosyst. Environ. 1999, 74, 157–165. [Google Scholar] [CrossRef]
- Romoser, W.S.; Stoffolano, J.G., Jr. The Science of Entomology, 3rd ed.; WMC Brown Communications Inc.: Dubuque, IA, USA, 1994. [Google Scholar]
- Hopkin, S.P. Biology of the Springtails (Insecta: Collembola); Oxford University Press: Oxford, UK, 1997; p. 330. [Google Scholar]
- Filser, J. The role of Collembola in carbon and nitrogen cycling in soil. Pedobiologia 2002, 46, 234–245. [Google Scholar] [CrossRef]
- Rusek, J. Biodiversity of Collembola and their functional role in the ecosystem. Biodivers. Conserv. 1998, 7, 1207–1219. [Google Scholar] [CrossRef]
- Christiansen, K.A.; Bellinger, P.; Janssens, F. Collembola: (Springtails, Snow Fleas). In Encyclopedia of Insects, 2nd ed.; Resh, V.H., Cardé, R.T., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 206–210. [Google Scholar]
- Ponge, J.F. Utilisation de la micromorphologie pour l’étude des relations trophiques dans le sol: La couche L d’un moder hydromorphe sous Pinus sylvestris (Forêt d’Orléans, France). Bull. Ecol. 1985, 16, 117–132. [Google Scholar]
- Orgiazzi, A.; Bardgett, R.D.; Barrios, E.; Behan-Pelletier, V.; Briones, M.J.I.; Chotte, J.-L.; De Deyn, G.B.; Eggleton, P.; Fierer, N.; Fraser, T.; et al. Global Soil Biodiversity Atlas; European Commission, Publications Office of the European Union: Luxembourg, 2016; p. 176. [Google Scholar]
- Greenslade, P.; Boyer, S.; Shields, M.W.; Wratten, S.D. First record of a possible predatory collembolan species, Dicyrtoma fusca (Collembola: Dicyrtomidae), in New Zealand. Austral. Entomol. 2016, 56, 332–338. [Google Scholar] [CrossRef]
- Chernova, N.M.; Kuznetsova, N.A. Collembolan community organization and its temporal predictability. Pedobiologia 2000, 44, 451–466. [Google Scholar] [CrossRef]
- Arbea, J.I.; Blasco-Zumeta, J. Ecología de los Colémbolos (Hexapoda, Collembola) en Los Monegros (Zaragoza, España). Bol. Soc. Entomol. Aragonesa 2001, 28, 35–48. [Google Scholar]
- Detsis, V. Vertical distribution of Collembola in deciduous forests under Mediterranean climatic conditions. Belg. J. Zool. 2000, 130, 57–61. [Google Scholar]
- Yadav, R.S.; Kerketta, D.; Kumar, D.; Prasad, S. Vertical Distribution of Collembola (Arthropoda: Collembola) at Varanasi, India. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 609–613. [Google Scholar] [CrossRef]
- Yin, R.; Gruss, I.; Eisenhauer, N.; Kardol, P.; Thakur, M.P.; Schmidt, A.; Xu, Z.; Siebert, J.; Zhang, C.; Wu, G.L.; et al. Land use modulates the effects of climate change on density but not community composition of Collembola. Soil Biol. Biochem. 2019, 138, 107598. [Google Scholar] [CrossRef]
- Yin, R.; Kardol, P.; Thakur, M.P.; Gruss, I.; Wu, G.L.; Eisenhauer, N.; Schädler, M. Soil functional biodiversity and biological quality under threat: Intensive land use outweighs climate change. Soil Biol. Biochem. 2020, 147, 107847. [Google Scholar] [CrossRef] [PubMed]
- Fountain, M.T.; Hopkin, S.P. Biodiversity of Collembola in urban soils and the use of Folsomia candida to assess soil ’quality’. Ecotoxicology 2004, 13, 555–572. [Google Scholar] [CrossRef]
- An, Y.-J.; Kim, S.W.; Lee, W.-M. The collembola Lobella sokamensis juvenile as a new soil quality indicator of heavy metal pollution. Ecol. Indic. 2013, 27, 56–60. [Google Scholar] [CrossRef]
- Zhu, D.; Chen, Q.-L.; An, X.L.; Yang, X.-R.; Christie, P.; Ke, X.; Wu, L.-H.; Zhu, Y.-G. Exposure of soil collembolans to microplastics perturbs their gut microbiota and alters their isotopic composition. Soil Biol. Biochem. 2018, 116, 302–310. [Google Scholar] [CrossRef]
- Frampton, G.K. The potential of Collembola as indicators of pesticide usage: Evidence and methods from the UK arable ecosystem. Pedobiologia 1997, 41, 179–184. [Google Scholar]
- Fiera, C.; Ulrich, W.; Popescu, D.; Buchholz, J.; Querner, P.; Bunea, C.-I.; Strauss, P.; Bauer, T.; Kratschmer, S.; Winter, S.; et al. Tillage intensity and herbicide application influence surface-active springtail (Collembola) communities in Romanian vineyards. Agric. Ecosyst. Environ. 2020, 300, 107006. [Google Scholar] [CrossRef]
- Sterzynska, M.; Nicia, P.; Zadrozny, P.; Fiera, C.; Shrubovych, J.; Ulrich, W. Urban springtail species richness decreases with increasing air pollution. Ecol. Indic. 2018, 94, 328–335. [Google Scholar] [CrossRef]
- Sendra, A.; Jiménez-Valverde, A.; Selfa, J.; Reboleira, A.S.P.S. Diversity, ecology, distribution and biogeography of Diplura. Insect Conserv. Divers. 2021, 14, 415–425. [Google Scholar] [CrossRef]
- Bachelier, G. La Vie Animale Dans les Sols; ORSTOM: Paris, France, 1963; p. 279. [Google Scholar]
- Beutel, R.G.; Friedrich, F.; Ge, S.-Q.; Yang, X.-K. Insect Morphology and Phylogeny: A Textbook for Students of Entomology; De Gruyter: Berlin, Germany, 2014; p. 516. [Google Scholar]
- Pass, G.; Szucsich, N.U. 100 years of research on the Protura: Many secrets still retained. Soil Org. 2011, 83, 309–334. [Google Scholar]
- Galli, L.; Capurro, M.; Molyneux, T.; Torti, C.; Zinni, M. Ecology of Italian Protura. Pedobiol. J. Soil Ecol. 2019, 73, 20–28. [Google Scholar] [CrossRef]
- Malmström, A.; Persson, T. Responses of Collembola and Protura to tree girdling—Some support for ectomycorrhizal feeding. Soil Org. 2011, 83, 279–285. [Google Scholar]
- Bluhm, S.L.; Potapov, A.M.; Shrubovych, J.; Ammerschubert, S.; Polle, A.; Scheu, S. Protura are unique: First evidence of specialized feeding on ectomycorrhizal fungi in soil invertebrates. BMC Ecol. 2019, 19, 10. [Google Scholar] [CrossRef]
- Christian, E.; Szeptycki, A. Distribution of Protura along an urban gradient in Vienna. Pedobiologia 2004, 48, 445–452. [Google Scholar] [CrossRef]
- Nakamura, O. Habitat preference of species of the family Eosentomidae (Hexapoda: Protura) in Kanto district, central Japan. Bull. Saitama Mus. Nat. Hist. 2014, 8, 15–18. [Google Scholar]
- Triplehorn, C.A.; Johnson, N.F. Borror and DeLong’s Introduction to the Study of Insects, 7th ed.; Thomson Brooks/Cole: Belmont, CA, USA, 2005. [Google Scholar]
- Ghannem, S.; Touaylia, S.; Boumaiza, M. Beetles (Insecta: Coleoptera) as bioindicators of the assessment of environmental pollution. Hum. Ecol. Risk Assess. 2018, 24, 456–464. [Google Scholar] [CrossRef]
- Lövei, G.L.; Sunderland, K.D. Ecology and behavior of ground beetles (Coleoptera: Carabidae). Annu. Rev. Entomol. 1996, 41, 231–256. [Google Scholar] [CrossRef]
- Holland, J.M.; Luff, M.L. The effects of agricultural practices on Carabidae in temperate agroecosystems. Integr. Pest. Manag. Rev. 2000, 5, 109–129. [Google Scholar] [CrossRef]
- Gailis, J.; Turka, I. Discussion on ground beetles and rove beetles as indicators of sustainable agriculture in Latvia: Review. Res. Rural Dev. 2013, 1, 56–62. [Google Scholar]
- Kotze, D.J.; Brandmayr, P.; Casale, A.; Dauffy-Richard, E.; Dekoninck, W.; Koivula, M.J.; Lövei, G.L.; Mossakowski, D.; Noordijk, J.; Paarmann, W.; et al. Forty years of carabid beetle research in Europe—from taxonomy, biology, ecology and population studies to bioindication, habitat assessment and conservation. Zookeys 2011, 100, 55–148. [Google Scholar] [CrossRef]
- Niemelä, J. Carabid beetles (Coleóptera: Carabidae) and habitat fragmentation: A review. Eur. J. Entomol. 2001, 98, 127–132. [Google Scholar] [CrossRef]
- Tőzsér, D.; Magura, T.; Simon, E.; Mizser, S.; Papp, D.; Tóthmérész, B. Pollution intensity-dependent metal accumulation in ground beetles: A meta-analysis. Environ. Sci. Pollut. Res. 2019, 26, 32092–32102. [Google Scholar] [CrossRef]
- Rainio, J.; Niemelä, J. Ground beetles (Coleoptera: Carabidae) as bioindicators. Biodivers. Conserv. 2003, 12, 487–506. [Google Scholar] [CrossRef]
- Avgın, S.S.; Luff, M.L. Ground beetles (Coleoptera: Carabidae) as bioindicators of human impact. Munis Entomol. Zool. 2010, 5, 209–215. [Google Scholar]
- Butovsky, R.O. Heavy metals in carabids (Coleoptera, Carabidae). Zookeys 2011, 100, 215–222. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A.; Ortuño, V.M. The history of endemic Iberian ground beetle description (Insecta, Coleoptera, Carabidae): Which species were described first? Acta Oecol. 2007, 31, 13–31. [Google Scholar] [CrossRef]
- Mifsud, D. Altica ampelophaga Guerin-Meneville, 1858—new record of Flea Beetle for Malta (Coleoptera, Chrysomelidae). Bull. Entomol. Soc. Malta 2012, 5, 185–187. [Google Scholar]
- El-Torkey, A.M.; Oshaibah, A.D.A.; Salem, M.M.H.; Hossni, M.T.; El-Zouk, A.A.A. Soft winged flower beetles (Coleoptera: Malachiidae) in Egypt. Bol. Soc. Entomol. Aragonesa 2012, 50, 285–294. [Google Scholar]
- Yildirim, E.; Bulak, Y. A contribution to the knowledge of the Malachiidae (Coleoptera: Cleroidea) fauna of Turkey. Türk. Entomol. Derg. 2012, 36, 231–238. [Google Scholar]
- Yavorskaya, M.I.; Leschen, R.A.B.; Polilov, A.A.; Beutel, R.G. Unique rostrate larvae and basidiomycophagy in the beetle family Corylophidae. Arthropod Struct. Dev. 2014, 43, 153–162. [Google Scholar] [CrossRef]
- Wagner, T. Influence of forest type and tree species on canopy-dwelling beetles in Budongo Forest, Uganda. Biotropica 2000, 32, 502–514. [Google Scholar] [CrossRef]
- Lord, N.P.; Hartley, C.S.; Lawrence, J.F.; Mchugh, J.V.; Whiting, M.F.; Miller, K.B. Phylogenetic analysis of the minute brown scavenger beetles (Coleoptera: Latridiidae), and recognition of a new beetle family, Akalyptoischiidae fam.n. (Coleoptera: Cucujoidea). Syst. Entomol. 2010, 35, 753–763. [Google Scholar] [CrossRef]
- Gimmel, M.L.; Aston, P. New records of the family Phalacridae from Hong Kong (Coleoptera: Cucujoidea). Hong Kong Entomol. Soc. 2010, 2, 11–12. [Google Scholar]
- Sousa, W.O.; Ribeiro-Costa, C.S.; Rosado-Neto, G.H. A preliminary overview of the Brazilian Apioninae (Coleoptera: Brentidae) with an illustrated key for genera, and a checklist with distribution information. Biota Neotrop. 2019, 19, e20190813. [Google Scholar] [CrossRef]
- Shah, F.A.; Ansari, M.A.; Prasad, M.; Butt, T.M. Evaluation of black vine weevil (Otiorhynchus sulcatus) control strategies using Metarhizium anisopliae with sublethal doses of insecticides in disparate horticultural growing media. Biol. Control 2007, 40, 246–252. [Google Scholar] [CrossRef]
- Alekseev, V.I.; Grzymala, T.L. New Aderidae (Coleoptera: Tenebrionoidea) from Baltic and Bitterfeld amber. Zootaxa 2015, 3956, 239–257. [Google Scholar] [CrossRef]
- Werner, F.G.; Chandler, D.S. Anthicidae (Insecta: Coleoptera). Fauna New Zeal. 1995, 34, 1–64. [Google Scholar]
- Bologna, M.A.; Pinto, J.D. The Old World genera of Meloidae (Coleoptera): A key and synopsis. J. Nat. Hist. 2002, 36, 2013–2102. [Google Scholar] [CrossRef]
- Pinto, J.D.; Bologna, M.A. Beetles (Coleoptera) of Peru. Survey of the Families. Meloidae Gyllenhal, 1810. J. Kansas Entomol. Soc. 2016, 89, 202–209. [Google Scholar] [CrossRef]
- Sánches-Vialas, A.; García-París, M.; Ruiz, J.L.; Recuero, E. Patterns of morphological diversification in giant Berberomeloe blister beetles (Coleoptera: Meloidae) reveal an unexpected taxonomic diversity concordant with mtDNA phylogenetic structure. Zool. J. Linn. Soc. 2020, 189, 1249–1312. [Google Scholar] [CrossRef]
- Michaels, K.F. Using staphylinid and tenebrionid beetles as indicators of sustainable landscape management in Australia: A review. Aust. J. Exp. Agric. 2007, 47, 435–449. [Google Scholar] [CrossRef]
- Ocete, R.; Armendáriz, I.; Ocete, C.A.; Maistrello, L.; Valle, J.A.; Rodríguez, A.; Usategui, L. Spread parameters of the borer Xylotrechus arvicola (Olivier) (Coleoptera: Cerambicidae) in a ‘Tempranillo’ Vineyard in la Rioja (Spain): A long-term study. Ciênc. Téc. Vitiv. 2020, 35, 148–166. [Google Scholar] [CrossRef]
- Kozlov, M.V.; Prosvirov, A.S.; Zvereva, E.L. Can larvae of forest click beetles (Coleoptera: Elateridae) feed on live plant roots? Insects 2020, 11, 850. [Google Scholar] [CrossRef] [PubMed]
- Vuts, J.; Imrei, Z.; Birkett, M.A.; Pickett, J.A.; Woodcock, C.M.; Tóth, M. Semiochemistry of the Scarabaeoidea. J. Chem. Ecol. 2014, 40, 190–210. [Google Scholar] [CrossRef]
- Kurbatov, S.A.; Egorov, L.V. Review of the fauna of the beetle families Scydmaenidae and Pselaphidae (Coleoptera, Staphylinoidea) of Chuvashia. Entomol. Rev. 2012, 92, 864–878. [Google Scholar] [CrossRef]
- Newton, A.F.; Franz, H. World catalog of the genera of Scydmaenidae (Coleoptera). Koleopterol. Rundsch. 1998, 68, 137–165. [Google Scholar]
- López-Pérez, J.J. NOTA NOTE—Corología de Palaeostigus palpalis (Latreille, 1804) (Coleoptera, Staphylinoidea, Scydmaenidae) en la provincia de Huelva (S.O. de Andalucía, España). Arq. Entomolóxicos 2015, 13, 145–148. [Google Scholar]
- Frank, J.H.; Thomas, M.C. Rove beetles of the World, Staphylinidae (Insecta: Coleoptera: Staphylinidae). Entomology and Nematology Department, UF/IFAS Extension. 2016. Available online: https://edis.ifas.ufl.edu/publication/IN271 (accessed on 12 July 2021).
- Bohac, J. Staphylinid beetles as bioindicators. Agric. Ecosyst. Environ. 1999, 74, 357–372. [Google Scholar] [CrossRef]
- Klimaszewski, J.; Crosby, T.K. A revision of the New Zealand species of the parasitoid genus Aleochara, with description of four new species (Coleoptera: Staphylinidae). J. R. Soc. N. Z. 1997, 27, 243–269. [Google Scholar] [CrossRef]
- Klimaszewski, J.; Pace, R.; Center, T.D.; Couture, J. A remarkable new species of Himalusa pace from Thailand (Coleoptera, Staphylinidae, Aleocharinae): Phytophagous aleocharine beetle with potential for biocontrol of skunkvine-related weeds in the United States. ZooKeys 2010, 35, 1–12. [Google Scholar] [CrossRef]
- Marcelino, J.A.P.; Giordano, R.; Borges, P.A.V.; Garcia, P.V.; Soto-Adames, F.N.; Soares, A.O. Distribution and genetic variability of Staphylinidae across a gradient of anthropogenically influenced insular landscapes. Bull. Insectology 2016, 69, 117–126. [Google Scholar]
- Weithmann, S.; Kuppler, J.; Degasperi, G.; Steiger, S.; Ayasse, M.; von Hoermann, C. Local and landscape effects on carrion-associated rove beetle (Coleoptera: Staphylinidae) Communities in German Forests. Insects 2020, 11, 828. [Google Scholar] [CrossRef]
- Lupi, D.; Colombo, M.; Zanetti, A. The rove beetles (Coleoptera Staphylinidae) of three horticultural farms in Lombardy (Northern Italy). Boll. Zool. Agr. Bachic. 2006, 38, 143–165. [Google Scholar]
- Donovan, S.; Eggleton, P.; Bignell, D. Gut content analysis and a new feeding group classification of termites. Ecol. Entomol. 2001, 26, 356–366. [Google Scholar] [CrossRef]
- Bourguignon, T.; Sobotník, J.; Lepoint, G.; Martin, J.-M.; Hardy, O.J.; Ean, A.D.; Roiin, Y. Feeding ecology and phylogenetic structure of a complex neotropical termite assemblage, revealed by nitrogen stable isotope ratios. Ecol. Entomol. 2011, 36, 261–269. [Google Scholar] [CrossRef]
- Jouquet, P.; Chaudhary, E.; Kumar, A.R.V. Sustainable use of termite activity in agro-ecosystems with reference to earthworms. A review. Agron. Sustain. Dev. 2018, 38, 3. [Google Scholar] [CrossRef]
- Eggleton, P.; Bignell, D.E.; Hauser, S.; Dibog, L.; Norgrove, L.; Madong, B. Termite diversity across an anthropogenic disturbance gradient in the humid forest zone of West Africa. Agric. Ecosyst. Environ. 2002, 90, 189–202. [Google Scholar] [CrossRef]
- Jones, D.T.; Susilo, F.X.; Bignell, D.E.; Hardiwinoto, S.; Gillison, A.N.; Eggleton, P. Termite assemblage collapse along a land-use intensification gradient in lowland central Sumatra, Indonesia. J. Appl. Ecol. 2003, 40, 380–391. [Google Scholar] [CrossRef]
- Pribadi, T. Termites community as environmental bioindicators in highlands: A case study in eastern slopes of Mount Slamet, Central Java. Biodivers. J. Biol. Divers. 2011, 12, 235–240. [Google Scholar] [CrossRef]
- Viana-Junior, A.B.; Souza, V.B.; Reis, Y.T.; Marques-Costa, A.P. Termite assemblages in dry tropical forests of Northeastern Brazil: Are termites bioindicators of environmental disturbances? Sociobiology 2014, 61, 324–331. [Google Scholar] [CrossRef][Green Version]
- Frouz, J. Use of soil dwelling Diptera (Insecta, Diptera) as bioindicators: A review of ecological requirements and response to disturbance. Agric. Ecosyst. Environ. 1999, 74, 167–186. [Google Scholar] [CrossRef]
- McMillan, D.; Hohu, K.; Edgerly, J.S. Choreography of silk spinning by webspinners (Insecta: Embioptera) reflects lifestyle and hints at phylogeny. Biol. J. Linn. Soc. 2016, 118, 430–442. [Google Scholar] [CrossRef]
- Gullan, P.J.; Cranston, P.S. The Insects: An Outline of Entomology, 5th ed.; Wiley-Blackwell: West Sussex, UK, 2014; p. 624. [Google Scholar]
- Song, Z.-S.; Bartlett, C.R.; O’Brien, L.B.; Liang, A.P.; Bourgoin, T. Morphological phylogeny of Dictyopharidae (Hemiptera: Fulgoromorpha). Syst. Entomol. 2018, 43, 637–658. [Google Scholar] [CrossRef]
- Carlos, C.C.R. Towards a Sustainable Control of Arthropod Pests in Douro Demarcated Region Vineyards with Emphasis on the Grape Berry Moth, Lobesia botrana (Denis & Schifermüller). Ph.D. Thesis, University of Trás-os-Montes and Alto Douro, Vila Real, Portugal, 2017. [Google Scholar]
- Krstić, O.; Cvrković, T.; Mitrović, M.; Toševski, I.; Jović, J. Dictyophara europaea (Hemiptera: Fulgoromorpha: Dictyopharidae): Description of immatures, biology and host plant associations. Bull. Entomol. Res. 2016, 106, 395–405. [Google Scholar] [CrossRef]
- Schwertner, C.F.; Nardi, C. Burrower Bugs (Cydnidae). In True Bugs (Heteroptera) of the Neotropics. Entomology in Focus; Panizzi, A., Grazia, J., Eds.; Springer: Dordrecht, Germany, 2015; Volume 2. [Google Scholar]
- Henry, T.J. Biodiversity of Heteroptera. In Insect Biodiversity: Science and Society, 2nd ed.; Foottit, R.G., Adler, P.H., Eds.; John Wiley & Sons Ltd.: New Jersey, NJ, USA, 2017; Volume I, pp. 279–335. [Google Scholar]
- Henry, T.J.; Dellapé, P.M.; de Paula, A.S. The Big-Eyed Bugs, Chinch Bugs, and Seed Bugs (Lygaeoidea). In True Bugs (Heteroptera) of the Neotropics. Entomology in Focus; Panizzi, A., Grazia, J., Eds.; Springer: Dordrecht, Germany, 2015; Volume 2, pp. 154–196. [Google Scholar]
- Koch, R.L.; Pezzini, D.T.; Michel, A.P.; Hunt, T.E. Identification, biology, impacts, and management of stink bugs (Hemiptera: Heteroptera: Pentatomidae) of soybean and corn in the Midwestern United States. J. Integr. Pest. Manag. 2017, 8, 1–14. [Google Scholar] [CrossRef]
- Taylor, M.E.; Bundy, C.S.; Mcpheron, J.E. Unusual Ovipositional Behavior of the Stink Bug Bagrada hilaris (Hemiptera: Heteroptera: Pentatomidae). Ann. Entomol. Soc. Am. 2014, 107, 872–877. [Google Scholar] [CrossRef]
- Fowles, T.M.; Coscarón, M.C.; Panizzi, A.R.; Carroll, S.P. Scentless Plant Bugs (Rhopalidae). In True Bugs (Heteroptera) of the Neotropics. Entomology in Focus; Panizzi, A., Grazia, J., Eds.; Springer: Dordrecht, Germany, 2015; Volume 2, pp. 607–637. [Google Scholar]
- Panizzi, A.R.; Hirose, E.; Chocorosqui, V.R. Unusual Oviposition Behavior by a Seed Feeding Bug (Heteroptera: Rhopalidae). Scientific Note. Neotrop. Entomol. 2002, 31, 477–479. [Google Scholar] [CrossRef]
- Wu, Y.-Z.; Rédei, D.; Eger, J., Jr.; Wang, Y.-H.; Wu, H.-Y.; Carapezza, A.; Kment., P.; Cai, B.; Sun, X.-Y.; Guo, P.-L.; et al. Phylogeny and the colourful history of jewel bugs (Insecta: Hemiptera: Scutelleridae). Cladistics 2018, 34, 502–516. [Google Scholar] [CrossRef]
- Rasplus, J.-Y.; Villemant, C.; Paiva, M.R.; Delvare, G.; Roques, A. Hymenoptera. Chapter 12. BioRisk 2010, 4, 669–776. [Google Scholar] [CrossRef]
- Guglielmino, A. Dryinidae (Hymenoptera Chrysidoidea): An interesting group among the natural enemies of the Auchenorrhyncha (Hemiptera). Denisia 2002, 4, 549–556. [Google Scholar]
- Macek, J. Chrysidoidea: Dryinidae (Lapkovití) and Embolemidae (Vejřenkovití). In Annotated checklist of the Aculeata (Hymenoptera) of the Czech Republic and Slovakia; Bogusch, P., Straka, J., Kment, P., Eds.; Acta Entomologica Musei Nationalis Pragae Supplementum: Prague, Czech Republic, 2007; pp. 65–84. [Google Scholar]
- Cerdá, X.; Dejean, A. Predation by Ants on Arthropods and Other Animals. Predation in the Hymenoptera: In An Evolutionary Perspective; Polidori, C., Ed.; Transworld Research Network: Kerala, India, 2011; pp. 39–78. [Google Scholar]
- Karhu, K.J. Effects of ant exclusion during outbreaks of a defoliator and a sap-sucker on birch. Ecol. Entomol. 1998, 23, 185–194. [Google Scholar] [CrossRef]
- Albert, M.J.; Escudero, A.; Iriondo, J.M. Assessing ant seed predation in threatened plants: A case study. Acta Oecol. 2005, 28, 213–220. [Google Scholar] [CrossRef]
- Rodriguez, J.; Calle, Z.; Montoya-Lerma, J. Herbivory of Atta cephalotes (Hymenoptera: Myrmicinae) on three plant substrates. Rev. Colomb. Entomol. 2008, 34, 156–162. [Google Scholar]
- Baraibar, B.; Torra, J.; Royo-Esnal, A.; Recasens, J.; Comas, C. Harvester ant nest distribution depends on soil disturbance regime. Biol. Control. 2019, 128, 1–5. [Google Scholar] [CrossRef]
- Perez, J.E.J.; Dupo, A.L.A.B. Arthropod community structure during the early stages of leaf litter decomposition. Asian J. Biodiv. 2013, 4, 84–98. [Google Scholar] [CrossRef]
- Detrain, C.; Verheggen, F.J.; Diez, L.; Wathelet, B.; Haubruge, E. Aphid-ant mutualism: How honeydew sugars influence the behaviour of ant scouts. Physiol. Entomol. 2010, 35, 168–174. [Google Scholar] [CrossRef]
- Urbani, C.B.; de Andrade, M.L. Pollen eating, storing, and spitting by ants. Naturwissenschaften 1997, 84, 256–258. [Google Scholar] [CrossRef]
- Kost, C.; Heil, M. Increased availability of extrafloral nectar reduces herbivory in Lima bean planted (Phaseolus lunatus, Fabaceae). Basic App. Ecol. 2005, 6, 237–248. [Google Scholar] [CrossRef]
- Stefani, V.; Pires, T.; Torezan-Silingardi, H.M.; Del Claro, K. Beneficial effects of ants and spiders on the reproductive value of Eriotheca gracilipes (Malvaceae) in a Tropical Savanna. PLoS ONE 2015, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Del-Claro, K.; Rico-Gray, V.; Torezan-Silingardi, H.M.; Alves-Silva, E.; Fagundes, R.; Lange, D.; Dáttilo, W.; Vilela, A.A.; Aguirre, A.; Rodriguez-Morales, D. Loss and gains in ant-plant interactions mediated by extrafloral nectar: Fidelity, cheats, and lies. Insectes Soc. 2016, 63, 207–221. [Google Scholar] [CrossRef]
- Majer, J.D. Ants: Bio-indicators of mine site rehabilitation, land-use, and land conservation. Environ. Manag. 1993, 7, 375–383. [Google Scholar] [CrossRef]
- Casimiro, M.S.; Sansevero, J.B.B.; Queiroz, J.M. What can ants tell us about ecological restoration? A global meta-analysis. Ecol. Indic. 2019, 102, 593–598. [Google Scholar] [CrossRef]
- Andersen, A.N.; Majer, J.D. Ants show the way down under: Invertebrates as bioindicators in land management. Front. Ecol. Environ. 2004, 2, 291–298. [Google Scholar] [CrossRef]
- Bogusch, P. Vespoidea: Mutillidae (Kodulkovití). In Annotated Checklist of the Aculeata (Hymenoptera) of the Czech Republic and Slovakia; Bogusch, P., Straka, J., Kment, P., Eds.; Acta Entomologica Musei Nationalis Pragae Supplementum: Prague, Czech Republic, 2007; pp. 93–104. [Google Scholar]
- Brothers, D.J. Alternative Life-History Styles of Mutillid Wasps (Insecta, Hymenoptera). In Alternative Life-History Styles of Animals; Bruton, M.N., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1989; pp. 279–291. [Google Scholar]
- Luz, D.R.; Rosa, B.B.; Williams, K.A.; Melo, G.A.R. An uncommon feeding habit: Mutillid wasps (Hymenoptera, Mutillidae) visiting extrafloral nectaries in Malpighiaceae. Braz. J. Biol. 2016, 76, 551–553. [Google Scholar] [CrossRef][Green Version]
- Bidau, C. Patterns in Orthoptera biodiversity. I. Adaptations in ecological and evolutionary contexts. J. Insect Biodiv. 2014, 2, 1–39. [Google Scholar] [CrossRef]
- Whitman, D.W.; Richardson, M.L. Necrophagy in grasshoppers: Taeniopoda eques feeds on mammal carrion. J. Orthoptera Res. 2010, 19, 377–380. [Google Scholar] [CrossRef]
- Capinera, J.L.; Sechrist, T.S. Grasshoppers (Acrididae) of Colorado: Identification, biology and management. Colo. State Univ. Exp. Stn. Collin Bull. 1982, 584, 1–161. [Google Scholar]
- López-Colón, J.I. Sciobia lusitanica Rambur, 1839, grillo endémico del área ibero-marroquí (Orthoptera, Gryllidae, Sciobiinae). Bol. Soc. Entomol. Aragonesa 2001, 28, 66. [Google Scholar]
- Miranda-Arabolaza, M.J.; Barranco, P. Os ortópteros da bacia do rio Sabor (Trás-os-Montes e Alto Douro, Portugal) (Insecta, Orthoptera). Bol. Soc. Entomol. Aragonesa 2005, 37, 173–200. [Google Scholar]
- Pérez-Bote, J.L.; Castaño, A.J.R.; Sanromán, J.M.T.; Jiménez, J.M.G. Nuevas citas de Sciobia lusitanica (Rambur, 1839) (Orthoptera, Gryllidae, Gryllinae) en Extremadura (Suroeste de la Península Ibérica). Bol. Soc. Entomol. Aragonesa 2006, 38, 290–291. [Google Scholar]
- Xu, Y.; Held, D.W.; Hu, X.P. Dietary choices and their implication for survival and development of omnivorous mole crickets (Orthoptera: Gryllotalpidae). App. Soil Ecol. 2013, 71, 65–71. [Google Scholar] [CrossRef]
- Bailey, D.L.; Held, D.W.; Kalra, A.; Twarakavi, N.; Arriaga, F. Biopores from mole crickets (Scapteriscus spp.) increase soil hydraulic conductivity and infiltration rates. Appl. Soil Ecol. 2015, 94, 7–14. [Google Scholar] [CrossRef]
- New, T.R. Neuroptera (Lacewings). In Insects of Australia; CSIRO, Ed.; Cornell University Press: Ithaca, NY, USA, 1991; Volume 2, pp. 525–542. [Google Scholar]
- Tauber, C.A.; Tauber, M.J.; Albuquerque, G.S. Neuroptera: (Lacewings, Antlions). In Encyclopedia of Insects, 2nd ed.; Resh, V.H., Cardé, R.T., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 695–707. [Google Scholar]
- Mound, L.A. Thysanoptera: Diversity and Interactions. Annu. Rev. Entomol. 2005, 50, 247–269. [Google Scholar] [CrossRef] [PubMed]
- Reynaud, P. Chapter 13.1—Thrips (Thysanoptera). BioRisk 2010, 4, 767–791. [Google Scholar] [CrossRef]
- Edgecombe, G.D.; Giribet, G. Evolutionary Biology of Centipedes (Myriapoda: Chilopoda). Annu. Rev. Entomol. 2007, 52, 151–170. [Google Scholar] [CrossRef]
- Kula, E.; Lazorik, M. Centipedes, millipedes, terrestrial isopods and their relationships to physical and chemical properties of forest soils. Entomol. Fennica 2016, 27, 33–51. [Google Scholar] [CrossRef][Green Version]
- Lensing, J.R.; Wise, D.H. Predicted climate change alters the indirect effect of predators on an ecosystem process. Proc. Natl. Acad. Sci. USA 2006, 103, 15502–15505. [Google Scholar] [CrossRef] [PubMed]
- Wolters, V.; Ekschmitt, K. Gastropods, isopods, diplopods, and chilopods: Neglected groups of the decomposer food web. In Fauna in Soil Ecosystems Recycling Processes, Nutrient Fluxes, and Agricultural Production, 1st ed.; Benckiser, G., Ed.; CRC Press: New York, NY, USA, 1997; pp. 265–306. [Google Scholar]
- Ferlian, O.; Scheu, S.; Pollierer, M.M. Trophic interactions in centipedes (Chilopoda, Myriapoda) as indicated by fatty acid patterns: Variations with life stage, forest age and season. Soil Biol. Biochem. 2012, 52, 33–42. [Google Scholar] [CrossRef]
- Tuf, I.H.; Tufová, J. Proposal of ecological classification of centipede, millipede and terrestrial isopod faunas for evaluation of habitat quality in Czech Republic. Cas. Slez. Muz. Opava 2008, 57, 37–44. [Google Scholar]
- Scheu, S.; Poser, G. The soil macrofauna (Diplopoda, Isopoda, Lumbricidae and Chilopoda) near tree trunks in a beechwood on limestone: Indications for stemfow induced changes in community structure. Appl. Soil Ecol. 1996, 3, 115–125. [Google Scholar] [CrossRef]
- Blackburn, J.; Arthur, W. Comparative abundance of centipedes on organic and conventional farms, and its possible relation to declines in farmland bird populations. Basic Appl. Ecol. 2001, 2, 373–381. [Google Scholar] [CrossRef]
- Klarner, B.; Winkelmann, H.; Krashevska, V.; Maraun, M.; Widyastuti, R.; Scheu, S. Trophic niches, diversity and community composition of invertebrate top predators (Chilopoda) as affected by conversion of tropical lowland rainforest in Sumatra (Indonesia). PLoS ONE 2017, 12, e0180915. [Google Scholar]
- Read, H.; Martin, M.H.; Rayner, J.M.V. Invertebrates in woodlands polluted by heavy metals. An evaluation using canonical correspondence analysis. Water Air Soil Pollut. 1998, 106, 17–42. [Google Scholar] [CrossRef]
- De Godoy, J.A.P.; Fontanetti, C.S. Diplopods as Bioindicators of Soils: Analysis of Midgut of Individuals Maintained in Substract Containing Sewage Sludge. Water Air Soil Pollut. 2010, 210, 389–398. [Google Scholar] [CrossRef]
- Redi, B.H.; Van Aarde, R.J.; Wassenaar, T.D. Coastal dune forest development and the regeneration of millipede communities. Restor. Ecol. 2005, 13, 284–291. [Google Scholar] [CrossRef]
- Scheller, U.; Berg, M.P.; Jansen, M.G.M. Pauropoda (Myriapoda), a class new to the Dutch fauna, with the description of a new species. Entomol. Ber. 2004, 64, 3–9. [Google Scholar]
- Scheller, U.; Minor, M. New records of Pauropoda (Myriapoda) from New Zealand with descriptions of four new species and a new family Eirmopauropodidae. N. Z. J. Zool. 2010, 37, 323–337. [Google Scholar] [CrossRef][Green Version]
- Rodriguez, M.T.D. Symphyla y Pauropoda (Myriapoda) de Suelo de España. Ph.D. Thesis, Complutense University of Madrid, Madrid, Spain, 1992. [Google Scholar]
- Ochoa-Hueso, R.; Rocha, I.; Stevens, C.J.; Manrique, E.; Luciañez, M.J. Simulated nitrogen deposition affects soil fauna from a semiarid Mediterranean ecosystem in central Spain. Biol. Fertil. Soils 2014, 50, 191–196. [Google Scholar] [CrossRef]
- Moritz, L.; Wesener, T. Symphylella patrickmuelleri sp. nov. (Myriapoda: Symphyla): The oldest known Symphyla and first fossil record of Scolopendrellidae from Cretaceous Burmese amber. Cretac. Res. 2018, 84, 258–263. [Google Scholar] [CrossRef]
- Camacho, M.D. Phylogeny of the Symphyla (Myriapoda). Ph.D. Thesis, Free University of Berlin, Berlin, Germany, 2009. [Google Scholar]
- Langor, D.W.; de Waard, J.R.; Snyder, B.A. Myriapoda of Canada. ZooKeys 2019, 819, 169–186. [Google Scholar] [CrossRef] [PubMed]
- Voigtländer, K.; Decker, P.; Burkhardt, U.; Spelda, J. The present knowledge of the Symphyla and Pauropoda (Myriapoda) in Germany—An annotated checklist. Acta Soc. Zool. Bohem. 2016, 80, 51–85. [Google Scholar]
- Millennium Ecosystem Assessment. Ecosystems and Human Well-being: Biodiversity Synthesis; World Resources Institute: Washington, DC, USA, 2005. [Google Scholar]
- Shackleton, C.M.; Ruwanza, S.; Sinasson Sanni, G.K.; Bennett, S.; De Lacy, P.; Modipa, R.; Mtati, N.; Sachikonye, M.; Thondhlana, G. Unpacking Pandora’s box: Understanding and categorising ecosystem disservices for environmental management and human wellbeing. Ecosystems 2016, 19, 587–600. [Google Scholar] [CrossRef]
- Gillespie, M.A.K.; Wratten, S.D. The role of ecosystem disservices in pest management. In Environmental Pest Management: Challenges for Agronomists, Ecologists, Economists and Policymakers; Coll, M., Wajnberg, E., Eds.; John Wiley & Sons: Oxford, UK, 2017; pp. 175–194. [Google Scholar]
- Frouz, J.; Roubíčková, A.; Heděnec, P.; Tajovský, K. Do soil fauna really hasten litter decomposition? A meta-analysis of enclosure studies. Eur. J. Soil Biol. 2015, 68, 18–24. [Google Scholar] [CrossRef]
- David, J.F. The role of litter-feeding macroarthropods in decomposition processes: A reappraisal of common views. Soil Biol. Biochem. 2014, 76, 109–118. [Google Scholar] [CrossRef]
- Snyder, B.A.; Hendrix, P.F. Current and potential roles of soil macroinvertebrates (earthworms, millipedes, and isopods) in ecological restoration. Rest. Ecol. 2008, 16, 629–636. [Google Scholar] [CrossRef]
- Sharma, R.M.; Chandra, K. Insecta: Embioptera. ZooI. Surv. India 2013, 21, 125–126. [Google Scholar]
- Noriega, J.A.; Hortal, J.; Azcárate, F.M.; Berg, M.P.; Bonada, N.; Briones, M.J.I.; Toro, I.D.; Goulson, D.; Ibanez, S.; Landis, D.A.; et al. Research trends in ecosystem services provided by insects. Basic Appl. Ecol. 2018, 26, 8–23. [Google Scholar] [CrossRef]
- Honek, A.; Martinkova, Z.; Jaroik, V. Ground beetles (Carabidae) as seed predators. Eur. J. Entomol. 2003, 100, 531–544. [Google Scholar] [CrossRef]
- Ichihara, M.; Matsuno, K.; Inagaki, H.; Saiki, C.; Mizumoto, S.; Yamaguchi, S.; Yamashita, M.; Sawada, H. Creation of paddy levees to enhance the ecosystem service of weed seed predation by crickets. Landsc. Ecol. Eng. 2015, 11, 227–233. [Google Scholar] [CrossRef]
- Blubaugh, C.K.; Hagler, J.R.; Machtley, S.A.; Kaplan, I. Cover crops increase foraging activity of omnivorous predators in seed patches and facilitate weed biological control. Agric. Ecosyst. Environ. 2016, 231, 264–270. [Google Scholar] [CrossRef]
- Kendall, D.A. Soil Tillage and Epigeal Predatory Insects. In Soil Tillage in Soil Agroecosystems; El Titi, A., Ed.; CRC Press: Boca Raton, FL, USA, 2003; pp. 297–342. [Google Scholar]
- Hoffmann, C.; Köckerling, J.; Biancu, S.; Gramm, T.; Michl, G.; Entling, M.H. Can flowering greencover crops promote biological control in German vineyards. Insects 2017, 8, 121. [Google Scholar] [CrossRef] [PubMed]
- Roltsch, W.R.; Hanna, F.; Zalom, H.; Shorey, H.; Mayse, M. Spiders and Vineyard Habitat Relationship in Central California. In Enhancing Natural Control of Arthropods Through Habitat Management; Pickett, C., Bugg, R., Eds.; University of California Press: Berkeley, CA, USA, 1998; pp. 311–338. [Google Scholar]
- Pétremand, G.; Fleury, D.; Castella, E.; Delabays, N. Influence de l’enherbement viticole sur les Carabidae (Coleoptera) et intérêt potentiel pour le contrôle de certains ravageurs de la vigne. Biotechnol. Agron. Soc. Environ. 2016, 20, 375–385. [Google Scholar]
- Blaise, C.; Mazzia, C.; Bischoff, A.; Millon, A.; Ponel, P.; Blight, O. The key role of inter-row vegetation and ants on predation in Mediterranean organic vineyards. Agric. Ecosyst. Environ. 2021, 311, 107327. [Google Scholar] [CrossRef]
- Agustí, N.; Shayler, P.S.; Harwood, J.D.; Vaughan, I.P.; Sunderland, K.D.; Symondson, W.O.C. Collembola as alternative prey sustaining spiders in arable ecosystems: Prey detection within predators using molecular markers. Mol. Ecol. 2003, 12, 3467–3475. [Google Scholar] [CrossRef]
- Bauer, T. Beetles which use a setal trap to hunt springtails: The hunting strategy and apparatus of Leistus (Coleoptera, Carabidae). Pedobiologia 1985, 28, 275–287. [Google Scholar]
- Oelbermann, K.; Langel, R.; Scheu, S. Utilization of prey from the decomposer system by generalist predators of grassland. Oecologia 2008, 155, 605–617. [Google Scholar] [CrossRef]
- Lavelle, P.; Bignell, D.; Lepage, M. Soil function in a changing world: The role of invertebrate ecosystem engineers. Eur. J. Soil Biol. 1997, 33, 159–193. [Google Scholar]
- Jouquet, P.; Dauber, J.; Lagerlöf, J.; Lavelle, P.; Lepage, M. Soil invertebrates as ecosystem engineers: Intended and accidental effects on soil and feedback loops. App. Soil Ecol. 2006, 32, 153–164. [Google Scholar] [CrossRef]
- Ashton, L.A.; Griffiths, H.M.; Parr, C.L.; Evans, T.A.; Didham, R.K.; Hasan, F.; The, Y.A.; Tin, H.S.; Vairappan, C.S.; Eggleton, P. Termites mitigate the effects of drought in tropical rainforest. Science 2019, 363, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.K.; Singh, H.; Rastogi, N.; Agarwal, V.M. Impact of abundant Pheidole ant species on soil nutrients in relation to the food biology of the species. App. Soil Ecol. 2013, 71, 15–23. [Google Scholar] [CrossRef]
- Cerda, A.; Jurgensen, M.F. The influence of ants on soil and water losses from an orange orchard in eastern Spain. J. Appl. Entomol. 2008, 132, 306–314. [Google Scholar] [CrossRef]
- Jouquet, P.; Janeau, J.L.; Pisano, A.; Hai, T.S.; Orange, D.; Minh, L.T.N.; Valentin, C. Influence of earthworms and termites on runoff and erosion in a tropical steep slope fallow in Vietnam: A rainfall simulation experiment. Appl. Soil Ecol. 2012, 61, 161–168. [Google Scholar] [CrossRef]
- Evans, T.A.; Dawes, T.Z.; Ward, P.R.; Lo, N. Ants and termites increase crop yield in a dry climate. Nat. Commun. 2011, 2, 262. [Google Scholar] [CrossRef]
- Ali, I.G.; Sheridan, G.; French, J.R.J.; Ahmed, B.M. Ecological benefits of termite soil interaction and microbial symbiosis in the soil ecosystem. J. Earth Sci. Geotech. Eng. 2013, 3, 63–85. [Google Scholar]
- Ginzburg, O.; Whitford, W.G.; Steinberger, Y. Effects of harvester ant (Messor spp.) activity on soil properties and microbial communities in a Negev Desert ecosystem. Biol. Fertil. Soil. 2008, 45, 165–173. [Google Scholar] [CrossRef]
- Issoufou, A.A.; ·Soumana, I.; Maman, G.; Konate, S.; Mahamane, A. Effects of termites growth on litter decomposition: A modeling approach. Int. J. Recycl. Org. Waste Agric. 2019, 8, S415–S421. [Google Scholar] [CrossRef]
- Prosdocimi, M.; Jordán, A.; Tarolli, P.; Keesstra, S.; Novara, A.; Cerdà, A. The immediate effectiveness of barley straw mulch in reducing soil erodibility and surface runoff generation in Mediterranean vineyards. Sci. Total Environ. 2016, 547, 323–330. [Google Scholar] [CrossRef]
- Bertone, M.A.; Green, J.T.; Washburn, S.P.; Poore, M.H.; Watson, D.W. The contribution of tunneling dung beetles to pasture soil nutrition. Forage Grazinglands 2006, 4, 1–12. [Google Scholar] [CrossRef]
- Byk, A.; Piętka, J. Dung beetles and their role in the nature. Eduk. Biol. Sr. 2018, 1, 17–26. [Google Scholar] [CrossRef]
- Brown, J.; Scholtz, C.H.; Janeau, J.-L.; Grellier, S.; Podwojewski, P. Dung beetles (Coleoptera: Scarabaeidae) can improve soil hydrological properties. Appl. Soil Ecol. 2010, 46, 9–16. [Google Scholar] [CrossRef]
- Richardson, A.M.M.; Morton, H.P. Terrestrial amphipods (crustacea, amphipoda, F. Talitridae) and soil respiration. Soil Biol. Biochem. 1986, 18, 197–200. [Google Scholar] [CrossRef]





| Taxa | N | % | Trophic Group(s) | Bioindicator | |||
|---|---|---|---|---|---|---|---|
| Chelicerata | |||||||
| Arachnida | 1149 | 9.169 | |||||
| Opiliones | 806 | 0.663 | omn, pred | Y | |||
| Pseudoscopiones | 1 | 0.001 | pred | N | |||
| Scorpiones | 7 | 0.006 | pred | N | |||
| Solifugae | 1 | 0.001 | pred | NA | |||
| Acari | 5812 | 4.780 | detr, pred | Y | |||
| Oribatida | 4054 | 3.334 | detr | ||||
| Other Acari | 1758 | 1.446 | |||||
| Araneae | 4522 | 3.719 | pred | Y | |||
| Crustacea | |||||||
| Malacostraca | 229 | 0.188 | |||||
| Amphipoda | 2 | 0.002 | detr | Y | |||
| Isopoda | 227 | 0.187 | detr, phyt | Y | |||
| Hexapoda | |||||||
| Entognatha | 68406 | 56.258 | |||||
| Collembola | 68406 | 56.258 | detr, omn, pred | Y | |||
| Insecta | 41563 | 34.182 | |||||
| Coleoptera | 5610 | 4.614 | detr, fung, phyt, pred | Y | |||
| Carabidae | 2075 | 1.706 | omn, phyt, pred | Y | |||
| Chrysomelidae | 252 | 0.207 | phyt | Y | |||
| Malachiidae | 7 | 0.006 | detr, omn, pred | NA | |||
| Corylophidae | 26 | 0.021 | fung | NA | |||
| Latridiidae | 14 | 0.012 | fung | NA | |||
| Phalacridae | 14 | 0.012 | fung | NA | |||
| Brentidae + Curculionidae | 234 | 0.192 | detr, phyt | NA | |||
| Aderidae | 78 | 0.064 | fung | NA | |||
| Anthicidae | 1321 | 1.086 | detr, pred | NA | |||
| Meloidae | 3 | 0.002 | phyt, pred | NA | |||
| Tenebrionidae | 577 | 0.475 | detr, fung | NA | |||
| Buprestidae | 3 | 0.002 | detr, phyt | NA | |||
| Elateridae | 139 | 0.114 | omn, phyt, pred | NA | |||
| Geotrupidae + Scarabaeidae | 141 | 0.116 | copro, detr, phyt | Y | |||
| Scydmaenidae | 86 | 0.071 | pred | NA | |||
| Staphylinidae | 382 | 0.314 | detr, fung, paras, phyt, pred | Y | |||
| Other Coleoptera | 258 | 0.212 | |||||
| Dictyoptera | 2 | 0.002 | detr, fung | NA | |||
| Embioptera Hemiptera | 5 | 0.004 | detr | NA | |||
| 597 | 0.491 | phyt, pred | Y | ||||
| Dictyopharidae | 15 | 0.012 | phyt | NA | |||
| Cydnidae | 189 | 0.155 | phyt | NA | |||
| Lygaeidae | 83 | 0.068 | phyt, pred | NA | |||
| Pentatomidae | 16 | 0.013 | phyt, pred, omn | NA | |||
| Rhopalidae | 288 | 0.237 | phyt | NA | |||
| Scutelleridae | 6 | 0.005 | phyt | NA | |||
| Hymenoptera | 34869 | 28.677 | detr, omn, paras, phyt, pred | Y | |||
| Formicidae | 34757 | 28.584 | detr, omn, phyt, pred, | Y | |||
| Mutillidae | 89 | 0.073 | paras, pred | NA | |||
| Dryinidae | 23 | 0.019 | paras, pred | NA | |||
| Orthoptera | 480 | 0.395 | detr, omn, phyt, pred | Y | |||
| Myriapoda | |||||||
| Chilopoda | 161 | 0.132 | pred | Y | |||
| Diplopoda | 86 | 0.071 | detr, omn, pred | Y | |||
| Total | 121594 | 100.000 | |||||
| Taxa | N | % | Trophic Group(s) | Bioindicator | ||
|---|---|---|---|---|---|---|
| Chelicerata | ||||||
| Arachnida | 20444 | 45.381 | ||||
| Opiliones | 2 | 0.004 | omn, pred | Y | ||
| Pseudoscopiones | 42 | 0.093 | pred | N | ||
| Acari | 20331 | 45.130 | detr, pred | Y | ||
| Araneae | 62 | 0.138 | pred | Y | ||
| Palpigradi | 7 | 0.016 | bact | NA | ||
| Crustacea | ||||||
| Malacostraca | 21 | 0.047 | ||||
| Isopoda | 21 | 0.047 | detr, phyt | Y | ||
| Hexapoda | ||||||
| Entognatha | 19126 | 42.453 | ||||
| Collembola | 18737 | 41.590 | detr, omn, pred | Y | ||
| Diplura | 345 | 0.766 | microb, pred | Y | ||
| Protura | 44 | 0.098 | fung | Y | ||
| Insecta | 4881 | 10.834 | ||||
| Coleoptera (adults and larvae) | 813 | 1.805 | detr, fung, phyt, pred | Y | ||
| Dictyoptera (Isoptera) | 47 | 0.104 | detr, fung | NA | ||
| Diptera (larvae) | 303 | 0.673 | detr, paras, phyt, pred | Y | ||
| Embioptera Hemiptera | 13 | 0.029 | detr | NA | ||
| 126 | 0.280 | phyt, pred | Y | |||
| Hymenoptera (Formicidae) | 3282 | 7.285 | detr, omn, phyt, pred | Y | ||
| Lepidoptera (larvae) | 254 | 0.564 | phyt, pred | Y | ||
| Neuroptera (larvae) | 5 | 0.011 | detr, omn phyt, pred | Y | ||
| Thysanoptera | 38 | 0.084 | fung, omn, phyt, pred | Y | ||
| Myriapoda | ||||||
| Chilopoda | 234 | 0.519 | pred | Y | ||
| Diplopoda | 31 | 0.069 | detr, omn, pred | Y | ||
| Pauropoda | 13 | 0.029 | detr, microb | Y | ||
| Symphyla | 301 | 0.668 | detr, phyt, pred | NA | ||
| Total | 45052 | 100.000 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, F.; Carlos, C.; Crespo, L.; Zina, V.; Oliveira, A.; Salvação, J.; Pereira, J.A.; Torres, L. Soil Arthropods in the Douro Demarcated Region Vineyards: General Characteristics and Ecosystem Services Provided. Sustainability 2021, 13, 7837. https://doi.org/10.3390/su13147837
Gonçalves F, Carlos C, Crespo L, Zina V, Oliveira A, Salvação J, Pereira JA, Torres L. Soil Arthropods in the Douro Demarcated Region Vineyards: General Characteristics and Ecosystem Services Provided. Sustainability. 2021; 13(14):7837. https://doi.org/10.3390/su13147837
Chicago/Turabian StyleGonçalves, Fátima, Cristina Carlos, Luís Crespo, Vera Zina, Amália Oliveira, Juliana Salvação, José Alberto Pereira, and Laura Torres. 2021. "Soil Arthropods in the Douro Demarcated Region Vineyards: General Characteristics and Ecosystem Services Provided" Sustainability 13, no. 14: 7837. https://doi.org/10.3390/su13147837
APA StyleGonçalves, F., Carlos, C., Crespo, L., Zina, V., Oliveira, A., Salvação, J., Pereira, J. A., & Torres, L. (2021). Soil Arthropods in the Douro Demarcated Region Vineyards: General Characteristics and Ecosystem Services Provided. Sustainability, 13(14), 7837. https://doi.org/10.3390/su13147837








