Effects of Resistance Training Program on Muscle Mass and Muscle Strength and the Relationship with Cognition in Older Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participants
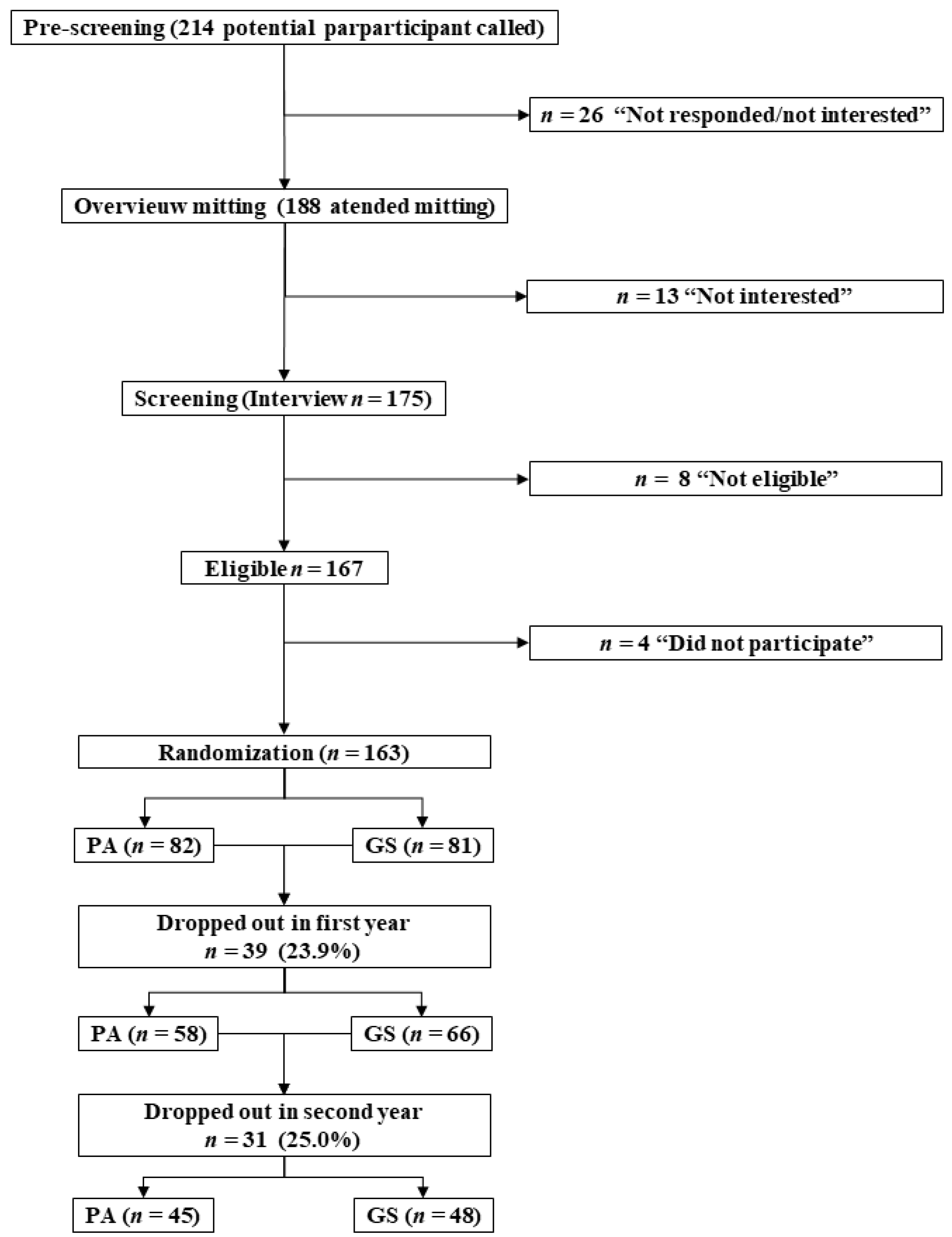
2.3. Procedure
2.4. Variables
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Damiano, S.; Muscariello, E.; La Rosa, G.; Di Maro, M.; Mondola, P.; Santillo, M. Dual Role of Reactive Oxygen Species in Muscle Function: Can Antioxidant Dietary Supplements Counteract Age-Related Sarcopenia? Int. J. Mol. Sci. 2019, 20, 3815. [Google Scholar] [CrossRef]
- Kumar, A.; Yegla, B.; Foster, T.C. Redox Signaling in Neurotransmission and Cognition During Aging. Antioxid. Redox Signal. 2018, 28, 1724–1745. [Google Scholar] [CrossRef]
- Hajjar, I.; Hayek, S.S.; Goldstein, F.C.; Martin, G.; Jones, D.P.; Quyyumi, A. Oxidative stress predicts cognitive decline with aging in healthy adults: An observational study. J. Neuroinflamm. 2018, 15, 17. [Google Scholar] [CrossRef]
- Molina-Sotomayor, E.; Castillo-Quezada, H.; Martínez-Salazar, C.; González-Orb, M.; Espinoza-Salinas, A.; Gonzalez-Jurado, J.A. Effects of progressive resistance training on cognition and igf-1 levels in elder women who live in areas with high air pollution. Int. J. Environ. Res. Public Health 2020, 17, 6203. [Google Scholar] [CrossRef]
- Lauretani, F.; Meschi, T.; Ticinesi, A.; Maggio, M. “Brain-muscle loop” in the fragility of older persons: From pathophysiology to new organizing models. Aging Clin. Exp. Res. 2017, 29, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Onder, G.; Russo, A.; Liperoti, R.; Tosato, M.; Martone, A.M.; Capoluongo, E.; Bernabei, R. Calf circumference, frailty and physical performance among older adults living in the community. Clin. Nutr. 2014, 33, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Miyamura, K.; Fhon, J.R.S.; Bueno, A.d.A.; Fuentes-Neira, W.L.; Silveira, R.C.d.C.P.; Rodrigues, R.A.P. Frailty syndrome and cognitive impairment in older adults: Systematic review of the literature. Rev. Lat. Am. Enferm. 2019, 27. [Google Scholar] [CrossRef] [PubMed]
- Lauretani, F.; Maggio, M.; Ticinesi, A.; Tana, C.; Prati, B.; Gionti, L.; Nouvenne, A.; Meschi, T. Muscle weakness, cognitive impairment and their interaction on altered balance in elderly outpatients: Results from the TRIP observational study. Clin. Interv. Aging 2018, 13, 1437. [Google Scholar] [CrossRef]
- Catalano, A.; Sardella, A.; Bellone, F.; Lasco, C.G.; Martino, G.; Morabito, N. Executive functions predict fracture risk in postmenopausal women assessed for osteoporosis. Aging Clin. Exp. Res. 2020, 32, 2251–2257. [Google Scholar] [CrossRef] [PubMed]
- Sui, S.X.; Williams, L.J.; Holloway-Kew, K.L.; Hyde, N.K.; Anderson, K.B.; Tembo, M.C.; Addinsall, A.B.; Leach, S.; Pasco, J.A. Skeletal Muscle Density and Cognitive Function: A Cross-Sectional Study in Men. Calcif. Tissue Int. 2021, 108, 165–175. [Google Scholar] [CrossRef]
- Wang, L.; Yin, L.; Zhao, Y.; Su, Y.; Sun, W.; Chen, S.; Liu, Y.; Yang, M.; Yu, A.; Guglielmi, G.; et al. Muscle Density, but Not Size, Correlates Well with Muscle Strength and Physical Performance. J. Am. Med. Dir. Assoc. 2020. [Google Scholar] [CrossRef]
- Sui, S.X.; Williams, L.J.; Holloway-Kew, K.L.; Hyde, N.K.; Pasco, J.A. Skeletal muscle health and cognitive function: A narrative review. Int. J. Mol. Sci. 2021, 22, 255. [Google Scholar] [CrossRef]
- Borde, R.; Hortobágyi, T.; Granacher, U. Dose-Response Relationships of Resistance Training in Healthy Old Adults: A Systematic Review and Meta-Analysis. Sports Med. 2015, 45, 1693–1720. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Y.; Lim, J.; Park, H.S. Relationship between low handgrip strength and quality of life in Korean men and women. Qual. Life Res. 2018, 27, 2571–2580. [Google Scholar] [CrossRef]
- Cabett Cipolli, G.; Sanches Yassuda, M.; Aprahamian, I. Sarcopenia Is Associated with Cognitive Impairment in Older Adults: A Systematic Review and Meta-Analysis. J. Nutr. Health Aging 2019, 23, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Rijk, J.M.; Roos, P.R.; Deckx, L.; van den Akker, M.; Buntinx, F. Prognostic value of handgrip strength in people aged 60 years and older: A systematic review and meta-analysis. Geriatr. Gerontol. Int. 2016, 16, 5–20. [Google Scholar] [CrossRef]
- Kim, J.; Yim, J. Effects of an Exercise Protocol for Improving Handgrip Strength and Walking Speed on Cognitive Function in Patients with Chronic Stroke. Med. Sci. Monit. 2017, 23, 5402–5409. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Kaneko, Y.; Sato, T.; Shimizu, S.; Kanetaka, H.; Hanyu, H. Sarcopenia and Muscle Functions at Various Stages of Alzheimer Disease. Front. Neurol. 2018, 9, 710. [Google Scholar] [CrossRef]
- Ishii, H.; Makizako, H.; Doi, T.; Tsutsumimoto, K.; Shimada, H. Associations of Skeletal Muscle Mass, Lower-Extremity Functioning, and Cognitive Impairment in Community-Dwelling Older People in Japan. J. Nutr. Health Aging 2019, 23, 35–41. [Google Scholar] [CrossRef]
- Ferreira, B.N.; Lopes, E.D.d.S.; Henriques, I.F.; Reis, M.d.M.; Pádua, A.M.d.; Figueiredo, K.d.; Magno, F.A.L.; Coelho, F.G.d.M. Dual Task Multimodal Physical Training in Alzheimer s Disease: Effect on Cognitive Functions and Muscle Strength. Rev. Bras. Cineantropometria Desempenho Hum. 2017, 19, 575–584. [Google Scholar] [CrossRef][Green Version]
- Kemmler, W.; von Stengel, S.; Schoene, D. Longitudinal Changes in Muscle Mass and Function in Older Men at Increased Risk for Sarcopenia—The FrOST-Study. J. Frailty Aging 2019, 8, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.H.; Lee, J.Y.; Song, W. Effects of Resistance Exercise Training on Cognitive Function and Physical Performance in Cognitive Frailty: A Randomized Controlled Trial. J. Nutr. Health Aging 2018, 22, 944–951. [Google Scholar] [CrossRef]
- Ramnath, U.; Rauch, L.; Lambert, E.V.; Kolbe-Alexander, T.L. The relationship between functional status, physical fitness and cognitive performance in physically active older adults: A pilot study. PLoS ONE 2018, 13, e0194918. [Google Scholar] [CrossRef]
- Morley, J.E. Editorial: Bidirectional Communication between Brain and Muscle. J. Nutr. Health Aging 2018, 22, 1144–1145. [Google Scholar] [CrossRef]
- Morishita, S.; Tsubaki, A.; Nakamura, M.; Nashimoto, S.; Fu, J.B.; Onishi, H. Rating of perceived exertion on resistance training in elderly subjects. Expert Rev. Cardiovasc. Ther. 2019, 17, 135–142. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Jones, R.N.; Gallo, J.J. Dimensions of the Mini-Mental State Examination among community dwelling older adults. Psychol. Med. 2000, 30, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, P.; Albala, C.; Klaasen, G. Validation of a screening test for age associated cognitive impairment, in Chile. Rev. Med. Chil. 2004, 132, 467–478. [Google Scholar] [CrossRef]
- Reyes-Ortiz, C.A.; Luque, J.S.; Eriksson, C.K.; Soto, L. Self-reported tooth loss and cognitive function: Data from the Hispanic Established Populations for Epidemiologic Studies of the Elderly (Hispanic EPESE). Colomb. Med. 2013, 44, 139–145. [Google Scholar] [CrossRef]
- Lourenço, R.A.; Veras, R.P. Mini-Mental State Examination: Psychometric characteristics in elderly outpatients. Rev. Saude Publica 2006, 40, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Cortés, N.A.R.; Villarreal, R.E.; Galicia, R.L.; Martínez, G.L.; Vargas, D.E.R. Evaluación geriátrica integral del adulto mayor. Rev. Med. Chil. 2011, 139, 725–731. [Google Scholar] [CrossRef]
- Muñoz Silva, C.A.; Rojas Orellana, P.A.; Marzuca-Nassr, G.N. Criterios de valoración geriátrica integral en adultos mayores con dependencia moderada y severa en Centros de Atención Primaria en Chile. Rev. Med. Chil. 2015, 143, 612–618. [Google Scholar] [CrossRef]
- Cavedon, V.; Milanese, C.; Zancanaro, C. Are body circumferences able to predict strength, muscle mass and bone characteristics in obesity? A preliminary study in women. Int. J. Med. Sci. 2020, 17, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, R.; Miyachi, M.; Sawada, S.S.; Torii, S.; Midorikawa, T.; Tanisawa, K.; Ito, T.; Usui, C.; Ishii, K.; Suzuki, K.; et al. Cut-offs for calf circumference as a screening tool for low muscle mass: Waseda’s Health Study. Geriatr. Gerontol. Int. 2020, 20, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Esteves, C.L.; Ohara, D.G.; Matos, A.P.; Ferreira, V.T.K.; Iosimuta, N.C.R.; Pegorari, M.S. Anthropometric indicators as a discriminator of sarcopenia in community-dwelling older adults of the Amazon region: A cross-sectional study. BMC Geriatr. 2020, 20, 518. [Google Scholar] [CrossRef]
- Selvaraj, K.; Jayalakshmy, R.; Yousuf, A.; Singh, A.K.; Ramaswamy, G.; Palanivel, C. Can mid-upper arm circumference and calf circumference be the proxy measures to detect undernutrition among elderly? Findings of a community-based survey in rural Puducherry, India. J. Fam. Med. Prim. Care 2017, 6, 356–359. [Google Scholar] [CrossRef]
- Knutzen, K.M.; Brilla, L.R.; Caine, D. Validity of 1RM prediction equations for older adults. J. Strength Cond. Res. 1999, 13, 242–246. [Google Scholar]
- García-Ramos, A.; Barboza-González, P.; Ulloa-Díaz, D.; Rodriguez-Perea, A.; Martinez-Garcia, D.; Guede-Rojas, F.; Hinojosa-Riveros, H.; Chirosa-Ríos, L.J.; Cuevas-Aburto, J.; Janicijevic, D. Reliability and validity of different methods of estimating the one-repetition maximum during the free-weight prone bench pull exercise. J. Sports Sci. 2019, 37, 2205–2212. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Wang, J.; Liu, S. Establishment of the prediction equations of 1RM skeletal muscle strength in 60- to 75-year-old Chinese men and women. J. Aging Phys. Act. 2015, 23, 640–646. [Google Scholar] [CrossRef][Green Version]
- Farrow, M.; Biglands, J.; Tanner, S.F.; Clegg, A.; Brown, L.; Hensor, E.M.A.; O’Connor, P.; Emery, P.; Tan, A.L. The effect of ageing on skeletal muscle as assessed by quantitative MR imaging: An association with frailty and muscle strength. Aging Clin. Exp. Res. 2021, 33, 291–301. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B. The loss of skeletal muscle strength, mass, and quality in older adults: The Health, Aging and Body Composition Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 1059–1064. [Google Scholar] [CrossRef]
- Oikawa, S.Y.; Holloway, T.M.; Phillips, S.M. The impact of step reduction on muscle health in aging: Protein and exercise as countermeasures. Front. Nutr. 2019, 6. [Google Scholar] [CrossRef] [PubMed]
- Sellami, M.; Gasmi, M.; Denham, J.; Hayes, L.D.; Stratton, D.; Padulo, J.; Bragazzi, N. Effects of acute and chronic exercise on immunological parameters in the elderly aged: Can physical activity counteract the effects of aging? Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.D.; Wewege, M.A.; Hackett, D.A.; Keogh, J.W.L.; Hagstrom, A.D. Sex Differences in Adaptations in Muscle Strength and Size Following Resistance Training in Older Adults: A Systematic Review and Meta-analysis. Sports Med. 2021, 51, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Fiogbé, E.; Carnavale, B.F.; Takahashi, A.C.D.M. Exercise training in older adults, what effects on muscle force control? A systematic review of randomized clinical trials. Arch. Gerontol. Geriatr. 2019, 83, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, A.; Hortobágyi, T.; Beurskens, R.; Granacher, U. Effects of Supervised vs. Unsupervised Training Programs on Balance and Muscle Strength in Older Adults: A Systematic Review and Meta-Analysis. Sports Med. 2017, 47, 2341–2361. [Google Scholar] [CrossRef]
- Snijders, T.; Leenders, M.; de Groot, L.C.P.G.M.; van Loon, L.J.C.; Verdijk, L.B. Muscle mass and strength gains following 6 months of resistance type exercise training are only partly preserved within one year with autonomous exercise continuation in older adults. Exp. Gerontol. 2019, 121, 71–78. [Google Scholar] [CrossRef]
- Guizelini, P.C.; de Aguiar, R.A.; Denadai, B.S.; Caputo, F.; Greco, C.C. Effect of resistance training on muscle strength and rate of force development in healthy older adults: A systematic review and meta-analysis. Exp. Gerontol. 2018, 102, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Quezada, H.C.; Martínez-Salazar, C.; Fuentealba-Urra, S.; Hernández-Mosqueira, C.; Garcés, N.A.; Rodríguez-Rodríguez, F.; Concha-Cisternas, Y.; Molina-Sotomayor, E. Effects of two physical training programs on the cognitive status of a group of older adults in chile. Int. J. Environ. Res. Public Health 2021, 18, 4186. [Google Scholar] [CrossRef]
- Coelho-Júnior, H.J.; Gonçalves, I.O.; Sampaio, R.A.C.; Sampaio, P.Y.S.; Cadore, E.L.; Calvani, R.; Picca, A.; Izquierdo, M.; Marzetti, E.; Uchida, M.C. Effects of combined resistance and power training on cognitive function in older women: A randomized controlled trial. Int. J. Environ. Res. Public Health 2020, 17, 3435. [Google Scholar] [CrossRef]
- Li, Z.; Peng, X.; Xiang, W.; Han, J.; Li, K. The effect of resistance training on cognitive function in the older adults: A systematic review of randomized clinical trials. Aging Clin. Exp. Res. 2018, 30, 1259–1273. [Google Scholar] [CrossRef]
- Yoshiko, A.; Kaji, T.; Sugiyama, H.; Koike, T.; Oshida, Y.; Akima, H. Twenty-Four Months’ Resistance and Endurance Training Improves Muscle Size and Physical Functions but Not Muscle Quality in Older Adults Requiring Long-Term Care. J. Nutr. Health Aging 2019, 23, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Madarame, H.; Ogasawara, R.; Nakazato, K.; Ishii, N. Effect of very low-intensity resistance training with slow movement on muscle size and strength in healthy older adults. Clin. Physiol. Funct. Imaging 2014, 34, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Bårdstu, H.B.; Andersen, V.; Fimland, M.S.; Aasdahl, L.; Raastad, T.; Cumming, K.T.; Sæterbakken, A.H. Effectiveness of a resistance training program on physical function, muscle strength, and body composition in community-dwelling older adults receiving home care: A cluster-randomized controlled trial. Eur. Rev. Aging Phys. Act. 2020, 17. [Google Scholar] [CrossRef]
- Zouita, S.; Zouhal, H.; Ferchichi, H.; Paillard, T.; Dziri, C.; Hackney, A.C.; Laher, I.; Granacher, U.; Ben Moussa Zouita, A. Effects of Combined Balance and Strength Training on Measures of Balance and Muscle Strength in Older Women with a History of Falls. Front. Physiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Gylling, A.T.; Bloch-Ibenfeldt, M.; Eriksen, C.S.; Ziegler, A.K.; Wimmelmann, C.L.; Baekgaard, M.; Boraxbekk, C.J.; Siebner, H.R.; Mortensen, E.L.; Kjaer, M. Maintenance of muscle strength following a one-year resistance training program in older adults. Exp. Gerontol. 2020, 139. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. Physical activity and muscle-brain crosstalk. Nat. Rev. Endocrinol. 2019, 15, 383–392. [Google Scholar] [CrossRef]
- Amiri, N.; Fathei, M.; Ziaaldini, M.M. Effects of resistance training on muscle strength, insulin-like growth factor-1, and insulin-like growth factor–binding protein-3 in healthy elderly subjects: A systematic review and meta-analysis of randomized controlled trials. Hormones 2021, 1–11. [Google Scholar] [CrossRef]
- Chen, W.; Wang, L.; You, W.; Shan, T. Myokines mediate the cross talk between skeletal muscle and other organs. J. Cell. Physiol. 2020, 236, 2393–2412. [Google Scholar] [CrossRef]
- Pedersen, B.; Febbraio, M. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef]
- Schumann, M.; Bloch, W.; Oberste, M. Effects of physical training on skeletal muscle and the central nervous system in older adults. Dtsch. Med. Wochenschr. 2019, 144, 1396–1399. [Google Scholar] [PubMed]
- Severinsen, M.C.; Pedersen, B. Muscle—Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef] [PubMed]



| Variables | Control Group (n = 48) | Experimental Group (n = 45) | ||
|---|---|---|---|---|
| Mean ± SD | 95% (CI) | Mean ± SD | 95% (CI) | |
| Age (years) | 77.71 ± 3.41 | (76.72 to 78.70) | 77.93 ± 3.54 | (76.87 to 79.00) |
| Body mass (kg) | 62.07 ± 6.19 | (60.27 to 63.86) | 61.45 ± 6.38 | (59.53 to 63.36) |
| Stature (cm) | 1.58 ± 0.06 | (1.56 to 1.59) | 1.57 ± 0.06 | (1.55 to 1.59) |
| Primary school completed | 100% | 100% | ||
| High school completed | 74.4% | 80.1% | ||
| Phase 1 | Phase 2 | Phase 3 (101 Weeks): Intervention Period | Phase 4 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week −1 | Week 0 | Weeks 1–17 | Weeks 18–34 | Weeks 35–51 | Weeks 52–68 | Weeks 63–85 | Weeks 86–101 | Week 102 | |||||
| Learning | Pre-Test | Training 16 weeks | Control Test 1 1 week | Training 16 weeks | Control Test 2 1 week | Training 16 weeks | Control Test 3 1 week | Training 16 weeks | Control Test 4 1 week | Training 16 weeks | Control Test 5 1 week | Training 16 weeks | Post-test |
 |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Weeks | 0 | 1–17 | 18–34 | 35–51 | 52–68 | 63–85 | 86–101 | 102 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intensity | 1RM § 30–40% | 1RM 35–40% | 1RM 40–45% | 1RM 40–45% | 1RM 45–50% | 1RM 50–55% | |||||||
| Bench press | Pre-test ¥ | 1–2 set; 6–8 rep * 30–60 s | Control test 1 ¥ | 2–3 set; 6–8 rep * 30–60 s | Control test 2 ¥ | 2–4 set; 5–7 rep * 1–2 min | Control test 3 ¥ | 3–4 set; 4–6 rep * 2–3 min | Control test 4 ¥ | 2–3 set; 3–5 rep * 3–4 min | Control test 5 ¥ | 2–3 set; 2–4 rep * 3–4 min | Post-test ¥ |
| Biceps curl | 1–2 set; 6–8 rep * 30–60 s | 2–3 set; 6–8 rep * 30–60 s | 2–4 set; 5–7 rep * 1–2 min | 3–4 set; 4–6 rep * 2–3 min | 2–3 set; 3–5 rep * 3–4 min | 2–3 set; 2–4 rep * 3–4 min | |||||||
| Triceps extension | 1–2 set; 6–8 rep * 30–60 s | 2–3 set; 6–8 rep * 30–60 s | 2–4 set; 4–6 rep * 1–2 min | 3–4 set; 4–6 rep * 2–3 min | 2–3 set; 3–5 rep * 3–4 min | 2–3 set; 2–4 rep * 3–4 min | |||||||
| Leg press | 1–2 set; 5–7 rep * 30–60 s | 2–3 set; 5–7 rep * 30–60 s | 2–4 set; 4–6 rep * 1–2 min | 3–4 set; 3–5 rep * 2–3 min | 2–3 set; 2–4 rep * 3–4 min | 2–3 set; 2–3 rep * 3–4 min | |||||||
| Leg extension | 1–2 set; 5–7 rep * 30–60 s | 2–3 set; 5–7 rep * 30–60 s | 2–4 set; 4–6 rep * 1–2 min | 3–4 set; 3–5 rep * 2–3 min | 2–3 set; 2–4 rep * 3–4 min | 2–3 set; 2–3 rep * 3–4 min | |||||||
| Leg curl | 1–2 set; 4–6 rep * 30–60 s | 2–3 set; 4–5 rep * 30–60 s | 2–4 set; 3–4 rep * 1–2 min | 3–4 set; 2–4 rep * 2–3 min | 2–3 set; 2–3 rep * 3–4 min | 2–3 set; 1–2 rep * 3–4 min | |||||||
| Sedentary Group (n = 48) | ||||||
|---|---|---|---|---|---|---|
| Variables | Pre-Test | Post-Test | p-Value * | Effect Size ¥ | ||
| Mean ± SD | 95% (CI) | Mean ± SD | 95% (CI) | |||
| BMI (kg/m2) | 25.09 ± 3.10 | (24.19 to 25.99) | 24.32 ± 3.26 | (23.3 to 25.27) | <0.001 | −0.24 |
| 1RM Chest Press (kg) | 13.25 ± 1.76 | (12.74 to 13.76) | 12.8 ± 1.79 | (12.2 to 13.33) | <0.001 | −0.25 |
| Arm Circ. (cm) | 27.33 ± 3.62 | (26.28 to 28.38) | 26.10 ± 3.48 | (25.09 to 27.12) | <0.001 | −0.35 |
| 1RM Leg Press (kg) | 44.48 ± 3.16 | (43.56 to 45.40) | 42.6 ± 3.46 | (41.68 to 43.69) | <0.001 | −0.54 |
| Calf Circ. (cm) | 29.9 ± 4.34 | (28.64 to 31.16) | 28.6 ± 4.29 | (27.44 to 29.93) | <0.001 | −0.28 |
| MMSE (max. score 30) | 23.25 ± 2.23 | (22.60 to 23.90) | 22.33 ± 1.75 | (21.82 to 22.84) | 0.003 | −0.46 |
| Physical Activity Group (n = 45) | ||||||
|---|---|---|---|---|---|---|
| Variables | Pre-Test | Post-Test | p-Value * | Effect Size ¥ | ||
| Mean ± SD | 95% (CI) | Mean ± SD | 95% (CI) | |||
| BMI (kg/m2) | 25.16 ± 3.70 | (24.04 to 26.27) | 25.32 ± 3.68 | (24.21 to 26.43) | 0.053 | 0.04 |
| 1RM Chest Press (kg) | 13.31 ± 1.76 | (12.78 to 13.84) | 13.76 ± 1.88 | (13.19 to 14.32) | 0.007 | 0.24 |
| Arm Circ. (cm) | 27.11 ± 3.24 | (26.14 to 28.09) | 27.62 ± 3.52 | (26.56 to 28-68) | <0.001 | 0.15 |
| 1RM Leg Press (kg) | 45.64 ± 4.35 | (44.34 to 46.95) | 47.18 ± 4.23 | (45.91 to 48.45) | <0.001 | 0.36 |
| Calf Circ. (cm) | 30.09 ± 4.41 | (28.76 to 31.41) | 30.78 ± 4.24 | (29.50 to 32.05) | <0.001 | 0.16 |
| MMSE (max. score 30) | 23.16 ± 1.88 | (22.59 to 23.72) | 23.93 ± 1.78 | (23.40 to 24.47) | 0.017 | 0.43 |
| Variables | Change Physical Activity Group (n = 45) | Change Sedentary Group (n = 48) | p-Value * | Effect Size ¥ | ||
|---|---|---|---|---|---|---|
| Mean ± SD | 95% (CI) | Mean ± SD | 95% (CI) | |||
| BMI (kg/m2) | 0.165 ± 0.555 | (−0.002 to 0.33) | −0.770 ± 0.97 | (−1.054 to 0.487) | <0.001 | −1.17 |
| 1RM Chest Press (kg) | 0.444 ± 1.056 | (1.127 to 0.762) | −0.438 ± 0.50 | (−0.583 to −0.292) | <0.001 | −1.08 |
| Arm Circ. (cm) | 0.511 ± 0.920 | (0.235 to 0.788) | −1.229 ± 0.88 | (−1.485 to −0.973) | <0.001 | −1.93 |
| 1RM Leg Press (kg) | 1.533 ± 1.236 | (1.162 to 1.905) | −1.792 ± 1.03 | (−2.091 to −1.492) | <0.001 | −2.93 |
| Calf Circ. (cm) | 0.689 ± 1.221 | (0.322 to 1.056) | −1.208 ± 0.77 | (−1.432 to −0.985) | <0.001 | −1.87 |
| MMSE (max. score 30) | 0.778 ± 2.099 | (0.147 to 1.408) | −0.917 ± 0.91 | (−1.472 to −0.362) | <0.001 | −0.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina-Sotomayor, E.; Espinoza-Salinas, A.; Arenas-Sánchez, G.; Pradas de la Fuente, F.; Leon-Prados, J.A.; Gonzalez-Jurado, J.A. Effects of Resistance Training Program on Muscle Mass and Muscle Strength and the Relationship with Cognition in Older Women. Sustainability 2021, 13, 7687. https://doi.org/10.3390/su13147687
Molina-Sotomayor E, Espinoza-Salinas A, Arenas-Sánchez G, Pradas de la Fuente F, Leon-Prados JA, Gonzalez-Jurado JA. Effects of Resistance Training Program on Muscle Mass and Muscle Strength and the Relationship with Cognition in Older Women. Sustainability. 2021; 13(14):7687. https://doi.org/10.3390/su13147687
Chicago/Turabian StyleMolina-Sotomayor, Edgardo, Alexis Espinoza-Salinas, Giovanny Arenas-Sánchez, Francisco Pradas de la Fuente, Juan Antonio Leon-Prados, and Jose Antonio Gonzalez-Jurado. 2021. "Effects of Resistance Training Program on Muscle Mass and Muscle Strength and the Relationship with Cognition in Older Women" Sustainability 13, no. 14: 7687. https://doi.org/10.3390/su13147687
APA StyleMolina-Sotomayor, E., Espinoza-Salinas, A., Arenas-Sánchez, G., Pradas de la Fuente, F., Leon-Prados, J. A., & Gonzalez-Jurado, J. A. (2021). Effects of Resistance Training Program on Muscle Mass and Muscle Strength and the Relationship with Cognition in Older Women. Sustainability, 13(14), 7687. https://doi.org/10.3390/su13147687








