Experimental and Informational Modeling Study of Sustainable Self-Compacting Geopolymer Concrete
Abstract
:1. Introduction
2. Experimental Program
2.1. Materials Characterization
2.2. Mixture Design and Specimen Preparation
2.3. Test Program
3. Results and Discussion
3.1. Fresh Properties
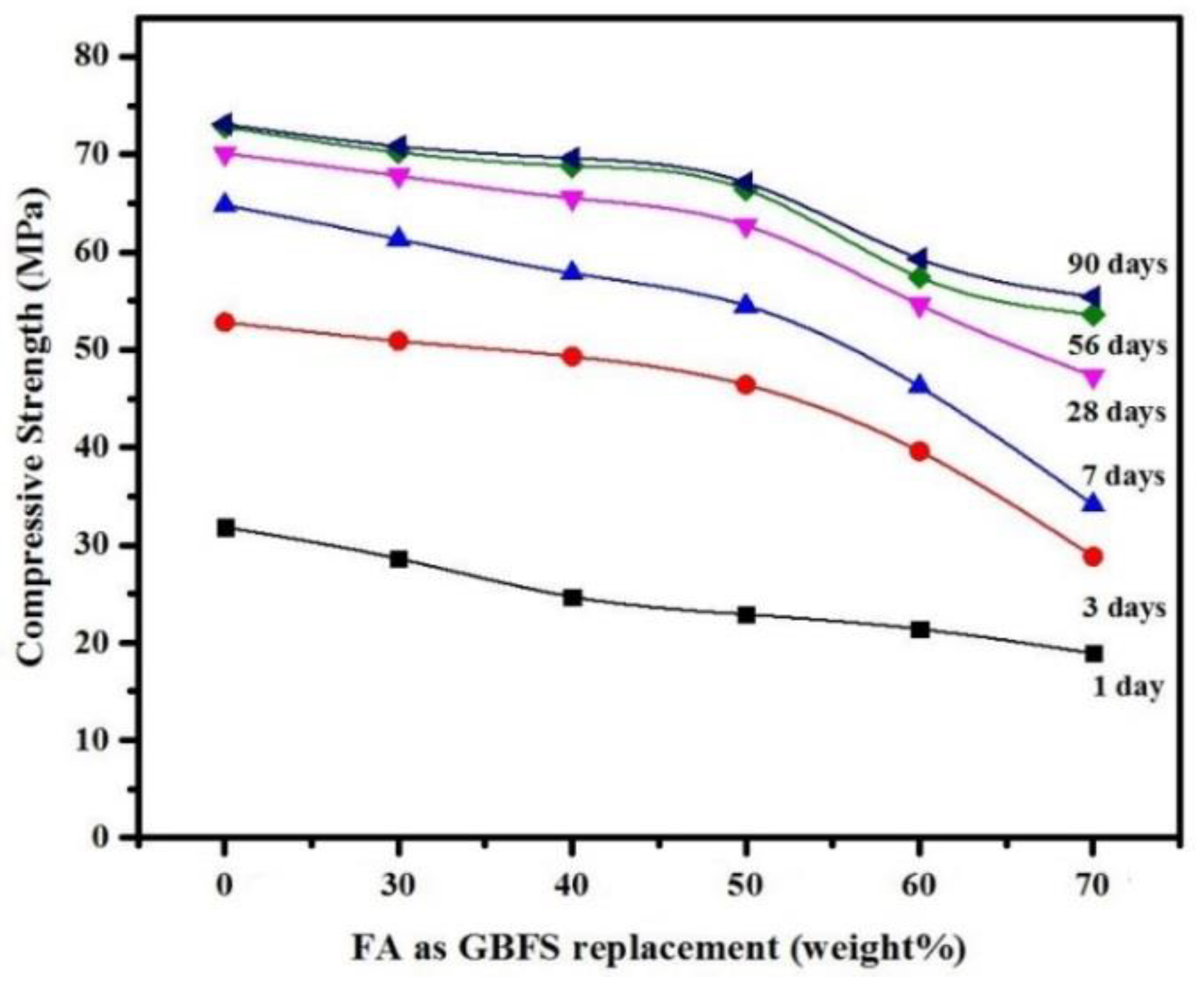
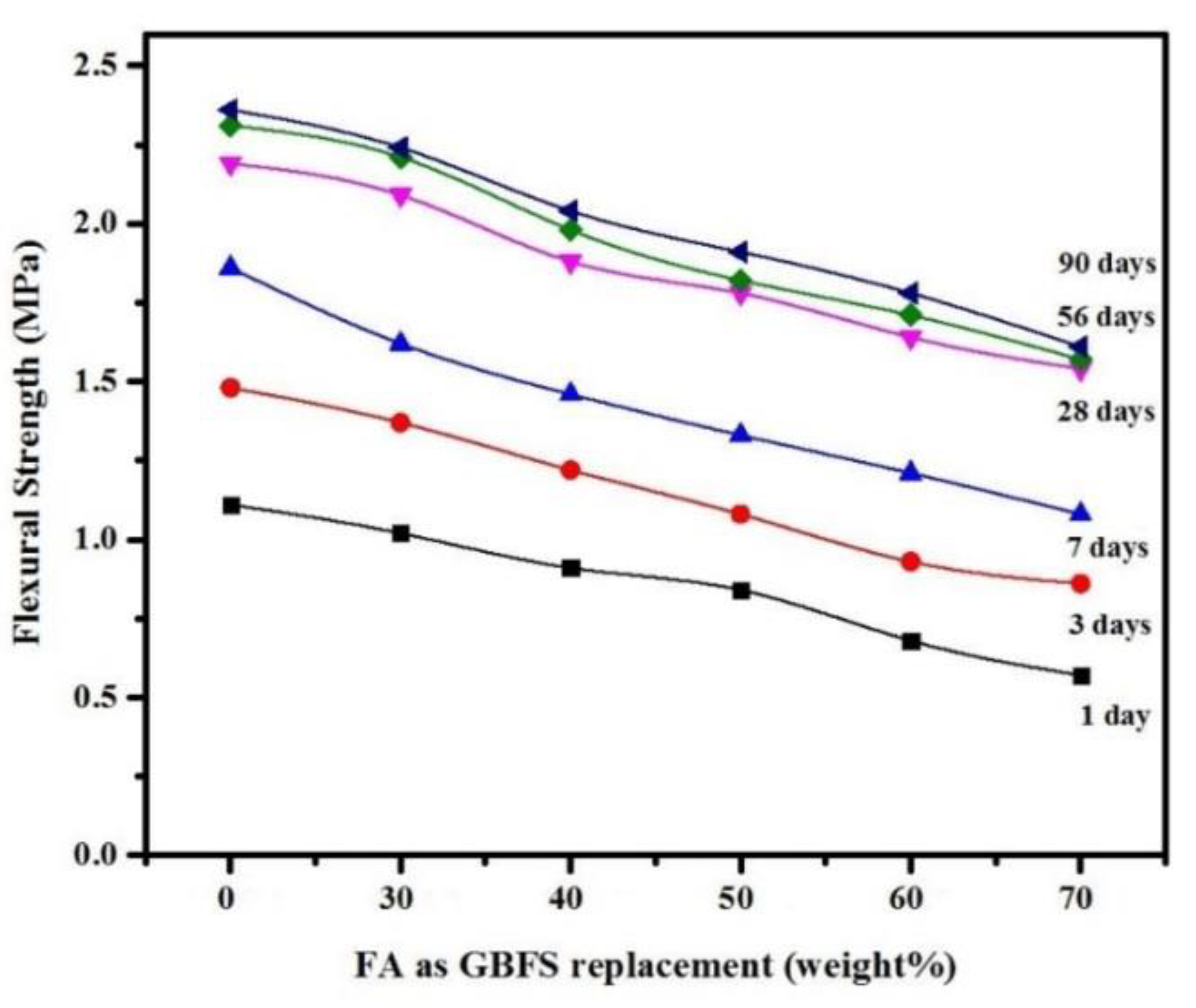
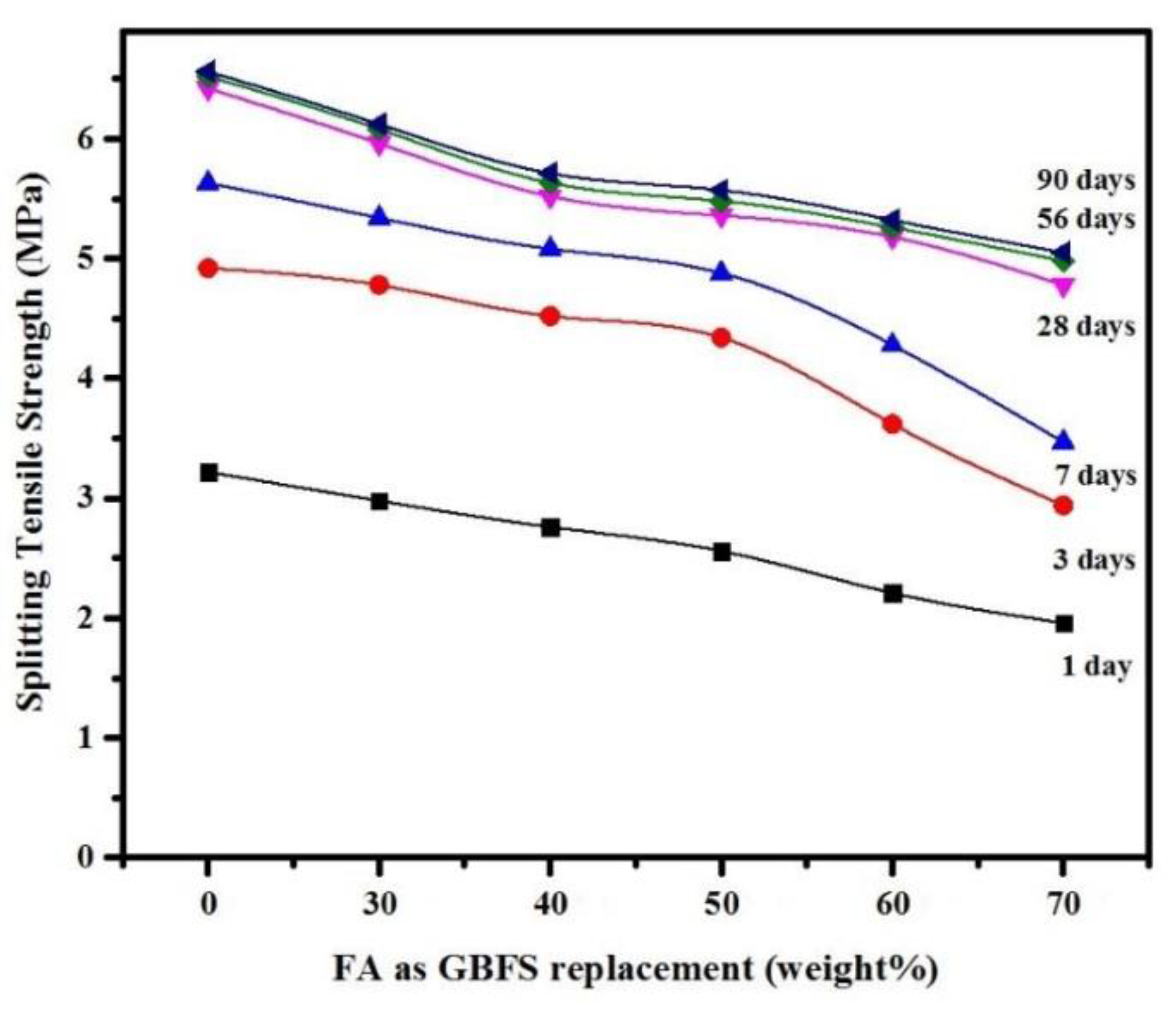
3.2. Mechanical Properties
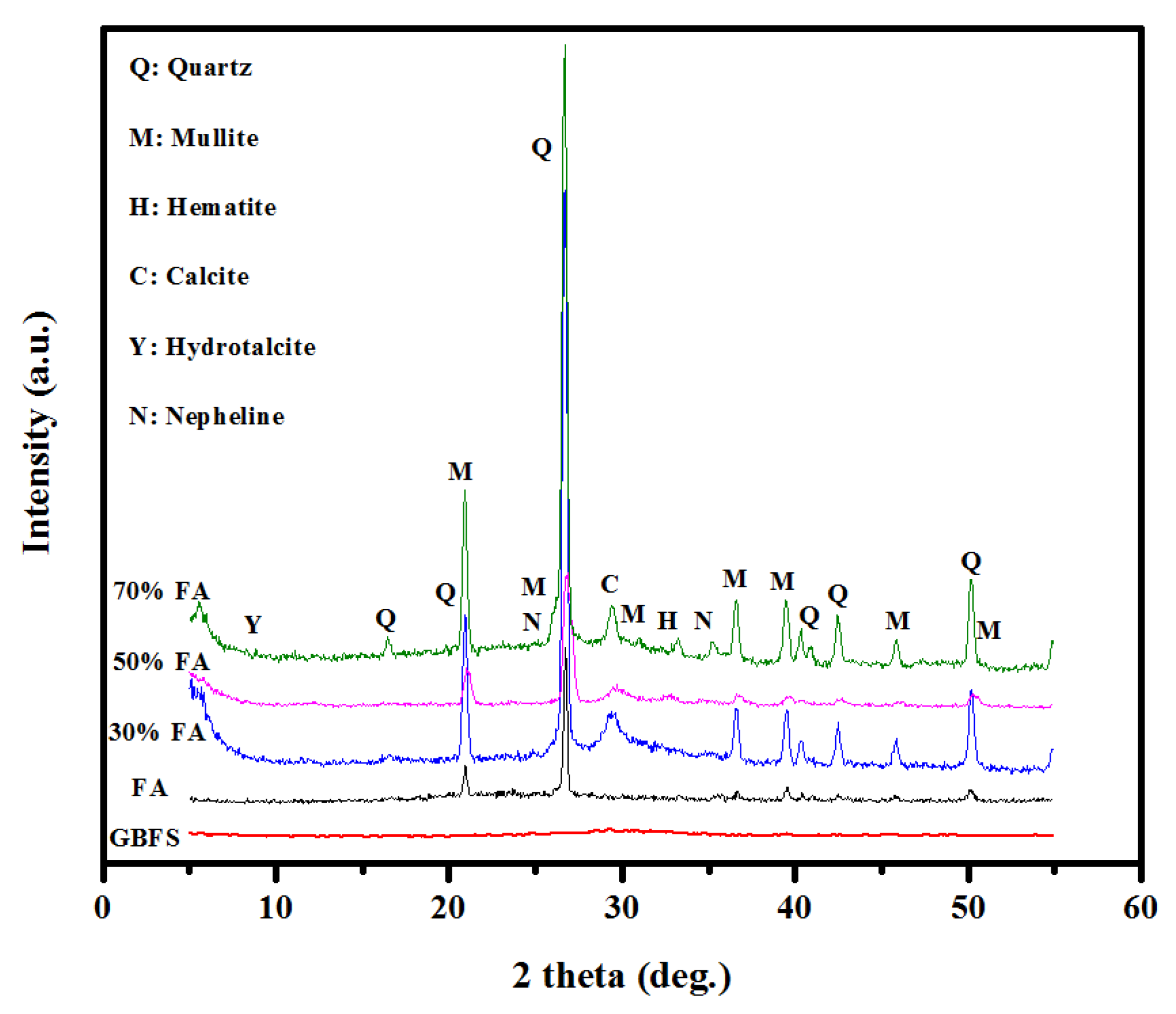
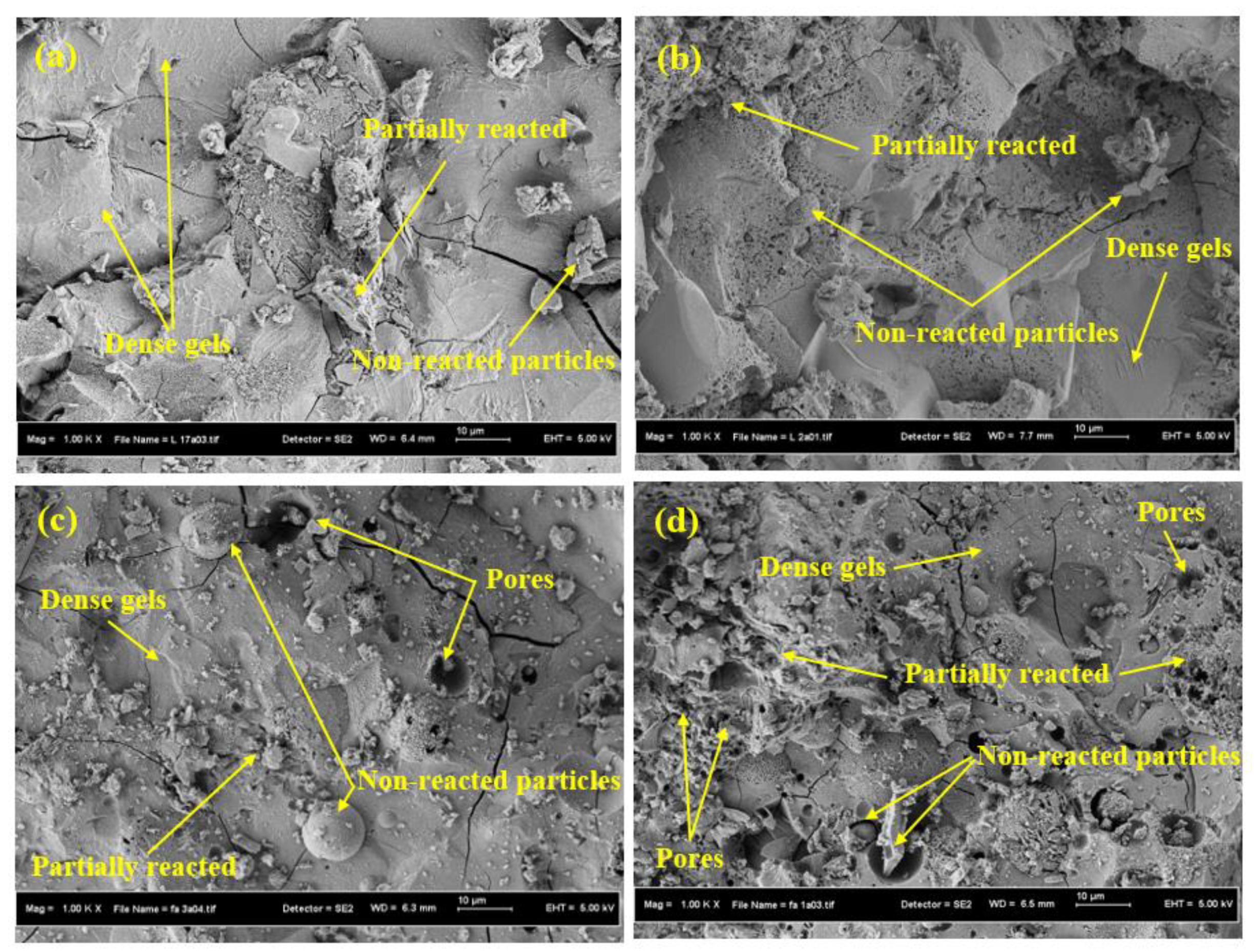
3.3. Structure and Morphology Analyses
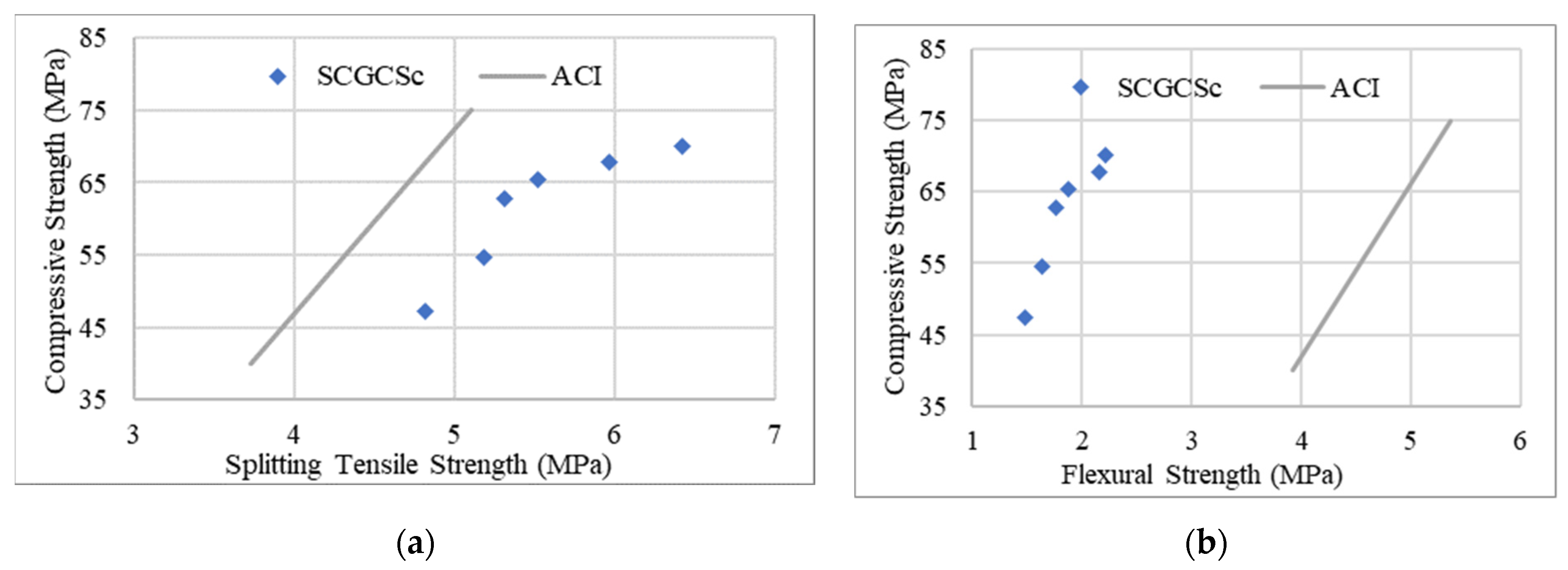
4. Strengths and UPV Correlation of SCGCs
5. Development of Informational Model to Estimate the CS of SCGCs
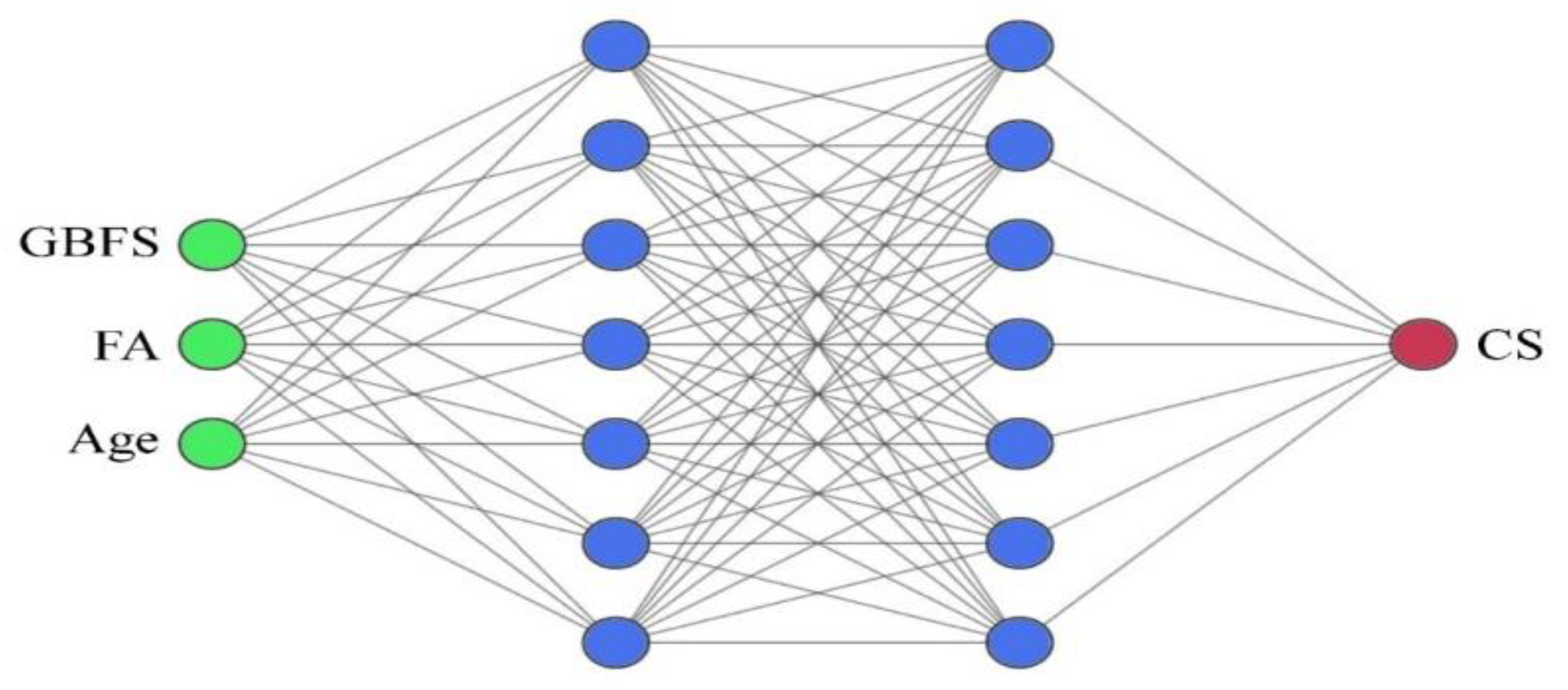
5.1. Feed-Forward ANN and Bat Optimization Algorithm
5.2. Input and Output Parameters
5.3. Topologies and Structure Development of the Feed-Forward ANN
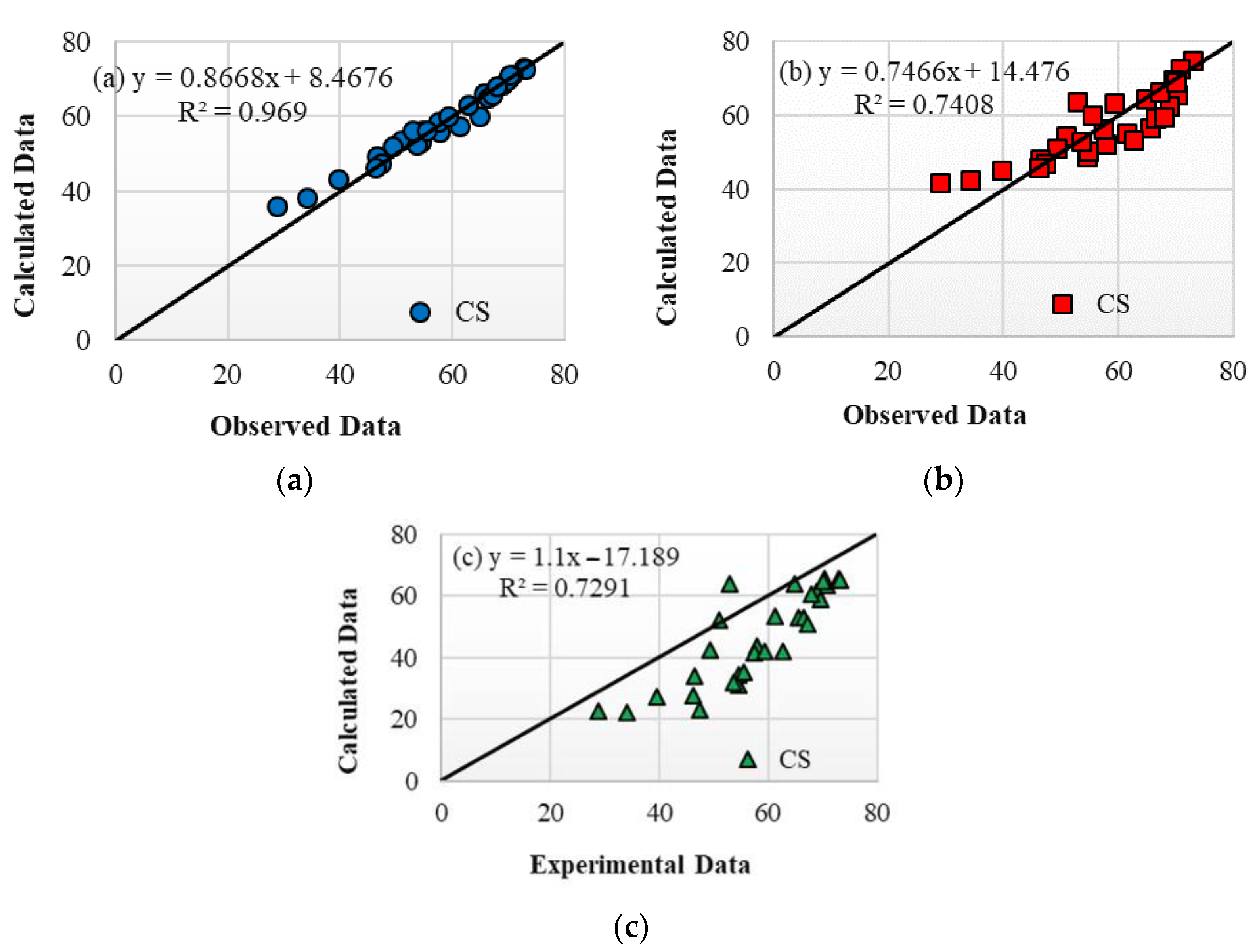
5.4. Bat-ANN Model Validation
6. Conclusions
- The results confirmed that partial replacement of GBFS with up to 50% FA as a precursor binder in SCGCs yielded excellent workability and mechanical properties, meeting the EFNARC criteria for SCC.
- SCGC mixtures made either with high volume of FA or GBFS resulted in high plastic viscosity values. For instance, the plastic viscosity of the mixture was increased from 79 to 86 cP with the increase of FA dosage from 60% to 70%. This increase in the plastic viscosity of mixtures made with high GBFS and FA levels was likely due to their chemical compositions and physical properties.
- The results of microstructural analysis of SCGCs including XRD and SEM showed an improvement in the number of non-reacted particles, cracks, and pores when the FA content was increased as partial substitution for GBFS. This in turn enhanced the porosity and reduced the density as well as the C,N-(A)-S-H gel.
- SCGC mixtures prepared with up to 50% of FA partial replacement for GBFS can mitigate the disposal cost and environmental footprint of such by-products. Consequently, carbon dioxide emissions can be reduced from the cement production, while eliminating the high energy and natural resource intake in the building sector and contributing to improved development and sustainability.
- The results confirmed that the proposed informational Bat-ANN model attained the most reliable and robust predictive results for estimating the CS of SCGC mixtures, as confirmed by various statistical metrics revealing its superior accuracy compared to other informational models. Accordingly, such an informational model can reduce the need for costly, time-consuming, and material wasteful trial batches in the laboratory.
- Aside from the positive environmental impacts, the developed SCGCs also offered a superior product in terms of mechanical properties, which is of great interest to concrete manufacturers. This alternative material for OPC-based self-compacting concrete has far-reaching suitability and may serve to fulfill sustainability goals for companies in the business of ecological construction, especially in precast concrete making.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SCC | Self-Compacting Concrete |
| SCGC | Self-compacting geopolymer concrete |
| GB | Geopolymer binder |
| CS | Compressive strength |
| TS | Splitting tensile strength |
| FS | Flexural strength |
| FA | Fly ash |
| GBFS | Ground blast furnace slag |
| XRF | X-ray fluorescence |
| XRD | X-ray diffraction |
| SEM | Scanning electron microscopy |
| ANN | Artificial neural network |
References
- Long, G.; Gao, Y.; Xie, Y. Designing more sustainable and greener self-compacting concrete. Constr. Build. Mater. 2015, 84, 301–306. [Google Scholar] [CrossRef]
- Domone, P. Self-compacting concrete: An analysis of 11 years of case studies. Cem. Concr. Compos. 2006, 28, 197–208. [Google Scholar] [CrossRef]
- Huseien, G.F.; Mirza, J.; Ismail, M. Effects of high volume ceramic binders on flexural strength of self-compacting geopolymer concrete. Adv. Sci. Lett. 2018, 24, 4097–4101. [Google Scholar] [CrossRef]
- Gülşan, M.E.; Alzeebaree, R.; Rasheed, A.A.; Niş, A.; Kurtoğlu, A.E. Development of fly ash/slag based self-compacting geopolymer concrete using nano-silica and steel fiber. Constr. Build. Mater. 2019, 211, 271–283. [Google Scholar] [CrossRef]
- Faridmehr, I.; Nehdi, M.L.; Nikoo, M.; Huseien, G.F.; Ozbakkaloglu, T. Life-Cycle Assessment of Alkali-Activated Materials Incorporating Industrial Byproducts. Materials 2021, 14, 2401. [Google Scholar] [CrossRef]
- Samadi, M.; Shah, K.W.; Huseien, G.F.; Lim, N.H.A.S. Influence of glass silica waste nano powder on the mechanical and microstructure properties of alkali-activated mortars. Nanomaterials 2020, 10, 324. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.K.; Khaudhair, Z.A.; Ahuja, A.K. A new method for proportioning recycled concrete. Struct. Concr. 2016, 17, 677–687. [Google Scholar] [CrossRef]
- Sivakrishna, A.; Adesina, A.; Awoyera, P.; Kumar, K.R. Green concrete: A review of recent developments. Mater. Today Proc. 2020, 27, 54–58. [Google Scholar] [CrossRef]
- Awoyera, P.; Adesina, A.; Sivakrishna, A.; Gobinath, R.; Kumar, K.R.; Srinivas, A. Alkali activated binders: Challenges and opportunities. Mater. Today Proc. 2020, 27, 40–43. [Google Scholar] [CrossRef]
- Adesina, A. Performance of fibre reinforced alkali-activated composites—A review. Materialia 2020, 12, 100782. [Google Scholar] [CrossRef]
- Juenger, M.; Winnefeld, F.; Provis, J.L.; Ideker, J. Advances in alternative cementitious binders. Cem. Concr. Res. 2011, 41, 1232–1243. [Google Scholar] [CrossRef]
- Xu, H.; Provis, J.L.; van Deventer, J.S.; Krivenko, P.V. Characterization of aged slag concretes. ACI Mater. J. 2008, 105, 131. [Google Scholar]
- Awoyera, P.; Adesina, A. Durability properties of alkali activated slag composites: Short overview. Silicon 2020, 12, 987–996. [Google Scholar] [CrossRef]
- Hassan, A.; Arif, M.; Shariq, M. Use of geopolymer concrete for a cleaner and sustainable environment—A review of mechanical properties and microstructure. J. Clean. Prod. 2019, 223, 704–728. [Google Scholar] [CrossRef]
- Pasupathy, K.; Berndt, M.; Sanjayan, J.; Rajeev, P.; Cheema, D.S. Durability performance of precast fly ash–based geopolymer concrete under atmospheric exposure conditions. J. Mater. Civ. Eng. 2018, 30, 04018007. [Google Scholar] [CrossRef]
- Amer, I.; Kohail, M.; El-Feky, M.; Rashad, A.; Khalaf, M.A. Characterization of alkali-activated hybrid slag/cement concrete. Ain Shams Eng. J. 2021, 12, 135–144. [Google Scholar] [CrossRef]
- Huseien, G.F.; Sam, A.R.M.; Shah, K.W.; Budiea, A.; Mirza, J. Utilizing spend garnets as sand replacement in alkali-activated mortars containing fly ash and GBFS. Constr. Build. Mater. 2019, 225, 132–145. [Google Scholar] [CrossRef]
- Tzevelekou, T.; Lampropoulou, P.; Giannakopoulou, P.P.; Rogkala, A.; Koutsovitis, P.; Koukouzas, N.; Petrounias, P. Valorization of Slags Produced by Smelting of Metallurgical Dusts and Lateritic Ore Fines in Manufacturing of Slag Cements. Appl. Sci. 2020, 10, 4670. [Google Scholar] [CrossRef]
- Mehta, A.; Siddique, R.; Ozbakkaloglu, T.; Shaikh, F.U.A.; Belarbi, R. Fly ash and ground granulated blast furnace slag-based alkali-activated concrete: Mechanical, transport and microstructural properties. Constr. Build. Mater. 2020, 257, 119548. [Google Scholar] [CrossRef]
- Fang, G.; Ho, W.K.; Tu, W.; Zhang, M. Workability and mechanical properties of alkali-activated fly ash-slag concrete cured at ambient temperature. Constr. Build. Mater. 2018, 172, 476–487. [Google Scholar] [CrossRef]
- Xuequan, W.; Hong, Z.; Xinkai, H.; Husen, L. Study on steel slag and fly ash composite Portland cement. Cem. Concr. Res. 1999, 29, 1103–1106. [Google Scholar] [CrossRef]
- Embong, R.; Kusbiantoro, A.; Shafiq, N.; Nuruddin, M.F. Strength and microstructural properties of fly ash based geopolymer concrete containing high-calcium and water-absorptive aggregate. J. Clean. Prod. 2016, 112, 816–822. [Google Scholar] [CrossRef]
- Kubba, Z.; Huseien, G.F.; Sam, A.R.M.; Shah, K.W.; Asaad, M.A.; Ismail, M.; Tahir, M.M.; Mirza, J. Impact of curing temperatures and alkaline activators on compressive strength and porosity of ternary blended geopolymer mortars. Case Stud. Constr. Mater. 2018, 9, e00205. [Google Scholar] [CrossRef]
- Atiş, C.; Görür, E.; Karahan, O.; Bilim, C.; İlkentapar, S.; Luga, E. Very high strength (120 MPa) class F fly ash geopolymer mortar activated at different NaOH amount, heat curing temperature and heat curing duration. Constr. Build. Mater. 2015, 96, 673–678. [Google Scholar] [CrossRef]
- Leong, H.Y.; Ong, D.E.L.; Sanjayan, J.G.; Nazari, A. The effect of different Na2O and K2O ratios of alkali activator on compressive strength of fly ash based-geopolymer. Constr. Build. Mater. 2016, 106, 500–511. [Google Scholar] [CrossRef] [Green Version]
- Huseien, G.F.; Ismail, M.; Mirza, J. Influence of curing methods and sodium silicate content on compressive strength and microstructure of multi blend geopolymer mortars. Adv. Sci. Lett. 2018, 24, 4218–4222. [Google Scholar] [CrossRef]
- Safiuddin, M.; West, J.; Soudki, K. Flowing ability of the mortars formulated from self-compacting concretes incorporating rice husk ash. Constr. Build. Mater. 2011, 25, 973–978. [Google Scholar] [CrossRef]
- Standard, ASTM. C618-15 Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- ASTM. C-618 (1993) Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use as a Mineral Admixture in Concrete; Annual Book of ASTM Standards; ASTM: Philadelphia, PA, USA, 1999. [Google Scholar]
- Salih, M.A.; Farzadnia, N.; Ali, A.A.A.; Demirboga, R. Development of high strength alkali activated binder using palm oil fuel ash and GGBS at ambient temperature. Constr. Build. Mater. 2015, 93, 289–300. [Google Scholar] [CrossRef]
- EFNARC. Specification and Guidelines for Self-Compacting Concrete; European Federation of Specialist Construction Chemicals and Concrete System: Gamberley, UK, 2002. [Google Scholar]
- ASTM International. ASTM D445-12: Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (and Calculation of Dynamic Viscosity); ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- ASTM. Standard Test Methods for Time of Setting of Hydraulic Cement by Vicat Needle. 2008. Available online: https://standards.globalspec.com/std/562203/astm-c191 (accessed on 30 May 2021).
- Standard, A. C109/C109M-16a Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (using 2-in. or [50-mm] Cube Specimens); Committee C-1 on Cement, Ed.; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- ASTM. Standard Test Method for Splitting Tensile Strength of Cylindrical Concrete Specimens. 2011. Available online: https://www.astm.org/Standards/C496 (accessed on 30 May 2021).
- Concrete, A.I.C.C.o.; Aggregates, C. Standard Test Method for Flexural Strength of Concrete (using Simple Beam with Center-point Loading); ASTM international: West Conshohocken, PA, USA, 2010. [Google Scholar]
- Duan, P.; Yan, C.; Zhou, W.; Luo, W. Fresh properties, mechanical strength and microstructure of fly ash geopolymer paste reinforced with sawdust. Constr. Build. Mater. 2016, 111, 600–610. [Google Scholar] [CrossRef]
- Felekoğlu, B.; Türkel, S.; Baradan, B. Effect of water/cement ratio on the fresh and hardened properties of self-compacting concrete. Build. Environ. 2007, 42, 1795–1802. [Google Scholar] [CrossRef]
- Boukendakdji, O.; Kenai, S.; Kadri, E.; Rouis, F. Effect of slag on the rheology of fresh self-compacted concrete. Constr. Build. Mater. 2009, 23, 2593–2598. [Google Scholar] [CrossRef]
- Belaidi, A.; Azzouz, L.; Kadri, E.; Kenai, S. Effect of natural pozzolana and marble powder on the properties of self-compacting concrete. Constr. Build. Mater. 2012, 31, 251–257. [Google Scholar] [CrossRef]
- Diamantonis, N.; Marinos, I.; Katsiotis, M.; Sakellariou, A.; Papathanasiou, A.; Kaloidas, V.; Katsioti, M. Investigations about the influence of fine additives on the viscosity of cement paste for self-compacting concrete. Constr. Build. Mater. 2010, 24, 1518–1522. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Effect of GGBFS on setting, workability and early strength properties of fly ash geopolymer concrete cured in ambient condition. Constr. Build. Mater. 2014, 66, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Yusuf, M.O.; Johari, M.A.M.; Ahmad, Z.A.; Maslehuddin, M. Evolution of alkaline activated ground blast furnace slag–ultrafine palm oil fuel ash based concrete. Mater. Des. 2014, 55, 387–393. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K.; Rangan, V.B. Early age properties of low-calcium fly ash geopolymer concrete suitable for ambient curing. Procedia Eng. 2015, 125, 601–607. [Google Scholar] [CrossRef] [Green Version]
- Vijai, K.; Kumutha, R.; Vishnuram, B. Effect of types of curing on strength of geopolymer concrete. Int. J. Phys. Sci. 2010, 5, 1419–1423. [Google Scholar]
- Kumar, S.; Kumar, R.; Mehrotra, S. Influence of granulated blast furnace slag on the reaction, structure and properties of fly ash based geopolymer. J. Mater. Sci. 2010, 45, 607–615. [Google Scholar] [CrossRef]
- Sugama, T.; Brothers, L.; Van de Putte, T. Acid-resistant cements for geothermal wells: Sodium silicate activated slag/fly ash blends. Adv. Cem. Res. 2005, 17, 65–75. [Google Scholar] [CrossRef]
- Puligilla, S.; Mondal, P. Role of slag in microstructural development and hardening of fly ash-slag geopolymer. Cem. Concr. Res. 2013, 43, 70–80. [Google Scholar] [CrossRef]
- British Standard. Testing hardened concrete. Compressive Strength Test Specim. BS EN 2009, 1, 12390-3. [Google Scholar]
- Mozgawa, W.; Deja, J. Spectroscopic studies of alkaline activated slag geopolymers. J. Mol. Struct. 2009, 924, 434–441. [Google Scholar] [CrossRef]
- Huseien, G.F.; Sam, A.R.M.; Shah, K.W.; Asaad, M.A.; Tahir, M.M.; Mirza, J. Properties of ceramic tile waste based alkali-activated mortars incorporating GBFS and fly ash. Constr. Build. Mater. 2019, 214, 355–368. [Google Scholar] [CrossRef]
- Mohammadhosseini, H.; Lim, N.H.A.S.; Tahir, M.M.; Alyousef, R.; Alabduljabbar, H.; Samadi, M. Enhanced performance of green mortar comprising high volume of ceramic waste in aggressive environments. Constr. Build. Mater. 2019, 212, 607–617. [Google Scholar] [CrossRef]
- Huseien, G.F.; Sam, A.R.M.; Mirza, J.; Tahir, M.M.; Asaad, M.A.; Ismail, M.; Shah, K.W. Waste ceramic powder incorporated alkali activated mortars exposed to elevated Temperatures: Performance evaluation. Constr. Build. Mater. 2018, 187, 307–317. [Google Scholar] [CrossRef]
- American Concrete Institute. Building Code Requirements for Structural Concrete (ACI 318-14): An ACI Standard: Commentary on Building Code Requirements for Structural Concrete (ACI 318R-14), an ACI Report; American Concrete Institute: Farmington Hills, MI, USA, 2012. [Google Scholar]
- Demirboğa, R.; Türkmen, İ.; Karakoc, M.B. Relationship between ultrasonic velocity and compressive strength for high-volume mineral-admixtured concrete. Cem. Concr. Res. 2004, 34, 2329–2336. [Google Scholar] [CrossRef]
- Bogas, J.A.; Gomes, M.G.; Gomes, A. Compressive strength evaluation of structural lightweight concrete by non-destructive ultrasonic pulse velocity method. Ultrasonics 2013, 53, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Nash’t, I.H.; A’bour, S.H.; Sadoon, A.A. Finding an unified relationship between crushing strength of concrete and non-destructive tests. In Middle East Nondestructive Testing Conference & Exhibition; Citeseer: Manama, Bahrain, 2005. [Google Scholar]
- Kubat, M. Neural networks: A comprehensive foundation by Simon Haykin, Macmillan, 1994, ISBN 0-02-352781-7. Knowl. Eng. Rev. 1999, 13, 409–412. [Google Scholar] [CrossRef]
- Bishop, C.M. Pattern Recognition and Machine Learning; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Awoyera, P.O.; Kirgiz, M.S.; Viloria, A.; Ovallos-Gazabon, D. Estimating strength properties of geopolymer self-compacting concrete using machine learning techniques. J. Mater. Res. Technol. 2020, 9, 9016–9028. [Google Scholar] [CrossRef]
- Najimi, M.; Ghafoori, N.; Nikoo, M. Modeling chloride penetration in self-consolidating concrete using artificial neural network combined with artificial bee colony algorithm. J. Build. Eng. 2019, 22, 216–226. [Google Scholar] [CrossRef]
- Sadowski, Ł.; Nikoo, M.; Shariq, M.; Joker, E.; Czarnecki, S. The nature-inspired metaheuristic method for predicting the creep strain of green concrete containing ground granulated blast furnace slag. Materials 2019, 12, 293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.S.; Gandomi, A.H. Bat algorithm: A novel approach for global engineering optimization. Eng. Comput. 2012. [Google Scholar] [CrossRef] [Green Version]


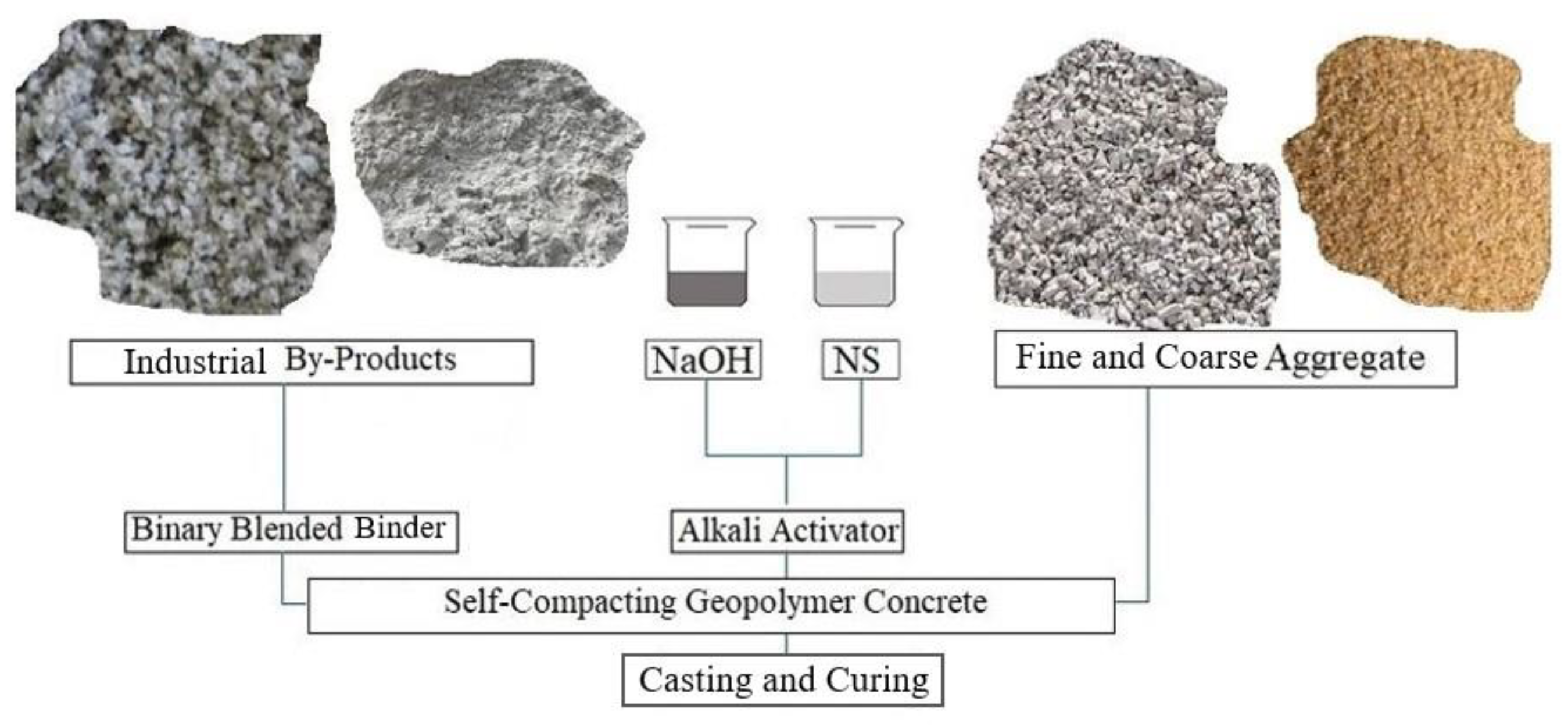
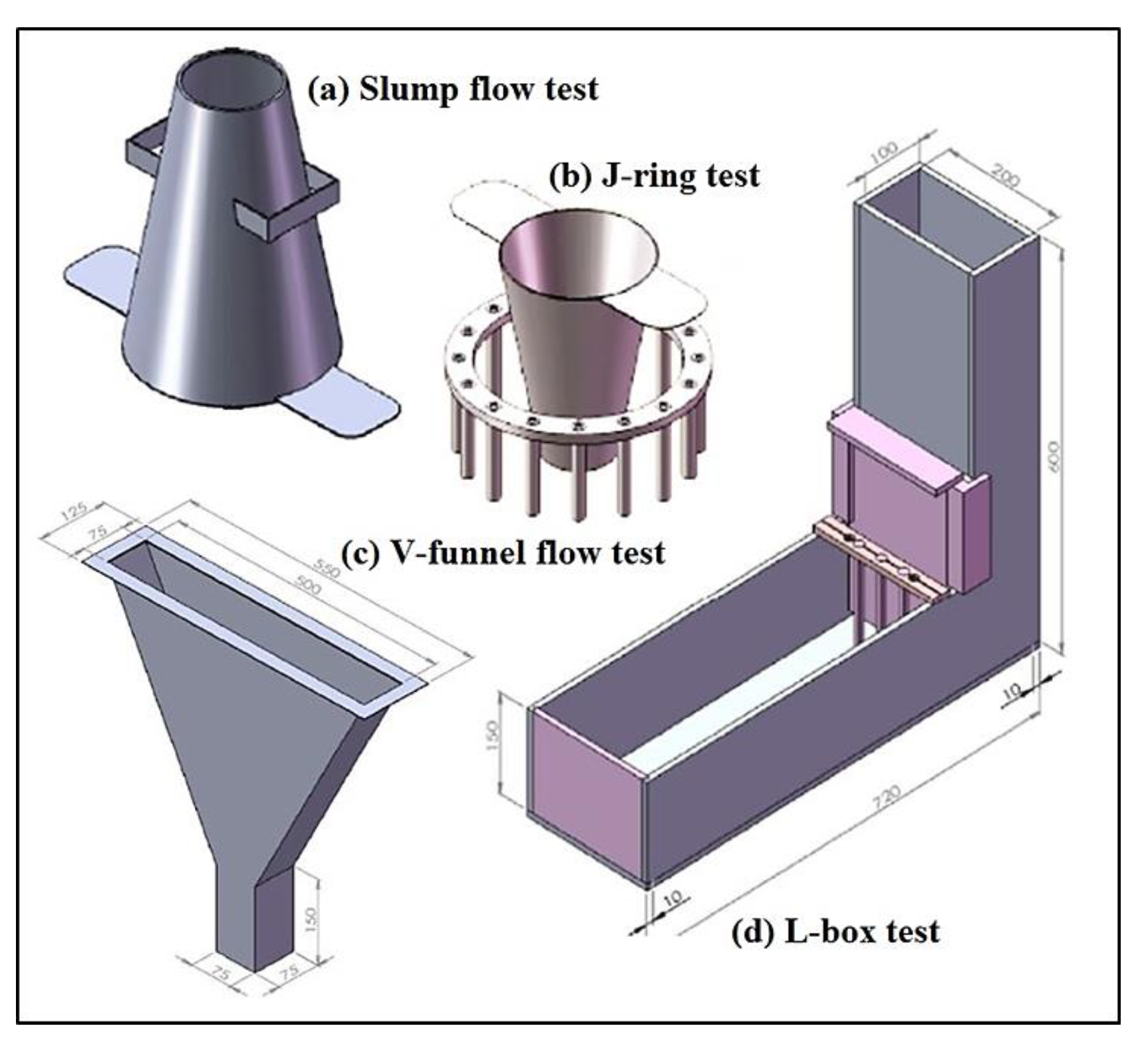






| Constituent or Property | FA | GBFS |
|---|---|---|
| Silicon oxide (SiO2) | 57.20 | 30.8 |
| Aluminum oxide (Al2O3) | 28.8 | 10.9 |
| Iron(III) oxide (Fe2O3) | 3.67 | 0.64 |
| Calcium oxide (CaO) | 5.16 | 51.8 |
| Magnesium oxide (MgO) | 1.48 | 4.57 |
| Potassium oxide (K2O) | 0.94 | 0.36 |
| Sodium oxide (Na2O) | 0.08 | 0.45 |
| Sulfur trioxide (SO3) | 0.10 | 0.06 |
| Loss on ignition (LOI) | 0.12 | 0.22 |
| Mix Code | Binders, kg/m3 | Aggregates, kg/m3 | Alkaline Solution, kg/m3 | Solution Modulus (Ms) | ||
|---|---|---|---|---|---|---|
| GBFS | FA | Fine | Coarse | |||
| MS1 | 484 | 0 | ||||
| MS2 | 338.8 | 145.2 | ||||
| MS3 | 290.4 | 193.6 | 844 | 756 | 242 | 1.2 |
| MS4 | 242 | 242 | ||||
| MS5 | 193.6 | 290.4 | ||||
| MS6 | 145.2 | 338.8 | ||||
| Test (Unit) | SCGCs Mixture Code | EFNARC Criteria | ||||||
|---|---|---|---|---|---|---|---|---|
| MS1 | MS2 | MS3 | MS4 | MS5 | MS6 | Min | Max | |
| Slump flow (mm) | 560 | 640 | 695 | 720 | 680 | 630 | 650 | 800 |
| T50 flow (s) | 6.0 | 5.5 | 4.0 | 3.5 | 4.5 | 5.5 | 2 | 5 |
| V-funnel (s) | 14 | 12.5 | 10 | 8.5 | 10.5 | 13 | 6 | 12 |
| L-box ratio (H2/H1) | 0.78 | 0.80 | 0.86 | 0.92 | 0.84 | 0.80 | 0.80 | 1.0 |
| J-ring (mm) | 12 | 10 | 8 | 6 | 7.5 | 10.5 | 0 | 10 |
| Plastic viscosity, cP | 91 | 82 | 74 | 63 | 79 | 86 | - | - |
| Initial setting, min | 6 | 16 | 20 | 24 | 32 | 38 | - | - |
| Final setting, min | 10 | 28 | 36 | 47 | 58 | 66 | - | - |
| Acceptance criteria | No | No | No | Yes | Yes | No | - | - |
| Mix Design | UPV (km/s) | CS (MPa) |
|---|---|---|
| SCAAC1 | 4.8 | 70.1 |
| SCAAC2 | 4.64 | 67.8 |
| SCAAC3 | 4.12 | 65.5 |
| SCAAC4 | 2.12 | 62.7 |
| SCAAC5 | 1.92 | 54.6 |
| SCAAC6 | 0.91 | 47.3 |
| Parameter | Unit | Type | MAX | MIN | Average | STD |
|---|---|---|---|---|---|---|
| GBFS | % | Input | 484.0 | 145.2 | 282.3 | 111.6 |
| FA | % | Input | 338.8 | 0.0 | 201.7 | 111.6 |
| Age | Days | Input | 90.0 | 3.0 | 36.8 | 33.1 |
| CS | MPa | Output | 73.1 | 28.8 | 58.0 | 11.6 |
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| Population size | 100 | Max generations | 200 |
| Loudness | 0.9 | Pulse rate | 0.5 |
| Minimum frequency | 0 | Maximum frequency | 2 |
| Alpha | 0.99 | Gamma | 0.01 |
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| Max generations | 100 | Crossover (%) | 50 |
| Recombination (%) | 15 | Crossover method | Single point |
| Lower bound | −1 | Selection mode | 1 |
| Upper bound | +1 | Population size | 150 |
| Model Name | Bat-ANN | MLR | GA-ANN | |
|---|---|---|---|---|
| Statistical index | MAE | 1.78 | 4.61 | 12.20 |
| MSE | 5.98 | 33.72 | 189.53 | |
| RMSE | 2.45 | 5.81 | 13.77 | |
| AAE | 0.04 | 0.09 | 0.22 | |
| EF | 0.96 | 0.75 | 0.04 | |
| VAF | 96 | 74 | 54 |
| IW | b1 | ||||||
|---|---|---|---|---|---|---|---|
| 0.3281 | 0.9040 | 0.6187 | 0.3616 | ||||
| 0.5118 | 0.6689 | 0.7581 | −1.0025 | ||||
| −0.0444 | −0.2850 | −0.2679 | −0.1930 | ||||
| −0.7793 | 0.8197 | 0.5175 | −0.8392 | ||||
| −0.0273 | −0.0039 | 1.1754 | 1.0871 | ||||
| −0.2892 | 0.4963 | −0.3293 | −0.1229 | ||||
| −1.0426 | −0.8094 | −0.6969 | 0.2663 | ||||
| LW1 | b2 | ||||||
| 0.3932 | 0.6504 | −0.2983 | 0.8155 | 0.7735 | 0.5149 | 0.5264 | 0.3523 |
| −0.8810 | 0.5294 | 0.2749 | 0.2058 | 0.3659 | −1.0303 | −0.7782 | 0.6929 |
| 0.1824 | 0.3343 | −0.5047 | −1.1157 | 0.9182 | −0.3391 | 0.2526 | 0.0045 |
| 0.3877 | −1.0380 | −0.0549 | −0.7666 | 0.9970 | −0.0396 | −0.2966 | 0.2352 |
| 0.4829 | −0.6795 | −0.1755 | 0.5596 | 1.1436 | −0.2803 | −0.6786 | 0.9843 |
| 0.3248 | 0.6792 | −0.5054 | 0.0690 | −0.4307 | 0.0636 | 0.3974 | −0.6749 |
| −0.4096 | −0.4026 | 0.5977 | 0.3671 | 0.1925 | 0.6574 | −0.1048 | −0.7101 |
| LW2 | b3 | ||||||
| −0.0775 | 0.1793 | 1.1223 | 0.2630 | 0.6937 | 0.5723 | 0.5534 | −0.2355 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faridmehr, I.; Nehdi, M.L.; Huseien, G.F.; Baghban, M.H.; Sam, A.R.M.; Algaifi, H.A. Experimental and Informational Modeling Study of Sustainable Self-Compacting Geopolymer Concrete. Sustainability 2021, 13, 7444. https://doi.org/10.3390/su13137444
Faridmehr I, Nehdi ML, Huseien GF, Baghban MH, Sam ARM, Algaifi HA. Experimental and Informational Modeling Study of Sustainable Self-Compacting Geopolymer Concrete. Sustainability. 2021; 13(13):7444. https://doi.org/10.3390/su13137444
Chicago/Turabian StyleFaridmehr, Iman, Moncef L. Nehdi, Ghasan Fahim Huseien, Mohammad Hajmohammadian Baghban, Abdul Rahman Mohd Sam, and Hassan Amer Algaifi. 2021. "Experimental and Informational Modeling Study of Sustainable Self-Compacting Geopolymer Concrete" Sustainability 13, no. 13: 7444. https://doi.org/10.3390/su13137444
APA StyleFaridmehr, I., Nehdi, M. L., Huseien, G. F., Baghban, M. H., Sam, A. R. M., & Algaifi, H. A. (2021). Experimental and Informational Modeling Study of Sustainable Self-Compacting Geopolymer Concrete. Sustainability, 13(13), 7444. https://doi.org/10.3390/su13137444











