A Sustainable and Global Health Perspective of the Dietary Pattern of French Population during the 1998–2015 Period from INCA Surveys
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data and Study Population
2.1.1. Data Collection
2.1.2. Study Population
2.2. Data Processing for the 3 V Index
2.2.1. Metric 1: “Végétal” (Plant-Based Foods)
2.2.2. Metric 2: “Vrai” (Non-UPF/Real Foods)
2.2.3. Metric 3: “Varié” (Varied Foods)
2.3. Data Analyses
3. Results
3.1. Adequacy Level in Meeting the 3 V Index
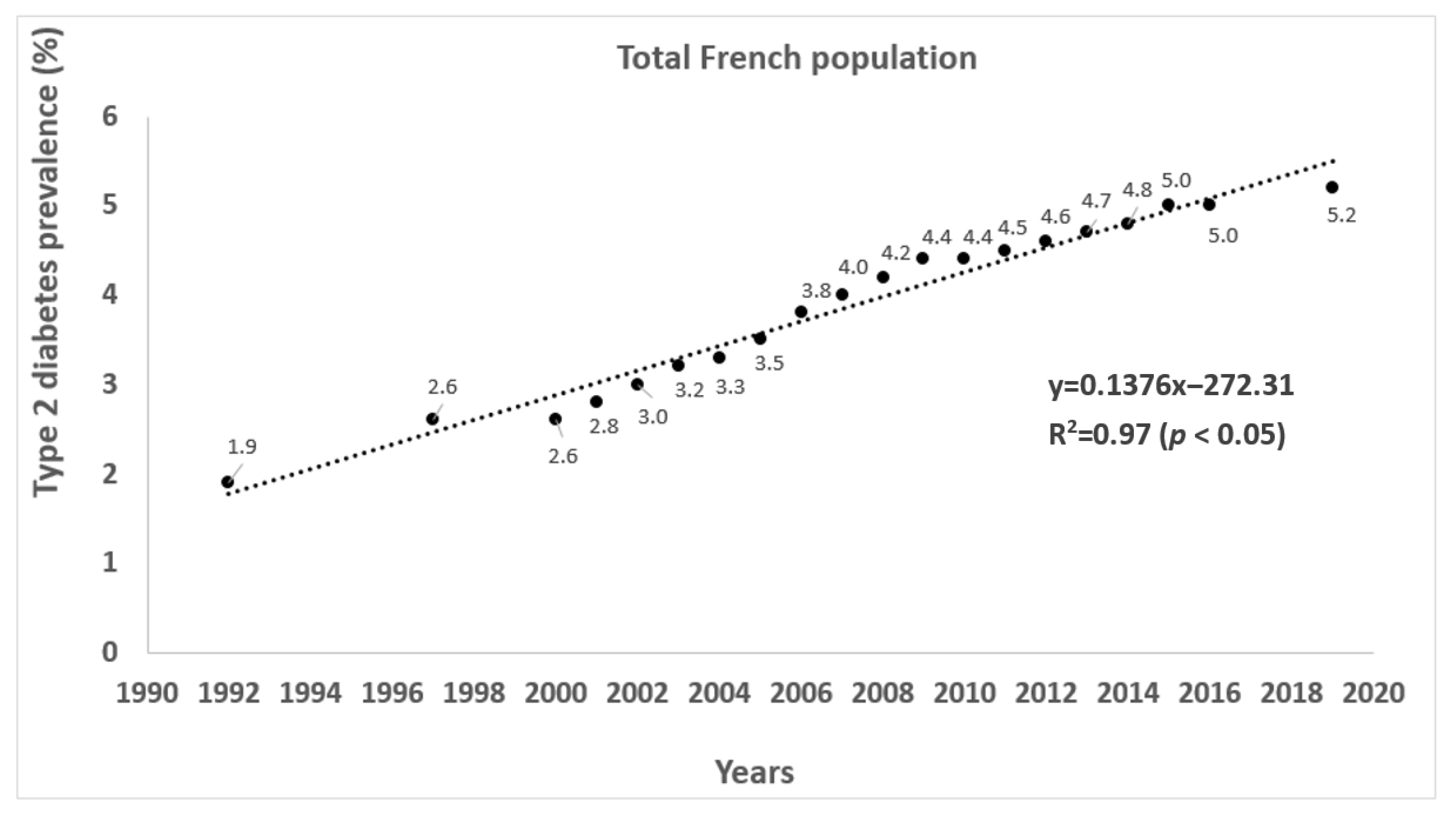
3.2. The Evolution of the Prevalence of Overweight, Obesity, Type 2 Diabetes, and Physical Activity
4. Discussion
4.1. Adequacy in Meeting the 3V Index
4.2. Association with Chronic Disease Prevalence
4.3. Impact of the French Programme National Nutrition Santé
4.4. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Baker, P.; Machado, P.; Santos, T.; Sievert, K.; Backholer, K.; Hadjikakou, M.; Russell, C.; Huse, O.; Bell, C.; Scrinis, G.; et al. Ultra-processed foods and the nutrition transition: Global, regional and national trends, food systems transformations and political economy drivers. Obes. Rev. 2020, 21, e13126. [Google Scholar] [CrossRef]
- Gouel, C.; Guimbard, H. Nutrition Transition and the Structure of Global Food Demand. Am. J. Agric. Econ. 2018, 386, 2145–2191. [Google Scholar] [CrossRef] [Green Version]
- Popkin, B.M. Contemporary nutritional transition: Determinants of diet and its impact on body composition. Proc. Nutr. Soc. 2011, 70, 82–91. [Google Scholar] [CrossRef] [Green Version]
- Popkin, B.M. The nutrition transition: An overview of world patterns of change. Nutr. Rev. 2004, 62, S140–S143. [Google Scholar] [CrossRef] [PubMed]
- Conde, W.L.; Monteiro, C.A. Nutrition transition and double burden of undernutrition and excess of weight in Brazil. Am. J. Clin. Nutr. 2014, 100, 1617S–1622S. [Google Scholar] [CrossRef] [PubMed]
- Sievert, K.; Lawrence, M.; Naika, A.; Baker, P. Processed foods and nutrition transition in the Pacific: Regional trends, patterns and food system drivers. Nutrients 2019, 11, 1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, P.; Friel, S. Processed foods and the nutrition transition: Evidence from Asia. Obes. Rev. 2014, 15, 564–577. [Google Scholar] [CrossRef] [Green Version]
- Du, S.; Lu, B.; Zhai, F.; Popkin, B.M. Chapter 11—The nutrition transition in China: A new stage of the Chinese diet. In The Nutrition Transition: Diet and Disease in the Developing World; Caballero, B., Popkin, B.C., Eds.; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Reardon, T.; Tschirley, D.; Liverpool-Tasie, L.S.O.; Awokuse, T.; Fanzo, J.; Minten, B.; Vos, R.; Dolislager, M.; Sauer, C.; Dhar, R.; et al. The processed food revolution in African food systems and the double burden of malnutrition. Glob. Food Sec. 2021, 28, 100466. [Google Scholar] [CrossRef] [PubMed]
- Mercer, A. Infections, Chronic Disease, and the Epidemiological Transition: A New Perspective; Boydell & Brewer: Suffolk, UK, 2014. [Google Scholar]
- Remais, J.V.; Zeng, G.; Li, G.; Tian, L.; Engelgau, M.M. Convergence of non-communicable and infectious diseases in low- and middle-income countries. Int. J. Epidemiol. 2013, 42, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, L.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [Green Version]
- Naghavi, M.; Abajobir, A.A.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abera, S.F.; Aboyans, V.; Adetokunboh, O.; Arnlov, J.; Afshin, A.; et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef] [Green Version]
- WHO. Facing the Facts #1: Chronic Diseases Are the Major Cause of Death and Disability Worldwide; WHO: Geneva, Switzerland, 2005. [Google Scholar]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef] [Green Version]
- Poti, J.M.; Slining, M.M.; Popkin, B.M. Where Are Kids Getting Their Empty Calories? Stores, Schools, and Fast-Food Restaurants Each Played an Important Role in Empty Calorie Intake among US Children During 2009–2010. J. Acad. Nutr. Diet. 2014, 114, 908–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirkpatrick, S.I.; Reedy, J.; Krebs-Smith, S.M. Sources of dietary guidance-based food groups and empty calories among the US population in relation to income and race/ethnicity. FASEB J. 2013, 27, 232.1. [Google Scholar] [CrossRef]
- Slining, M.M.; Popkin, B.M. Trends in sources of empty calories for 2–18 year olds in the US: 1977–2008. FASEB J. 2012, 26. [Google Scholar] [CrossRef]
- Dixon, J. From the imperial to the empty calorie: How nutrition relations underpin food regime transitions. Agric. Hum. Val. 2009, 26, 321–333. [Google Scholar] [CrossRef]
- FAO; Monteiro, C.A.; Cannon, G.; Lawrence, M.; Louzada, M.L.d.C.; Machado, P.P. Ultra-Processed Foods, Diet Quality, and Health Using the NOVA Classification System; FAO: Rome, Italy, 2019. [Google Scholar]
- McLean, E.; Cogswell, M.; Egli, I.; Wojdyla, D.; de Benoist, B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr. 2009, 12, 444–454. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, M.B.; Andersson, M. Update on iodine status worldwide. Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 382–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosanoff, A.; Weaver, C.M.; Rude, R.K. Suboptimal magnesium status in the United States: Are the health consequences underestimated? Nutr. Rev. 2012, 70, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.L.; Dodd, K.W.; Goldman, J.A.; Gahche, J.J.; Dwyer, J.T.; Moshfegh, A.J.; Sempos, C.T.; Picciano, M.F. Estimation of total usual calcium and vitamin D intakes in the United States. J. Nutr. 2010, 140, 817–822. [Google Scholar] [CrossRef] [Green Version]
- Pawlak, R.; Lester, S.E.; Babatunde, T. The prevalence of cobalamin deficiency among vegetarians assessed by serum vitamin B12: A review of literature. Eur. J. Clin. Nutr. 2014, 68, 541–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrès, E.; Loukili, N.H.; Noel, E.; Kaltenbach, G.; Abdelgheni, M.B.; Perrin, A.E.; Noblet-Dick, M.; Maloisel, F.; Schlienger, J.L.; Blicklé, J.F. Vitamin B12 (cobalamin) deficiency in elderly patients. CMAJ 2004, 171, 251–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar] [CrossRef] [Green Version]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; García-Honduvilla, N.; Asúnsolo, A.; et al. Nutritional components in Western diet versus Mediterranean diet at the gut microbiota–immune system interplay. Implications for health and disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef] [PubMed]
- Kopp, W. How Western diet and lifestyle drive the pandemic of obesity and civilization diseases. Diabetes Metab. Syndr. Obes. 2019, 12, 2221–2236. [Google Scholar] [CrossRef] [Green Version]
- Zinöcker, M.K.; Lindseth, I.A. The Western diet-microbiome-host interaction and its role in metabolic disease. Nutrients 2018, 10, 365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakersain, B.; Santoni, G.; Larsson, S.C.; Faxen-Irving, G.; Fastbom, J.; Fratiglioni, L.; Xu, W. Prudent diet may attenuate the adverse effects of Western diet on cognitive decline. Alzheimers Dement. 2016, 12, 100–109. [Google Scholar] [CrossRef] [Green Version]
- Manzel, A.; Muller, D.N.; Hafler, D.A.; Erdman, S.E.; Linker, R.A.; Kleinewietfeld, M. Role of “Western Diet” in Inflammatory Autoimmune Diseases. Curr. Allergy Asthma Rep. 2014, 14, 404. [Google Scholar] [CrossRef] [Green Version]
- Oddy, W.H.; Herbison, C.E.; Jacoby, P.; Ambrosini, G.L.; O’Sullivan, T.A.; Ayonrinde, O.T.; Olynyk, J.K.; Black, L.J.; Beilin, L.J.; Mori, T.A.; et al. The Western dietary pattern is prospectively associated with nonalcoholic fatty liver disease in adolescence. Am. J. Gastroenterol. 2013, 108, 778–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrera-Bastos, P.; Fontes-Villalba, M.; O’Keefe, J.H.; Lindeberg, S.; Cordain, L. The western diet and lifestyle and diseases of civilization. Res. Rep. Clin. Cardiol. 2011, 2011, 15–35. [Google Scholar] [CrossRef] [Green Version]
- Castello, A.; de Larrea, N.F.; Martin, V.; Davila-Batista, V.; Boldo, E.; Guevara, M.; Moreno, V.; Castano-Vinyals, G.; Gomez-Acebo, I.; Fernandez-Tardon, G.; et al. High adherence to the Western, Prudent, and Mediterranean dietary patterns and risk of gastric adenocarcinoma: MCC-Spain study. Gastric Cancer 2018, 21, 372–382. [Google Scholar] [CrossRef] [Green Version]
- Fabiani, R.; Minelli, L.; Bertarelli, G.; Bacci, S. A Western dietary pattern increases prostate cancer risk: A systematic review and meta-analysis. Nutrients 2016, 8, 626. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Qiu, Y.; Yang, H.S.; Li, M.Y.; Zhuang, X.J.; Zhang, S.H.; Feng, R.; Chen, B.L.; He, Y.; Zeng, Z.R.; et al. Systematic review and meta-analysis: Association of a pre-illness Western dietary pattern with the risk of developing inflammatory bowel disease. J. Digest. Dis. 2020, 21, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, S.; Djafarian, K.; Alizadeh, M.; Shab-Bidar, S. The relation of healthy and Western dietary patterns to the risk of endometrial and ovarian cancers: A systematic review and meta-analysis. Int. J. Vitam. Nutr. Res. 2020, 90, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.L.; Fanzo, J.C.; Cogill, B. Understanding Sustainable Diets: A Descriptive Analysis of the Determinants and Processes That Influence Diets and Their Impact on Health, Food Security, and Environmental Sustainability. Adv. Nutr. 2014, 5, 418–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fardet, A.; Rock, E. How to protect both health and food system sustainability? A holistic ‘global health’-based approach via the 3V rule proposal. Public Health Nutr. 2020, 23, 3028–3044. [Google Scholar] [CrossRef]
- Tilman, D.; Clark, M. Global diets link environmental sustainability and human health. Nature 2014, 515, 518–522. [Google Scholar] [CrossRef]
- Fardet, A.; Rock, E. Ultra-processed foods and food system sustainability: What are the links? Sustainability 2020, 12, 6280. [Google Scholar] [CrossRef]
- FAO. The State of Food Security and Nutrition in the World 2020; FAO: Roma, Italy, 2020. [Google Scholar]
- FAO; WHO. Sustainable Healthy Diets: Guiding Principles; FAO: Roma, Italy, 2019. [Google Scholar]
- Crippa, M.; Solazzo, E.; Guizzardi, D.; Monforti-Ferrario, F.; Tubiello, F.N.; Leip, A. Food systems are responsible for a third of global anthropogenic GHG emissions. Nat. Food 2021, 2, 198–209. [Google Scholar] [CrossRef]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 2–8. [Google Scholar] [CrossRef]
- Mora, O.; Le Mouël, C.; de Lattre-Gasquet, M.; Donnars, C.; Dumas, P.; Réchauchère, O.; Brunelle, T.; Manceron, S.; Marajo-Petitzon, E.; Moreau, C.; et al. Exploring the future of land use and food security: A new set of global scenarios. PLoS ONE 2020, 15, e0235597. [Google Scholar] [CrossRef]
- Détang-Dessendre, C.; Guyomard, H.; Réquillart, V.; Soler, L.-G. Changing agricultural systems and food diets to prevent and mitigate global health shocks. Sustainability 2020, 12, 6462. [Google Scholar] [CrossRef]
- Aubert, P.-M.; Gardin, B.; Alliot, C. Vers une Transition Juste des Systèmes Alimentaires. Enjeux et Leviers Politiques Pour la France; IDDRI: Paris, France, 2021. [Google Scholar]
- Solagro. Afterres2050: Un Scénario Soutenable Pour L’agriculture et L’utilisation des Terres en France à L’horizon 2050; Solagro: Toulouse, France, 2014. [Google Scholar]
- Fardet, A.; Rock, E. Reductionist nutrition research has meaning only within the framework of holistic thinking. Adv. Nutr. 2018, 9, 655–670. [Google Scholar] [CrossRef]
- Dubuisson, C.; Dufour, A.; Carrillo, S.; Drouillet-Pinard, P.; Havard, S.; Volatier, J.L. The Third French Individual and National Food Consumption (INCA3) Survey 2014–2015: Method, design and participation rate in the framework of a European harmonization process. Public Health Nutr. 2019, 22, 584–600. [Google Scholar] [CrossRef]
- Lioret, S.; Dubuisson, C.; Dufour, A.; Touvier, M.; Calamassi-Tran, G.; Maire, B.; Volatier, J.L.; Lafay, L. Trends in food intake in French children from 1999 to 2007: Results from the INCA (étude Individuelle Nationale des Consommations Alimentaires) dietary surveys. Br. J. Nutr. 2010, 103, 585–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubuisson, C.; Lioret, S.; Touvier, M.; Dufour, A.; Calamassi-Tran, G.; Volatier, J.-L.; Lafay, L. Trends in food and nutritional intakes of French adults from 1999 to 2007: Results from the INCA surveys. Br. J. Nutr. 2009, 103, 1035–1048. [Google Scholar] [CrossRef] [Green Version]
- ANSES. Étude Individuelle Nationale des Consommations Alimentaires 3 (INCA 3); ANSES: Maison-Alfort, France, 2017.
- ANSES-Afssa. Étude Individuelle Nationale des Consommations Alimentaires 2 (INCA 2) (2006–2007); ANSES-Afssa: Maison-Alfort, France, 2009. [Google Scholar]
- Afssa; INPES. Étude Individuelle Nationale des Consommations Alimentaires 1 (INCA 1) (1998–1999); ANSES-Afssa: Maison-Alfort, France, 2000. [Google Scholar]
- Haute Autorité de Santé. Surpoids et Obésité de L’adulte: Prise en Charge Médicale de Premier Recours—Recommandations Pour la Pratique Clinique; Haute Autorité de Santé: Saint-Denis La Plaine, France, 2011. [Google Scholar]
- Haute Autorité de Santé. Surpoids et Obésité de L’enfant et de L’adolescent (Actualisation des Recommandations 2003)—Argumentaire Scientifique; HAS: Saint-Denis La Plaine, France, 2011; Volume 219. [Google Scholar]
- Fosse-Edorh, S.; Mandereau-Bruno, L.; Piffaretti, C. Le poids du Diabète en France en 2016. Synthèse Epidémiologique; Santé Publique France: Saint-Maurice, France, 2018. [Google Scholar]
- Fagot-Campagna, A.; Romon, I.; Fosse, S.; Roudier, C. Prévalence et Incidence du Diabète, et Mortalité Liée au Diabète en France—Synthèse Epidémiologique; Institut de Veille Sanitaire: Saint-Maurice, France, 2010. [Google Scholar]
- ANAES. Service évaluation des technologies et service évaluation économique. In Principes de Dépistage du Diabète de Type 2; ANAES: Saint-Denis La Plaine, France, 2003. [Google Scholar]
- Fuentes, S.; Mandereau-Bruno, L.; Regnault, N.; Bernillon, P.; Bonaldi, C.; Cosson, E.; Fosse-Edorh, S. Is the type 2 diabetes epidemic plateauing in France? A nationwide population-based study. Diabetes Metab. 2020, 46, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Santé Publique France. Prévalence et Incidence du Diabète; Santé Publique France: Saint-Maurice, France, 2019. Available online: https://www.santepubliquefrance.fr/maladies-et-traumatismes/diabete/articles/prevalence-et-incidence-du-diabete (accessed on 30 June 2021).
- ANSES. Table CIQUAL. Composition Nutritionnelle des Aliments; ANSES: Maison-Alfort, France, 2017.
- Davidou, S.; Christodoulou, A.; Fardet, A.; Frank, K. The holistico-reductionist Siga classification according to degree of food processing: An evaluation of ultra-processed foods in French supermarkets. Food Func. 2020, 11, 2026–2039. [Google Scholar] [CrossRef] [PubMed]
- Herpin, N.; Verger, D. Consommation et Modes de vie en France. Une Approche Economique et Sociologique Sur un Demi-Siècle; La Découverte: Paris, France, 2008; p. 264. [Google Scholar]
- Julia, C.; Martinez, L.; Alles, B.; Touvier, M.; Hercberg, S.; Mejean, C.; Kesse-Guyot, E. Contribution of ultra-processed foods in the diet of adults from the French NutriNet-Sante study. Public Health Nutr. 2018, 21, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Salomé, M.; Arrazat, L.; Wang, J.; Dufour, A.; Dubuisson, C.; Volatier, J.-L.; Huneau, J.-F.; Mariotti, F. Contrary to ultra-processed foods, the consumption of unprocessed or minimally processed foods is associated with favorable patterns of protein intake, diet quality and lower cardiometabolic risk in French adults (INCA3). Eur. J. Nutr. 2021. [Google Scholar] [CrossRef]
- Moubarac, J.-C.; Cannon, G. Ultra-Processed Foods in Canada: Consumption, Impact on Diet Quality and Policy Implications; Université de Montréal: Montréal, QC, Canada, 2017. [Google Scholar]
- Rauber, F.; Campagnolo, P.D.B.; Hoffman, D.J.; Vitolo, M.R. Consumption of ultra-processed food products and its effects on children’s lipid profiles: A longitudinal study. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Canella, D.S.; Levy, R.B.; Martins, A.P.B.; Claro, R.M.; Moubarac, J.C.; Baraldi, L.G.; Cannon, G.; Monteiro, C.A. Ultra-processed food products and obesity in Brazilian households (2008–2009). PLoS ONE 2014, 9, e92752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sparrenberger, K.; Friedrich, R.R.; Schiffner, M.D.; Schuch, I.; Wagner, M.B. Ultra-processed food consumption in children from a Basic Health Unit. J. Pediatr. 2015, 91, 535–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allemandi, L.; Castronuovo, L.; Tiscornia, M.V.; Ponce, M.; Schoj, V. Food advertising on Argentinean television: Are ultra-processed foods in the lead? Public Health Nutr. 2017, 21, 238–246. [Google Scholar] [CrossRef] [Green Version]
- Mallarino, C.; Gomez, L.F.; Gonzalez-Zapata, L.; Cadena, Y.; Parra, D.C. Advertising of ultra-processed foods and beverages: Children as a vulnerable population. Rev. Saude Publica 2013, 47, 1006–1010. [Google Scholar] [CrossRef] [Green Version]
- Fagerberg, P.; Langlet, B.; Oravsky, A.; Sandborg, J.; Löf, M.; Ioakimidis, I. Ultra-processed food advertisements dominate the food advertising landscape in two Stockholm areas with low vs. high socioeconomic status. Is it time for regulatory action? BMC Public Health 2019, 19, 1717. [Google Scholar] [CrossRef] [PubMed]
- Solberg, S.L.; Terragni, L.; Granheim, S.I. Ultra-processed food purchases in Norway: A quantitative study on a representative sample of food retailers. Public Health Nutr. 2016, 19, 1990–2001. [Google Scholar] [CrossRef] [Green Version]
- Devia, G.; Forli, S.; Vidal, L.; Rosa Curutchet, M.; Ares, G. References to home-made and natural foods on the labels of ultra-processed products increase healthfulness perception and purchase intention: Insights for policy making. Food Qual. Pref. 2020, 104110. [Google Scholar] [CrossRef]
- Moran, A.J.; Khandpur, N.; Polacsek, M.; Rimm, E.B. What factors influence ultra-processed food purchases and consumption in households with children? A comparison between participants and non-participants in the Supplemental Nutrition Assistance Program (SNAP). Appetite 2019, 134, 1–8. [Google Scholar] [CrossRef]
- Gehring, J.; Touvier, M.; Baudry, J.; Julia, C.; Buscail, C.; Srour, B.; Hercberg, S.; Péneau, S.; Kesse-Guyot, E.; Allès, B. Consumption of ultra-processed foods by pesco-vegetarians, vegetarians, and vegans: Associations with duration and age at diet initiation. J. Nutr. 2020, 151, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Askari, M.; Heshmati, J.; Shahinfar, H.; Tripathi, N.; Daneshzad, E. Ultra-processed food and the risk of overweight and obesity: A systematic review and meta-analysis of observational studies. Int. J. Obes. 2020, 2080–2091. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Moubarac, J.-C.; Levy, R.B.; Canella, D.S.; Louzada, M.L.d.C.; Cannon, G. Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr. 2017, 21, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Canhada, S.; Luft, V.C.; Giatti, L.; Duncan, B.B.; Chor, D.; Fonseca, M.J.M.; Matos, S.M.; Bisi Molina, M.D.C.; Barreto, S.M.; Bertazzi Levy, R.; et al. Ultra-processed foods, incident overweight and obesity, and longitudinal changes in weight and waist circumference: The Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Public Health Nutr. 2019, 23, 1076–1086. [Google Scholar] [CrossRef] [Green Version]
- Fardet, A.; Boirie, Y. Associations between diet-related diseases and impaired physiological mechanisms: A holistic approach based on meta-analyses to identify targets for preventive nutrition. Nutr. Rev. 2013, 71, 643–656. [Google Scholar] [CrossRef] [PubMed]
- Duval, C.; Rouillier, M.-A.; Rabasa-Lhoret, R.; Karelis, A.D. High intensity exercise: Can it protect you from a fast food diet? Nutrients 2017, 9, 943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, A.J.; Henley, W.E.; Williams, C.A.; Hurst, A.J.; Logan, S.; Wyatt, K.M. Systematic review and meta-analysis of the association between childhood overweight and obesity and primary school diet and physical activity policies. Int. J. Behav. Nutr. Phys. Act. 2013, 10, 101. [Google Scholar] [CrossRef] [Green Version]
- Shurong, L.; Jian, S.; Quanyong, X.; Ming, W. Association of dietary pattern and physical activity level with triglyceride to high-density lipoprotein cholesterol ratio among adults in Jiangsu, China: A cross-sectional study with sex-specific differences. Nutr. Res. 2014, 34, 674–681. [Google Scholar]
- Castetbon, K.; Lafay, L.; Volatier, J.L.; Escalon, H.; Delamaire, C.; Chauliac, M.; Ledesert, B.; Hercberg, S. The French national nutrition and health program (PNNS): Report of the studies and observed results.Le Programme National Nutrition Sante (PNNS): Bilan des études et résultats observés. Cah. Nutr. Diét. 2011, 46, S11–S25. [Google Scholar] [CrossRef]
- Ministère des Solidarités et de la Santé. Programme National Nutrition Santé 2019–2023; Ministère des Solidarités et de la Santé: Paris, France, 2019. [Google Scholar]
- Monteiro, C. The big issue is ultra-processing. World Nutr. 2010, 1, 237–269. [Google Scholar]
| INCA1 (1998–1999) | INCA2 (2006–2007) | INCA3 (2014–2015) | INCA1 (1998–1999) | INCA2 (2006–2007) | INCA3 (2014–2015) | |
|---|---|---|---|---|---|---|
| Children (0–17 1) | Adults (18 1–79) | |||||
| Kcal/day | 1759 | 1777 | 1765 | 2285 | 2162 | 2114 |
| Minimally processed food category calorie (%) 2 | 47.2 | 42.8 | 41.7 | 49.4 | 48.0 | 48.9 |
| Plant calories (%) | 54.5 | 56.2 | 61.2 | 59.6 | 59.7 | 64.1 |
| Real food (i.e., non-UPF) calories (%) | 57.2 | 56.8 | 54.5 | 60.8 | 59.8 | 65.0 |
| Low level of physical activity (%) | - 3 | 20 | 38 | - 3 | 25 | 37 |
| Overweight (%) | 15 4 | 14 | 17 | 30 4 | 43 | 51 |
| Obesity (%) | 4 2 | 3 | 4 | 9 4 | 12 | 17 |
| INCA1 | INCA2 | INCA3 | |||
|---|---|---|---|---|---|
| Nutrients | Unit | DRI/Day | % DRI | ||
| Free sugars | g | 10% calories max | - 1 | 12 | 13 |
| Fiber | 10% kcal | 30 | 71 | 58 | 66 |
| EPA | mg | 250 | - | - | 47 |
| DHA | mg | 250 | - | - | 68 |
| Salt | g | 5 | - | - | 160 |
| SFA | 12% kcal | 12% calories max | 15 | 14 | 13 |
| Sodium | mg | 1900 | 168 | 156 | 167 |
| Magnesium | mg | 420 | - | 69 | 81 |
| Phosphorus | mg | 700 | - | 181 | 177 |
| Potassium | mg | 2000 | - | 148 | 155 |
| Calcium | mg | 900 | 95 | 102 | 103 |
| Manganese | mg | 3 | - | 104 | 111 |
| Iron | mg | 11 | 119 | 119 | 95 |
| Copper | mg | 1 | - | 115 | 131 |
| Zinc | mg | 8 | - | 143 | 127 |
| Selenium | mg | 70 | - | 76 | 179 |
| Iodine | mg | 150 | - | 84 | 99 |
| Retinol | mg | 750 | - | 94 | 63 |
| Vitamin B1 | mg | 1 | - | 92 | 92 |
| Vitamin B2 | mg | 2 | - | 106 | 100 |
| Vitamin B3 | mg | 17 | - | 107 | 118 |
| Vitamin B5 | mg | 6 | - | 97 | 98 |
| Vitamin B6 | mg | 2 | - | 94 | 94 |
| Vitamin B9 | mg | 330 | 80 | 87 | 92 |
| Vitamin B12 | mg | 4 | - | 145 | 135 |
| Vitamin C | mg | 110 | 70 | 84 | 82 |
| Vitamin D | mg | 15 | - | 17 | 21 2 |
| Vitamin E | mg | 11 | - | 110 | 93 |
| INCA1 (1998–1999) | INCA2 (2006–2007) | INCA3 (2014–2015) | INCA1 (1998–1999) | INCA2 (2006–2007) | INCA3 (2014–2015) | |
|---|---|---|---|---|---|---|
| Children (0–17 1) | Adults (18 1–79) | |||||
| Bread and dry breadmaking | 10.0 | 9.3 | 8.7 | 15.3 | 14.7 | 15.4 |
| Milk | 5.9 | 5.0 | 3.3 | 5.6 | 5.1 | 4.8 |
| Pasta, rice, and semolina | 4.8 | 4.4 | 5.4 | 4.7 | 4.5 | 4.2 |
| Meats | 4.8 | 4.4 | 3.7 | 3.7 | 3.4 | 4.1 |
| Potatoes and their derivatives | 4.3 | 3.7 | 2.5 | 3.5 | 3.2 | 3.1 |
| Cheeses | 4.1 | 3.5 | 3.2 | 3.2 | 3.3 | 3.4 |
| Ultra-fresh dairy | 3.9 | 3.7 | 4.4 | 3.1 | 2.7 | 2.2 |
| Poultry and game | 2.8 | 2.0 | 2.1 | 2.9 | 2.9 | 3.1 |
| Fruits | 2.6 | 2.0 | 2.3 | 2.5 | 2.0 | 1.6 |
| Fish, crustaceans, and mollusks | 1.4 | 1.7 | 1.5 | 1.6 | 2.0 | 1.9 |
| Vegetables (except potatoes) | 1.3 | 1.4 | 1.6 | 1.6 | 1.9 | 2.6 |
| Eggs and derivatives | 0.9 | 1.0 | 1.1 | 1.2 | 1.2 | 1.1 |
| Legumes | 0.4 | 0.4 | 1.6 | 0.5 | 0.4 | 0.4 |
| Nuts, seeds, and oleaginous fruits | - 2 | 0.2 | 0.2 | - 2 | 0.5 | 0.7 |
| Offal | - 2 | 0.1 | 0.1 | - 2 | 0.2 | 0.3 |
| Total | 47.2 | 42.8 | 41.7 | 49.4 | 48.0 | 48.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fardet, A.; Thivel, D.; Gerbaud, L.; Rock, E. A Sustainable and Global Health Perspective of the Dietary Pattern of French Population during the 1998–2015 Period from INCA Surveys. Sustainability 2021, 13, 7433. https://doi.org/10.3390/su13137433
Fardet A, Thivel D, Gerbaud L, Rock E. A Sustainable and Global Health Perspective of the Dietary Pattern of French Population during the 1998–2015 Period from INCA Surveys. Sustainability. 2021; 13(13):7433. https://doi.org/10.3390/su13137433
Chicago/Turabian StyleFardet, Anthony, David Thivel, Laurent Gerbaud, and Edmond Rock. 2021. "A Sustainable and Global Health Perspective of the Dietary Pattern of French Population during the 1998–2015 Period from INCA Surveys" Sustainability 13, no. 13: 7433. https://doi.org/10.3390/su13137433
APA StyleFardet, A., Thivel, D., Gerbaud, L., & Rock, E. (2021). A Sustainable and Global Health Perspective of the Dietary Pattern of French Population during the 1998–2015 Period from INCA Surveys. Sustainability, 13(13), 7433. https://doi.org/10.3390/su13137433







