Membrane Filtration as Post-Treatment of Rotating Biological Contactor for Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wastewater Preparation and Characterization
2.2. Membrane Preparation and Characterization
2.2.1. Determination of Filtration Performance
2.2.2. Membrane Fouling Analysis
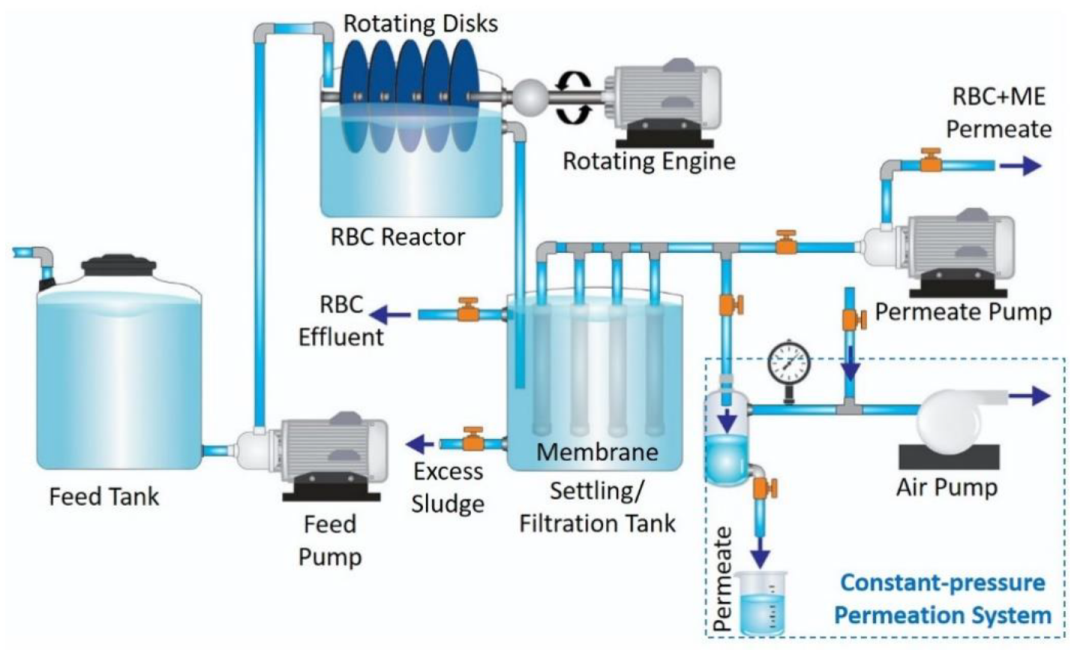
2.3. Bioreactor Set-Up and Operation
2.3.1. Hydraulic Retention Time
2.3.2. Scanning Electron Microscope
3. Results
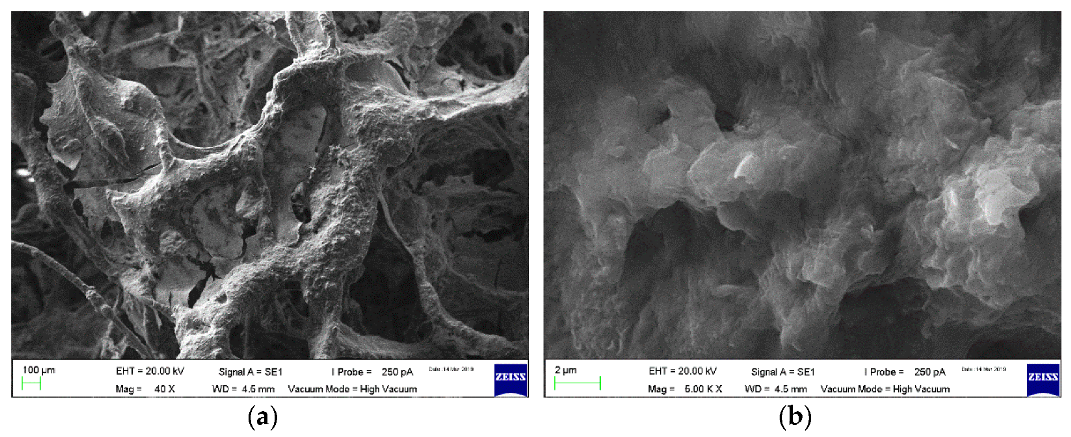
3.1. Biofilm Analysis
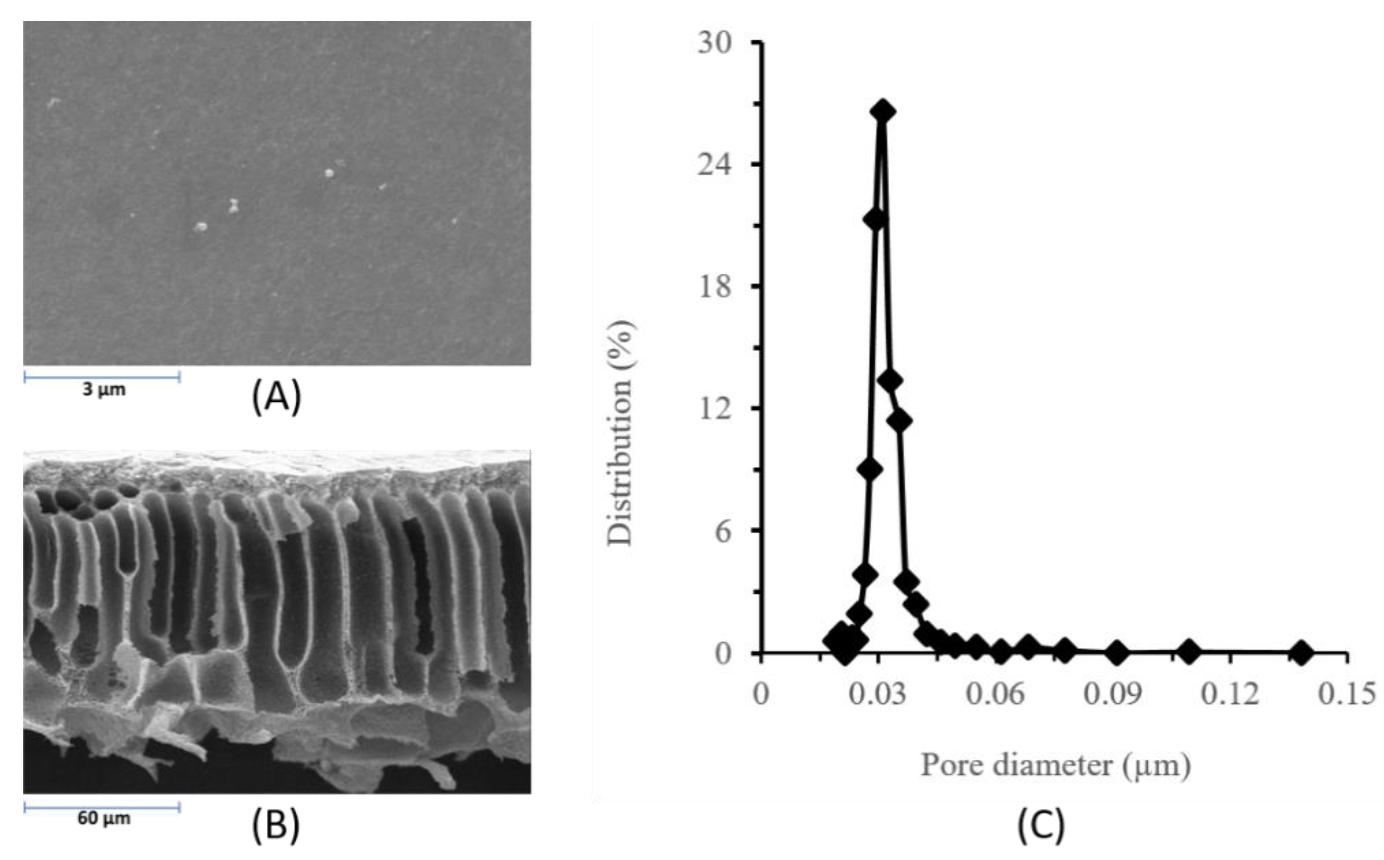
3.2. Membrane Characterization
3.3. Biological Performance
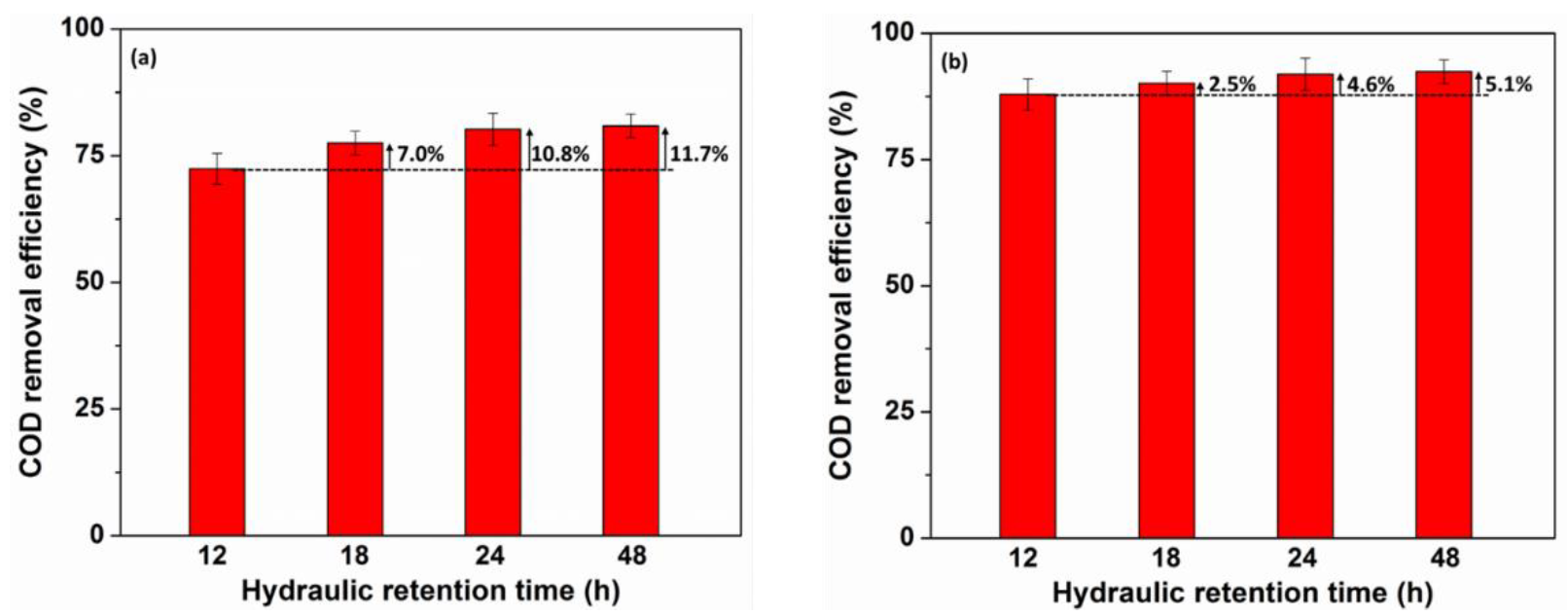
3.4. Effect of Hydraulic Retention Time on COD Removal
3.5. Effect of Hydraulic Retention Time on TN Removal
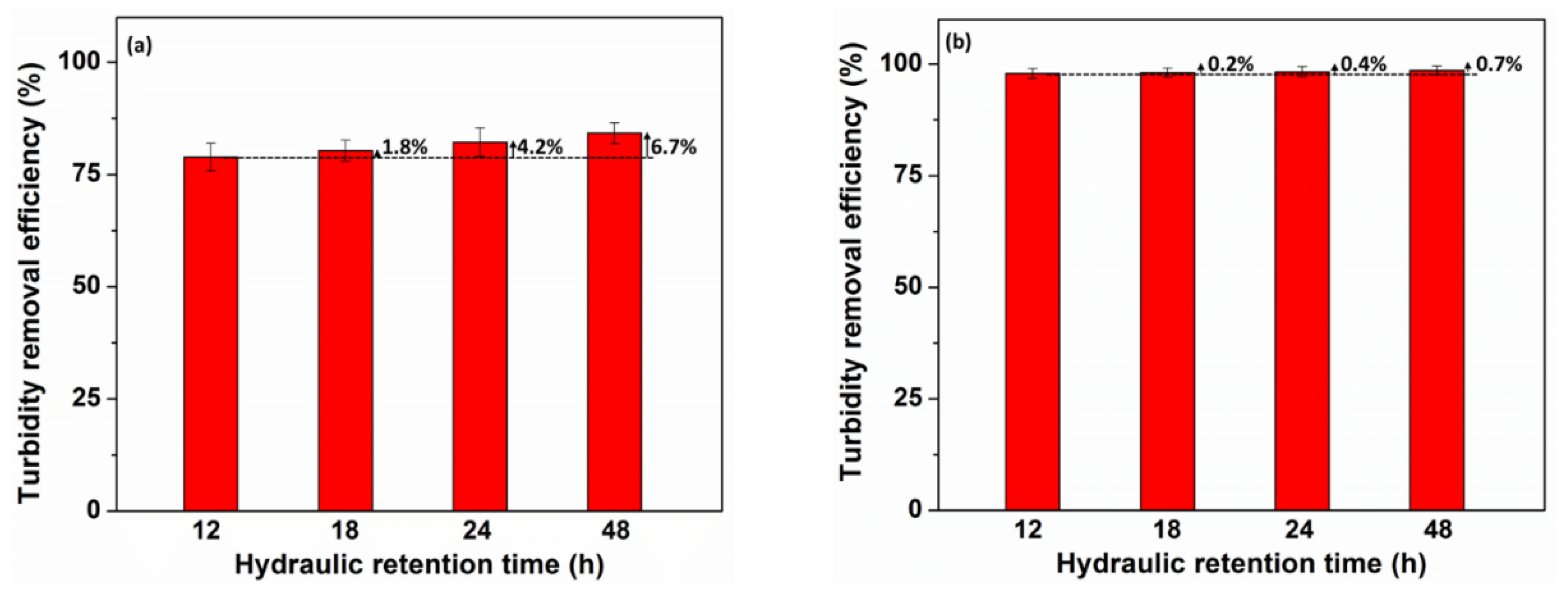
3.6. Effect of Hydraulic Retention Time on Turbidity
3.7. Membrane Permeability versus Hydraulic Retention Time in RBC–ME
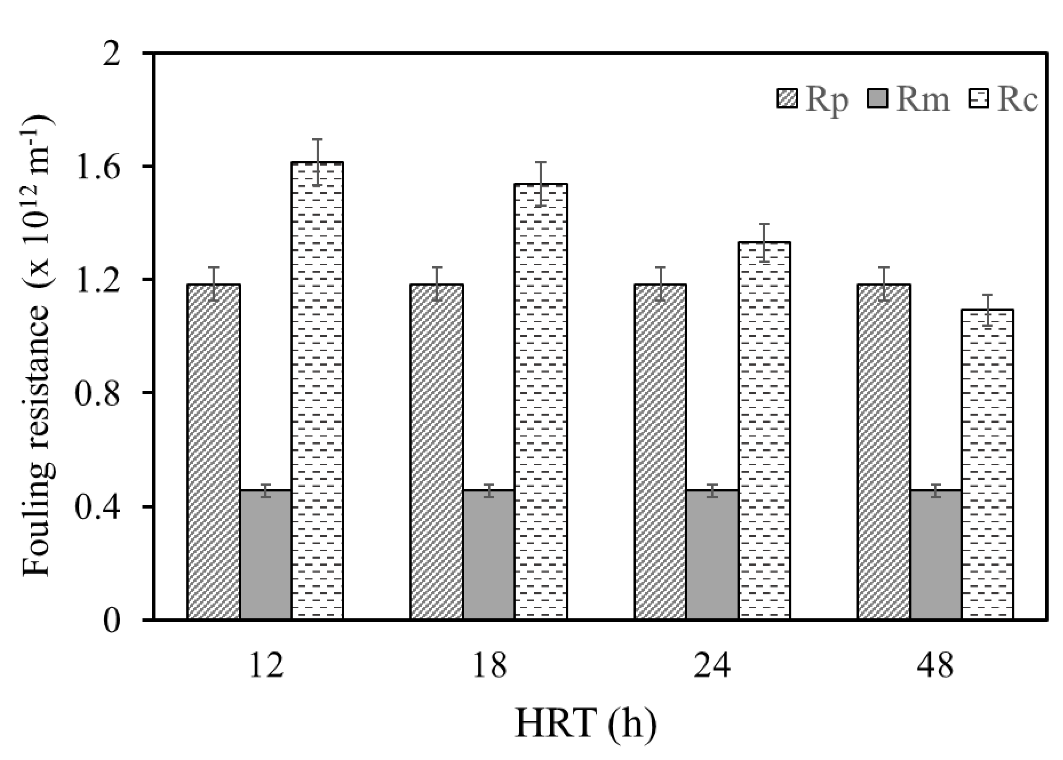
3.8. Membrane Fouling Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ziembińska-Buczyńska, A.; Ciesielski, S.; Żabczyński, S.; Cema, G. Bacterial community structure in rotating biological contactor treating coke wastewater in relation to medium composition. Environ. Sci. Pollut. Res. 2019, 26, 19171–19179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.; Shi, Y.; El-Din, M.G.; Liu, Y. Performance of flocs and biofilms in integrated fixed-film activated sludge (IFAS) systems for the treatment of oil sands process-affected water (OSPW). Chem. Eng. J. 2017, 314, 368–377. [Google Scholar] [CrossRef]
- Waqas, S.; Bilad, M.R.; Man, Z.; Wibisono, Y.; Jaafar, J.; Mahlia, T.M.I.; Khan, A.L.; Aslam, M. Recent progress in integrated fixed-film activated sludge process for wastewater treatment: A review. J. Environ. Manag. 2020, 268, 110718. [Google Scholar] [CrossRef] [PubMed]
- Waqas, S.; Bilad, M.R.; Aqsha, A.; Harun, N.Y.; Ayoub, M.; Wirzal, M.D.H.; Jaafar, J.; Mulyati, S.; Elma, M. Effect of membrane properties in a membrane rotating biological contactor for wastewater treatment. J. Environ. Chem. Eng. 2021, 9, 104869. [Google Scholar] [CrossRef]
- Hassard, F.; Biddle, J.; Cartmell, E.; Jefferson, B.; Tyrrel, S.; Stephenson, T. Rotating biological contactors for wastewater treatment—A review. Process Saf. Environ. Prot. 2015, 94, 285–306. [Google Scholar] [CrossRef] [Green Version]
- Findlay, G. The selection and design of rotating biological contactors and reed beds for small sewage treatment plants. Proc. Inst. Civ. Eng. Water Marit. Energy 1993, 101, 237–246. [Google Scholar] [CrossRef]
- Griffin, P.; Findlay, G. Process and engineering improvements to rotating biological contactor design. Water Sci. Technol. 2000, 41, 137–144. [Google Scholar] [CrossRef]
- Najafpour, G.; Yieng, H.A.; Younesi, H.; Zinatizadeh, A. Effect of organic loading on performance of rotating biological contactors using palm oil mill effluents. Process Biochem. 2005, 40, 2879–2884. [Google Scholar] [CrossRef]
- Patwardhan, A. Rotating biological contactors: A review. Ind. Eng. Chem. Res. 2003, 42, 2035–2051. [Google Scholar] [CrossRef]
- Waqas, S.; Bilad, M.R.; Man, Z.B. Performance and Energy Consumption Evaluation of Rotating Biological Contactor for Domestic Wastewater Treatment. Indones. J. Sci. Technol. 2021, 6, 101–112. [Google Scholar] [CrossRef]
- Cheng, S.-F.; Lee, Y.-C.; Kuo, C.-Y.; Wu, T.-N. A case study of antibiotic wastewater treatment by using a membrane biological reactor system. Int. Biodeterior. Biodegr. 2015, 102, 398–401. [Google Scholar] [CrossRef]
- Waqas, S.; Bilad, M.R. A review on rotating biological contactors. Indones. J. Sci. Technol. 2019, 4, 241–256. [Google Scholar] [CrossRef]
- Safa, M.; Alemzadeh, I.; Vossoughi, M. Biodegradability of oily wastewater using rotating biological contactor combined with an external membrane. J. Environ. Health Sci. 2014, 12, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rana, S.; Gupta, N.; Rana, R. Removal of organic pollutant with the use of rotating biological contactor. Mater. Today Proc. 2018, 5, 4218–4224. [Google Scholar] [CrossRef]
- Waqas, S.; Bilad, M.R.; Man, Z.B. Effect of organic and nitrogen loading rate in a rotating biological contactor for wastewater treatment. J. Phys. Conf. Ser. 2021, 1793, 012063. [Google Scholar] [CrossRef]
- Cortez, S.; Teixeira, P.; Oliveira, R.; Mota, M. Rotating biological contactors: A review on main factors affecting performance. Rev. Environ. Sci. Biotechnol. 2008, 7, 155–172. [Google Scholar] [CrossRef] [Green Version]
- Dutta, S.; Hoffmann, E.; Hahn, H. Study of rotating biological contactor performance in wastewater treatment using multi-culture biofilm model. Water Sci. Technol. 2007, 55, 345–353. [Google Scholar] [CrossRef]
- Han, Y.; Ma, J.; Xiao, B.; Huo, X.; Guo, X. New integrated self-refluxing rotating biological contactor for rural sewage treatment. J. Clean. Prod. 2019, 217, 324–334. [Google Scholar] [CrossRef]
- Waqas, S.; Bilad, M.R.; Man, Z.B.; Klaysom, C.; Jaafar, J.; Khan, A.L. An integrated rotating biological contactor and membrane separation process for domestic wastewater treatment. Alex. Eng. J. 2020, 59, 4257–4265. [Google Scholar] [CrossRef]
- Mohammadi, M.; Mohammadi, P.; Karami, N.; Barzegar, A.; Annuar, M.S.M. Efficient hydrogen gas production from molasses in hybrid anaerobic-activated sludge-rotating biological contactor. Int. J. Hydrogen Energy 2019, 44, 2592–2602. [Google Scholar] [CrossRef]
- Barambu, N.U.; Bilad, M.R.; Huda, N.; Nordin, N.A.H.M.; Bustam, M.A.; Doyan, A.; Roslan, J. Effect of Membrane Materials and Operational Parameters on Performance and Energy Consumption of Oil/Water Emulsion Filtration. Membranes 2021, 11, 370. [Google Scholar] [CrossRef]
- Barambu, N.U.; Peter, D.; Yusoff, M.H.M.; Bilad, M.R.; Shamsuddin, N.; Marbelia, L.; Nordin, N.A.H.; Jaafar, J. Detergent and Water Recovery from Laundry Wastewater Using Tilted Panel Membrane Filtration System. Membranes 2020, 10, 260. [Google Scholar] [CrossRef]
- Hanhan, O.; Orhon, D.; Krauth, K.; Günder, B. Evaluation of denitrification potential of rotating biological contactors for treatment of municipal wastewater. Water Sci. Technol. 2005, 51, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Najafpour, G.; Zinatizadeh, A.; Lee, L. Performance of a three-stage aerobic RBC reactor in food canning wastewater treatment. Biochem. Eng. J. 2006, 30, 297–302. [Google Scholar] [CrossRef]
- Costley, S.; Wallis, F. Effect of flow rate on heavy metal accumulation by rotating biological contactor (RBC) biofilms. J. Ind. Microbiol. Biotechnol. 2000, 24, 244–250. [Google Scholar] [CrossRef]
- Ghalehkhondabi, V.; Fazlali, A.; Fallah, B. Performance analysis of four-stage rotating biological contactor in nitrification and COD removal from petroleum refinery wastewater. Chem. Eng. Process. Process Intensif. 2021, 159, 108214. [Google Scholar] [CrossRef]
- Win, T.T.; Kim, H.; Cho, K.; Song, K.G.; Park, J. Monitoring the microbial community shift throughout the shock changes of hydraulic retention time in an anaerobic moving bed membrane bioreactor. Bioresour. Technol. 2016, 202, 125–132. [Google Scholar] [CrossRef]
- Zhang, M.; Peng, Y.; Wang, C.; Wang, C.; Zhao, W.; Zeng, W. Optimization denitrifying phosphorus removal at different hydraulic retention times in a novel anaerobic anoxic oxic-biological contact oxidation process. Biochem. Eng. J. 2016, 106, 26–36. [Google Scholar] [CrossRef]
- Kharraz, J.A.; Bilad, M.; Arafat, H.A. Simple and effective corrugation of PVDF membranes for enhanced MBR performance. J. Membr. Sci. 2015, 475, 91–100. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 9th ed.; American Public Health Association: Washington, DC, USA, 1997. [Google Scholar]
- Eliseus, A.; Bilad, M.; Nordin, N.; Khan, A.L.; Putra, Z.; Wirzal, M.; Aslam, M.; Aqsha, A.; Jaafar, J. Two-way switch: Maximizing productivity of tilted panel in membrane bioreactor. J. Environ. Manag. 2018, 228, 529–537. [Google Scholar] [CrossRef]
- Bilad, M.R.; Nawi, N.I.M.; Subramaniam, D.D.; Shamsuddin, N.; Khan, A.L.; Jaafar, J.; Nandiyanto, A.B.D. Low-pressure submerged membrane filtration for potential reuse of detergent and water from laundry wastewater. J. Water Process. Eng. 2020, 36, 101264. [Google Scholar] [CrossRef]
- Bilad, M.R.; Declerck, P.; Piasecka, A.; Vanysacker, L.; Yan, X.; Vankelecom, I.F. Treatment of molasses wastewater in a membrane bioreactor: Influence of membrane pore size. Sep. Purif. Technol. 2011, 78, 105–112. [Google Scholar] [CrossRef]
- AlMarzooqi, F.A.; Bilad, M.; Mansoor, B.; Arafat, H.A. A comparative study of image analysis and porometry techniques for characterization of porous membranes. J. Mater. Sci. 2016, 51, 2017–2032. [Google Scholar] [CrossRef]
- Vázquez-Padín, J.R.; Mosquera-Corral, A.; Campos, J.L.; Méndez, R.; Carrera, J.; Pérez, J. Modelling aerobic granular SBR at variable COD/N ratios including accurate description of total solids concentration. Biochem. Eng. J. 2010, 49, 173–184. [Google Scholar] [CrossRef]
- Brazil, B.L. Performance and operation of a rotating biological contactor in a tilapia recirculating aquaculture system. Aquac. Eng. 2006, 34, 261–274. [Google Scholar] [CrossRef]
- Hewawasam, C.; Matsuura, N.; Maharjan, N.; Hatamoto, M.; Yamaguchi, T. Oxygen transfer dynamics and nitrification in a novel rotational sponge reactor. Biochem. Eng. J. 2017, 128, 162–167. [Google Scholar] [CrossRef]
- Van Kessel, M.A.; Speth, D.R.; Albertsen, M.; Nielsen, P.H.; den Camp, H.J.O.; Kartal, B.; Jetten, M.S.; Lücker, S. Complete nitrification by a single microorganism. Nature 2015, 528, 555–559. [Google Scholar] [CrossRef] [Green Version]
- Zha, X.; Ma, J.; Lu, X. Performance of a coupling device combined energy-efficient rotating biological contactors with anoxic filter for low-strength rural wastewater treatment. J. Clean. Prod. 2018, 196, 1106–1115. [Google Scholar] [CrossRef]
- Waqas, S.; Bilad, M.R.; Man, Z.B.; Suleman, H.; Nordin, N.A.H.; Jaafar, J.; Othman, M.H.D.; Elma, M. An Energy-Efficient Membrane Rotating Biological Contactor for Wastewater Treatment. J. Clean. Prod. 2020, 282, 124544. [Google Scholar] [CrossRef]
- Wang, D.; Wang, G.; Yang, F.; Liu, C.; Kong, L.; Liu, Y. Treatment of municipal sewage with low carbon-to-nitrogen ratio via simultaneous partial nitrification, anaerobic ammonia oxidation, and denitrification (SNAD) in a non-woven rotating biological contactor. Chemosphere 2018, 208, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wu, D.; Hu, Z. Impact of hydraulic retention time on organic and nutrient removal in a membrane coupled sequencing batch reactor. Water Res. 2014, 55, 12–20. [Google Scholar] [CrossRef]
- Tadkaew, N.; Hai, F.I.; McDonald, J.A.; Khan, S.J.; Nghiem, L.D. Removal of trace organics by MBR treatment: The role of molecular properties. Water Res. 2011, 45, 2439–2451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boonnorat, J.; Techkarnjanaruk, S.; Honda, R.; Prachanurak, P. Effects of hydraulic retention time and carbon to nitrogen ratio on micro-pollutant biodegradation in membrane bioreactor for leachate treatment. Bioresour. Technol. 2016, 219, 53–63. [Google Scholar] [CrossRef]
- Nawi, M.; Izati, N.; Bilad, M.R.; Zolkhiflee, N.; Nordin, N.A.H.; Lau, W.J.; Narkkun, T.; Faungnawakij, K.; Arahman, N.; Mahlia, T.M.I. Development of a novel corrugated polyvinylidene difluoride membrane via improved imprinting technique for membrane distillation. Polymers 2019, 11, 865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marbelia, L.; Bilad, M.R.; Bertels, N.; Laine, C.; Vankelecom, I.F. Ribbed PVC–silica mixed matrix membranes for membrane bioreactors. J. Membr. Sci. 2016, 498, 315–323. [Google Scholar] [CrossRef]
- Liao, Y.; Bokhary, A.; Maleki, E.; Liao, B. A review of membrane fouling and its control in algal-related membrane processes. Bioresour. Technol. 2018, 264, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Guo, W.; Ngo, H.H.; Du, B.; Wei, Q.; Tran, N.H.; Nguyen, N.C.; Chen, S.-S.; Li, J. Effects of hydraulic retention time and bioflocculant addition on membrane fouling in a sponge-submerged membrane bioreactor. Bioresour. Technol. 2016, 210, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Mannina, G.; Capodici, M.; Cosenza, A.; Di Trapani, D.; Ekama, G.A. The effect of the solids and hydraulic retention time on moving bed membrane bioreactor performance. J. Cleaner Prod. 2018, 170, 1305–1315. [Google Scholar] [CrossRef]


| Influent | |
|---|---|
| COD (mg/L) | 298 ± 45.6 |
| TN (mg/L) | 2.4 ± 0.2 |
| Ammonium (mg/L) | 0.92 ± 0.07 |
| Nitrate (mg/L) | 0.52 ± 0.08 |
| Turbidity (NTU) | 15.2 ± 0.6 |
| pH | 6.35 ± 0.18 |
| IUPAC Name | Abbreviation | Avg. Molecular Mass | Purity * |
|---|---|---|---|
| Polysulfone | PSF | 22,000 Da | 100 wt% |
| Polyethylene glycol | PEG | 9000–12,500 Da | 100 wt% |
| N,N-Dimethylacetamide | DMAc | - | 99.7 vol% |
| Water | H2O | - | ~100 vol% |
| Properties (Unit) | Values |
|---|---|
| Materials | Polysulfone |
| Thickness (mm) | 0.28 ± 0.22 |
| Mean flow pore size (µm) | 0.03 µm |
| Surface contact angle (°) | 61.8 ± 1.0 |
| Cross-section morphology | Asymmetric |
| Clean water permeability (L/(m2 h bar) | 817 ± 35 |
| RBC Effluent | RBC % Removal Efficiency | RBC–ME Effluent | RBC–ME % Removal Efficiency | |
|---|---|---|---|---|
| COD (mg/L) | 78.2 ± 7.5 | 72.4 ± 2.5 | 35 ± 8.9 | 87.9 ± 3.2 |
| TN (mg/L) | 1.54 ± 0.05 | 38.3 ± 1.9 | 1.41 ± 0.05 | 45.2 ± 0.7 |
| Ammonium (mg/L) | 0.03 ± 0.01 | 95.6 ± 0.8 | 0.01 ± 0.01 | 98.9 ± 1.1 |
| Nitrate (mg/L) | 1.9 ± 0.3 | - | 1.8 ± 0.2 | - |
| Turbidity (NTU) | 3.3 ± 0.3 | 78.9 ± 0.3 | 0.32 ± 0.03 | 97.9 ± 0.1 |
| pH | 6.82 ± 0.03 | - | 6.95 ± 0.11 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waqas, S.; Bilad, M.R.; Huda, N.; Harun, N.Y.; Md Nordin, N.A.H.; Shamsuddin, N.; Wibisono, Y.; Khan, A.L.; Roslan, J. Membrane Filtration as Post-Treatment of Rotating Biological Contactor for Wastewater Treatment. Sustainability 2021, 13, 7287. https://doi.org/10.3390/su13137287
Waqas S, Bilad MR, Huda N, Harun NY, Md Nordin NAH, Shamsuddin N, Wibisono Y, Khan AL, Roslan J. Membrane Filtration as Post-Treatment of Rotating Biological Contactor for Wastewater Treatment. Sustainability. 2021; 13(13):7287. https://doi.org/10.3390/su13137287
Chicago/Turabian StyleWaqas, Sharjeel, Muhammad Roil Bilad, Nurul Huda, Noorfidza Yub Harun, Nik Abdul Hadi Md Nordin, Norazanita Shamsuddin, Yusuf Wibisono, Asim Laeeq Khan, and Jumardi Roslan. 2021. "Membrane Filtration as Post-Treatment of Rotating Biological Contactor for Wastewater Treatment" Sustainability 13, no. 13: 7287. https://doi.org/10.3390/su13137287











