Optimization of the Biomethane Production Process by Anaerobic Digestion of Wheat Straw Using Chemical Pretreatments Coupled with Ultrasonic Disintegration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate and Inocula
2.2. Biochemical Methane Potential (BMP) Tests
2.3. Ultrasound Pretreatment
2.4. Experimental Procedure
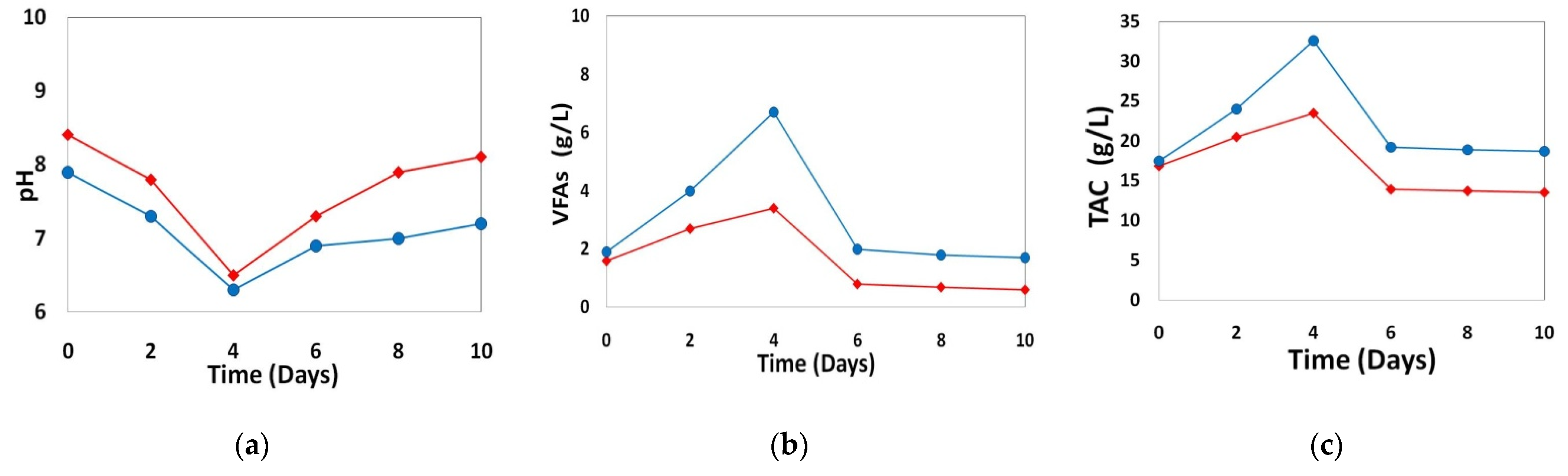
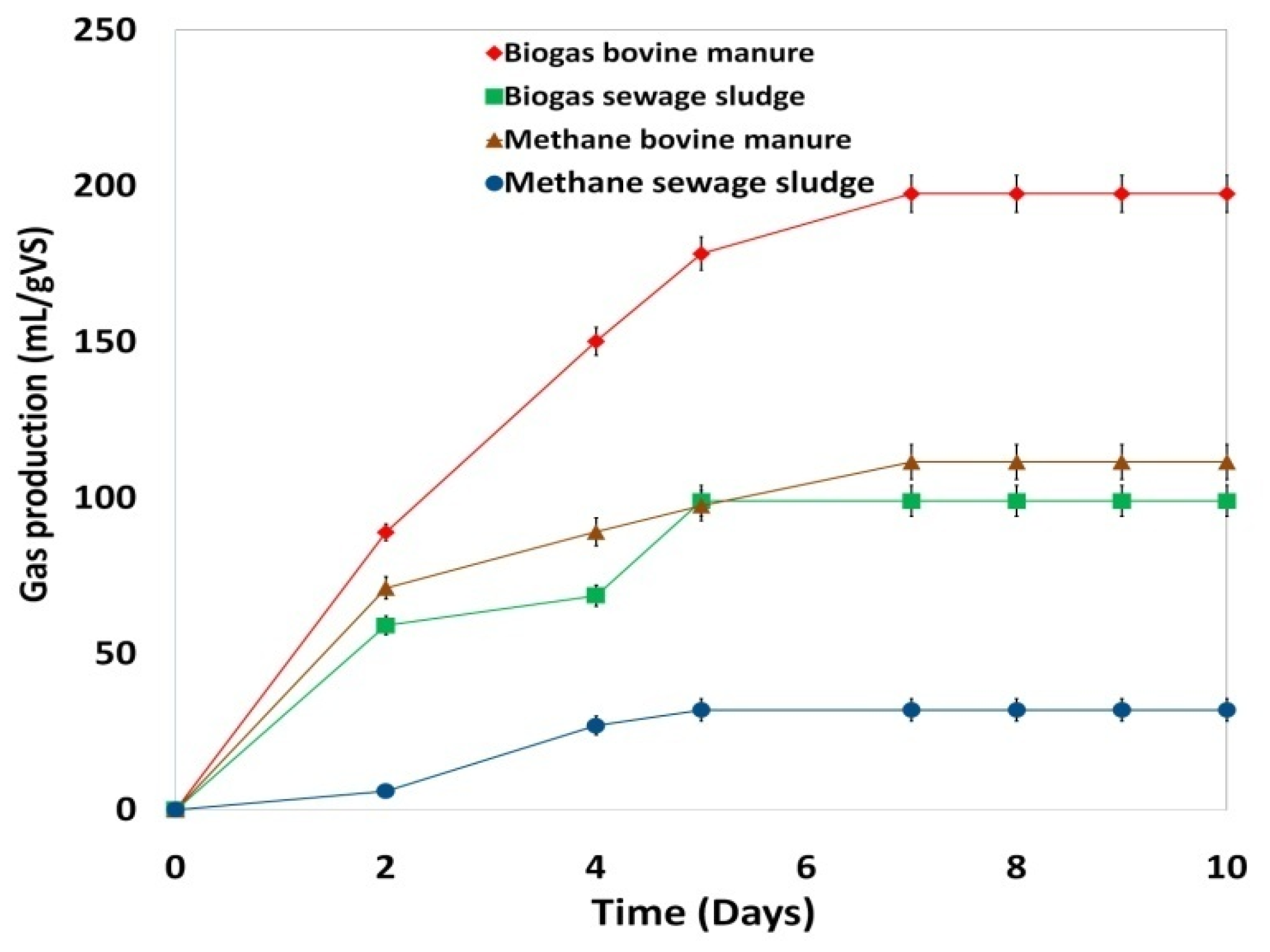
- First, for bovine manure and sewage sludge, we performed two BMP tests. AD was monitored daily by measuring biogas and methane volume, pH, volatile fatty acids (VFA), and total alkalinity (TA).
- Then, two BMP tests were performed with treated and untreated WS as substrate and cattle manure as inoculum.
- We used a ratio VS inoculum/VS substrate = 2.
- Finally, we performed two BMP tests with filter-treated WS and unfiltered WS under the same conditions as the previous BMP tests, with the filtrate being the liquid residue from the WS pretreatment.
2.5. Statistical Analysis
3. Results and Discussions
3.1. Effect of Heavy Metals Present in the Considered Inocula on Anaerobic Digestion
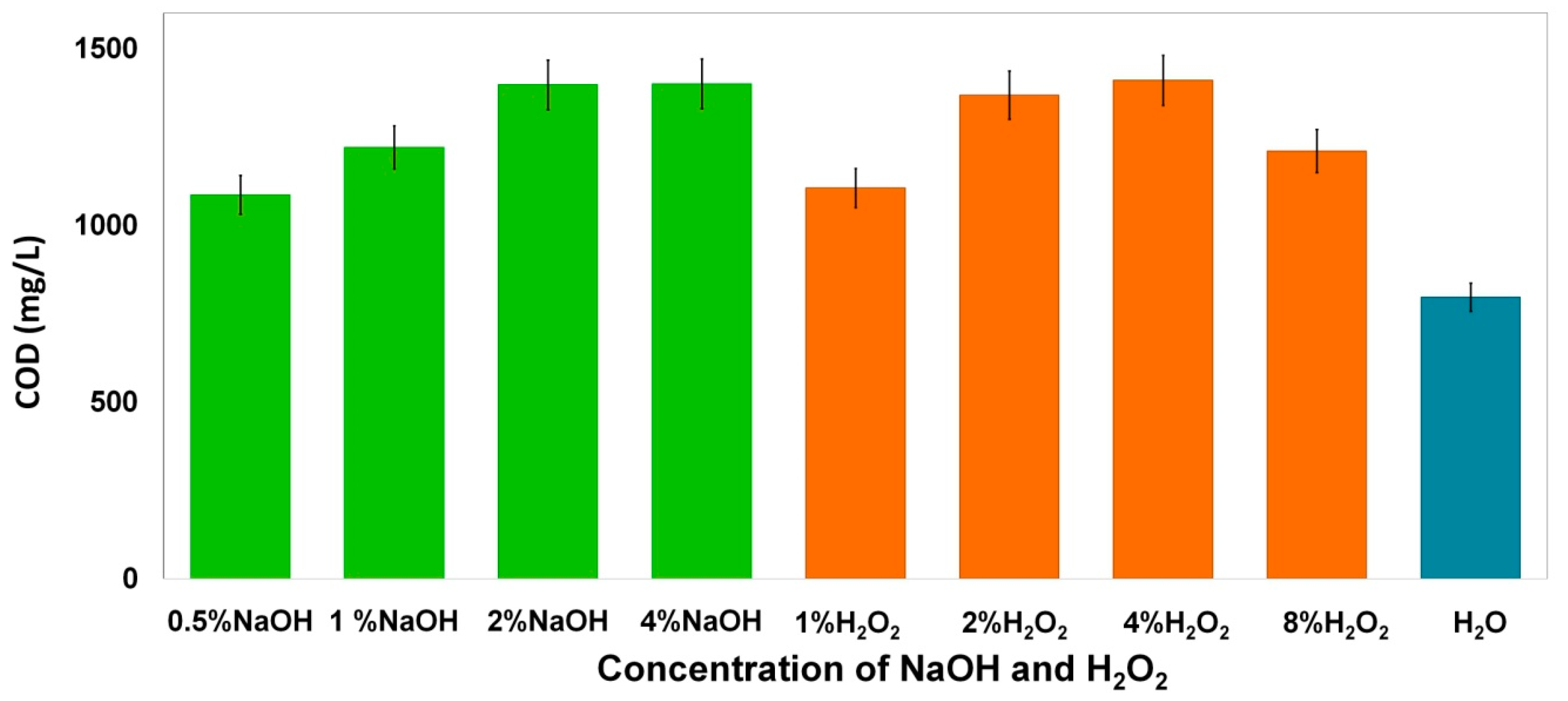
3.2. Effect of NaOH and H2O2 Concentration on WS Pretreatment
3.3. Effect of Coupling US with NaOH and H2O2 Pretreatment
3.4. Effect of Reaction Time for H2O2 Coupled with US Pretreatment
3.5. Scanning Electron Microscope (SEM) Observations
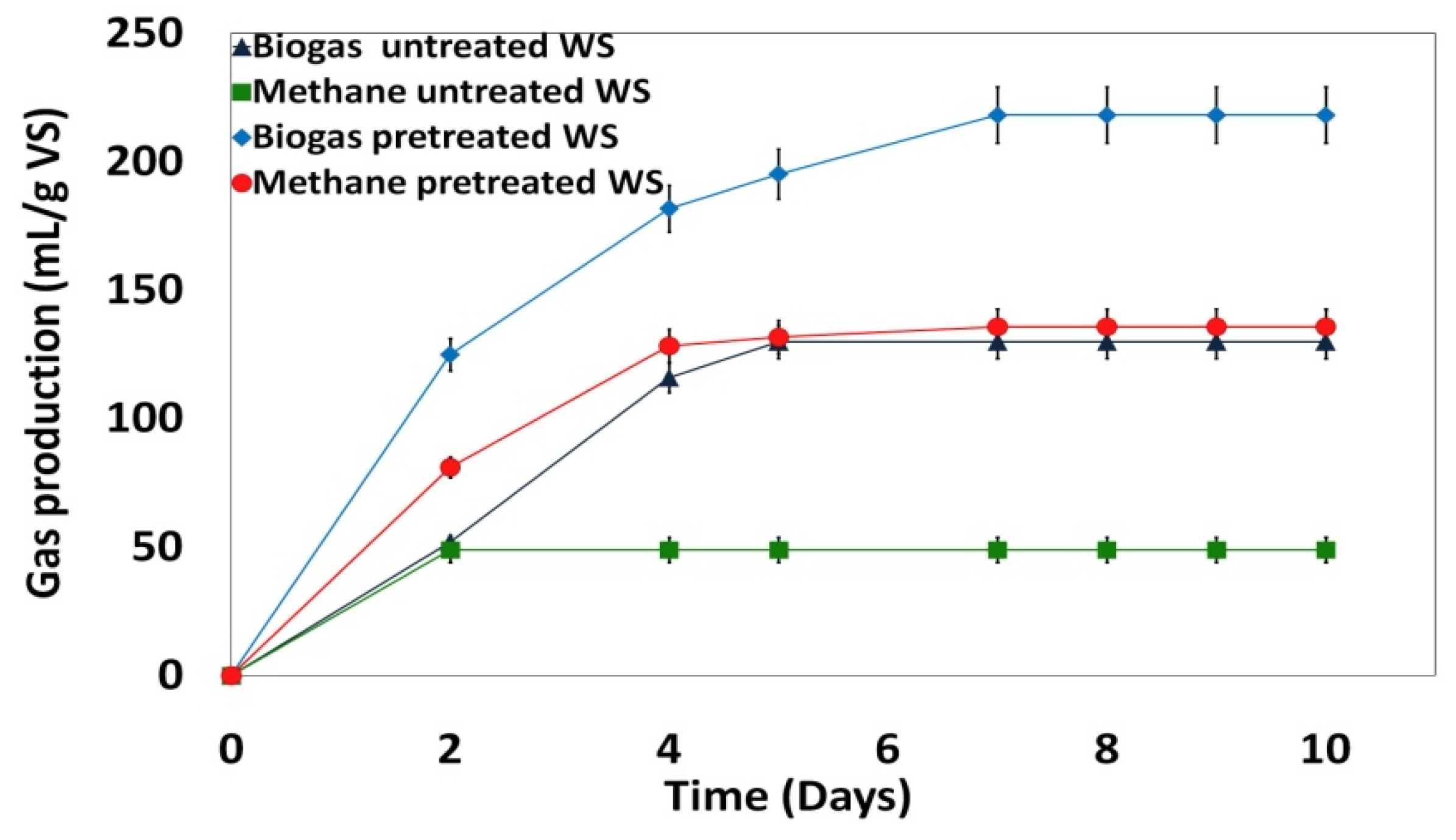
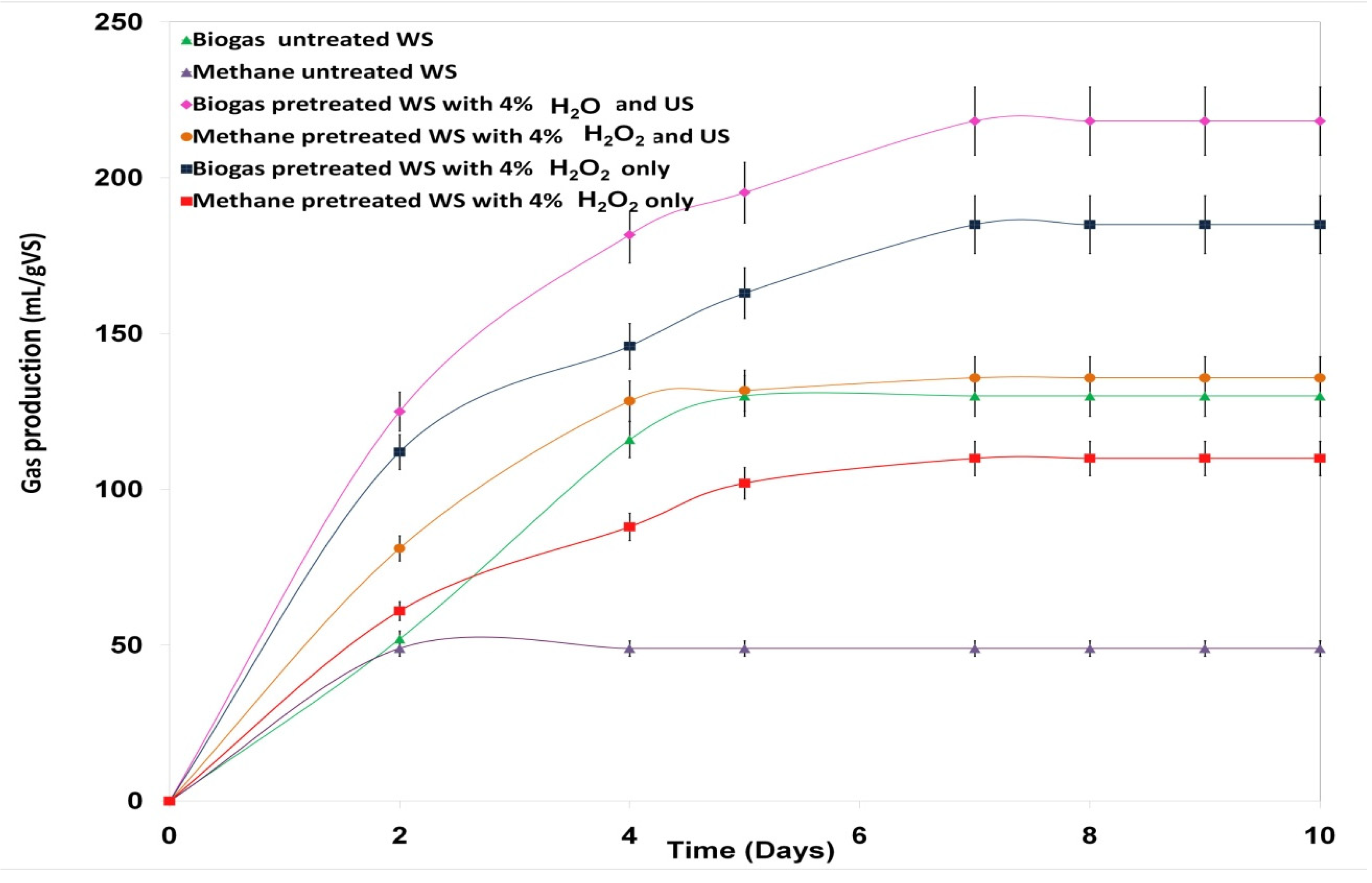
3.6. Impact of H2O2 Pretreatment Coupled with the US on Biogas Production
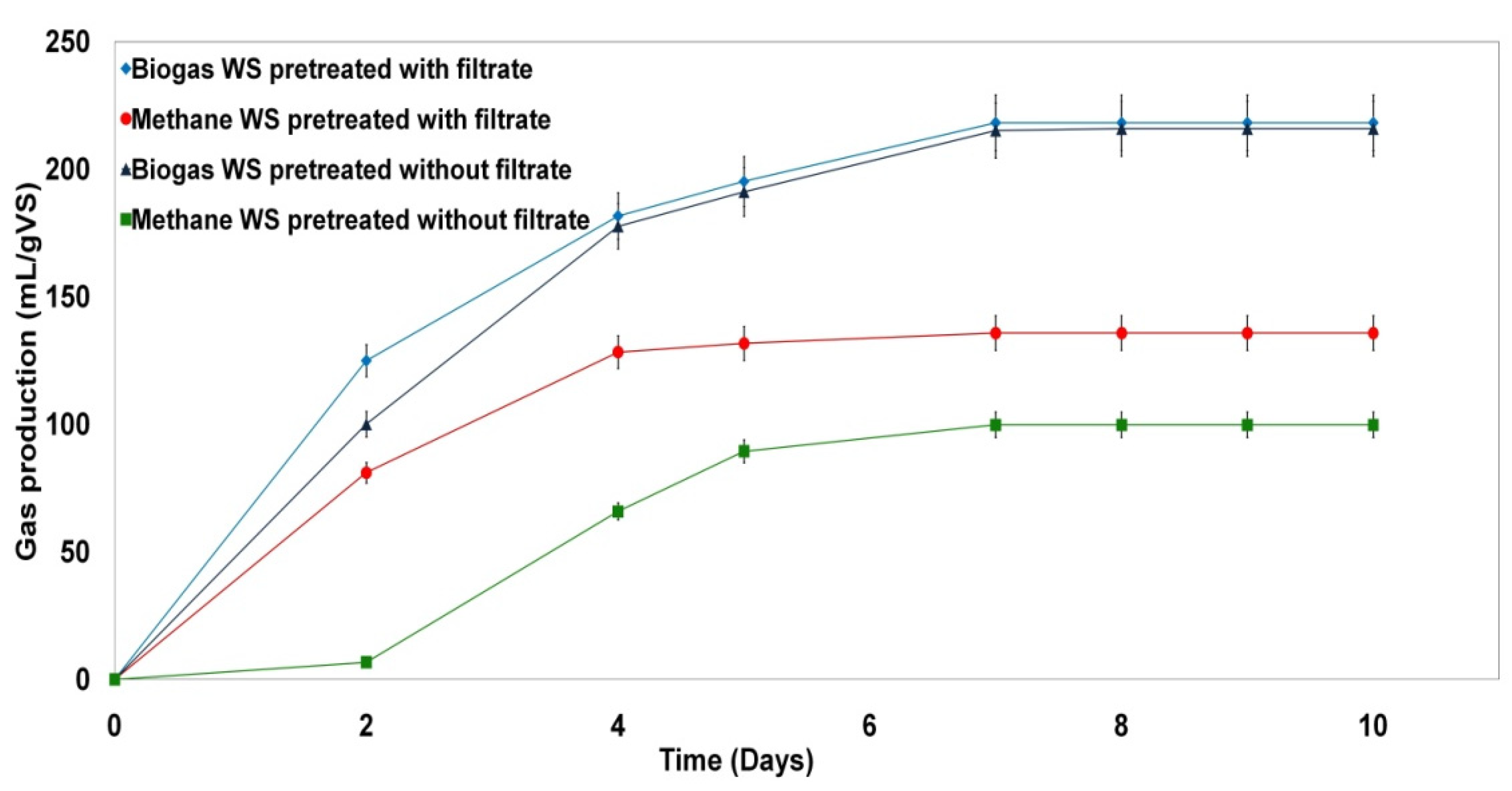
3.7. Effect of the Filtrate Resulting from the Pretreatment of WS with H2O2 Coupled with the US on the Biogas Production
3.8. Some Considerations about Ultrasonic Pretreatment
4. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, X.; Wachemo, A.C.; Zhang, L.; Bai, T.; Li, X.; Zuo, X.; Yuan, H. Effect of hydrothermal pretreatment severity on the pretreatment characteristics and anaerobic digestion performance of corn stove. Bioresour. Technol. 2019, 289, 121646. [Google Scholar] [CrossRef] [PubMed]
- Marks-Bielska, R.; Bielski, S.; Novikova, A.; Romaneckas, K. Straw Stocks as a Source of Renewable Energy. A Case Study of a District in Poland. Sustainability 2019, 11, 4714. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://ec.europa.eu/eurostat/fr/web/energy/data/energy-balances (accessed on 25 March 2021).
- Negro, S.O.; Hekkert, M.P.; Smits, R.E. Explaining the failure of the Dutch innovation system for biomass digestion—A functional analysis. Energy Policy 2007, 2, 925–938. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Q.; Zhou, T.; Wang, Y.; Cao, X.; Wu, S.; Zhao, M.; Wang, H.; Xu, M.; Zheng, B.; Zheng, J.; et al. Pretreatment of wheat straw leads to structural changes and improved enzymatic hydrolysis. Sci. Rep. 2018, 8, 1321–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sprafke, J.; Ekanthalu, V.S.; Nelles, M. Continuous Anaerobic Co-Digestion of Biowaste with Crude Glycerol under Mesophilic Conditions. Sustainability 2020, 12, 9512. [Google Scholar] [CrossRef]
- Farrukh, R.A.; Habiba, K.; Han, Z.; Sajidu, R.; Ruihong, Z.; Guangqing, L.; Chang, C. Pretreatment methods of lignocellulosic biomass for anaerobic digestion. AMB Express 2017, 7, 72–84. [Google Scholar]
- Paul, S.; Dutta, A. Challenges and opportunities of lignocellulosic biomass for anaerobic digestion. Resour. Conserv. Recycl. 2018, 130, 164–174. [Google Scholar] [CrossRef]
- Molaey, R.; Bayrakdar, A.; Sürmeli, R.Ö.; Çalli, B. Anaerobic digestion of chicken manure: Influence of trace element supplementation. Eng. Life Sci. 2019, 19, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Silva, G.G.; Couturier, M.; Berrin, J.G.; Buléon, A.; Rouau, X. Effects of grinding processes on enzymatic degradation of wheat straw. Bioresour. Technol. 2012, 1, 192–200. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Fu, E.H.; Liang, J.H. Effect of Ultrasonic Waves on the Saccharification Processes of Lignocellulose. Chem. Eng. Technol. 2008, 10, 1510–1515. [Google Scholar] [CrossRef]
- Bussemaker, J.M.; Zhang, D. Ultrasonic Pretreatment of Wheat Straw in Oxidative and Nonoxidative Conditions Aided with Microwave Heating. Ind. Eng. Chem. Res. 2013, 52, 12514–12522. [Google Scholar] [CrossRef]
- Maamir, W.; Ouahabi, Y.R.; Poncin, S.; Li, H.Z.; Bensadok, K. Effect of Fenton Pretreatment on Anaerobic Digestion of Olive Mill Wastewater and Olive Mill Solid Waste in Mesophilic Conditions. Int. J. Green Energy 2017, 14, 555–560. [Google Scholar] [CrossRef]
- Mancini, G.; Papirio, S.; Lens, P.; Esposito, G. Increased biogas production from wheat straw by chemical pretreatments. Renew. Energy 2018, 119, 608–614. [Google Scholar] [CrossRef]
- Guan, R.; Li, X.; Wachemo, A.C.; Yuan, H.; Liu, Y.; Zou, D.; Zuo, X.; Gu, J. Enhancing anaerobic digestion performance and degradation of lignocellulosic components of rice straw by combined biological and chemical pretreatment. Sci. Total Environ. 2018, 17, 637–638. [Google Scholar] [CrossRef]
- Rouches, E.; Escudié, R.; Latrille, E.; Carrère, H. Solid-state anaerobic digestion of wheat straw: Impact of S/I ratio and pilot scale fungal pretreatment. Waste Manag. 2019, 85, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.W.; Zhu, M.Q.; Li, M.F.; Wei, Q.; Sun, R.C. Effects of hydrothermal treatment on enhancing enzymatic hydrolysis of rapeseed straw. Renew. Energy 2019, 134, 446–452. [Google Scholar] [CrossRef]
- Barakat, A.; Mayer-Laigle, C.; Solhy, A.; Arancon, R.A.D.; De Vries, H.; Luque, R. Mechanical pretreatments of lignocellulosic biomass: Towards facile and environmentally sound technologies for biofuels production. RSC Adv. 2014, 4, 48109–48127. [Google Scholar] [CrossRef]
- Jaffar, M.; Pang, Y.; Yuan, H.; Zou, D.; Liu, Y.; Zhu, B.; Korai, M.R.; Li, X. Wheat straw pretreatment with KOH for enhancing biomethane production and fertilizer value in anaerobic digestion. Energy Resour. Environ. Technol. 2016, 3, 404–409. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. A comprehensive review on pretreatment strategy for lignocellulosic food industry waste: Challenges and opportunities. Bioresour. Technol. 2016, 199, 92–102. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.; Yag, G.; Feng, Y.; Ren, G.; Han, X. Pretreatment of rice straw by hydrogen peroxide for enhanced methane yield. J. Integr. Agric. 2013, 7, 1258–1266. [Google Scholar] [CrossRef]
- Jeon, Y.; Kim, H.; Shin, M.; Pak, S. Ultrasonic treatment of waste livestock blood for enhancement of solubilization. Environ. Eng. Res. 2016, 1, 22–28. [Google Scholar] [CrossRef]
- Arbaoui, A.; Ouahabi, A.; Jacques, S.; Hamiane, M. Concrete Cracks Monitoring using Deep Learning-based Multiresolution Analysis. Electronics 2021. [Google Scholar] [CrossRef]
- Labat, V.; Remenieras, J.P.; Matar, O.B.; Ouahabi, A.; Patat, F. Harmonic propagation of finite amplitude sound beams: Experimental determination of the nonlinearity parameter B/A. Ultrasonics 2000, 38, 292–296. [Google Scholar] [CrossRef]
- Ouahabi, A.; Femmam, S. Wavelet-based multifractal analysis of 1-D and 2-D signals: New results. Analog Integr. Circuits Signal Process. 2011, 69, 3–15. [Google Scholar] [CrossRef]
- Girault, J.M.; Ossant, F.; Ouahabi, A.; Kouame, D.; Patat, F. Time-varying autoregressive spectral estimation for ultrasound attenuation in tissue characterization. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 1998, 45, 650–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djeddi, M.; Ouahabi, A.; Batatia, H.; Basarab, A.; Kouamé, D. Discrete wavelet transform for multifractal texture classification: Application to ultrasound imaging. In Proceedings of the IEEE International Conference on Image Processing (IEEE ICIP 2010), Hong Kong, China, 12–15 September 2010; pp. 637–640. [Google Scholar]
- Girault, J.M.; Kouamé, D.; Ouahabi, A.; Patat, F. Estimation of the blood Doppler frequency shift by a time-varying parametric approach. Ultrasonics 2000, 38, 682–687. [Google Scholar] [CrossRef] [Green Version]
- APHA Association; Federation, Water Environmental. Standards Methods for the Examination of Water and Wastewater; United Book Press Inc.: Baltimor, MD, USA, 1998. [Google Scholar]
- Van Soest, J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Brown, A.M. A new software for carrying out one-way ANOVA post hoc tests. Comput. Methods Programs Biomed. 2005, 79, 89–95. [Google Scholar] [CrossRef]
- Bolan, N.; Adriano, D.; Mahimairaja, S. Distribution and Bioavailability of Trace Elements in Livestock and Poultry Manure By-Products. Crit. Rev. Environ. Sci. Technol. 2004, 34, 291–338. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.; Yang, M.; Wei, L. Content of Heavy Metals in Animal Feeds and Manures from Farms of Different Scales in Northeast China. Int. J. Environ. Public Health 2012, 9, 2658–2668. [Google Scholar] [CrossRef] [Green Version]
- Mudhoo, A.; Kumar, S. Effects of heavy metals as stress factors on anaerobic digestion processes and biogas production from biomass. Int. J. Environ. Sci. Technol. 2013, 6, 1383–1398. [Google Scholar] [CrossRef] [Green Version]
- Schattauer, A.; Abdoun, E.; Weiland, P.; Plochl, M.; Heiermann, M. Abundance of trace elements in demonstration biogas plants. Biosyst. Eng. 2011, 108, 57–65. [Google Scholar] [CrossRef]
- Chernicharo, C.A.L. Anaerobic Reactors. In Biological Wastewater Treatment Series; IWA Publishing: London, UK, 2007. [Google Scholar]
- Montusiewicz, A.; Szaja, A.; Musielewicz, I.; Cydzik-Kwiatkowska, A.; Lebiocka, M. Effect of bioaugmentation on digestate metal concentrations in anaerobic digestion of sewage sludge. PLoS ONE 2020, 7, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Paulo, L.M.; Stams, A.J.M.; Sousa, D.Z. Methanogens, sulphate and heavy metals: A complex system. Rev. Environ. Sci. Biotechnol. 2015, 14, 537–553. [Google Scholar] [CrossRef] [Green Version]
- Atlas, L. Inhibitory effect of heavy metals on methane-producing anaerobic granular sludge. J. Hazard. Mater. 2009, 162, 1551–1556. [Google Scholar]
- Chen, J.L.; Ortiz, R.; Steele, T.; Stuckey, D.C. Toxicants Inhibiting Anaerobic Digestion: A Review. Biotechnol. Adv. 2014, 8, 1523–1534. [Google Scholar] [CrossRef] [PubMed]
- Dokulilová, T.; Koutný, T.; Vítěz, T. Effect of Zinc and Copper on Anaerobic Stabilization of Sewage Sludge. Acta Univ. Agric. Silvic. Mendel. Brun. 2018, 2, 357–363. [Google Scholar] [CrossRef] [Green Version]
- Nandi, R.; Saha, C.K.; Sarker, S.; Huda, M.S.; Alam, M.M. Optimization of Reactor Temperature for Continuous Anaerobic Digestion of Cow Manure: Bangladesh Perspective. Sustainability 2020, 12, 8772. [Google Scholar] [CrossRef]
- Mota, V.T.; Zaiat, M. Two- vs. single-stage anaerobic reactors: Evaluation of effluent quality and energy production potential using sucrose-based wastewater. Water Sci. Technol. 2018, 9, 1966–1979. [Google Scholar] [CrossRef]
- Zhai, N.; Zhang, T.; Yin, D.; Yang, G.; Wang, X.; Ren, G.; Feng, Y. Effect of initial pH on anaerobic co-digestion of kitchen waste and cow manure. Waste Manag. 2015, 38, 126–131. [Google Scholar] [CrossRef]
- Rocamora, I.; Wagland, S.T.; Villa, R.; Simpson, E.W.; Fernández, O.; Bajón-Fernández, Y. Dry anaerobic digestion of organic waste: A review of operational parameters and their impact on process performance. Bioresour. Technol. 2020, 299, 122681. [Google Scholar] [CrossRef] [PubMed]
- Blasco, L.; Kahala, M.; Tampio, E.; Vainio, M.; Ervasti, S.; Rasi, S. Effect of inoculum pretreatment on the composition of microbial communities in anaerobic digesters producing volatile fatty acids. Microorganisms 2020, 8, 581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinco, B.; Heaven, S.; Banks, C.J.; Zhang, Y. Anaerobic digestion of whole-crop winter wheat silage for renewable energy production. Energy Fuel 2012, 26, 2357–2364. [Google Scholar] [CrossRef] [Green Version]
- Gallegos, D.; Wedwitschk, H.; Moeller, L.; Zehnsdorf, A.; Stinner, W. Effects of particle size reduction and ensiling fermentation on biogas formation and silage quality of wheat straw. Bioresour. Technol. 2017, 245, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Taherdanak, M.; Zilouei, H. Improving biogas production from wheat plant using alkaline pretreatment. Fuel 2014, 115, 714–719. [Google Scholar] [CrossRef]
- Komemoto, K.; Lim, Y.G.; Nagao, N.; Onoue, Y.; Niwa, C.; Toda, T. Effect of temperature on VFA’s and biogas production in anaerobic solubilization of food waste. Waste Manag. 2009, 29, 2950–2955. [Google Scholar] [CrossRef]
- Kumar, S.; Paritosh, K.; Pareek, N.; Chawade, A.; Vivekanand, V. De-construction of major Indian cereal crop residues through chemical pretreatment for improved biogas production: An overview. Renew. Sustain. Energy Rev. 2018, 90, 160–170. [Google Scholar] [CrossRef]
- Cinar, S.; Cinar, S.O.; Wieczorek, N.; Sohoo, I.; Kuchta, K. Integration of Artificial Intelligence into Biogas Plant Operation. Processes 2021, 9, 85. [Google Scholar] [CrossRef]
- Sakiewicz, P.; Piotrowski, K.; Ober, J.; Karwot, J. Innovative artificial neural network approach for integrated biogas—Wastewater treatment system modelling: Effect of plant operating parameters on process intensification. Renew. Sustain. Energy Rev. 2020, 124, 109784. [Google Scholar] [CrossRef]
- Ramachandran, A.; Rustum, R.; Adeloye, A.J. Review of Anaerobic Digestion Modeling and Optimization Using Nature-Inspired Techniques. Processes 2019, 7, 953. [Google Scholar] [CrossRef] [Green Version]



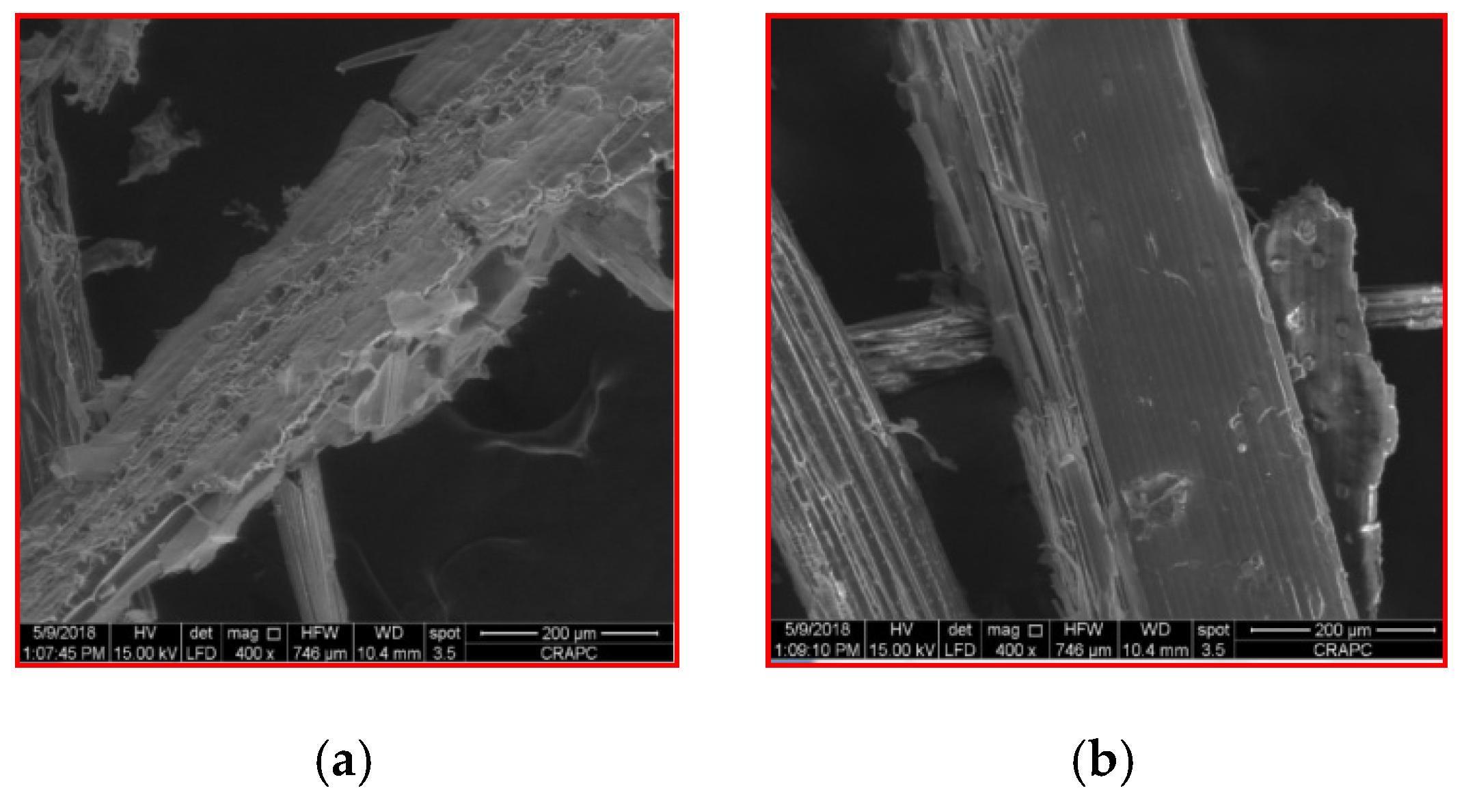
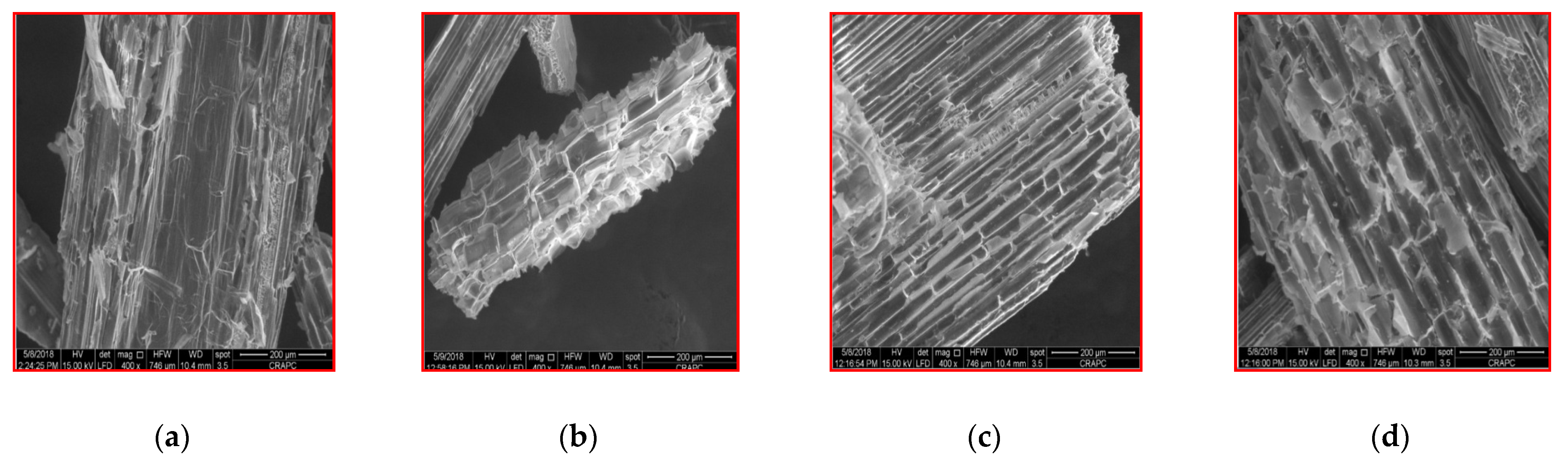
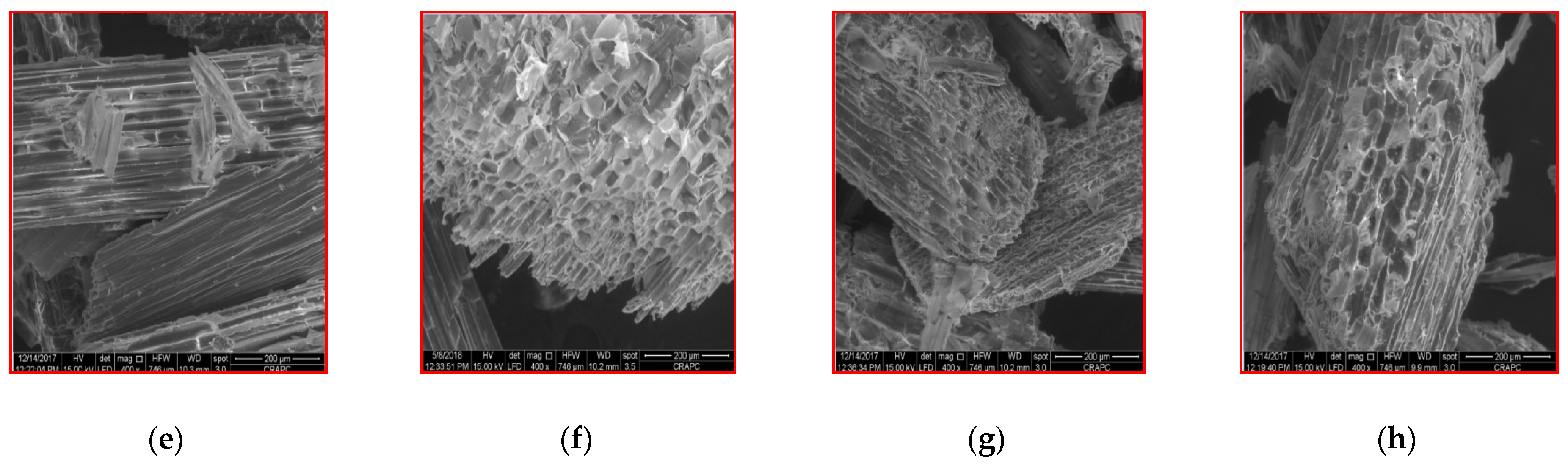
| Parameter | Raw Wheat Straw | Bovine Manure | Sewage Sludge |
|---|---|---|---|
| Total solids (TS) (g/L) | 91.33 | 24.14 | 82.02 |
| Volatile solids (VS) (g/L) | 6.15 | 20.10 | 2.50 |
| pH | / | / | 7.90 |
| Volatile fatty acid (VFA) (g/L) | 0.05 | / | 1.60 |
| Total alkalinity (TA) (g/L) | 0.01 | / | 1.00 |
| Hemicellulose (%) | 51.60 | / | / |
| Cellulose (%) | 41.30 | / | / |
| Lignin (%) | 10.30 | / | / |
| Metal Concentrations mg/kg | Bovine Manure Our Study | Bovine Manure Bolan et al. [32] | Bovine Manure Zhang et al. [33] | Sewage Sludge Our study | Sewage Sludge Bolan et al. [32] | Sewage Sludge Mudhoo et al. [34] | Required for AD Schattauer et al. [35] | Required for AD Chernicharo et al. [36] |
|---|---|---|---|---|---|---|---|---|
| Cu | 18.27 | 21.00 | 31.04 | 72.50 | 54.70 | 44.70 | 64 | 10 |
| Zn | 43.40 | 115.00 | 126.33 | 491.24 | 294.00 | 360.00 | - | 60 |
| Pb | 2.01 | 2.10 | 2.24 | 5.00 | 2.47 | 10.40 | 200 | - |
| Ni | 12.09 | 9.00 | - | 15.76 | 15.00 | 3.80 | 30 | 100 |
| Mn | 25.88 | 111.00 | - | 30.14 | 142.00 | 136.00 | 55 | 3 |
| Cr | 32.64 | 20.00 | 1.09 | 50.40 | 75.10 | 8.00 | 52 | - |
| Co | 2.34 | 1.70 | - | 2.11 | 4.10 | 1.80 | 20 | 75 |
| Bovine Manure | Sewage Sludge | |
|---|---|---|
| Biogas (mL/gVS) | 197.50 | 99.00 |
| Methane (mL/gVS) | 111.50 | 32.00 |
| Methane (%) in biogas | 56.46 | 32.32 |
| Pretreated Wheat Straw | Untreated Wheat Straw | |
|---|---|---|
| Biogas (mL/g VS) | 218 | 130 |
| Methane (mL/g VS) | 136 | 49 |
| Methane (%) in biogas | 62 | 38 |
| Methane improvement (%) for pretreated WS | 64 | / |
| Pretreated Wheat Straw | Untreated Wheat Straw | Degradation Rate | |
|---|---|---|---|
| Hemicellulose (%) | 51.60 | 23.20 | 55.04 |
| Cellulose (%) | 41.30 | 25.40 | 38.50 |
| Lignin (%) | 10.30 | 8.60 | 16.50 |
| Pretreated Wheat Straw with Filtrate | Pretreated Wheat Straw without Filtrate | |
|---|---|---|
| Biogas (mL/g VS) | 218 | 215 |
| Methane (mL/g VS) | 136 | 100 |
| Methane (%) in biogas | 62 | 46 |
| Methane improvement (%) for pretreated WS with filtrate | 26 | / |
| Pretreated Wheat Straw with 4% w/w H2O2 and US | Pretreated Wheat Straw with 4% w/w H2O2 only | Untreated Wheat Straw | |
|---|---|---|---|
| Biogas (mL/g VS) | 218 | 185 | 130 |
| Methane (mL/g VS) | 136 | 110 | 49 |
| Methane (%) in biogas | 62 | 59 | 38 |
| Methane improvement (%) for pretreated WS | 64 | 55 | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouahabi, Y.R.; Bensadok, K.; Ouahabi, A. Optimization of the Biomethane Production Process by Anaerobic Digestion of Wheat Straw Using Chemical Pretreatments Coupled with Ultrasonic Disintegration. Sustainability 2021, 13, 7202. https://doi.org/10.3390/su13137202
Ouahabi YR, Bensadok K, Ouahabi A. Optimization of the Biomethane Production Process by Anaerobic Digestion of Wheat Straw Using Chemical Pretreatments Coupled with Ultrasonic Disintegration. Sustainability. 2021; 13(13):7202. https://doi.org/10.3390/su13137202
Chicago/Turabian StyleOuahabi, Yasmine Ryma, Kenza Bensadok, and Abdeldjalil Ouahabi. 2021. "Optimization of the Biomethane Production Process by Anaerobic Digestion of Wheat Straw Using Chemical Pretreatments Coupled with Ultrasonic Disintegration" Sustainability 13, no. 13: 7202. https://doi.org/10.3390/su13137202
APA StyleOuahabi, Y. R., Bensadok, K., & Ouahabi, A. (2021). Optimization of the Biomethane Production Process by Anaerobic Digestion of Wheat Straw Using Chemical Pretreatments Coupled with Ultrasonic Disintegration. Sustainability, 13(13), 7202. https://doi.org/10.3390/su13137202







