Rangeland Biodiversity and Climate Variability: Supporting the Need for Flexible Grazing Management
Abstract
1. Introduction
2. Materials and Methods
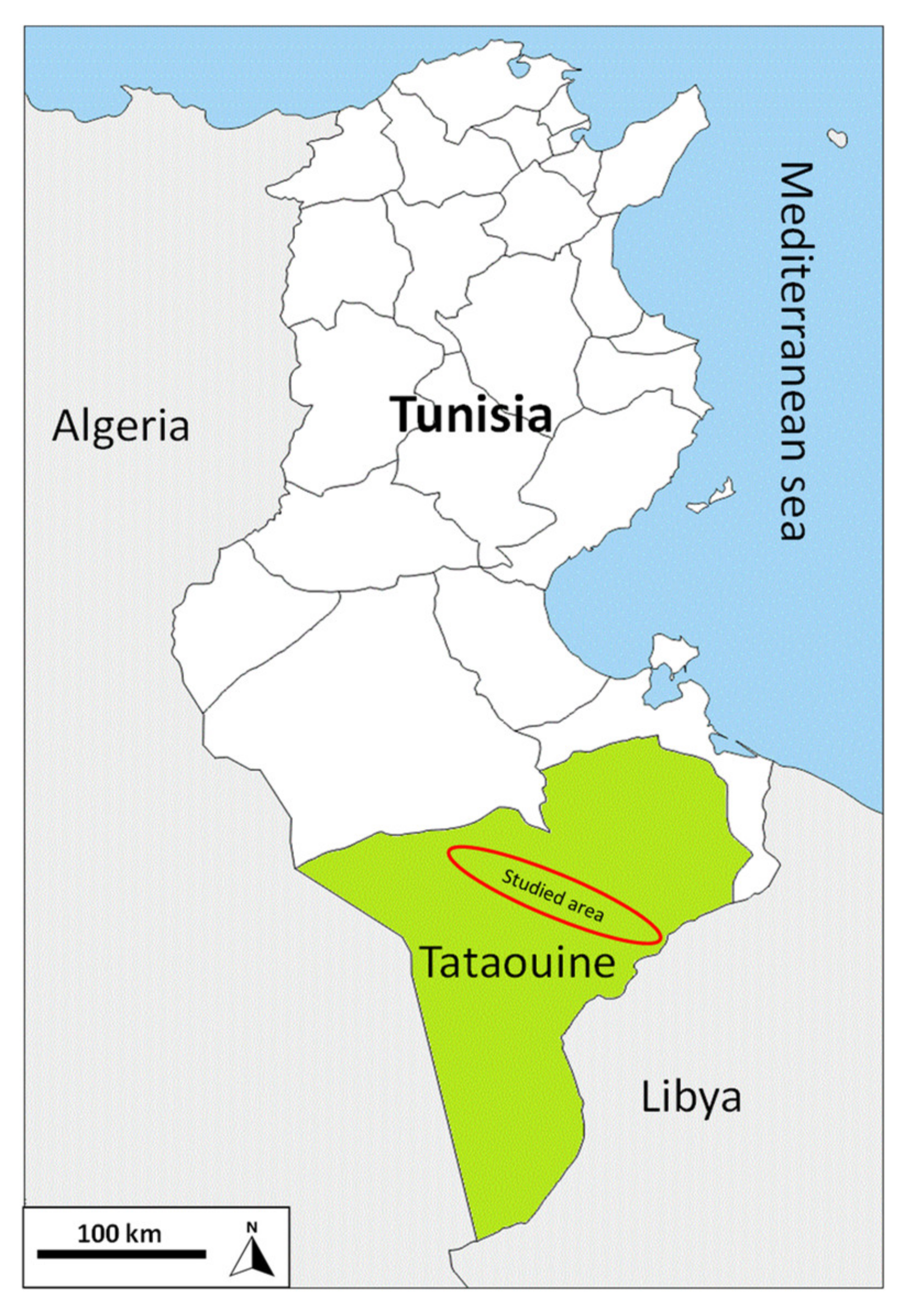
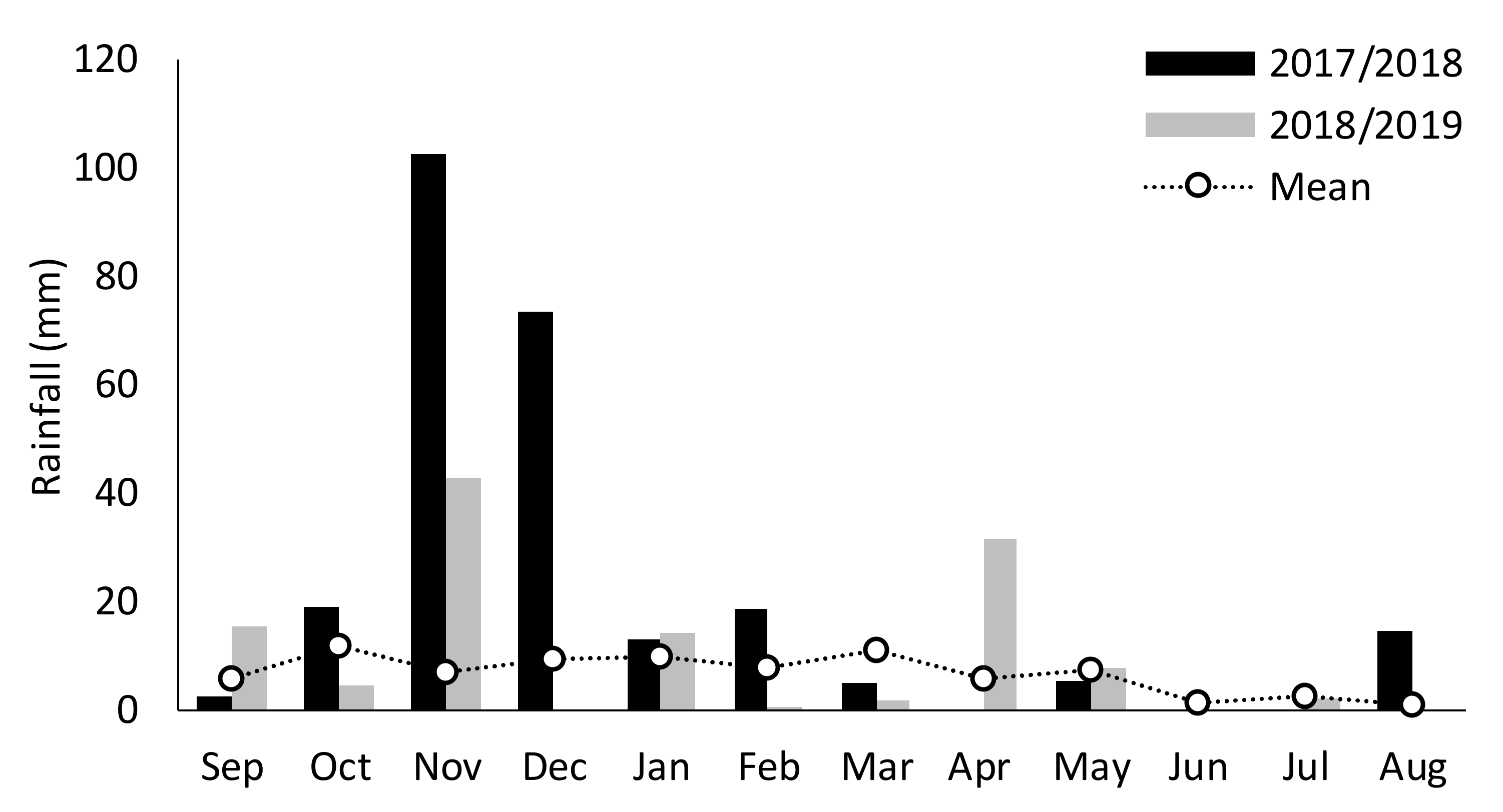
2.1. Site Description
2.2. Experimental Design and Field Measurements
- o
- Anthyllis henoniana community: growing on limestone soil (Regosol) with high calcium carbonate content, high pH, and low organic matter content.
- o
- Haloxylon schmittianum community: it colonizes shallow to deep sands or silty soils and is also found on gravel plains characterized by deep, loose soil with low moisture content, very low organic matter (0.8%), and low soil salinity.
- o
- Stipagrostis pungens community: it is located on sandy soil classified as sierozem dominated by mobile sand dunes and characterized by very high-water infiltration and low water retention. The organic matter content is low (less than 1%), pH exceeds 7, and calcium carbonate content is about 10–20%. Sand grains vary from coarse to medium size and are rarely fine.
- o
- Retama raetam community: it is found mainly on wadi beds dominated by sand accumulation. This soil is isohumic (subdesertic called sierozem) with a low water retention capacity (range 6–9%) and contains little organic matter (0.5–0.7%). Excessive amounts of calcium carbonate are present, and pH is approximately 8 down the whole profile.
2.3. Statistical Analysis
3. Results
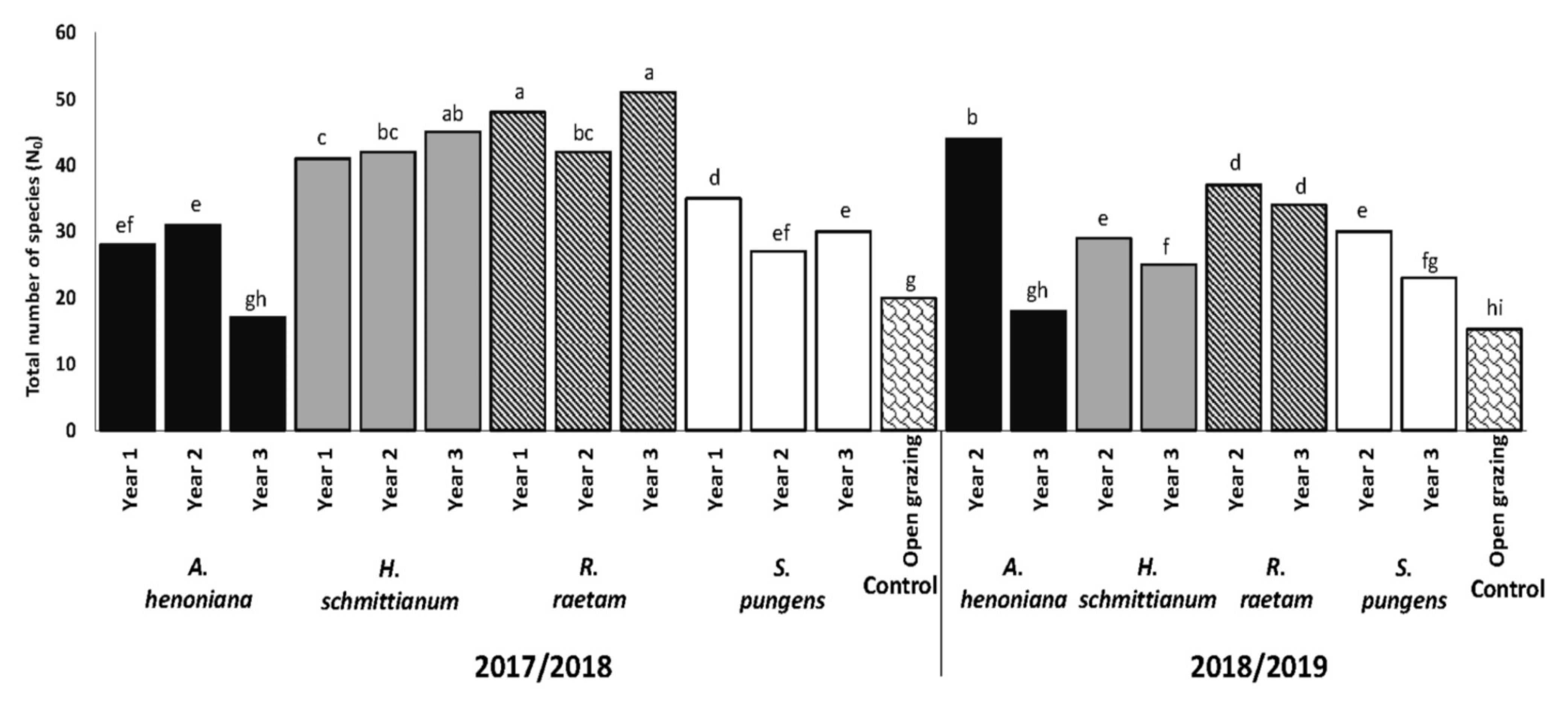
3.1. Total Number of Species (N0)
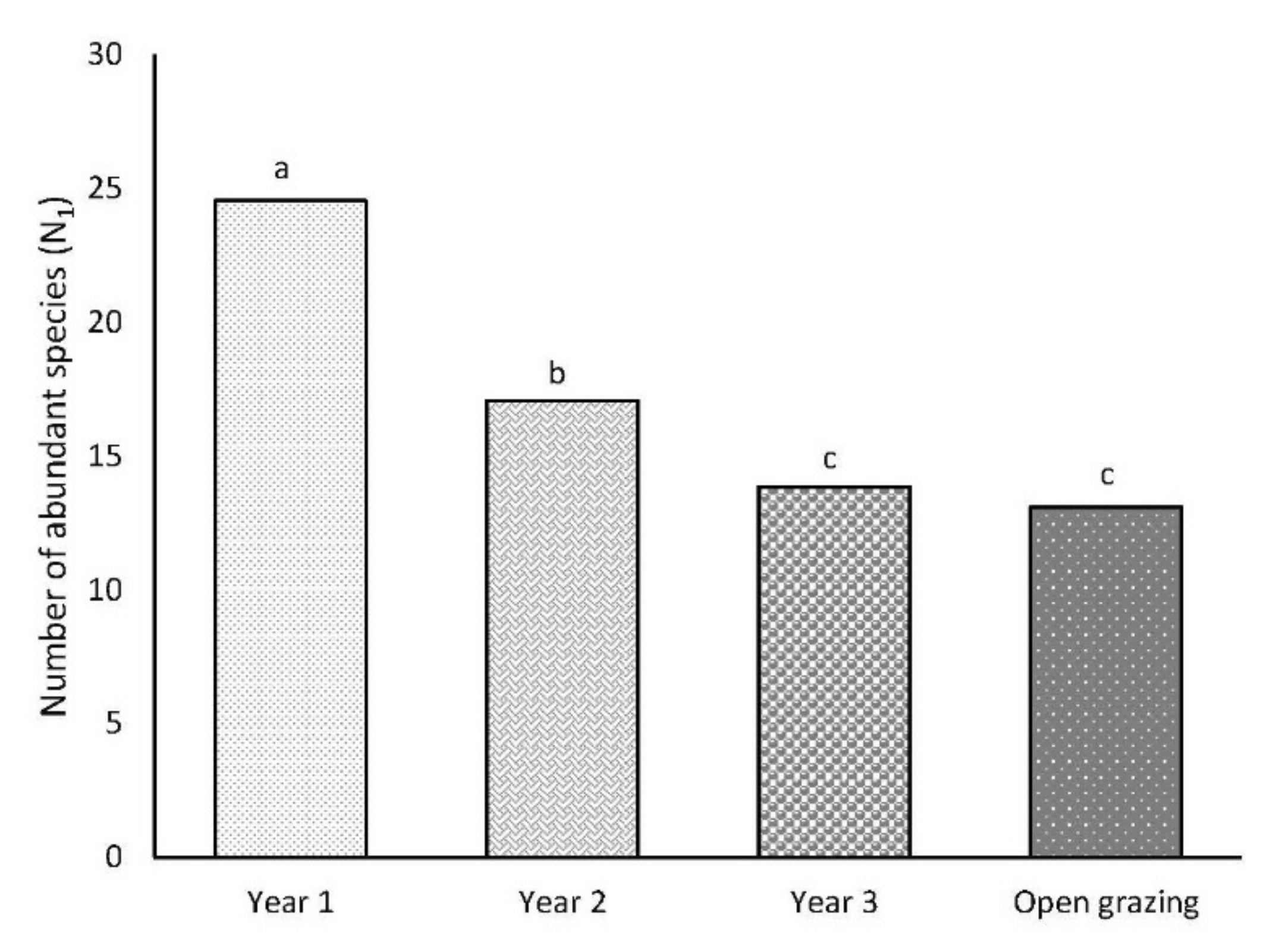
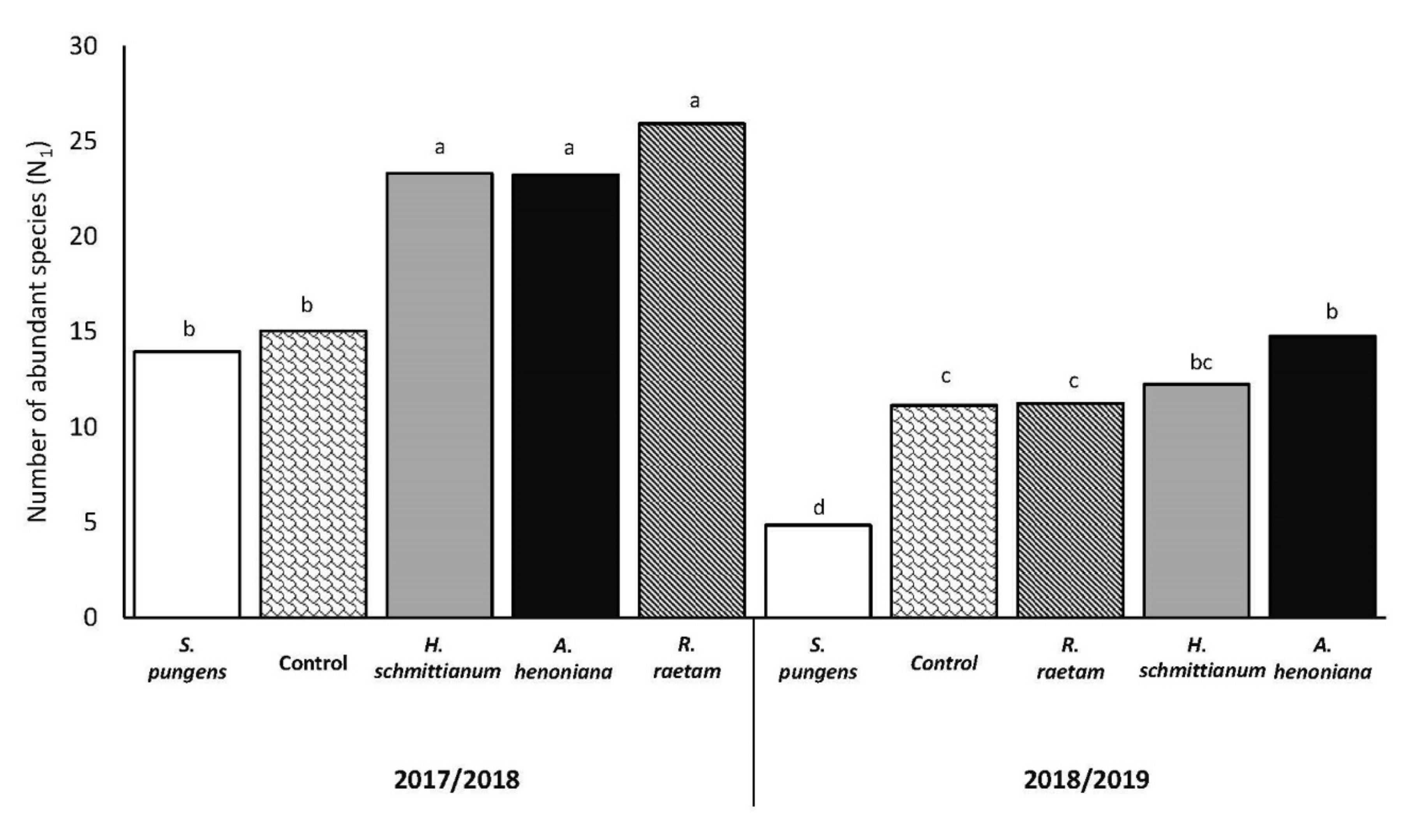
3.2. Number of Abundant Species (N1)
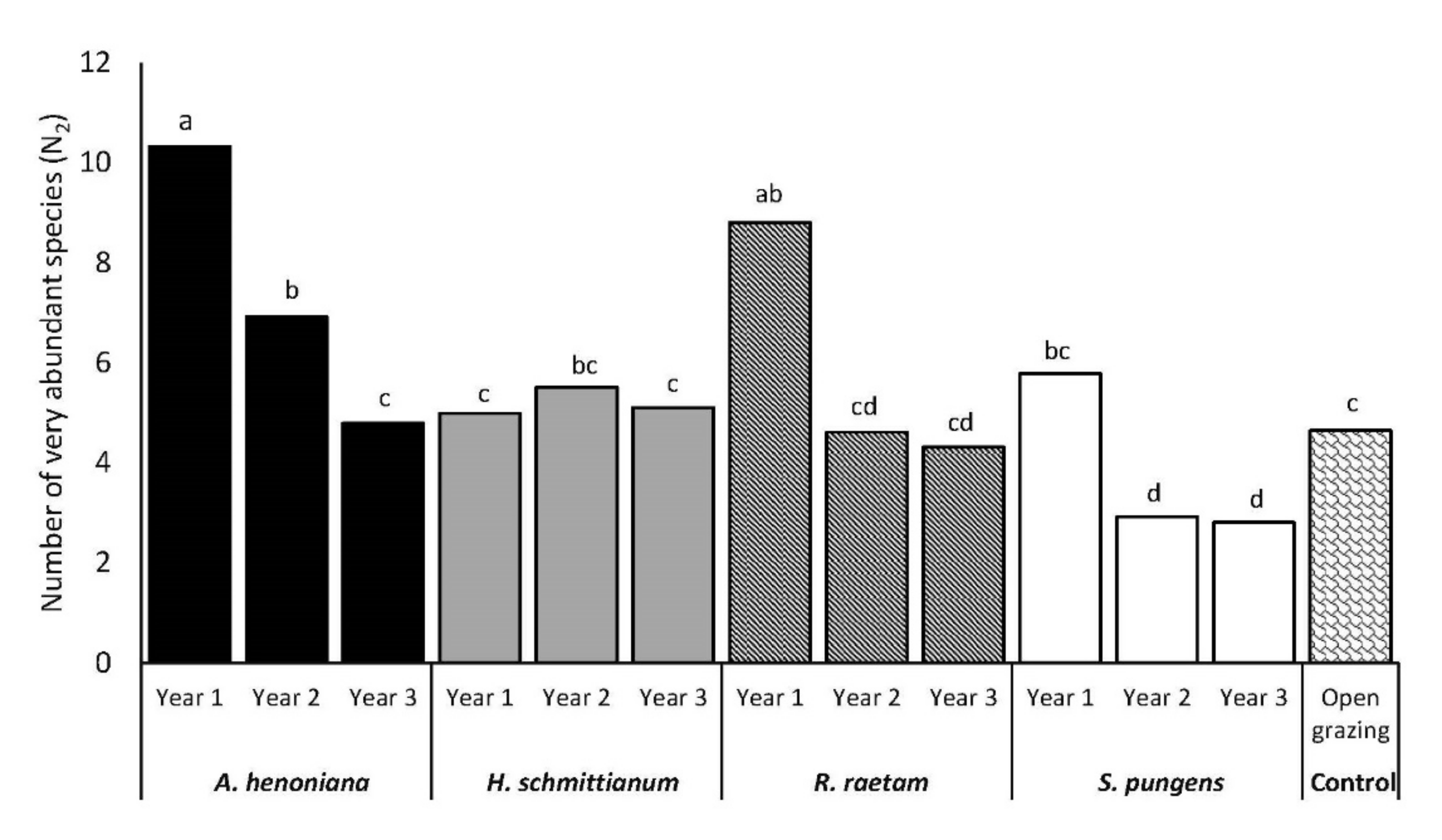
3.3. Number of Very Abundant Species (N2)
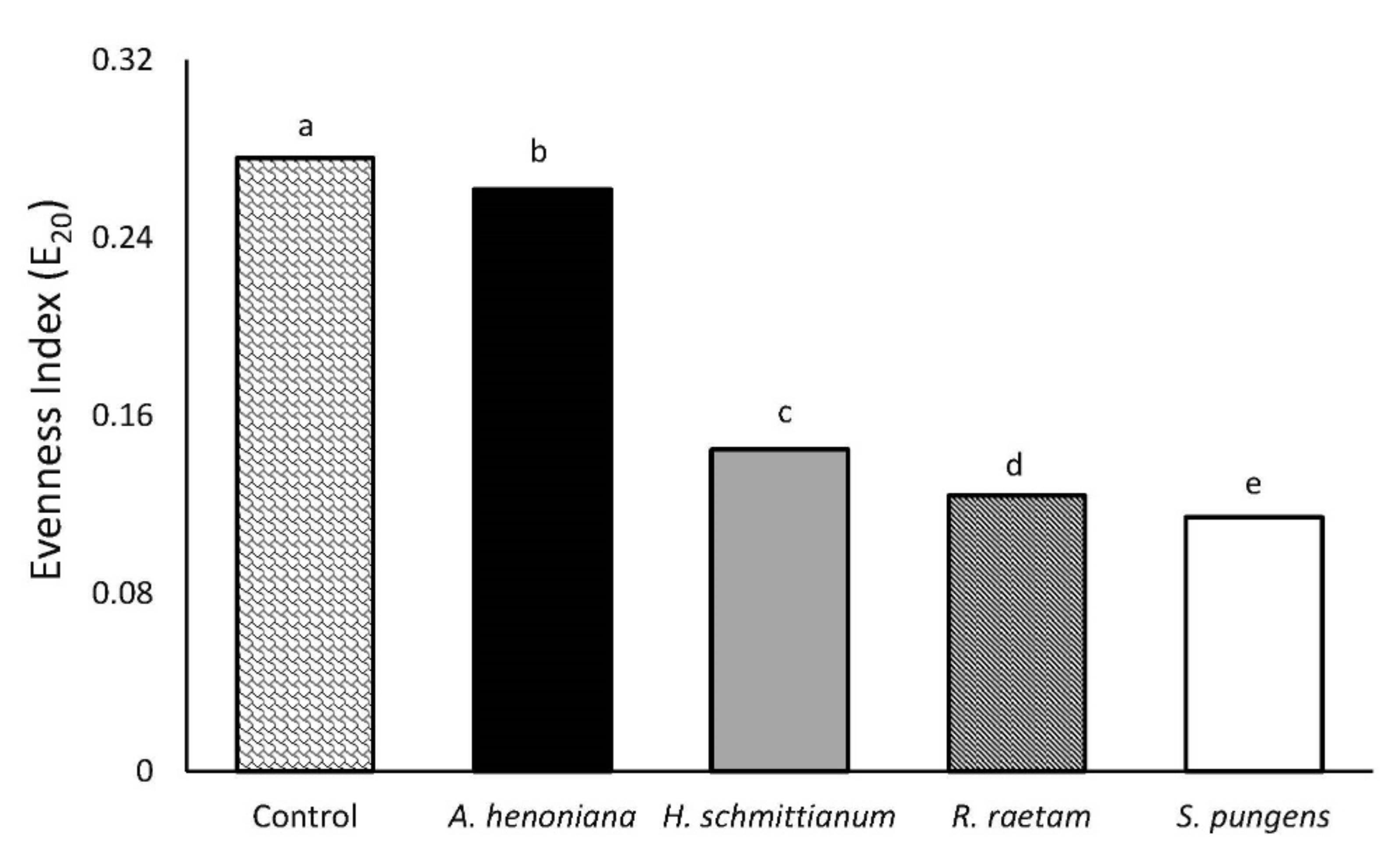
3.4. Evenness Index (E20)
3.5. Correlations Between Hill’s Diversity Indices
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species | A. henoniana | H. schmittianum | S. pungens | R. raetam | Control |
| Aegilops ventricosa | + | - | - | - | - |
| Anacyclus clavatus | +++ | +++ | - | ++ | + |
| Anacyclus monanthos | ++ | ++ | - | + | - |
| Anthyllis henoniana | +++ | + | + | + | ++ |
| Argyrolobium uniflorum | - | ++ | + | + | ++ |
| Arnebia decumbens | - | - | - | + | - |
| Artemisia campestris | - | - | + | - | - |
| Artemisia herba-alba | + | - | - | - | - |
| Asphodelus tenuifolius | - | ++ | ++ | ++ | - |
| Astragalus caprinus | - | - | + | - | - |
| Astragalus corrugatus | ++ | + | ++ | + | - |
| Atractylis cancellata | + | - | - | - | - |
| Atractylis carduus | - | + | + | + | - |
| Atractylis serratuloides | +++ | - | - | - | - |
| Avena sterilis | + | - | - | - | - |
| Bassia muricata | - | - | - | + | - |
| Bromus rubens | + | - | - | - | - |
| Calendula arvensis | + | + | + | + | - |
| Centaurea furfuracea | - | ++ | - | + | - |
| Cleome amblyocarpa | + | + | - | + | - |
| Convolvulus supinus | - | + | - | + | - |
| Cuscuta epithymum | + | - | - | - | - |
| Cutandia dichotoma | - | +++ | +++ | +++ | - |
| Cynara cardunculus | + | - | - | - | - |
| Cynodon dactylon | - | - | - | +++ | - |
| Daucus sahariensis | - | ++ | +++ | ++ | + |
| Deverra denudata | + | - | - | - | - |
| Deverra tortuosa | - | - | - | + | - |
| Diplotaxis harra | + | + | - | - | - |
| Diplotaxis simpelx | - | - | + | - | - |
| Echium humile | ++ | - | ++ | - | - |
| Enarthrocarpus clavatus | + | - | - | - | - |
| Erodium arborescens | ++ | - | - | - | - |
| Erodium crassifolium | + | - | - | - | - |
| Erodium laciniatum | + | ++ | + | + | - |
| Erucaria pinnata | ++ | - | - | - | - |
| Euphorbia retusa | - | + | - | - | - |
| Euphorbia terracina | + | - | - | - | - |
| Fagonia cretica | - | + | - | + | - |
| Fagonia glutinosa | - | ++ | + | + | ++ |
| Filago germanica | - | ++ | - | ++ | +++ |
| Gymnocarpos decander | +++ | + | + | + | ++ |
| Haloxylon schmittianum | - | +++ | + | ++ | ++ |
| Haloxylon scoparium | - | - | - | - | +++ |
| Hedysarum spinosissimum | + | + | ++ | + | - |
| Helianthemum kahiricum | +++ | ++ | - | + | +++ |
| Helianthemum nummularium | + | - | - | - | - |
| Helianthemum sessiliflorum | + | +++ | ++ | + | - |
| Hippocrepis areolata | - | - | ++ | + | - |
| Hordeum marinum | + | - | - | - | - |
| Ifloga spicata | - | +++ | - | ++ | +++ |
| Kickxia aegyptiaca | ++ | ++ | - | + | - |
| Koelpinia linearis | - | + | ++ | + | - |
| Launaea angustifolia | - | - | ++ | - | - |
| Launaea fragilis | +++ | ++ | +++ | ++ | ++ |
| Launaea glomerata | - | + | - | + | - |
| Launaea nudicaulis | - | - | - | + | - |
| Limonium pruinosum | - | - | - | - | +++ |
| Linaria laxiflora | - | + | - | - | - |
| Lotus halophilus | - | +++ | ++ | +++ | - |
| Lygeum spartum | + | - | - | - | - |
| Matthiola longipetala | - | - | ++ | ++ | ++ |
| Medicago minima | - | ++ | +++ | ++ | + |
| Neurada procumbens | - | ++ | - | ++ | - |
| Nonea calycina | + | - | - | - | - |
| Pallenis hierochuntica | - | ++ | - | ++ | - |
| Paronychia arabica | - | - | ++ | ++ | + |
| Picris asplenioides | ++ | - | - | - | - |
| Plantago albicans | ++ | +++ | +++ | ++ | ++ |
| Plantago coronopus | - | + | - | + | - |
| Plantago ovata | - | ++ | ++ | + | + |
| Polygonum equisetiforme | + | - | - | - | - |
| Reaumuria vermiculata | ++ | - | - | - | - |
| Reichardia tingitana | ++ | - | - | - | - |
| Reseda alba | - | - | + | - | - |
| Retama raetam | - | - | ++ | +++ | - |
| Rhanterium suaveolens | - | - | + | - | - |
| Salsola vermiculata | + | - | + | - | - |
| Salvia aegyptiaca | - | + | - | + | - |
| Savignya parviflora | - | ++ | - | - | ++ |
| Schismus barbatus | ++ | + | ++ | +++ | ++ |
| Scorzonera undulata | + | + | - | + | - |
| Senecio glaucus | + | + | - | + | - |
| Silene villosa | - | + | ++ | ++ | - |
| Stipa capensis | ++ | ++ | - | - | - |
| Stipa lagascae | - | - | - | + | - |
| Stipa tenacissima | - | - | - | + | - |
| Stipagrostis pungens | - | - | +++ | - | - |
| Thesium humile | + | + | - | + | - |
References
- Millennium Ecosystem Assessment. Dryland systems. In Ecosystems and Human Well-Being: Current State and Trends; Hassan, R., Scholes, R.J., Ash, N., Eds.; Earthscan: London, UK, 2005; pp. 623–662. [Google Scholar]
- Galvin, K.A.; Reid, R.; Behnke, R.H.; Hobbs, N.T. Fragmentation in Semi-Arid and Arid Landscapes: Consequences for Human and Natural Systems; Springer: Dordrecht, The Netherlands, 2008; pp. 25–44. [Google Scholar]
- Nicholson, S.E. Dryland Climatology; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Louhaichi, M.; Ouled, B.A.; Hassan, S.; Petersen, L.S. Effects of Climate change and grazing practices on shrub communities of West Asian rangelands. Int. J. Clim. Chang. Strateg. Manag. 2019, 11, 660–671. [Google Scholar] [CrossRef]
- Holechek, J.L.; Geli, H.M.E.; Cibils, A.F.; Sawalhah, M.N. Climate Change, Rangelands, and Sustainability of Ranching in the Western United States. Sustainability 2020, 12, 4942. [Google Scholar] [CrossRef]
- Ramawat, K.G. Desert Plants: Biology and Biotechnology; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Gamoun, M.; Ouled Belgacem, A.; Louhaichi, M. Diversity of desert rangelands of Tunisia. Plant Divers. 2018, 40, 217–225. [Google Scholar] [CrossRef]
- Hudson, L.N.; Newbold, T.; Contu, S.; Hill, S.L.; Lysenko, I.; de Palma, A.; Phillips, H.R.; Senior, R.A.; Bennett, D.J.; Booth, H.; et al. The PREDICTS database: A global database of how local terrestrial biodiversity responds to human impacts. Ecol. Evol. 2014, 4, 4701–4735. [Google Scholar] [CrossRef] [PubMed]
- Ouled Belgacem, A.; Louhaichi, M. The vulnerability of native rangeland plant species to global climate change in the West Asia and North African regions. Clim. Chang. 2013, 119, 451–463. [Google Scholar] [CrossRef]
- Gamoun, M. Grazing intensity effects on the vegetation in desert rangelands of Southern Tunisia. J. Arid Land 2014, 6, 324–333. [Google Scholar] [CrossRef]
- Bedunah, D.J.; Angerer, J.P. Rangeland degradation, poverty, and conflict: How can rangeland scientists contribute to effective responses and solutions? Rangel. Ecol. Manag. 2012, 6, 606–612. [Google Scholar] [CrossRef]
- Martínez-Valderrama, J.; Ibáñez, J.; Del Barrio, G.; Alcalá, F.J.; Sanjuán, M.E.; Ruiz, A.; Hirche, A.; Puigdefábregas, J. Doomed to collapse: Why Algerian steppe rangelands are overgrazed and some lessons to help land-use transitions. Sci. Total Environ. 2018, 613–614, 1489–1497. [Google Scholar] [CrossRef]
- Gamoun, M.; Essifi, B.; Dickens, C.; Hanchi, B. Interactive effects of grazing and drought on desert rangelands of Tunisia. Biologija 2016, 62, 105–115. [Google Scholar] [CrossRef]
- Ouled Belgacem, A.; Ben Salem, F.; Gamoun, M.; Chibani, R.; Louhaichi, M. Revival of traditional best practices for rangeland restoration under climate change in the dry areas: A case study from Southern Tunisia. Int. J. Clim. Chang. Strateg. Manag. 2019, 11, 643–659. [Google Scholar] [CrossRef]
- Gamoun, M.; Patton, B.; Hanchi, B. Assessment of vegetation response to grazing management in arid rangelands of southern Tunisia. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2015, 11, 106–113. [Google Scholar] [CrossRef]
- Ash, A.J.; Corfield, J.P.; McIvor, J.G.; Ksiksi, T.S. Grazing management in tropical savannas: Utilization and rest strategies to manipulate rangeland condition. Rangel. Ecol. Manag. 2011, 64, 223–239. [Google Scholar] [CrossRef]
- Tarhouni, M.; Ben Hmida, W.; Neffati, M. Long-term changes in plant life forms as a consequence of grazing exclusion under arid climatic conditions. Land Degrad. Dev. 2015, 28, 1199–1211. [Google Scholar] [CrossRef]
- Tarhouni, M.; Ben Hmida, W.; Ouled Belgacem, A.; Louhaichi, M.; Neffati, M. Is long-term protection useful for the regeneration of disturbed plant communities in dry areas? Afr. J. Ecol. 2017, 55, 509–517. [Google Scholar] [CrossRef]
- Ammar, H.; Bodas, R.; Younes, M.B.; López, S. Goat breeding systems in the South of Tunisia (Tataouine). In Economic, Social and Environmental Sustainability in Sheep and Goat Production Systems; CIHEAM/FAO/CITADGA: Zaragoza, Spain, 2011; pp. 283–288. [Google Scholar]
- OEP. Office de l’Elevage et des Pâturages, Tunisie. 2017. Available online: http://www.oep.nat.tn/index.php/en/open-data/liste-des-donnees/cat_view/12-elevage (accessed on 17 June 2021).
- Dutilly-Diane, C. Pastoral Economics and Marketing in North Africa: A Literature Review. Nomad. People 2007, 11, 69–90. [Google Scholar] [CrossRef]
- Gamoun, M.; Tarhouni, M.; Ouled Belgacem, A.; Hanchi, B.; Neffati, M. Response of different arid rangelands to protection and drought. Arid Land Res. Manag. 2011, 25, 372–378. [Google Scholar] [CrossRef]
- Gamoun, M.; Louhaichi, M. Botanical Composition and Species Diversity of Arid and Desert Rangelands in Tataouine. Tunis. Land 2021, 10, 313. [Google Scholar] [CrossRef]
- Daget, P.; Poissonet, J. Une méthode d’analyse phytologique des prairies. Critères d’application. Ann. Agronom. 1971, 22, 5–41. [Google Scholar]
- Hill, M. Diversity and evenness: A unifying notation and its consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Gillet, F.; Murisier, B.; Buttler, A.; Gallandat, J.D.; Gobat, J.M. Influence of tree cover on the diversity of herbaceous communities in subalpine wooded pastures. Appl. Veg. Sci. 1999, 2, 47–54. [Google Scholar] [CrossRef]
- Gamoun, M.; Ouled Belgacem, A.; Hanchi, B.; Neffati, M.; Gillet, F. Impact of grazing on the floristic diversity of arid rangelands in South Tunisia. Rev. d’Ecol. 2012, 67, 271–282. [Google Scholar]
- Gillet, F.; Mauchamp, L.; Badot, P.M.; Mouly, A. Recent changes in mountain grasslands: A vegetation resampling study. Ecol. Evol. 2016, 6, 2333–2345. [Google Scholar] [CrossRef] [PubMed]
- Birks, H.J.B.; Felde, V.A.; Bjune, A.E.; Grytnes, J.-A.; Seppä, H.; Giesecke, T. Does pollen-assemblage richness reflect floristic richness? A review of recent developments and future challenges. Rev. Palaeobot. Palynol. 2016, 228, 1–25. [Google Scholar] [CrossRef]
- Finsinger, W.; Morales-Molino, C.; Gałka, M.; Valsecchi, V.; Bojovic, S.; Tinner, W. Holocene vegetation and fire dynamics at Crveni Potok, a small mire in the Dinaric Alps (Tara National Park, Serbia). Quat. Sci. Rev. 2017, 167, 63–77. [Google Scholar] [CrossRef]
- Hurlbert, S.H. Pseudoreplication and the design of ecological field experiments. Ecol. Monogr. 1984, 54, 187–211. [Google Scholar] [CrossRef]
- Hiernaux, P. Effects of grazing on plant species composition and spatial distribution in rangelands of the Sahel. Plant Ecol. 1998, 33, 387–399. [Google Scholar]
- Nosberger, J.; Messerli, M.; Carlen, C. Biodiversity in grassland. Ann. Zootech. 1998, 47, 383–393. [Google Scholar] [CrossRef]
- Ondier, J.O.; Okach, D.O.; Onyango, J.C.; Otieno, D.O. Interactive influence of rainfall manipulation and livestock grazing on species diversity of the herbaceous layer community in a humid savannah in Kenya. Plant Divers. 2019, 41, 198–205. [Google Scholar] [CrossRef]
- Wang, C.; Tang, Y. A global meta-analysis of the response of multi-taxa diversity to grazing intensity in grasslands. Environ. Res. Lett. 2019, 14, 114003. [Google Scholar] [CrossRef]
- Salgado-Luarte, C.; Escobedo, V.M.; Stotz, G.C.; Rios, R.S.; Arancio, G.; Gianoli, E. Goat grazing reduces diversity and leads to functional, taxonomic, and phylogenetic homogenization in an arid shrubland. Land Degrad. Dev. 2018, 30, 178–189. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Wang, Z.W.; Han, G.D.; Schellenberg Michael, P.; Wu, Q.; Gu, C. Grazing induced changes in plant diversity is a critical factor controlling grassland productivity in the desert steppe, northern China. Agric. Ecosyst. Environ. 2018, 265, 73–83. [Google Scholar] [CrossRef]
- Ellis, J.E.; Swift, D.M. Stability of African pastoral ecosystems-alternate paradigms and implications for development. J. Range Manag. Arch. 1988, 41, 450–459. [Google Scholar] [CrossRef]
- Škornik, S.; Vidrih, M.; Kaligarič, M. The effect of grazing pressure on species richness, composition and productivity in North Adriatic Karst pastures. Plant Biosyst. 2010, 144, 355–364. [Google Scholar] [CrossRef]
- Baggio, R.; Medeiros, R.B.; Focht, T.; Boavista, L.; Pillar, V.D.; Müller, S.C. Effects of initial disturbances and grazing regime on native grassland invasion by Eragrostis plana in southern Brazil. Perspect. Ecol. Conserv. 2018, 16, 158–165. [Google Scholar] [CrossRef]
- Ould Sidi Mohamed, Y.; Neffati, M.; Henchi, B. Effect of the management mode on plant dynamics in Tunisia: Case of the national park of Sidi Toui and its surrounding. Sécheresse 2002, 13, 195–203. [Google Scholar]
- Zubair, M.; Louhaichi, M.; Rischkowsky, B.; Hassan, S.; Islam, M.; Razzaq, A.; Ibrahim, M.; Moyo, H.P.; Gul, S.; Saleem, A.; et al. The influence of protection from grazing on Cholistan Desert vegetation, Pakistan. Rangelands 2018, 5, 136–145. [Google Scholar] [CrossRef]
- Török, P.; Penksza, K.; Tóth, E.; Kelemen, A.; Sonkoly, J.; Tóthmérész, B. Vegetation type and grazing intensity jointly shape grazing effects on grassland biodiversity. Ecol. Evol. 2018, 8, 10326–10335. [Google Scholar] [CrossRef]
- Wang, S.; Fan, J.; Li, Y.; Huang, L. Effects of grazing exclusion on biomass growth and species diversity among various grassland types of the Tibetan Plateau. Sustainability 2019, 11, 1705. [Google Scholar] [CrossRef]
- Grime, J.P. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am. Nat. 1977, 111, 1169–1194. [Google Scholar] [CrossRef]
- Grime, J.P. Plant Strategies and Vegetation Processes; Wiley: Chichester, UK, 1979. [Google Scholar]
- Huston, M. A general hypothesis of species diversity. Am. Nat. 1979, 113, 81–101. [Google Scholar] [CrossRef]
- Levins, R. Evolution in Changing Environments; Princeton University Press: Princeton, NJ, USA, 1968. [Google Scholar]
- Vandermeer, J.H. The community matrix and the number of species in a community. Am. Nat. 1970, 104, 73–83. [Google Scholar] [CrossRef]
- Gamoun, M. Vegetation change in variable rangeland environments: The relative contribution of drought and soil type in arid rangelands. Ekológia 2013, 32, 148–157. [Google Scholar] [CrossRef][Green Version]
- Adjabi, A.; Sidi, H.; Bounar, R.; Naseri, H.R. Floristic distribution according to the edaphic parameters of a steppe zone, case of study: The nature reserve “El-Mergueb” M’sila, Algeria. Ekológia 2019, 38, 336–352. [Google Scholar] [CrossRef]
- Louhaichi, M. Grazing Management for Improving Soil Stability, Plant Health and Ecosystem Integrity; International Center for Agricultural Research in the Dry Areas (ICARDA): Beirut, Lebanon, 2020; Available online: https://hdl.handle.net/20.500.11766/11918 (accessed on 17 June 2021).
- Tielbörger, K.; Bilton, M.C.; Metz, J.; Kigel, J.; Holzapfel, C.; Lebrija-Trejos, E.; Konsens, I.; Panrag, H.A.; Sternberg, M. Middle-Eastern plant communities tolerate 9 years of drought in a multi-site climate manipulation experiment. Nat. Commun. 2014, 5, 5102. [Google Scholar] [CrossRef]
- Adler, P.B.; Levine, J.M. Contrasting relationships between precipitation and species richness in space and time. Oikos 2007, 116, 221–232. [Google Scholar] [CrossRef]
- Yan, H.; Liang, C.; Li, Z.; Liu, Z.; Miao, B.; He, C.; Sheng, L. Impact of precipitation patterns on biomass and species richness of annuals in a dry steppe. PLoS ONE 2015, 10, e0125300. [Google Scholar] [CrossRef]
- Floret, C.; Pontanier, R. Aridité climatique et aridité édaphique. Bull. Soc. Bot. Franc. Actual. Bot. 1984, 131, 265–276. [Google Scholar]
- Gamoun, M.; Chaieb, M.; Ouled Belgacem, A. Evolution of ecological characteristics along a gradient of soil degradation in the South Tunisian rangelands. Ecol. Mediterr. 2010, 36, 5–16. [Google Scholar] [CrossRef]
- Floret, C.; Pontanier, R. L’aridité en Tunisie Présaharienne: Climat, Sol, Végétation et Aménagement. In Travaux et Documents de l’ORSTOM; Orstom Éditions: Paris, France, 1982. [Google Scholar]
- Guevara, J.C.; Cavagnaro, J.B.; Estevez, O.R.; Le Houérou, H.N.; Stasi, C.R. Productivity and management problems in the arid rangelands of the central Mendoza plains (Argentina). J. Arid Environ. 1997, 35, 575–600. [Google Scholar] [CrossRef]
- Le Houérou, H.N. Estudios y Investigaciones Ecologicas de las Zonas Aridas y Semi-Aridas de Argentina; Instituto Argentino de Investigaciones de Zonas Aridas (IADIZA): Mendoza, Argentina, 1999. [Google Scholar]
- Bendali, F.; Floret, C.; Le Floc’h, E.; Pontanier, R. The dynamics of vegetation and sand mobility in arid regions of Tunisia. J. Arid Environ. 1990, 18, 21–32. [Google Scholar] [CrossRef]
- Brooker, R.W.; Maestre, F.T.; Callaway, R.M.; Lortie, C.L.; Cavieres, L.A.; Kunstler, G.; Liancourt, P.; Tielbörger, K.; Travis, J.M.J.; Anthelme, F.; et al. Facilitation in plant communities: The past, the present, and the future. J. Ecol. 2008, 96, 18–34. [Google Scholar] [CrossRef]
- Hillebrand, H.; Bennet, D.M.; Cadotte, M.W. Consequences of dominance: A review of evenness effects on local and regional ecosystem processes. Ecology 2008, 89, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Metzger, K.L.; Coughenour, M.B.; Reich, R.M.; Boone, R.B. Effects of seasonal grazing on plant species diversity and vegetation structure in a semi-arid ecosystem. J. Arid Environ. 2005, 61, 147–160. [Google Scholar] [CrossRef]
- Aguiar, M.R.; Sala, O.E. Patch structure, dynamics and implications for the functioning of arid ecosystems. Trends Ecol. Evol. 1999, 14, 273–277. [Google Scholar] [CrossRef]
- Brown, G.W. Desert Biology–Special Topics on the Physical and Biological Aspects of Arid Regions, 2nd ed.; Academic Press: New York, NY, USA, 1974. [Google Scholar]
- Noy-Meir, I.; Gutman, M.; Kaplan, Y. Responses of Mediterranean grassland plants to grazing and protection. J. Ecol. 1989, 77, 290–310. [Google Scholar] [CrossRef]
- Karami, P.; Bandak, I.; Karaji, M.G. Comparing the effects of continuous grazing and long term exclosure on floristic composition and plant diversity in rangeland ecosystems of Saral, Iran. Int. J. Environ. Sci. Technol. 2019, 16, 7769–7776. [Google Scholar] [CrossRef]

| Community | Area (ha) | 2017/2018 | Code | 2018/2019 | Code |
|---|---|---|---|---|---|
| A. henoniana | 120 | 1-year rest | 1 | 2 years rest | 14 |
| 170 | 2 years rest | 2 | 3 years rest | 15 | |
| 20 | 3 years rest | 3 | Open to grazing | ||
| H. schmittianum | 30 | 1-year rest | 4 | 2 years rest | 16 |
| 170 | 2years rest | 5 | 3 years rest | 17 | |
| 20 | 3 years rest | 6 | Open to grazing | ||
| S. pungens | 100 | 1-year rest | 7 | 2 years rest | 18 |
| 170 | 2 years rest | 8 | 3 years rest | 19 | |
| 20 | 3 years rest | 9 | Open to grazing | ||
| R. raetam | 30 | 1-year rest | 10 | 2 years rest | 20 |
| 140 | 2 years rest | 11 | 3 years rest | 21 | |
| 20 | 3 years rest | 12 | Open to grazing | ||
| Control | Continuously grazed | 13 | Continuously grazed | 22 |
| Source | df Effect | Total Number of Species (N0) | Number of Abundant Species (N1) | Number of Very Abundant Species (N2) | Evenness Index (E20) | ||||
|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | ||
| Plant community (A) | 3 | 47.47 | <0.0001 | 6.28 | 0.0009 | 9.65 | <0.0001 | 13.58 | <0.0001 |
| Resting durations (B) | 2 | 13.95 | <0.0001 | 3.18 | 0.0488 | 6.26 | 0.0034 | 1.68 | 0.1946 |
| Year (C) | 1 | 42.58 | <0.0001 | 25.56 | <0.0001 | 16.45 | 0.0001 | 1.49 | 0.227 |
| A × B | 6 | 14.68 | <0.0001 | 3.5 | 0.4641 | 2.65 | 0.024 | 1.12 | 0.364 |
| A × C | 3 | 27.01 | <0.0001 | 0.87 | 0.0049 | 0.48 | 0.695 | 0.65 | 0.5867 |
| B × C | 1 | 26.63 | <0.0001 | 0.18 | 0.6755 | 0.6 | 0.4427 | 1.3 | 0.2589 |
| A × B × C | 3 | 0.35 | <0.0001 | 1.81 | 0.1546 | 1.81 | 0.1554 | 1.11 | 0.3532 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Louhaichi, M.; Gamoun, M.; Ben Salem, F.; Ouled Belgacem, A. Rangeland Biodiversity and Climate Variability: Supporting the Need for Flexible Grazing Management. Sustainability 2021, 13, 7124. https://doi.org/10.3390/su13137124
Louhaichi M, Gamoun M, Ben Salem F, Ouled Belgacem A. Rangeland Biodiversity and Climate Variability: Supporting the Need for Flexible Grazing Management. Sustainability. 2021; 13(13):7124. https://doi.org/10.3390/su13137124
Chicago/Turabian StyleLouhaichi, Mounir, Mouldi Gamoun, Farah Ben Salem, and Azaiez Ouled Belgacem. 2021. "Rangeland Biodiversity and Climate Variability: Supporting the Need for Flexible Grazing Management" Sustainability 13, no. 13: 7124. https://doi.org/10.3390/su13137124
APA StyleLouhaichi, M., Gamoun, M., Ben Salem, F., & Ouled Belgacem, A. (2021). Rangeland Biodiversity and Climate Variability: Supporting the Need for Flexible Grazing Management. Sustainability, 13(13), 7124. https://doi.org/10.3390/su13137124








