Thermal Performance Measurement Procedure and Its Accuracy for Shape-Stabilized Phase-Change Material and Microcapsule Phase-Change Material Combined with Building Materials
Abstract
:Highlights
- Paraffin-based PCM combined with building materials were measured for thermal performance in a thermochamber.
- The samples were a sheet of shape-stabilized phase-change material (SSPCM) and a microencapsulated PCM-impregnated gypsum board.
- The effect of thermochamber temperature change and PCM capacity on thermal performance was confirmed using a dynamic method.
- The proposed approach can be used to measure the specific heat and enthalpy of various types of PCMs and building materials.
1. Introduction
2. Overview of the Experiment
2.1. PCM Sample
2.2. Measurement Method
3. Specific Heat Measurement of PCM
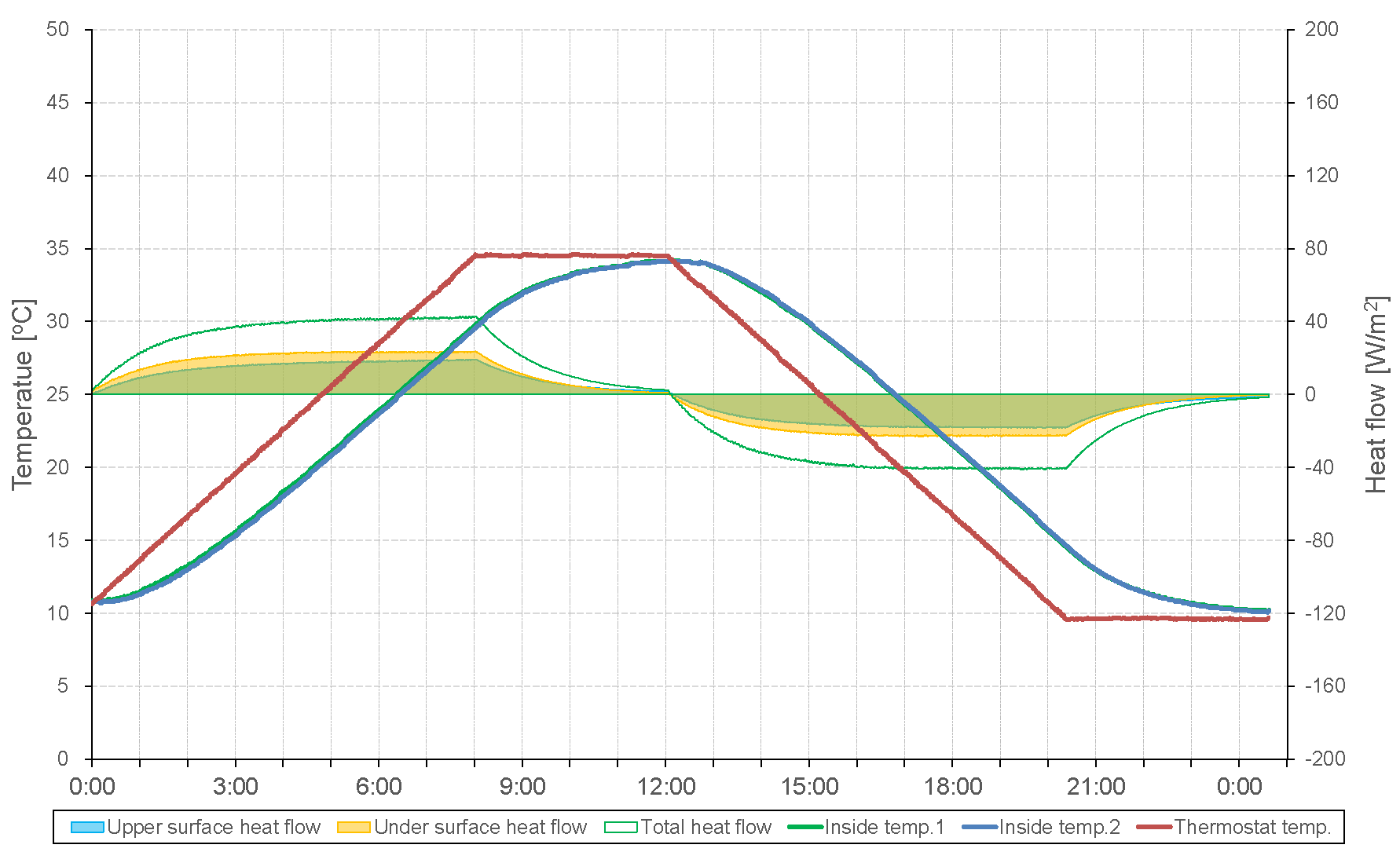
3.1. Adjustment of Measured Value of Thermochamber
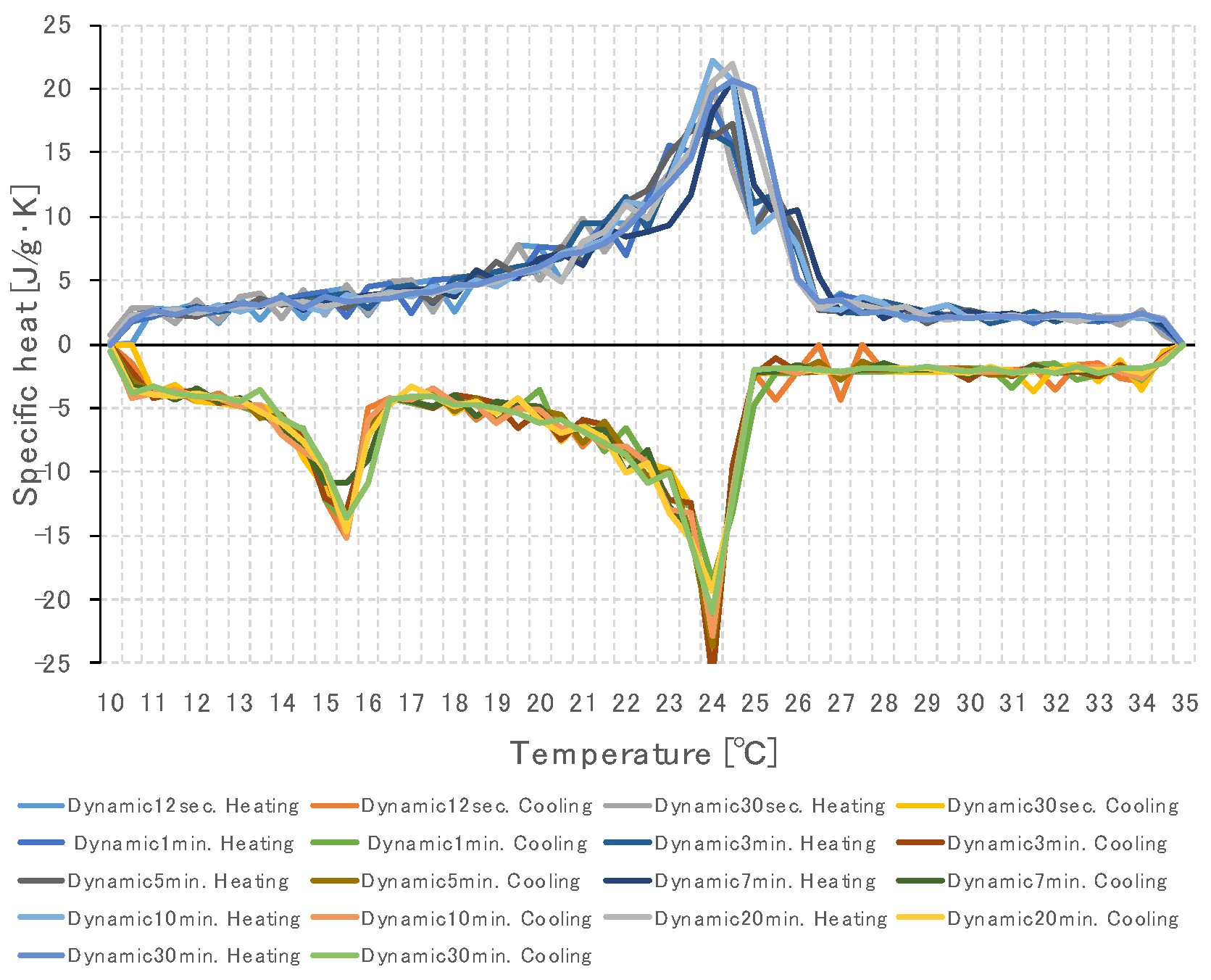
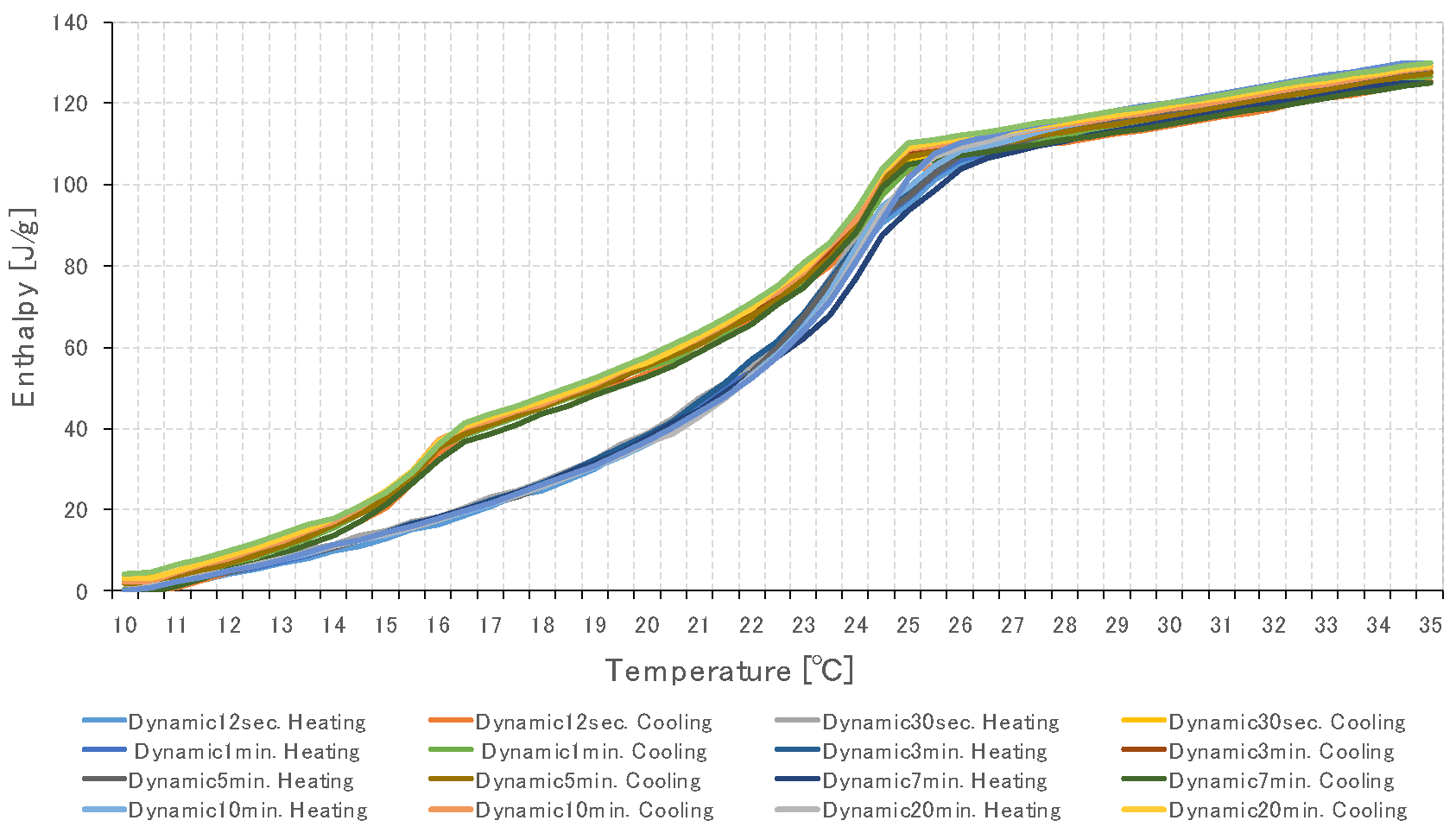
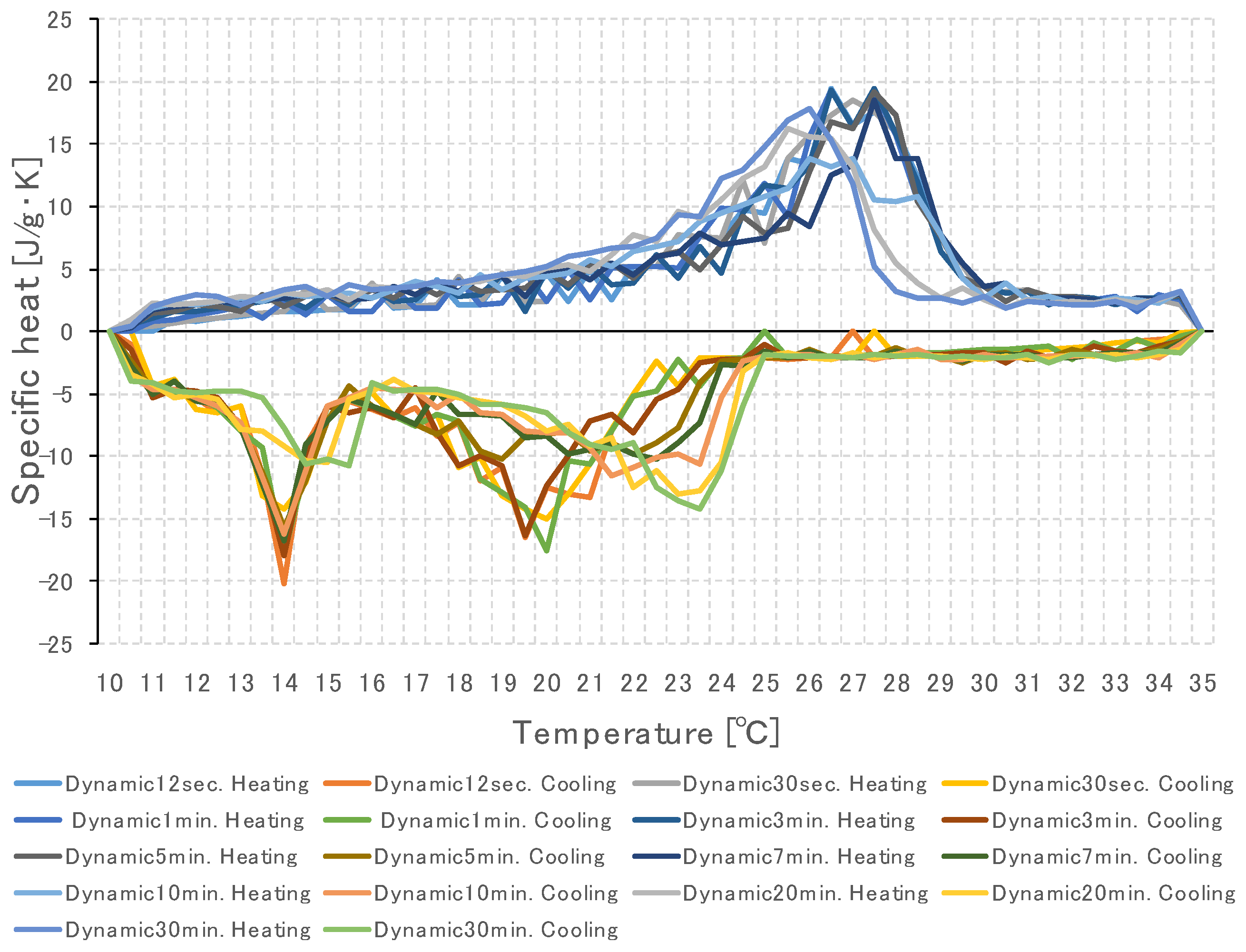
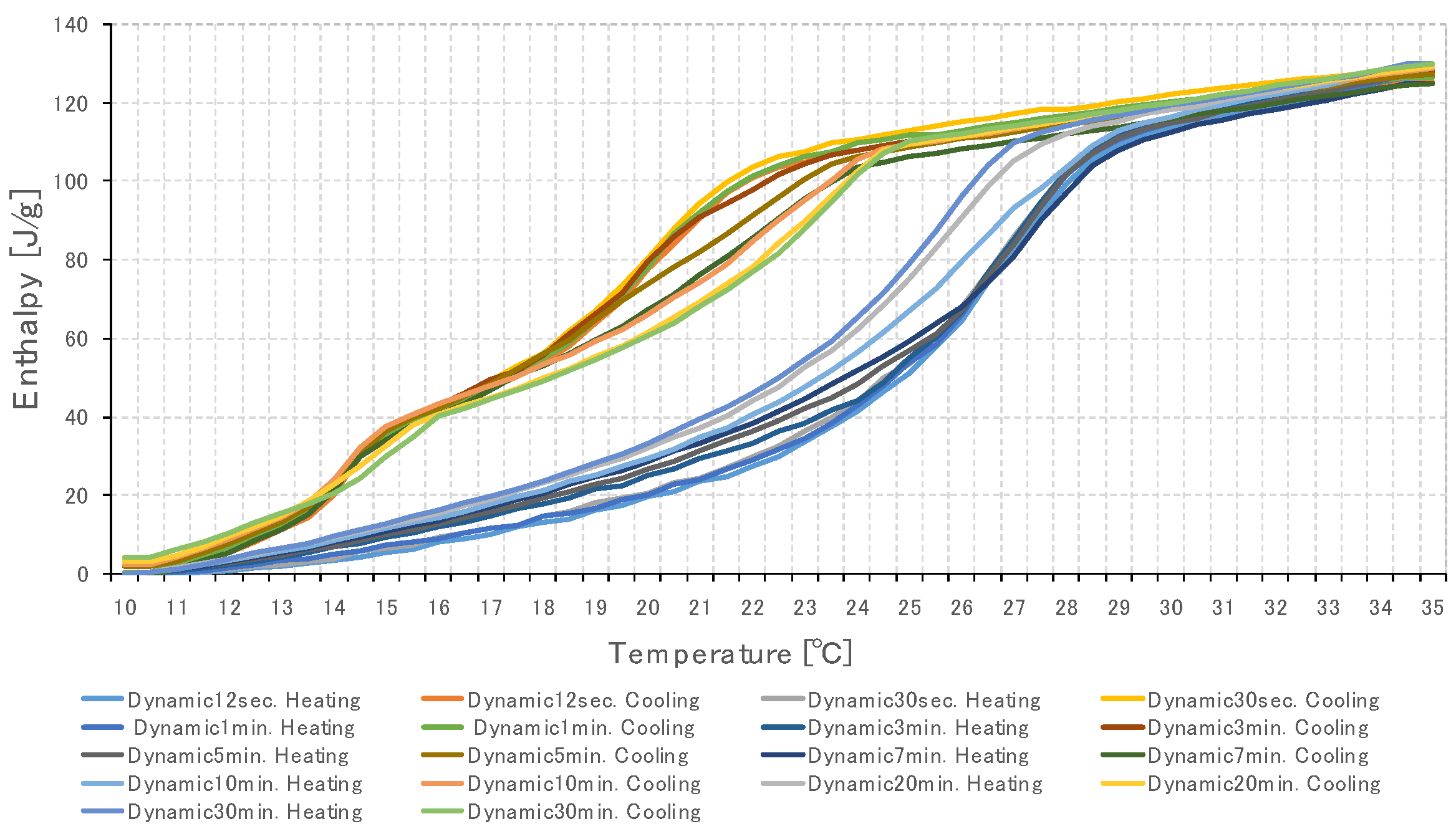
3.2. Specific Heat Measurement of PCM Using Dynamic Method
3.2.1. Temperature Change Setting Time of Thermochamber
3.2.2. Methods of Measuring Internal Temperature of PCM
3.2.2.1. Use of Internal Temperature of PCM
3.2.2.2. Use of Average Temperature of Upper and Lower Surfaces of PCM
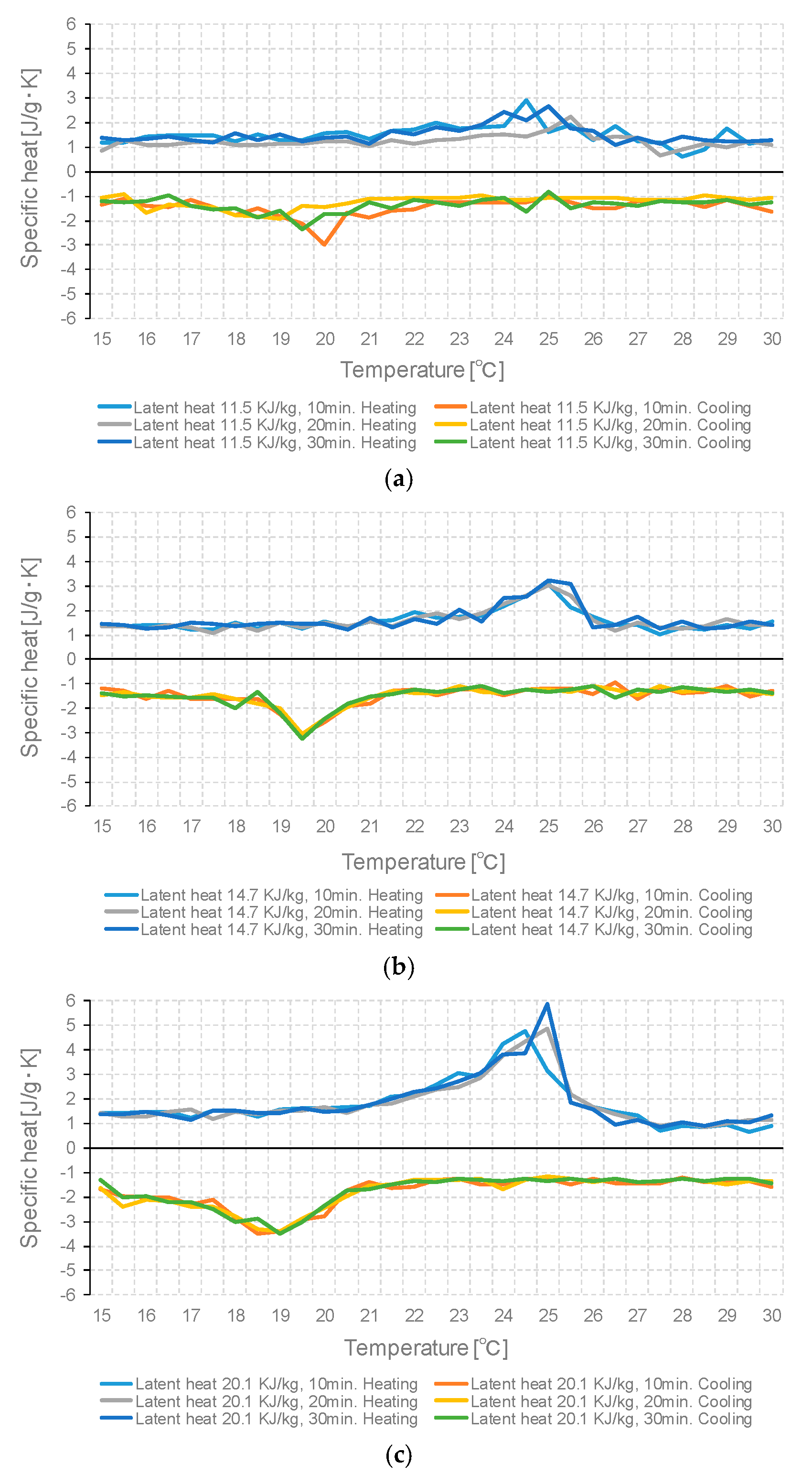
3.3. Specific Heat Measurement According to PCM Capacity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arce, P.; Medrano, M.; Gil, A.; Oró, E.; Cabeza, L.F. Overview of thermal energy storage (TES) potential energy savings and climate change mitigation in Spain and Europe. Appl. Energy 2011, 88, 2764–2774. [Google Scholar] [CrossRef]
- Heier, J.; Bales, C.; Martin, V. Combining thermal energy storage with buildings—A review. Renew. Sustain. Energy Rev. 2015, 42, 1305–1325. [Google Scholar] [CrossRef]
- John, A.; Duffie, W.A.B. Solar Engineering and Thermal Processes; Wiley: Wisconsin–Madison, Madison, WI, USA, 1980. [Google Scholar]
- Ibrahim Dincer, M.A.R. Thermal Energy Storage Systems and Applications; Wiley: Oshawa, ON, Canada, 2011. [Google Scholar]
- Chang, S.J.; Wi, S.; Jeong, S.G.; Kim, S. Thermal performance evaluation of macro-packed phase change materials (PCMs) using heat transfer analysis device. Energy Build. 2016, 117, 120–127. [Google Scholar] [CrossRef]
- Jeon, J.; Lee, J.H.; Seo, J.; Jeong, S.G.; Kim, S. Application of PCM thermal energy storage system to reduce building energy consumption. J. Therm. Anal. Calorim. 2013, 111, 279–288. [Google Scholar] [CrossRef]
- Kim, H.B.; Mae, M.; Choi, Y. Application of shape-stabilized phase-change material sheets as thermal energy storage to reduce heating load in Japanese climate. Build. Environ. 2017, 125, 1–14. [Google Scholar] [CrossRef]
- Kim, H.B.; Mae, M.; Choi, Y.; Kiyota, T. Experimental analysis of thermal performance in buildings with shape-stabilized phase change materials. Energy Build. 2017, 152, 524–533. [Google Scholar] [CrossRef]
- Franquet, E.; Gibout, S.; Bédécarrats, J.P.; Haillot, D.; Dumas, J.P. Inverse method for the identification of the enthalpy of phase change materials from calorimetry experiments. Thermochim. Acta 2012, 546, 61–80. [Google Scholar] [CrossRef]
- Jin, X.; Xu, X.; Zhang, X.; Yin, Y. Determination of the PCM melting temperature range using DSC. Thermochim. Acta 2014, 595, 17–21. [Google Scholar] [CrossRef]
- Zhou, G.; Zhang, Y.; Wang, X.; Lin, K.; Xiao, W. An assessment of mixed type PCM-gypsum and shape-stabilized PCM plates in a building for passive solar heating. Sol. Energy 2007, 81, 1351–1360. [Google Scholar] [CrossRef]
- Li, L.; Yu, H.; Wang, X.; Zheng, S. Thermal analysis of melting and freezing processes of phase change materials (PCMs) based on dynamic DSC test. Energy Build. 2016, 130, 388–396. [Google Scholar] [CrossRef]
- Zhu, N.; Ma, Z.; Wang, S. Dynamic characteristics and energy performance of buildings using phase change materials: A review. Energy Convers. Manag. 2009, 50, 3169–3181. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, X.; Xu, X.; Zhao, Y.; Zhang, S. The research progress on phase change hysteresis affecting the thermal characteristics of PCMs: A review. J. Mol. Liq. 2020, 317, 113760. [Google Scholar] [CrossRef]
- Yinping, Z.; Yi, J. A simple method, the T-history method, of determining the heat of fusion, specific heat and thermal conductivity of phase-change materials. Meas. Sci. Technol. 1999, 10, 201–205. [Google Scholar] [CrossRef]
- Kousksou, T.; Jamil, A.; Zeraouli, Y. Enthalpy and apparent specific heat capacity of the binary solution during the melting process: DSC modeling. Thermochim. Acta 2012, 541, 31–41. [Google Scholar] [CrossRef]
- Rady, M. Study of phase changing characteristics of granular composites using differential scanning calorimetry. Energy Convers. Manag. 2009, 50, 1210–1217. [Google Scholar] [CrossRef]
- Dumas, J.P.; Gibout, S.; Zalewski, L.; Johannes, K.; Franquet, E.; Lassue, S.; Bédécarrats, J.P.; Tittelein, P.; Kuznik, F. Interpretation of calorimetry experiments to characterise phase change materials. Int. J. Therm. Sci. 2014, 78, 48–55. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Xu, X.; Lu, M. Derivation of thermal properties of phase change materials based on T-history method. J. Energy Storage 2020, 27, 101062. [Google Scholar] [CrossRef]
- Silva, T.; Vicente, R.; Soares, N.; Ferreira, V. Experimental testing and numerical modelling of masonry wall solution with PCM incorporation: A passive construction solution. Energy Build. 2012, 49, 235–245. [Google Scholar] [CrossRef]
- Lai, C.M.; Chen, R.H.; Lin, C.Y. Heat transfer and thermal storage behaviour of gypsum boards incorporating micro-encapsulated PCM. Energy Build. 2010, 42, 1259–1266. [Google Scholar] [CrossRef]
- Castellón, C.; Günther, E.; Mehling, H.; Hiebler, S.; Cabeza, L.F. Determination of the enthalpy of PCM as a function of temperature using a heat-flux DSC—A study of different measurement procedures and their accuracy. Int. J. Energy Res. 2008, 33, 1258–1265. [Google Scholar] [CrossRef]
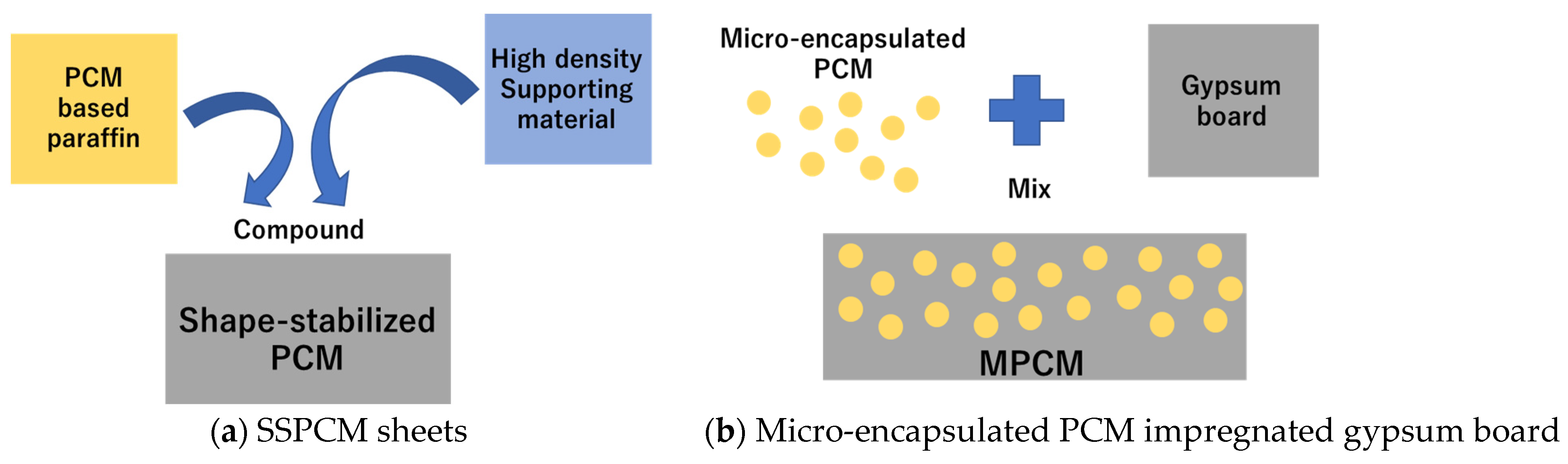


| PCM Type | Number | PCM Sample |
|---|---|---|
| SSPCM sheet | 1 | Phase change temp. 19–25 °C, latent heat (62.8 KJ/kg) |
| Microencapsulated PCM impregnated gypsum board (MPCM) | 2 | Phase change temp. 25 °C, latent heat (0 KJ/kg) |
| 3 | Phase change temp. 25 °C, latent heat (11.5 KJ/kg) | |
| 4 | Phase change temp. 25 °C, latent heat (14.7 KJ/kg) | |
| 5 | Phase change temp. 25 °C, latent heat (20.1 KJ/kg) |
| Dynamic Method: Temp. Change per 1 °C | Heat Absorption [KJ] | Heat Release [KJ] |
|---|---|---|
| 19–25 °C SSPCM Sheet | ||
| 30 min | 1126.2 | −1090.2 |
| 20 min | 1120.9 | −1094.6 |
| 10 min | 1115.2 | −1095.1 |
| 7 min | 1083.4 | −1086.6 |
| 5 min | 1102.2 | −1086.9 |
| 3 min | 1108.0 | −1086.7 |
| 1 min | 1097.2 | −1092.7 |
| 30 s | 1109.8 | −1076.9 |
| 12 s | 1086.5 | −1086.0 |
| Dynamic Method: Temp. Change per 1 °C | Heat Absorption [KJ] | Heat Release [KJ] |
|---|---|---|
| Sample type | 25 °C, 0 KJ/kg | |
| 30 min | 566.9 | −528.8 |
| 20 min | 560.6 | −533.2 |
| 10 min | 556.2 | −538.2 |
| Sample type | 25 °C, 11.5 KJ/kg | |
| 30 min | 777.7 | −716.5 |
| 20 min | 764.4 | −725.4 |
| 10 min | 763.3 | −735.5 |
| Sample type | 25 °C, 14.7 KJ/kg | |
| 30 min | 815.1 | −751.9 |
| 20 min | 802.7 | −761.4 |
| 10 min | 799.7 | −774.5 |
| Sample type | 25 °C, 20.1 KJ/kg | |
| 30 min | 884.3 | −833.1 |
| 20 min | 869.7 | −838.9 |
| 10 min | 868.4 | −847.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.B.; Mae, M.; Choi, Y. Thermal Performance Measurement Procedure and Its Accuracy for Shape-Stabilized Phase-Change Material and Microcapsule Phase-Change Material Combined with Building Materials. Sustainability 2021, 13, 6671. https://doi.org/10.3390/su13126671
Kim HB, Mae M, Choi Y. Thermal Performance Measurement Procedure and Its Accuracy for Shape-Stabilized Phase-Change Material and Microcapsule Phase-Change Material Combined with Building Materials. Sustainability. 2021; 13(12):6671. https://doi.org/10.3390/su13126671
Chicago/Turabian StyleKim, Hyun Bae, Masayuki Mae, and Youngjin Choi. 2021. "Thermal Performance Measurement Procedure and Its Accuracy for Shape-Stabilized Phase-Change Material and Microcapsule Phase-Change Material Combined with Building Materials" Sustainability 13, no. 12: 6671. https://doi.org/10.3390/su13126671
APA StyleKim, H. B., Mae, M., & Choi, Y. (2021). Thermal Performance Measurement Procedure and Its Accuracy for Shape-Stabilized Phase-Change Material and Microcapsule Phase-Change Material Combined with Building Materials. Sustainability, 13(12), 6671. https://doi.org/10.3390/su13126671






