Peat as a Raw Material for Plant Nutrients and Humic Substances
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation for Analysis
2.2. Methods of Analysis
2.3. Data Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- EU Nitrogen Expert Panel (EUNEP). Available online: https://www.fertilizerseurope.com/initiatives/eu-nitrogen-expert-panel-eu-nep/ (accessed on 16 March 2020).
- Pettit, R.E. Organic Matter, Humus, Humate, Humic Acid, Fulvic Acid and Humin: Their Importance in Soil Fertility and Plant Health. Available online: https://humates.com/wp-content/uploads/2020/04/ORGANICMATTERPettit.pdf (accessed on 10 April 2020).
- Melo, B.A.; Motta, F.L.; Santana, M.H. Humic acids: Structural properties and multiple functionalities for novel technological developments. Mater. Sci. Eng C 2016, 62, 967–974. [Google Scholar] [CrossRef]
- Asing, J.; Wong, N.C.; Lau, S. Optimization of extraction method and characterization of humic acid derived from coals and composts. J. Trop. Agric. Food Sci. 2009, 37, 211–223. [Google Scholar]
- Fuentes, M.; Baigorri, R.; González-Gaitano, G.; García-Mina, J.M. New methodology to assess the quantity and quality of humic substances in organic materials and commercial products for agriculture. J Soils Sediments 2018, 18, 1389–1399. [Google Scholar] [CrossRef]
- Senesi, N.; Miano, T.M.; Brunetti, G. Humic-like substances in organic amendments and effects on native soil humic substances. In Humic Substances in Terrestrial Ecosystems; Piccolo, A., Ed.; Elsevier Science: Amsterdam, The Netherlands, 1996; pp. 531–593. [Google Scholar]
- Peña-Méndez, E.M.; Havel, J.; Patočka, J. Humic substances-compounds of still unknown structure: Applications in agriculture, industry, environment, and biomedicine. J. Appl. Biomed. 2005, 3, 13–24. [Google Scholar] [CrossRef]
- Sariyildiz, T. Effects of leonardite and mineral fertilizer applications on plant growth and soil quality of garlic (Allium sativum L.). Turk. J. Agric. Food Sci. Technol. 2020, 8, 1763–1772. [Google Scholar] [CrossRef]
- Mosa, A.A.; Taha, A.; Elsaeid, M. Agro-environmental applications of humic substances: A critical review. Egypt. J. Soil Sci. 2020, 60, 211–229. [Google Scholar] [CrossRef]
- Canıeren, Ö.; Karaguzel, C.; Aydın, A. Effect of physical pre-enrichment on humic substance recovery from leonardite. Physicochem. Probl. Miner. Process. 2017, 53, 502–514. [Google Scholar] [CrossRef]
- Akimbekov, N.; Qiao, X.; Digel, I.; Abdieva, G.; Ualieva, P.; Zhubanova, A. The effect of leonardite-derived amendments on soil microbiome structure and potato yield. Agriculture 2020, 10, 147. [Google Scholar] [CrossRef]
- Huat, B.B.; Kazemian, S.; Prasad, A.; Barghchi, M.A. A State of art review of peat: Geotechnical engineering perspective. Int. J. Phys. Sci. 2011, 6, 1974–1981. [Google Scholar] [CrossRef]
- Agafonova, L.; Alsina, I.; Sokolov, G.; Kovrik, S.; Bambalov, N.; Apse, J.; Rak, M. New kinds of sapropel and peat based fertilizers. In Proceedings of the 10th International Scientific and Practical Conference, Rezekne, Latvia, 18–20 June 2015; Volume 2, pp. 20–26. [Google Scholar] [CrossRef]
- Orrua, M.; Übnerc, M.; Orrud, H. Chemical properties of peat in three peatlands with balneological potential in Estonia. Estonian J. Earth Sci. 2011, 60, 43–49. [Google Scholar] [CrossRef]
- Saito, B.; Seckler, M.M. Alkaline extraction of humic substances from peat applied to organic-mineral fertilizer production. Braz. J. Chem. Eng. 2014, 31, 675–682. [Google Scholar] [CrossRef]
- Peat Industry. Available online: https://peat.lt/durpiu-pramone (accessed on 10 April 2018).
- EUR-Lex. Regulation (EC) No 2003/2003 of the European Parliament and of the Council of 13 October 2003 Relating to Fertilisers. 2003. Available online: https://eur-lex.europa.eu/legal-content/GA/TXT/?uri=CELEX:32003R2003 (accessed on 16 March 2021).
- Lithuanian Standard. Peats and Peat Products for Horticulture and Horticultural and Agricultural Engineering—Test Methods, Properties, Specifications; LST 1957:2006/P:2010; Lithuanian Standard: Vilnius, Lithuania, 2010. [Google Scholar]
- Teirumnieka, E.; Kļaviņš, E.; Teirumnieks, M. Major and trace elements in peat from bogs of East Latvia. In Mires Peat; Klavins, M., Ed.; University of Latvia Press: Riga, Latvia, 2010; pp. 115–123. [Google Scholar]
- Chambers, F.; Beilman, D.; Yu, Z. Methods for determining peat humification and for quantifying peat bulk density, organic matter and carbon content for palaeostudies of climate and peatland carbon dynamics. Mires Peat 2010, 7, 1–10. [Google Scholar]
- Lithuanian Standard. Soil Improvers and Growing Media—Sample Preparation for Chemical and Physical Tests, Determination of Dry Matter Content, Moisture Content and Laboratory Compacted Bulk Density; LST EN 13040: 2008; Lithuanian Standard: Vilnius, Lithuania, 2008. [Google Scholar]
- Lithuanian Standard. Soil Improvers and Growing Media—Determination of pH; LST EN 13037: 2012; Lithuanian Standard: Vilnius, Lithuania, 2012. [Google Scholar]
- Lithuanian Standard. Fertilizers—Determination of Nitric and Ammoniacal Nitrogen According to Devarda; LST EN 15476:2009; Lithuanian Standard: Vilnius, Lithuania, 2009. [Google Scholar]
- Šlepetienė, A.; Šlepetys, J.; Liaudanskienė, I. Standard and modified methods for soil organic carbon determination in an agricultural soils. Agron. Res. 2008, 6, 543–554. [Google Scholar]
- SAS Institute Inc. SAS/STAT® 9.3 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2011. [Google Scholar]
- Neina, D. The role of soil pH in plant nutrition and soil remediation. Appl. Environ. Soil Sci. 2019, 2019, 5794869. [Google Scholar] [CrossRef]
- Inisheva, L.I. Agrochemical nature of peat. Chem. Plant Raw Mater. 1998, 4, 17–22. (In Russian) [Google Scholar]
- Porokhina, E.V.; Sergeeva, M.A.; Golubina, O.A. Chemical composition and biological activity of peat deposits of oligotrophic bog. Samara Sci. Bull. 2018, 7, 82–87. (In Russian) [Google Scholar] [CrossRef]
- Amalevičiūtė, K.; Šlepetienė, A.; Liaudanskienė, I.; Šlepetys, J. Chemical composition of peat bog soil and its influencing factors. J. Lithuan. Acad. Sci. 2014, 21, 1–8. [Google Scholar]
- Růžičková, J.; Kucbel, M.; Raclavská, H.; Švédová, B.; Raclavský, K.; Šafář, M.; Kantor, P. Chemical and mineralogical composition of soot and ash from the combustion of peat briquettes in household boilers. Energies 2019, 12, 3784. [Google Scholar] [CrossRef]
- Vincevica-Gaile, Z.; Stankevica, K. Impact of micro- and macroelement content on potential use of freshwater sediments (gyttja) derived from lakes of eastern Latvia. Environ. Geochem. Health 2018, 40, 1725–1738. [Google Scholar] [CrossRef]
- Romão, L.P.C.; Lead, J.R.; Rocha, J.C.; De Oliveira, L.C.; Rosa, A.H.; Mendonça, A.G.R.; Ribeiro, A.D.S. Structure and properties of brazilian peat: Analysis by spectroscopy and microscopy. J. Braz. Chem. Soc. 2007, 18, 714–720. [Google Scholar] [CrossRef][Green Version]
- Sjöström, J.K.; Bindler, R.; Granberg, T.; Kylander, M.E. Procedure for organic matter removal from peat samples for XRD mineral analysis. Wetland 2019, 39, 473–481. [Google Scholar] [CrossRef]
- Song, J.; Huang, W.; Peng, P.; Xiao, B.; Ma, Y. Humic acid molecular weight estimation by high-performance size-exclusion chromatography with ultraviolet absorbance detection and refractive index detection. Soil Sci. Soc. Am. J. 2010, 74, 2013–2020. [Google Scholar] [CrossRef]
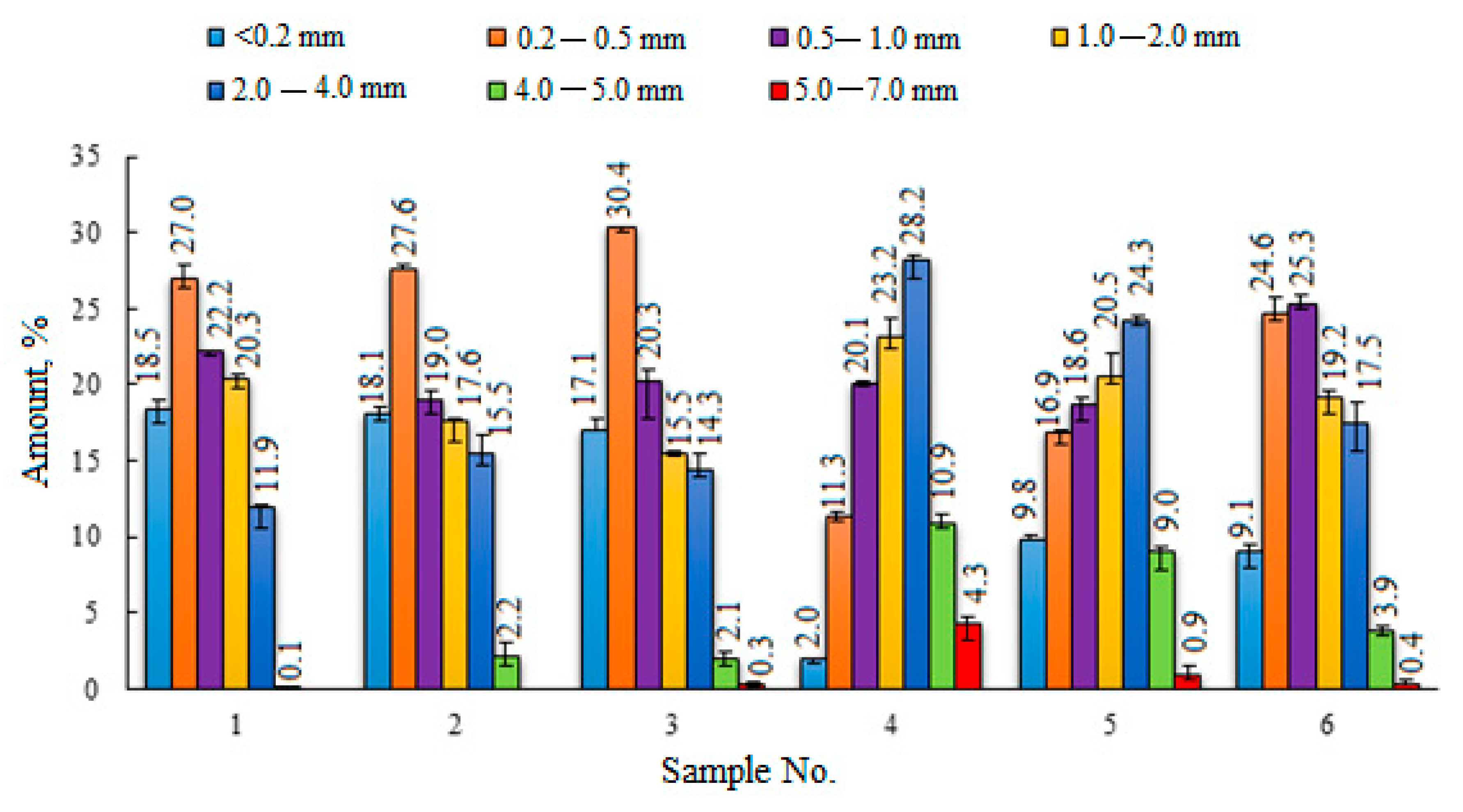


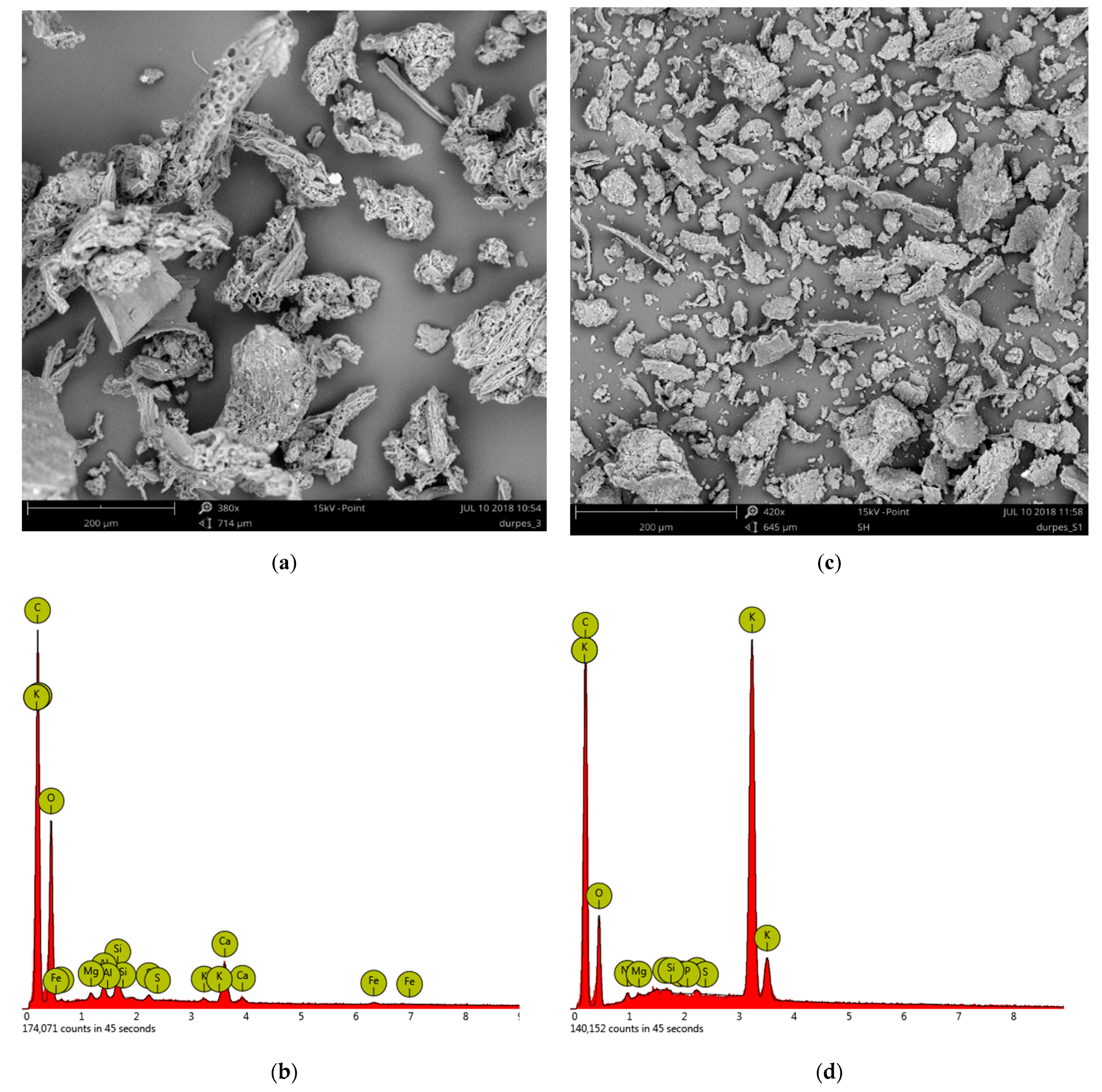
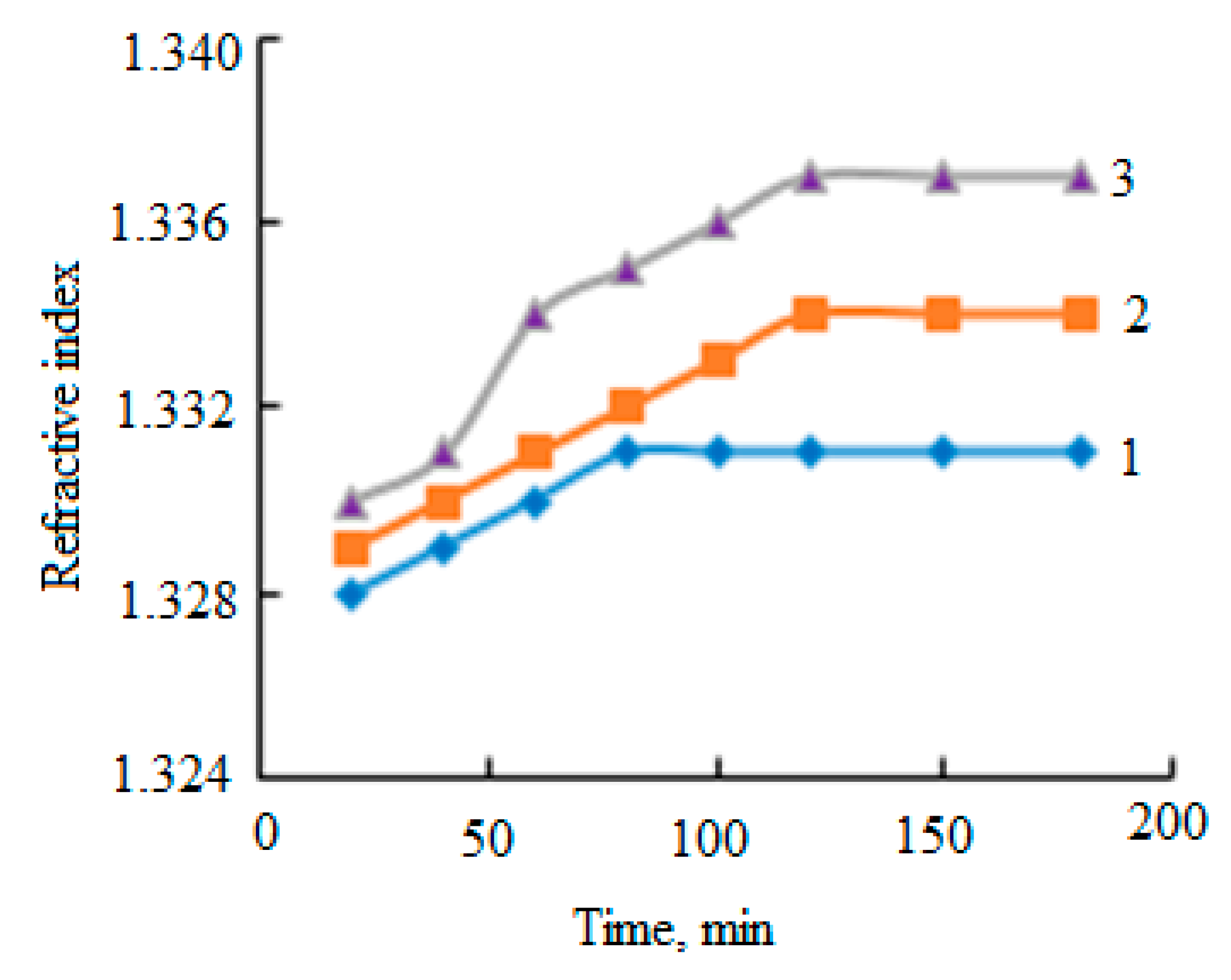
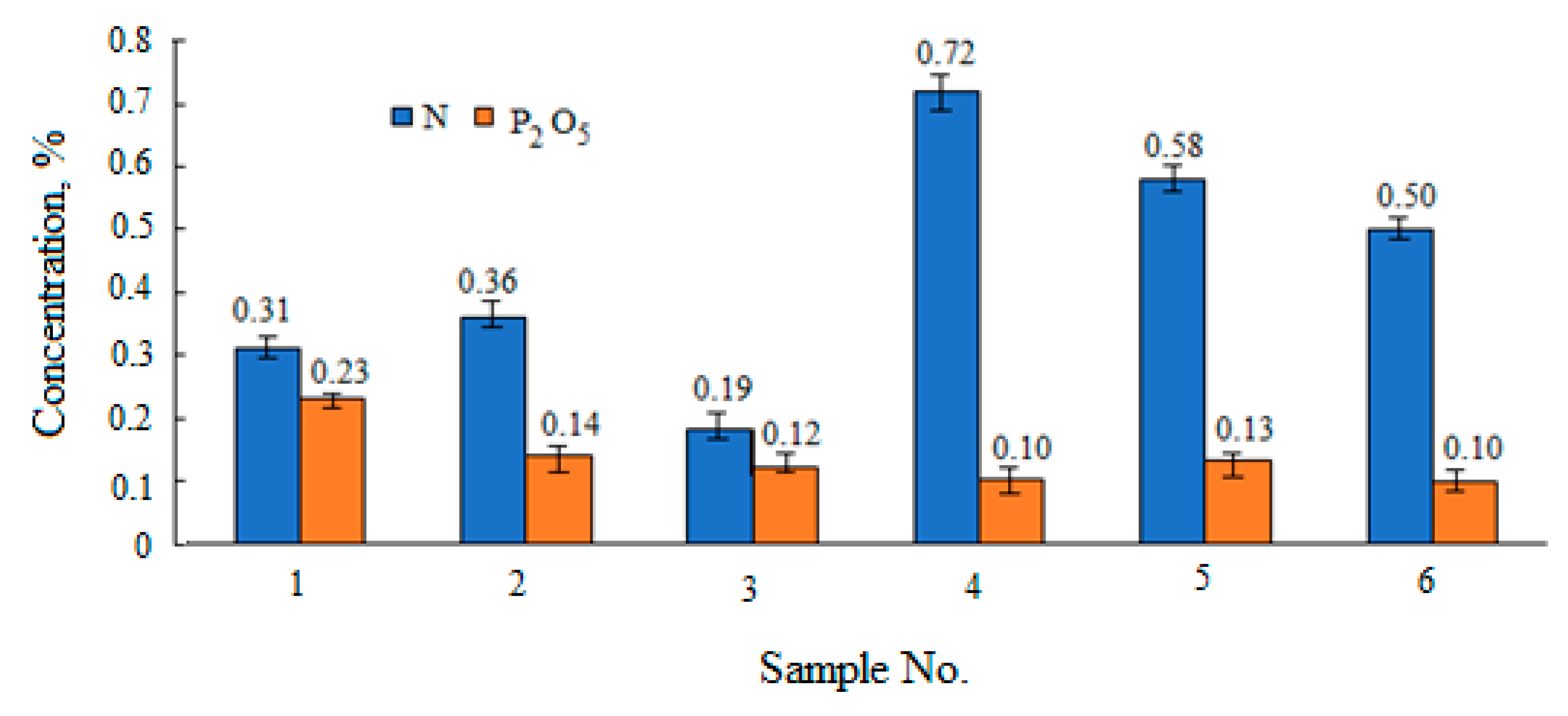
| Sample No. | Peatlands/Company | Abbreviations | pH | pHKCl | Bulk Density, kg·m−3 | Degree of Humification (von Post Scale) | |
|---|---|---|---|---|---|---|---|
| Loose | Packaged | ||||||
| 1 | Sepetos/JCS “Durpeta” | SD | 4.15 | 3.66 | 246 | 409 | H6 |
| 2 | Didziojo Tyrulio JCS “Didysis Tyrulis” | DT | 4.00 | 3.33 | 157 | 302 | H7 |
| 3 | Rekyvos SC “Rėkyva” | RR | 3.28 | 2.87 | 171 | 285 | H6 |
| 4 | Ezerelio JCS “Klasmann—Deilmann” | EKD | 4.40 | 3.29 | 245 | 409 | H8 |
| 5 | Aukštumalos pelke JCS “Klasmann—Deilmann” | AKD | 4.54 | 2.89 | 209 | 345 | H7 |
| 6 | Musos tyrelio JCS “Laveksa” | MTL | 4.45 | 2.70 | 192 | 295 | H5 |
| Extract | Macronutrients Concentration, % | Microelements Concentration by Method AAS, mg·kg−1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P2O5 | K2O * | Mn | Cu | Fe | Cd | Zn | Ni | Cr | Co | |
| Sample 1 SD | |||||||||||
| aqua | 0.03 ± 0.003* | 0.06 ± 0.005 * | 0.01 ± 0.002 | 0.43 ± 0.07 | 0.88 ± 0.08 | 8.78 ± 0.55 | - | 4.14 ± 0.12 | 1.85 ± 0.11 | - | 0.01 ± 0.002 |
| hydrochloric acid | 1.05 ± 0.06 | 0.37 ± 0.005 | 0.05 ± 0.004 | 12.11 ± 0.78 | 24.26 ± 1.05 | 1749.26 ± 52.05 | 0.01 ± 0.002 | 7.09 ± 0.13 | 12.48 ± 0.18 | 0.11 ± 0.012 | 0.03 ± 0.002 |
| aqua regia | – | 0.39 ± 0.006 | 0.06 ± 0.005 | 14.88 ± 0.84 | 28.85 ± 1.55 | 2179.43 ± 48.09 | 0.14 ± 0.012 | 20.24 ± 0.21 | 18.48 ± 0.27 | 0.39 ± 0.022 | 0.04 ± 0.003 |
| Sample 2 DT | |||||||||||
| aqua | 0.04 ± 0.004 * | 0.02 ± 0.002 * | 0.02 ± 0.002 | 0.55 ± 0.08 | 0.62 ± 0.06 | 13.89 ± 0.65 | 0.02 ± 0.002 | 6.23 ± 0.15 | 2.81 ± 0.16 | 0.01 ± 0.011 | 0.01 ± 0.002 |
| hydrochloric acid | 1.87 ± 0.07 | 0.19 ± 0.02 | 0.06 ± 0.006 | 17.21 ± 0.89 | 18.52 ± 0.91 | 2136.54 ± 44.74 | 0.04 ± 0.003 | 11.26 ± 0.12 | 13.79 ± 0.89 | 0.05 ± 0.007 | 0.04 ± 0.006 |
| aqua regia | – | 0.20 ± 0.02 | 0.07 ± 0.008 | 24.73 ± 1.00 | 19.67 ± 0.98 | 2630.44 ± 48.68 | 0.12 ± 0.012 | 21.16 ± 0.19 | 20.15 ± 0.92 | 0.16 ± 0.009 | 0.05 ± 0.007 |
| Sample 3 RR | |||||||||||
| aqua | 0.02 ± 0.002 * | 0.04 ± 0.003 * | 0.02 ± 0.002 | 0.45 ± 0.07 | 0.75 ± 0.07 | 12.99 ± 0.85 | 0.04 ± 0.006 | 4.21 ± 0.13 | 2.51 ± 0.18 | 0.04 ± 0.006 | 0.03 ± 0.005 |
| hydrochloric acid | 0.7 ± 0.09 | 0.27 ± 0.003 | 0.06 ± 0.006 | 19.43 ± 0.91 | 19.87 ± 0.96 | 2080.95 ± 32.44 | 0.08 ± 0.008 | 10.16 ± 0.14 | 13.16 ± 0.14 | 0.16 ± 0.008 | 0.07 ± 0.008 |
| aqua regia | – | 0.28 ± 0.03 | 0.06 ± 0.005 | 24.81 ± 0.99 | 22.22 ± 1.05 | 2734.41 ± 35.25 | 0.15 ± 0.022 | 12.12 ± 0.25 | 19.74 ± 0.22 | 0.68 ± 0.006 | 0.10 ± 0.01 |
| Sample 4 EKD | |||||||||||
| aqua | 0.05 ± 0.005 * | 0.01 ± 0.002 * | 0.02 ± 0.002 | 0.35 ± 0.05 | 1.59 ± 0.12 | 19.70 ± 0.87 | 0.01 ± 0.002 | 4.15 ± 0.12 | 3.60 ± 0.19 | 0.08 ± 0.004 | 0.04 ± 0.003 |
| hydrochloric acid | 2.11 -±0.21 | 0.11 ± 0.01 | 0.06 ± 0.006 | 16.08 ± 0.88 | 33.5 ± 1.21 | 3120.91 ± 35.59 | 0.03 ± 0.004 | 12.40 ± 0.14 | 21.50 ± 0.98 | 0.95 ± 0.006 | 0.61 ± 0.055 |
| aqua regia | – | 0.14 ± 0.01 | 0.07 ± 0.006 | 21.33 ± 0.97 | 35.7 ± 1.28 | 3918.26 ± 32.28 | 0.12 ± 0.02 | 18.03 ± 0.13 | 23.2 ± 1.01 | 1.12 ± 0.016 | 0.82 ± 0.085 |
| Sample 5 AKD | |||||||||||
| aqua | 0.04 ± 0.004 * | 0.02 ± 0.002 * | 0.01 ± 0.002 | 0.31 ± 0.04 | 1.07 ± 0.11 | 11.26 ± 0.65 | 0.08 ± 0.009 | 8.44 ± 0.13 | 2.30 ± 0.22 | 0.06 ± 0.004 | 0.05 ± 0.005 |
| hydrochloric acid | 1.43 ± 0.11 | 0.17 ± 0.02 | 0.05 ± 0.004 | 13.00 ± 0.81 | 20.11 ± 0.98 | 1972.63 ± 20.48 | 0.12 ± 0.02 | 12.88 ± 0.17 | 9.60 ± 0.52 | 0.46 ± 0.005 | 0.48 ± 0.056 |
| aqua regia | – | 0.20 ± 0.02 | 0.06 ± 0.006 | 21.49 ± 0.98 | 27.16 ± 0.97 | 2630.47 ± 28.00 | 0.17 ± 0.02 | 24.89 ± 0.92 | 13.79 ± 0.72 | 0.53 ± 0.065 | 0.72 ± 0.075 |
| Sample 6 MTL | |||||||||||
| aqua | 0.03 * | 0.07 ± 0.005 * | 0.01 ± 0.002 | 0.42 ± 0.06 | 1.41 ± 0.14 | 9.76 ± 0.55 | 0.07 ± 0.009 | 5.61 ± 0.12 | 2.20 | 0.06 ± 0.005 | 0.02 ± 0.003 |
| hydrochloric acid | 1.28 ± 0.11 | 0.41 ± 0.05 | 0.05 ± 0.006 | 20.17 ± 0.93 | 28.54 ± 1.06 | 1804.62 ± 25.20 | 0.12 ± 0.01 | 7.65 ± 0.18 | 7.86 | 0.48 ± 0.051 | 0.35 ± 0.042 |
| aqua regia | – | 0.42 ± 0.06 | 0.06 ± 0.008 | 26.38 ± 1.01 | 30.14 ± 1.02 | 2274.58 ± 22.87 | 0.14 ± 0.02 | 8.69 ± 0.17 | 14.28 | 0.69 ± 0.058 | 0.83 ± 0.086 |
| Sample | pH | Refractive Index | Electrical Conductivity, mS·cm−1 | Density, kg·m−3 | Viscosity | Humic Acids, % | Fulvic Acids, % | Sediment Content, % | |
|---|---|---|---|---|---|---|---|---|---|
| Kinematic, mm2·s−1 | Dynamic, mPa·s | ||||||||
| 1 | 13.05 | 1.337 ± 0.001 | 40.2 | 1016 | 1.452 | 1.475 | 14.38 ± 0.11 | 0.31 ± 0.05 | 79.57 |
| 2 | 13.12 | 1.338 ± 0.001 | 46.7 | 1016 | 1.393 | 1.415 | 16.56 ± 0.15 | 0.32 ± 0.07 | 86.83 |
| 3 | 12.91 | 1.338 ± 0.001 | 40.1 | 1016 | 1.367 | 1.389 | 12.32 ± 0.10 | 0.22 ± 0.08 | 81.25 |
| 4 | 12.94 | 1.337 ± 0.001 | 33.3 | 1015 | 1.468 | 1.490 | 25.87 ± 0.19 | 0.76 ± 0.14 | 66.99 |
| 5 | 13.01 | 1.337 ± 0.001 | 42.1 | 1018 | 1.421 | 1.447 | 15.31 ± 0.37 | 0.60 ± 0.17 | 81.65 |
| 6 | 13.04 | 1.338 ± 0.001 | 47.8 | 1017 | 1.407 | 1.431 | 13.18 ± 0.14 | 0.55 ± 0.19 | 88.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paleckiene, R.; Navikaite, R.; Slinksiene, R. Peat as a Raw Material for Plant Nutrients and Humic Substances. Sustainability 2021, 13, 6354. https://doi.org/10.3390/su13116354
Paleckiene R, Navikaite R, Slinksiene R. Peat as a Raw Material for Plant Nutrients and Humic Substances. Sustainability. 2021; 13(11):6354. https://doi.org/10.3390/su13116354
Chicago/Turabian StylePaleckiene, Rasa, Raminta Navikaite, and Rasa Slinksiene. 2021. "Peat as a Raw Material for Plant Nutrients and Humic Substances" Sustainability 13, no. 11: 6354. https://doi.org/10.3390/su13116354
APA StylePaleckiene, R., Navikaite, R., & Slinksiene, R. (2021). Peat as a Raw Material for Plant Nutrients and Humic Substances. Sustainability, 13(11), 6354. https://doi.org/10.3390/su13116354







