Efficiency of Natural Clay Mineral Adsorbent Filtration Systems in Wastewater Treatment for Potential Irrigation Purposes
Abstract
1. Introduction
2. Materials and Method
2.1. Case Study and Sampling
2.2. Adsorbents Preparation
2.3. Adsorbents Characterization
2.4. Experimental Device and Adsorption Methodology
2.5. Regeneration of Adsorbents
2.6. Removal Efficiency (RE)
2.7. Laboratory Analyses for Water Samples
2.8. Statistical Analysis
3. Results and Discussions
3.1. Characterizations of Natural Adsorbents
3.2. Characteristics of Wastewater
3.3. Characterizations of Natural Adsorbents
3.4. Regeneration of Spent Adsorbent Materials
3.5. Evaluation of Treated Water for Reuse in Irrigation
3.6. Cost-Benefit Analysis
4. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ibrahim, L.A.; Asaad, A.A.; Khalifa, E.A. Laboratory approach for wastewater treatment utilizing chemical addition case study: El-Rahway Drain, Egypt. Life Sci. J. 2019, 16, 56–67. [Google Scholar]
- Hsueh, C.L.; Huang, Y.H.; Wang, C.C.; Chen, C.Y. Degradation of Azo Dyes Using Low Iron Concentration of Fenton and Fenton-Like System. Chemosphere 2005, 58, 1409. [Google Scholar] [CrossRef] [PubMed]
- Jester, T.L.; Taylor, T.H. Industrial Waste Treatment at Scovill. Manufacturing Company. In Proceedings of the 28th Purdue Industrial Waste Conference, Lafayette, IN, USA, 1–3 May 1973. [Google Scholar]
- Kim, S.H.; Chung, H.; Jeong, S.; Nam, K. Identification of pH-dependent removal mechanisms of lead and arsenic by basic oxygen furnace slag: Relative contribution of precipitation and adsorption. J. Clean. Prod. 2021, 279, 123451. [Google Scholar] [CrossRef]
- Rossi, G.; Mainardis, M.; Aneggi, E.; Weavers, L.K.; Goi, D. Combined ultrasound-ozone treatment for reutilization of primary effluent—A preliminary study. Environ. Sci. Pollut. Res. 2021, 28, 700. [Google Scholar] [CrossRef]
- Mo, J.H.; Lee, Y.H.; Kim, J.; Jeong, J.Y.; Jegal, J. Treatment of dye aqueous solutions using nanofiltration polyamide composite membranes for the dye wastewater reuse. Dyes Pigm. 2008, 76, 429. [Google Scholar] [CrossRef]
- Chen, G. Electrochemical technologies in wastewater treatment. Sep. Purif. Technol. 2004, 38, 11–41. [Google Scholar] [CrossRef]
- Ibrahim, L.A.; El Gammal, H.A.A.; Mahran, B.N. In Vitro, Appraisal and abatement of drainage wastewater pollution in light of utilizing fly ash. Nat. Sci. 2017, 15, 2017. [Google Scholar]
- Goswami, R.K.; Mehariya, S.; Verma, P.; Lavecchia, R.; Zuorro, A. Microalgae-based biorefineries for sustainable resource recovery from wastewater. J. Water Process. Eng. 2021, 40, 101747. [Google Scholar] [CrossRef]
- Nan, X.; Lavrnić, S.; Toscano, A. Potential of wastewater treatment systems based on constructed wetlands for agricultural reuse under the EU framework. J. Environ. Manag. 2020, 275, 111219. [Google Scholar] [CrossRef]
- Maamoun, I.; Eljamal, O.; Falyouna, O.; Eljamal, R.; Sugihara, Y. Multi-objective optimization of permeable reactive barrier design for Cr (VI) removal from groundwater. Ecotoxicol. Environ. Saf. 2020, 200, 110773. [Google Scholar] [CrossRef]
- Maamoun, I.; Eljamal, O.; Khalil, A.M.E.; Sugihara, Y.; Matsunaga, N. Phosphate removal through the nano-zero-valent iron permeable reactive barrier; column experiment and reactive solute transport modeling. Transport Porous Med. 2018, 125, 395. [Google Scholar] [CrossRef]
- Swayampakula, K.; Boddu, V.M.; Nadavala, S.K.; Abburi, K. Competitive adsorption of Cu (II), Co (II) and Ni (II) from their binary and tertiary aqueous solutions using chitosan-coated perlite beads as biosorbent. J. Hazard. Mater. 2009, 170, 680–689. [Google Scholar] [CrossRef]
- Abdallah, S.M.; El-hussaini, O.M.; Mahdy, R.M. Towards a more safe environment (2) Characterization of some clay sediments in Egypt for safe environmental applications. Aust. J. Basic Appl. Sci. 2007, 1, 813–823. [Google Scholar]
- Abdallah, S.M.; El-hussaini, O.M.; Mahdy, R.M. Towards a more safe environment (4) Sorpability of vanadium by some clay sediments in Egypt. J. Appl. Sci. Res. 2005, 1, 168–175. [Google Scholar]
- Abdallah, S.M.; El-hussaini, O.M.; Mahdy, R.M. Towards a more safe environment: (6) Monitoring the recovery of boron adsorbed by some clay sediments in Egypt by FTIR spectroscopy. Aust. J. Basic Appl. Sci. 2009, 3, 1450–1460. [Google Scholar]
- Ali, M.M.A.; El-Sayed, E.E.; Kamel, M.Z. Removal of Hazardous Contaminants from Wastewater Using Natural Zeolite. J. Water Res. 2017, 138, 333–347. [Google Scholar]
- Ferretti, G.; Galamini, G.; Medoro, V.; Coltorti, M.; Giuseppe, D.D.; Faccin, B. Impact of Sequential Treatments with Natural and Na-Exchanged Chabazite Zeolite-Rich TuffonPig-Slurry Chemical Composition. Water 2020, 12, 310. [Google Scholar] [CrossRef]
- Erdem, E.; Karapinar, N.; Donat, R. The removal of heavy metal cations by natural zeolites. J. Colloid Interface Sci. 2004, 280, 309–314. [Google Scholar] [CrossRef]
- Shubaira, T.; Eljamala, O.; Taharab, A.; Sugiharaa, Y.; Matsunaga, N. Preparation of new magnetic zeolite nanocomposites for removal of strontium from polluted waters. J. Mol. Liq. 2019, 288, 111026. [Google Scholar] [CrossRef]
- Falyounaa, O.; Eljamala, O.; Maamouna, I.; Taharab, A.; Sugiharaa, Y. Magnetic zeolite synthesis for efficient removal of cesium in a lab-scale continuous treatment system. J. Colloid Interface Sci. 2020, 571, 66. [Google Scholar] [CrossRef]
- Dye, A.; Hriljac, J.; Evans, N. The use of columns of the zeolite clinoptilolite in the remediation of aqueous nuclear waste streams. J. Radioanal. Nucl. Chem. 2018, 318, 2473–2491. [Google Scholar]
- Demir, A.; Gunay, A.; Debik, E. Ammonium removal from aqueous solution by ion-exchange using packed bed natural zeolite. Water SA 2002, 28, 329–335. [Google Scholar] [CrossRef]
- Bakr, H.E.G.M.M. Diatomite: Its Characterization, Modifications and Applications. Asian J. Mater. Sci. 2010, 2, 121–136. [Google Scholar]
- Lemonas, J.F. Diatomite. Am. Ceramic Soc. Bull. 1997, 76, 92–95. [Google Scholar]
- Aytas, S.; Akyil, S.; Aslani, M.; Aytekin, U. Removal of uranium from aqueous solution by diatomite (Kieselguhr). J. Radioanal. Nucl. Chem. 1999, 240, 973–976. [Google Scholar] [CrossRef]
- Osmanlioglu, A.E. Natural diatomite process for removal of radioactivity from liquid waste. Appl. Radiat. Isot. 2007, 65, 17–2018. [Google Scholar] [CrossRef]
- Luckham, P.F.; Rossi, S. The colloidal and rheological properties of bentonite suspensions. Adv. Colloid Interface Sci. 1999, 82, 43–92. [Google Scholar] [CrossRef]
- Tabak, A.; Afşin, B.; Aygün, S.F.; Köksal, E. Structural characteristics of organomodified bentonites of different origin. J. Therm. Anal. Calorim. 2007, 87, 375–381. [Google Scholar] [CrossRef]
- Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association Inc.: New York, NY, USA, 2005.
- Reddy, K.R.; Dastgheibi, T.X.S. Removal of heavy metals from urban stormwater runoff using different filter materials. J. Environ. Chem. Eng. 2014, 2, 282–292. [Google Scholar] [CrossRef]
- Al-Essa, K. Activation of Jordanian Bentonite by Hydrochloric Acid and Its Potential for Olive Mill Wastewater Enhanced Treatment. J. Chem. 2018, 2018. [Google Scholar] [CrossRef]
- Agbenin, J.O. Laboratory Manual for Soil and Plant Analysis; Ahmadu Bello University: Zaria, Nigeria, 1995; pp. 30–72. [Google Scholar]
- Egyptian Governmental Law No. 48/1982-Decision 92. The Implementer Regulations for Law 48/1982, Decision 92/2013 Regarding the Protection of the River Nile and Water Ways from Pollution. Map Periodical Bull. 2013, 21, 21–30.
- Wastewater Treatment and Use in Agriculture; FAO Irrigation and Drainage Paper no. 47; Pescod, M.B., Ed.; Food and Agriculture Organization (FAO): Rome, Italy, 1992; p. 125. [Google Scholar]
- WHO Guidelines for the Safe Use of Wastewater, Excreta and Greywater. In Wastewater Use in Agriculture; World Health Organization: Geneva, Switzerland, 2006; Volume 2.
- Al-Wakeel, M.I. Characterization and process development of the Nile diatomaceous sediment. Int. J. Miner. Process. 2009, 92, 128–136. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Selim, A.Q. Evaluation of Egyptian diatomite for filter aid applications. Physicochem. Probl. Miner. Process. 2011, 47, 113–122. [Google Scholar]
- Rezvantalab, S.; Bahadori, F. Application of Natural Zeolites on Wastewater Treatment. Asian J. Agric. Res. 2015, 9, 343–349. [Google Scholar]
- Jarboui, R.; Sellami, F.; Kharroubi, A.; Gharsallah, N.; Ammar, E. Olive millwastewater stabilizationin open-airponds:impacton clay-sandysoil. Bioresour. Technol. 2008, 99, 7699–7708. [Google Scholar] [CrossRef] [PubMed]
- Sumanjit; Walia, T.P.S.; Ravneet, K. Removal of health hazards causing acidic dyes from aqueous solutions by the process of adsorption. J. Health Allied Sci. 2008, 6, 3. [Google Scholar]
- Namasivayam, C.; Radhika, R.; Suba, S. Uptake of dyes by a promising locally available agricultural solid waste: Coir pith. Waste Manag. 2001, 21, 381–387. [Google Scholar] [CrossRef]
- Oladoja, N.A.; Ademoroti, C.M.A.; Asia, I.O. Treatment of industrial effluent using fortified soil clay. Desalination 2006, 197, 247–261. [Google Scholar] [CrossRef]
- Patel, H.; Vashi, R.T. COD and BOD Removal from Textile Wastewater using Natural Materials. Int. J. Appl. Environ. Sci. 2010, 5, 179–188. [Google Scholar]
- Manz, D.H. Slow Sand Filtration—Demand Operated and Cleaned Using Backwash; Oasis Filter International Ltd.: Calgary, AB, Canada, 2010. [Google Scholar]
- Sobsey, M.D. Managing Water in the Home: Accelerated Gains from Improved Water Supply; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Kang, S.J.; Olmstead, K.; Takacs, K.; Collins, J. Municipal Nutrient Removal Technologies Reference Document; United State Environmental Protection Agency (EPA): Washington, DC, USA, 2008; Volume 1.
- Richards, L.A. Diagnosis and Improvement of Saline and Alkaline Soils; Agriculture Handbook; U.S. Department of Agriculture: Washington, DC, USA, 1954; p. 60.
- Gupta, S.K.; Gupta, I.C. Management of Saline Soils and Waters; Oxford and IBH Publishing Company: New Delhi, India, 1987. [Google Scholar]
- Harivandi, M. nterpreting Turfgrass Irrigation Water Test Results; UCANR Publications: Davis, CA, USA, 1999. [Google Scholar]
- Dec, N. Environmental Guidelines—Use of Effluent by Irrigation; NSW Department of Environment and Conservation: Sydney, Australia, 2004.
- Dhirendra, M.; Alok, K.; Namita, A. Assessment of the irrigation water quality of river Ganga in Haridwar district. Rasayan J. Chem. 2009, 2, 285–292. [Google Scholar]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture. FAO Irrigation and Drainage Paper 29. Revision; FAO: Rome, Italy, 1994. [Google Scholar]
- Rahman, M.M.; Hagare, D.; Maheshwari, B. Benefits and Risks. In Balanced Urban Development: Options and Strategies for Liveable Cities; Maheshwari, B., Singh, V.P., Thoradeniya, B., Eds.; Springer: Cham, Switzerland, 2016; Volume 72. [Google Scholar]
- Todd, D.K. Groundwater Hydrology; John Wiley and Sons Publication: Hoboken, NJ, USA, 2001; pp. 280–281. [Google Scholar]
- Doneen, L.D. Notes on Water Quality in Agriculture; Water Science and Engineering, University of California: Davis, CA, USA, 1964. [Google Scholar]
- Abakpa, G.O.; Umoh, V.J.; Ameh, J.B.; Yakubu, S.E. Microbial quality of irrigation water and irrigated vegetables in Kano State, Nigeria. Int. Food Res. J. 2013, 20, 2933–2938. [Google Scholar]
- Jahin, H.S.; Abuzaid, A.S.; Abdellatif, A.D. Using multivariate analysis to develop irrigation water quality index for surface water in Kafr El-Sheikh Governorate, Egypt. Environ. Technol. Innov. 2020, 17, 100532. [Google Scholar] [CrossRef]
- Ghieth, M.I.; Soliman, K.G.; Abu-Hashim, M.S. Quality of Surface Water in Some Selected Drains and Canals in Fyoum Governorate. Zagazig J. Agric. Res. 2019, 46, 2271–2285. [Google Scholar] [CrossRef]
- Abd-Elaty, I.; Zelenakova, M.; Straface, S.; Vranayova, Z.; Abu-hashim, M. Integrated Modelling for Groundwater Contamination from Polluted Streams Using New Protection Process Techniques. Water 2019, 11, 2321. [Google Scholar] [CrossRef]
- Zelenakova, M.; Purcz, P.; Pintilii, R.; Hlustik, P.; Oracova, A.; Abu-hashim, M. Spatio-temporal Variations in Water Quality Parameter Trends in River Waters. Rev. Chim. 2018, 69, 2940–2974. [Google Scholar] [CrossRef]
- Zelenakova, M.Z.; Repel, A.; Kaposztasova, D. Surface Runoff in Urban Area—Case Study. In New Trends in Urban Drainage Modelling. UDM 2018. Green Energy and Technology; Mannina, G., Ed.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Markovič, G.; Zelenakova, M.; Vranayova, Z.; Kaposztasova, D. Research of Infiltration Facility Efficiency and Quality of Rainwater Harvested from Surface Runoff in Real Conditions. In Storm Water Management; Hlavínek, P., Zeleňáková, M., Eds.; Springer Hydrogeology; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Senante, M.M.; Sancho, F.H.; Garrido, R.S. Economic feasibility study for wastewater treatment: A cost–benefit analysis. Sci. Total Environ. 2010, 408, 4396–4402. [Google Scholar] [CrossRef]
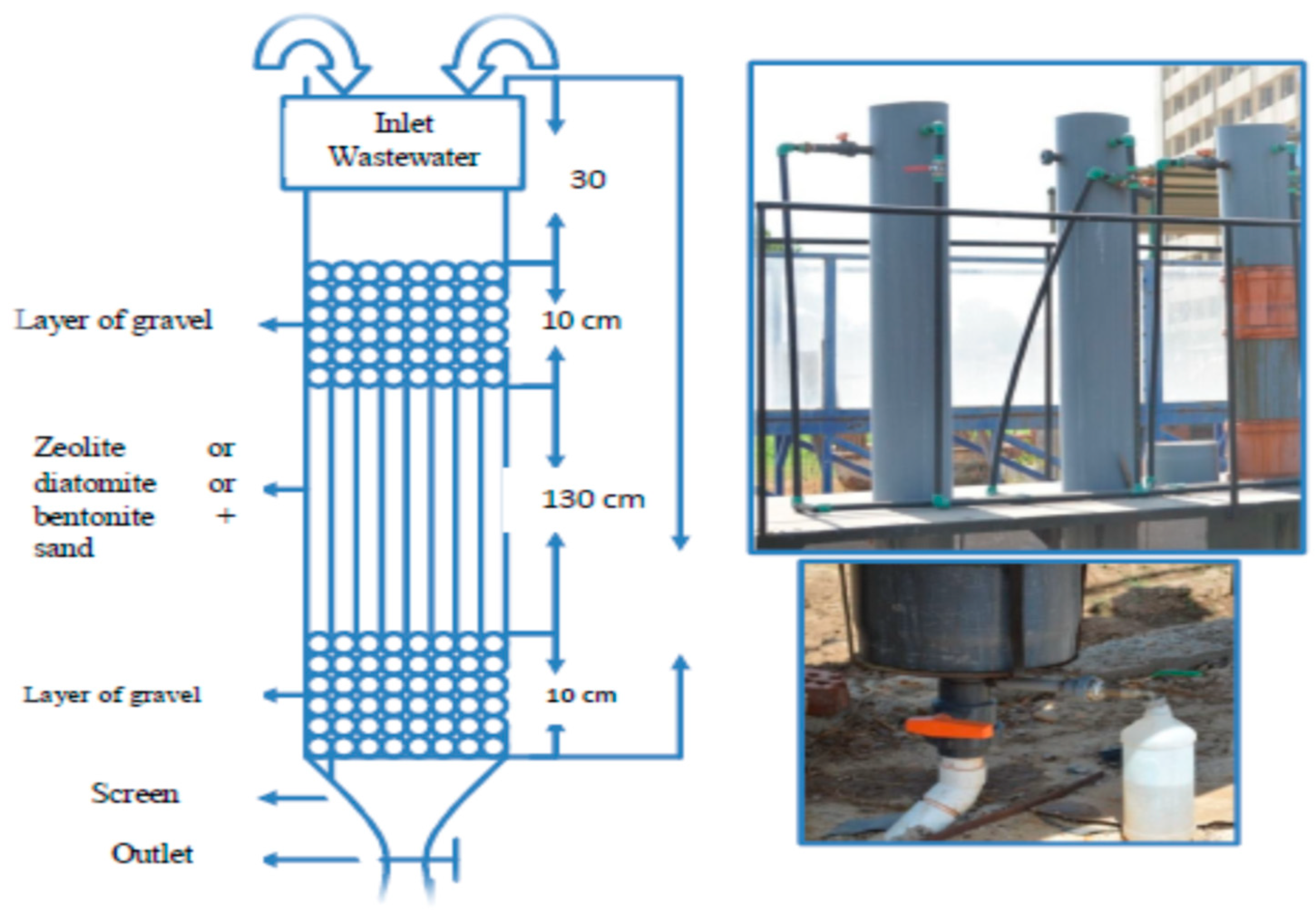
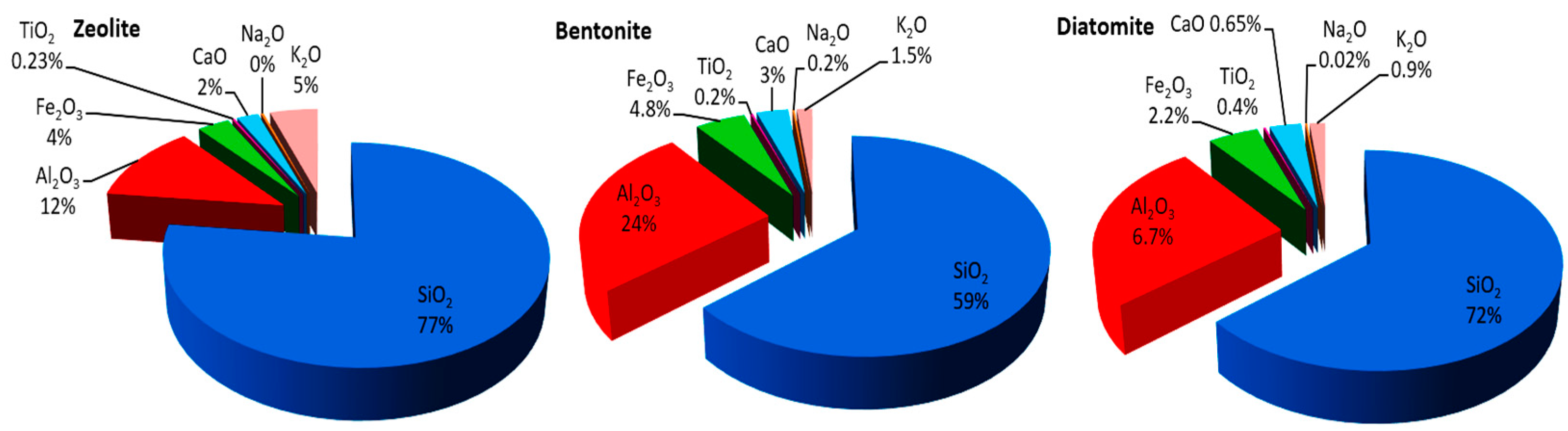
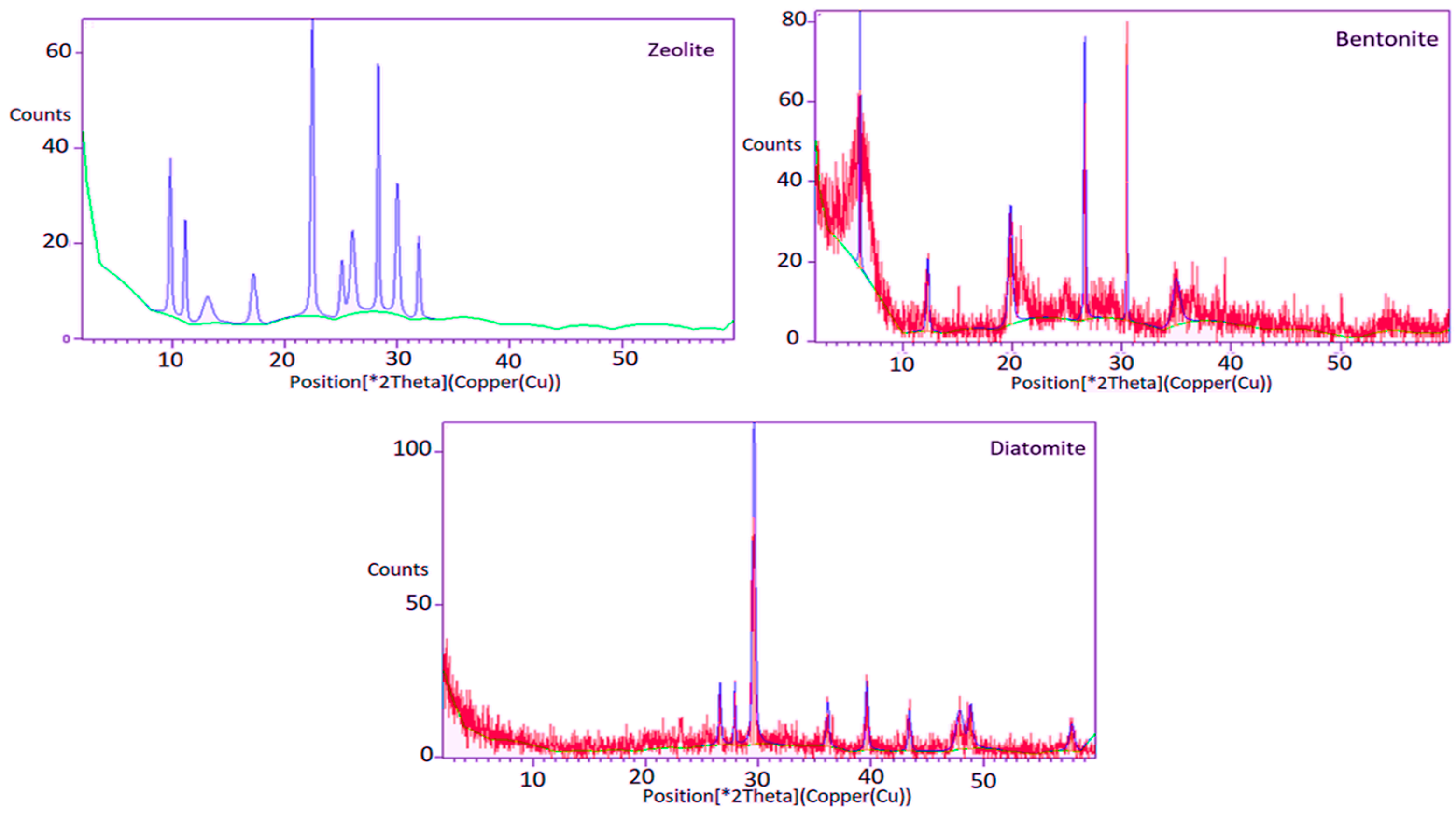
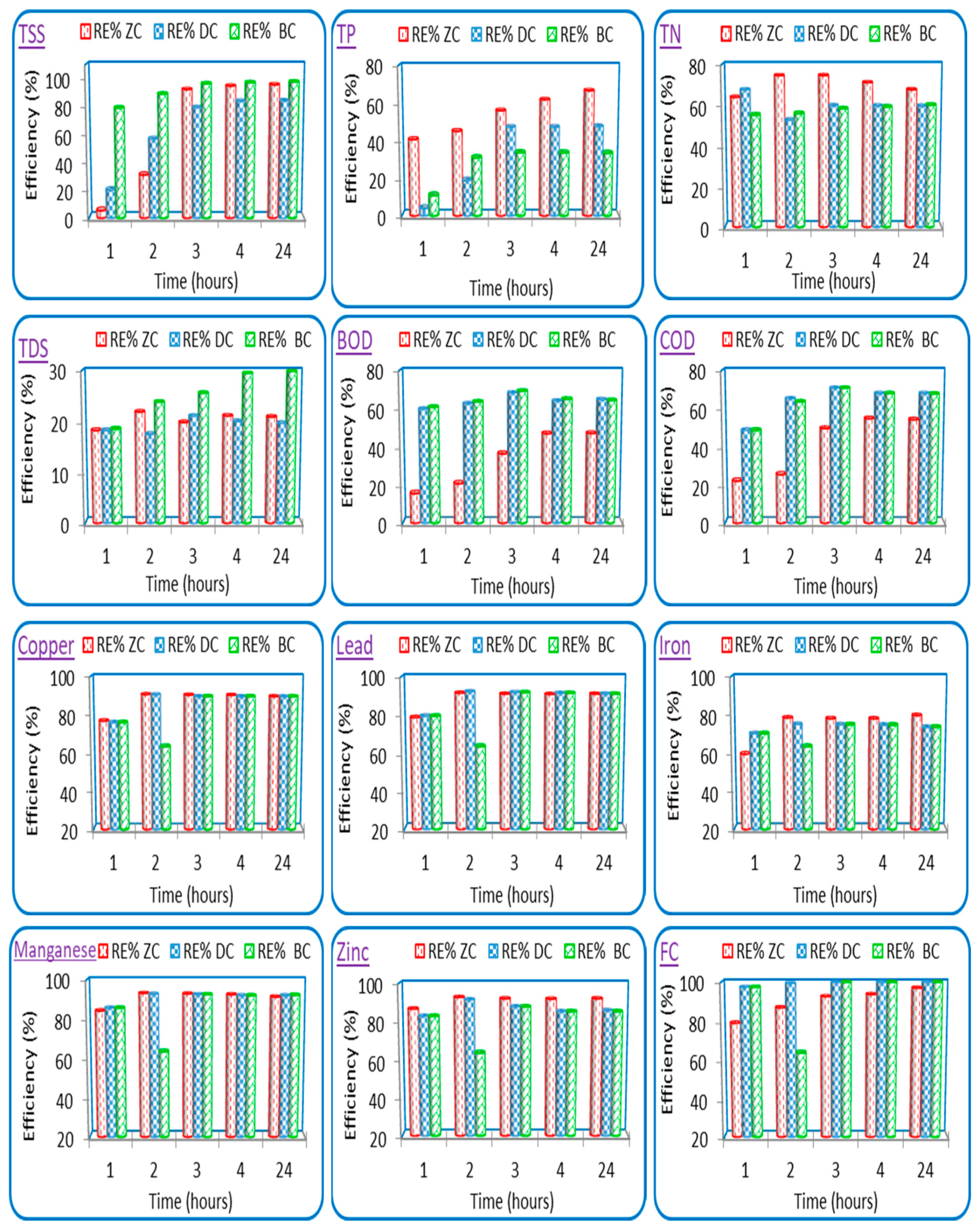
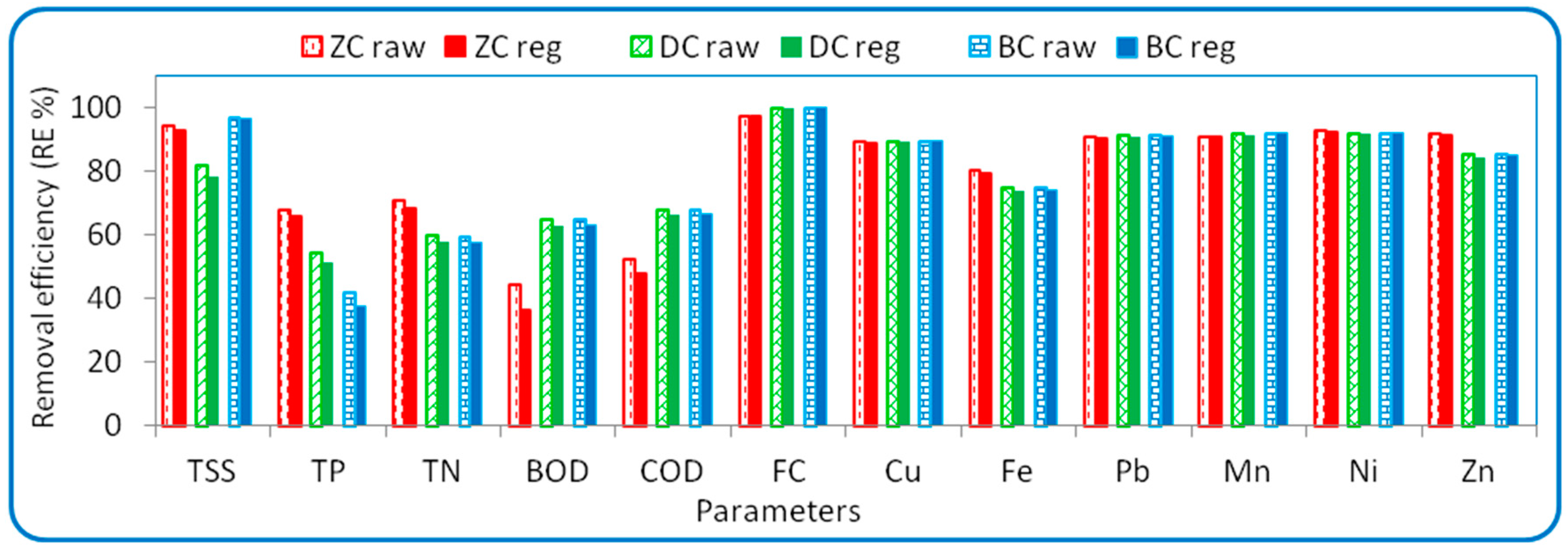
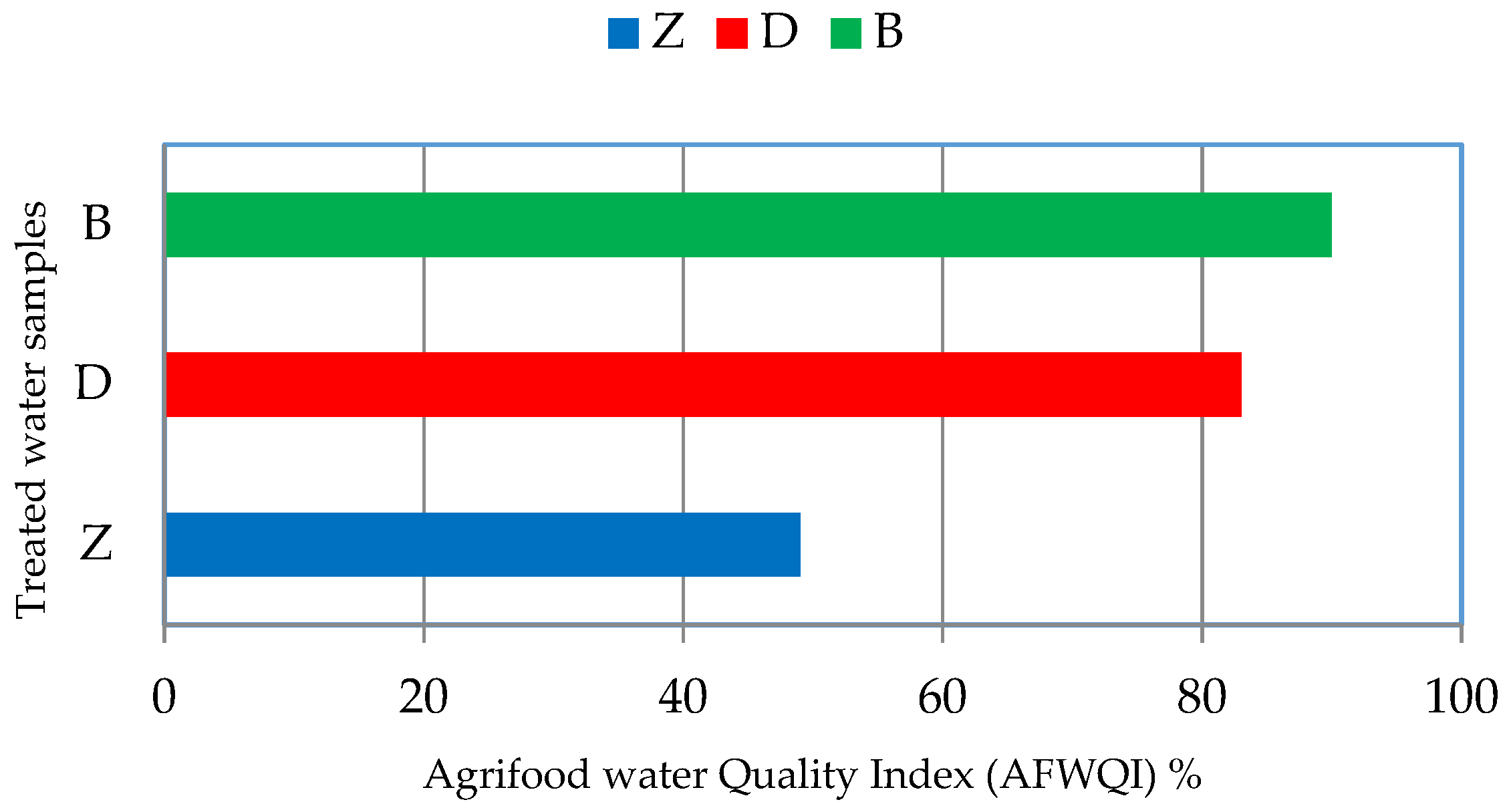
| Parameters | Min | Max | Mean ± SD | Law 48, Decision 91 (2013) Article 50 | FAO [35] and WHO [36] | |
|---|---|---|---|---|---|---|
| Concentration (mg/L) | Rosetta and Damietta Branch | River Nile | ||||
| pH * | 7.29 | 7.41 | 7.35 ± 0.06 | 6–9 | 6–9 | 6.5–8.4 |
| Total suspended solids (TSS) | 193 | 220 | 211 ± 15 | 30 | 30 | - |
| Total phosphorus (TP) | 0.341 | 0.462 | 0.4 ± 0.1 | 1 | 1 | - |
| Total nitrogen (TN) | 4.01 | 9.42 | 6.09 ± 2.9 | 5 | 5 | - |
| Biological oxygen demand (BOD) | 36 | 38 | 36.67 ± 1.2 | 30 | 20 | - |
| Chemical oxygen demand (COD) | 55 | 58 | 56 ± 1.7 | 40 | 30 | - |
| Copper (Cu) | 0.947 | 1.029 | 0.97 ± 0.05 | 1 | 1 | 0.2 |
| Iron (Fe) | 1.05 | 1.16 | 1.12 ± 0.06 | 1 | 1 | 5 |
| Lead (Pb) | 1.014 | 1.02 | 1.02 ± 0.003 | 0.1 | 0.1 | 5 |
| Manganese (Mn) | 1.005 | 1.19 | 1.07 ± 0.11 | 0.5 | 0.5 | 0.2 |
| Zinc (Zn) | 1.02 | 1.12 | 1.05 ± 0.06 | 1 | 1 | 2 |
| Total dissolved solids TDS | 1659 | 1741 | 1700 ± 41 | 1200 | 800 | 450–2000 |
| Calcium | 142 | 192 | 151 ± 8.04 | - | - | 400 |
| Potassium | 17 | 61 | 20.30 ± 2.62 | - | - | 78 |
| Magnesium | 41 | 88 | 47.60 ± 5.89 | - | - | 60 |
| Sodium | 366 | 427 | 386.00 ± 19.22 | - | - | 620 |
| Chloride | 486 | 543 | 502.00 ± 15.47 | - | - | 1065 |
| Nitrate | 0.32 | 41 | 0.34 ± 0.02 | - | - | 10 |
| Sulphate | 241 | 291 | 250 ± 8.92 | - | - | 960 |
| Bicarbonate | 239 | 286 | 245 ± 5.58 | - | - | 610 |
| Fecal coliforms (FC) * (CFU/100 mL) | 46 × 103 | 114 × 103 | 91 × 103 ± 39 × 103 | 1000 | 1000 | < 1000 |
| Treated Water (Z) | Treated Water (D) | Treated Water (B) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean ± SD | Min | Max | Mean ± SD | Min | Max | Mean ± SD | |
| dimensionless | |||||||||
| pH | 7.31 | 7.38 | 7.34 ± 0.03 | 7.32 | 7.33 | 7.33 ± 0.001 | 7.30 | 7.34 | 7.31 ± 0.01 |
| SAR | 4.89 | 4.97 | 4.94 ± 0.03 | 4.38 | 5.07 | 4.75 ± 0.29 | 4.42 | 4.63 | 4.52 ± 0.11 |
| MH | 30.20 | 30.80 | 30.5 ± 0.27 | 32.29 | 32.92 | 32.3 ± 0.17 | 31.54 | 36.46 | 35.1 ± 1.6 |
| meq/L | |||||||||
| RSC | −6.89 | −6.53 | −6.63 ± 0.14 | −8.05 | −6.86 | −7.42 ± 0.50 | −6.55 | −5.77 | −6.29 ± 0.30 |
| RSBC | −3.60 | −3.40 | −3.44 ± 0.08 | −4.35 | −3.40 | −3.78 ± 0.37 | −2.69 | −2.59 | −2.64 ± 0.05 |
| % | |||||||||
| Na% | 50.50 | 51.04 | 50.86 ± 0.21 | 46.53 | 50.95 | 48.92 ± 1.85 | 48.15 | 49.75 | 48.93 ± 0.82 |
| PI | 60.3 | 61.0 | 61.0 ± 0.4 | 56.0 | 61.0 | 58.7 ± 2.1 | 58.7 | 60.9 | 59.6 ± 1 |
| mg/L | |||||||||
| TSS | 4.00 | 17.00 | 9.20 ± 5.13 | 25.00 | 31.00 | 25.00 ± 7.89 | 4.00 | 7.00 | 5.05 ± 1.01 |
| TP | 0.14 | 0.15 | 0.15 ± 0.001 | 0.21 | 0.21 | 0.21 ± 0.001 | 0.25 | 0.29 | 0.26 ± 0.01 |
| TN | 1.59 | 2.02 | 1.84 ± 017 | 2.50 | 2.51 | 2.51 ± 0.01 | 2.48 | 2.59 | 2.52 ± 0.04 |
| BOD | 16.00 | 24.00 | 19.20 ± 3.16 | 11.00 | 14.00 | 12.80 ± 1.18 | 11.00 | 14.00 | 12.95 ± 1.01 |
| COD | 23.00 | 29.00 | 25.40 ± 2.37 | 16.00 | 19.00 | 17.80 ± 1.18 | 16.00 | 19.00 | 17.95 ± 1.01 |
| TDS | 1339 | 1361 | 1343 ±9.03 | 1340 | 1382 | 1363 ± 17.21 | 1175 | 1199 | 1193 ± 7.74 |
| Copper | 0.098 | 0.100 | 0.099 ± 0.001 | 0.097 | 0.097 | 0.097 ± 0.001 | 0.097 | 0.097 | 0.097 ± 0.001 |
| Iron | 0.227 | 0.229 | 0.228 ± 0.001 | 0.287 | 0.291 | 0.289 ± 0.002 | 0.287 | 0.291 | 0.290 ± 0.001 |
| Lead | 0.091 | 0.094 | 0.093 ± 0.001 | 0.083 | 0.091 | 0.088 ± 0.003 | 0.083 | 0.091 | 0.088 ± 0.003 |
| Manganese | 0.084 | 0.093 | 0.089 ± 0.004 | 0.074 | 0.084 | 0.080 ± 0.004 | 0.074 | 0.084 | 0.081 ± 0.003 |
| Zinc | 0.081 | 0.084 | 0.083 ± 0.001 | 0.124 | 0.174 | 0.154 ± 0.02 | 0.124 | 0.174 | 0.157 ± 0.017 |
| meq/L | |||||||||
| Bicarbonate | 3.79 | 3.88 | 3.85 ± 0.04 | 3.79 | 3.88 | 3.85 ± 0.04 | 4.05 | 4.15 | 4.08 ± 0.03 |
| Calcium | 7.24 | 7.39 | 7.29 ± 0.06 | 7.29 | 8.13 | 7.62 ± 0.33 | 6.69 | 6.79 | 6.72 ± 0.03 |
| Potassium | 0.41 | 0.49 | 0.45 ± 0.03 | 0.41 | 0.56 | 0.50 ± 0.06 | 0.36 | 0.41 | 0.37 ± 0.02 |
| Magnesium | 3.13 | 3.29 | 3.19 ± 0.06 | 3.45 | 3.78 | 3.64 ± 0.16 | 3.13 | 3.87 | 3.64 ± 0.26 |
| Sodium | 11.31 | 11.31 | 11.31 ± 0.001 | 10.66 | 11.74 | 11.26 ± 0.44 | 10.18 | 10.44 | 10.29 ± 0.13 |
| Chloride | 13.82 | 14.13 | 13.98 ± 0.14 | 13.40 | 14.67 | 14.17 ± 0.50 | 10.92 | 10.97 | 10.96 ± 0.02 |
| Nitrate | 0.22 | 0.22 | 0.22 ± 0.001 | 0.22 | 0.22 | 0.22 ± 0.001 | 0.22 | 0.22 | 0.22 ± 0.001 |
| Phosphate | 0.03 | 0.03 | 0.03 ± 0.001 | 0.03 | 0.03 | 0.03 ± 0.001 | 0.03 | 0.03 | 0.03 ± 0.001 |
| Sulphate− | 4.93 | 5.35 | 5.10 ± 0.16 | 4.93 | 5.35 | 5.10 ± 0.16 | 4.39 | 4.83 | 4.70 ± 0.15 |
| CFU/mL | |||||||||
| FC | 2400 | 3400 | 2800 ± 300 | 180 | 245 | 200 ± 20 | 180 | 230 | 190 ± 20 |
| Items | Variables | ZC | BC | DC |
|---|---|---|---|---|
| Variable cost (USD) | Average natural clay consumption per year | 4 | 0.2 | 1 |
| Cost of sand per year | 4 | 4 | 4 | |
| Cost of gravel per year | 1.2 | 1.2 | 1.2 | |
| Fixed Cost (USD) | Cost of PVC column | 8 | 8 | 8 |
| Cost of column accessories | 7 | 7 | 7 | |
| Variable cost (USD) | Annual cost for spent clay recovery per year | 5 | 5 | 5 |
| Operating cost including utilities, transportation, power, and maintenance per year | 15 | 15 | 15 | |
| Total annual cost | 44.2 | 40.4 | 41.2 | |
| Amount of treated water (m3/year) | 220 | 220 | 220 | |
| Total cost for 2 million m3/day | 401,818 | 367,273 | 374,545 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
ElBastamy, E.; Ibrahim, L.A.; Ghandour, A.; Zelenakova, M.; Vranayova, Z.; Abu-Hashim, M. Efficiency of Natural Clay Mineral Adsorbent Filtration Systems in Wastewater Treatment for Potential Irrigation Purposes. Sustainability 2021, 13, 5738. https://doi.org/10.3390/su13105738
ElBastamy E, Ibrahim LA, Ghandour A, Zelenakova M, Vranayova Z, Abu-Hashim M. Efficiency of Natural Clay Mineral Adsorbent Filtration Systems in Wastewater Treatment for Potential Irrigation Purposes. Sustainability. 2021; 13(10):5738. https://doi.org/10.3390/su13105738
Chicago/Turabian StyleElBastamy, ElSayed, Lubna A. Ibrahim, Atef Ghandour, Martina Zelenakova, Zuzana Vranayova, and Mohamed Abu-Hashim. 2021. "Efficiency of Natural Clay Mineral Adsorbent Filtration Systems in Wastewater Treatment for Potential Irrigation Purposes" Sustainability 13, no. 10: 5738. https://doi.org/10.3390/su13105738
APA StyleElBastamy, E., Ibrahim, L. A., Ghandour, A., Zelenakova, M., Vranayova, Z., & Abu-Hashim, M. (2021). Efficiency of Natural Clay Mineral Adsorbent Filtration Systems in Wastewater Treatment for Potential Irrigation Purposes. Sustainability, 13(10), 5738. https://doi.org/10.3390/su13105738











