Manure Flushing vs. Scraping in Dairy Freestall Lanes Reduces Gaseous Emissions
Abstract
1. Introduction
2. Materials and Methods
2.1. Environmental Chamber Design
2.2. Animals and Diets
2.3. Treatments
2.4. Equipment
2.5. Emissions Calculations
2.6. Data Analysis
3. Results and Discussion
3.1. Milk Production
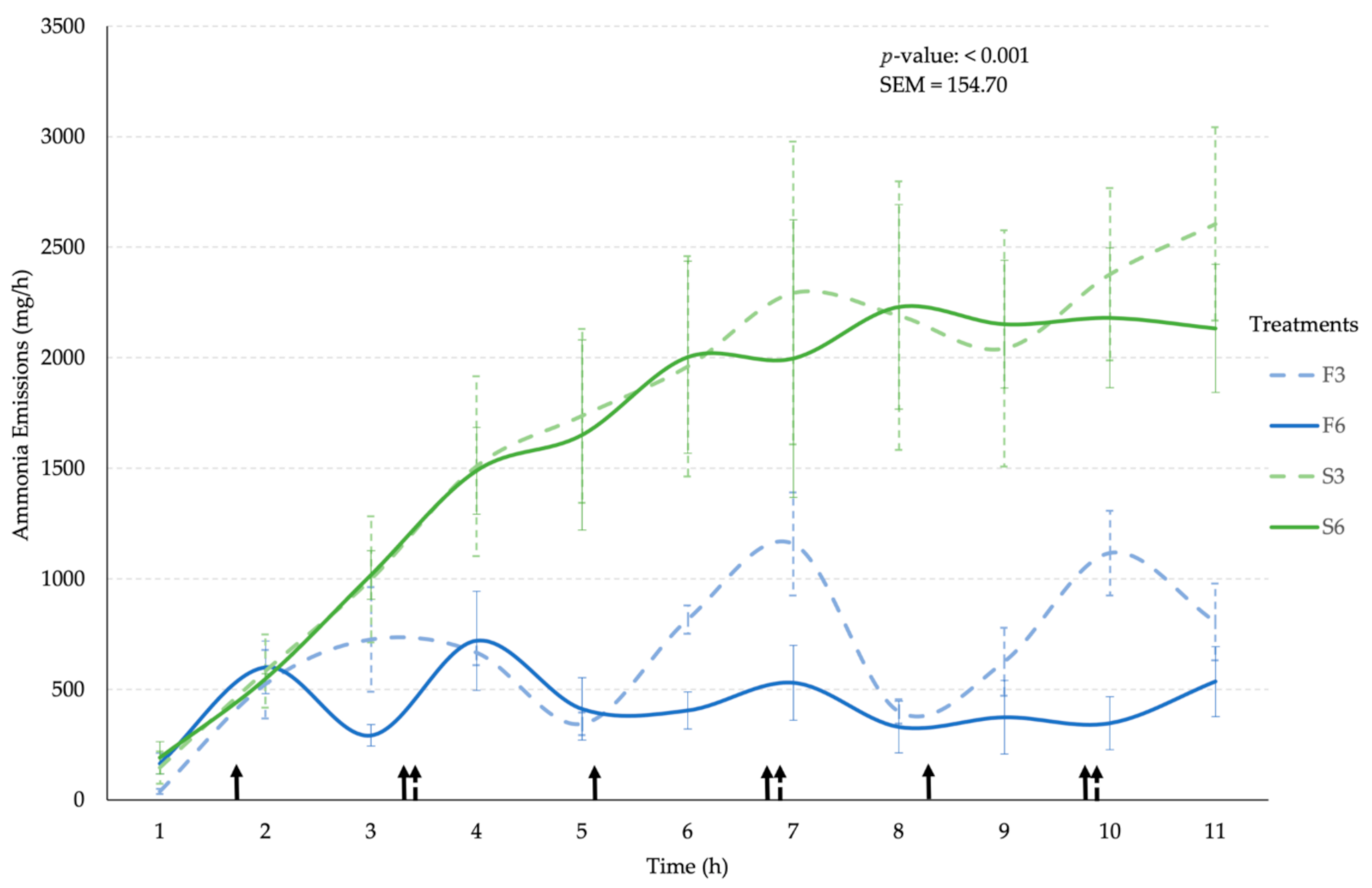
3.2. Ammonia Emissions
3.3. Methane Emissions
3.4. Hydrogen Sulfide Emissions
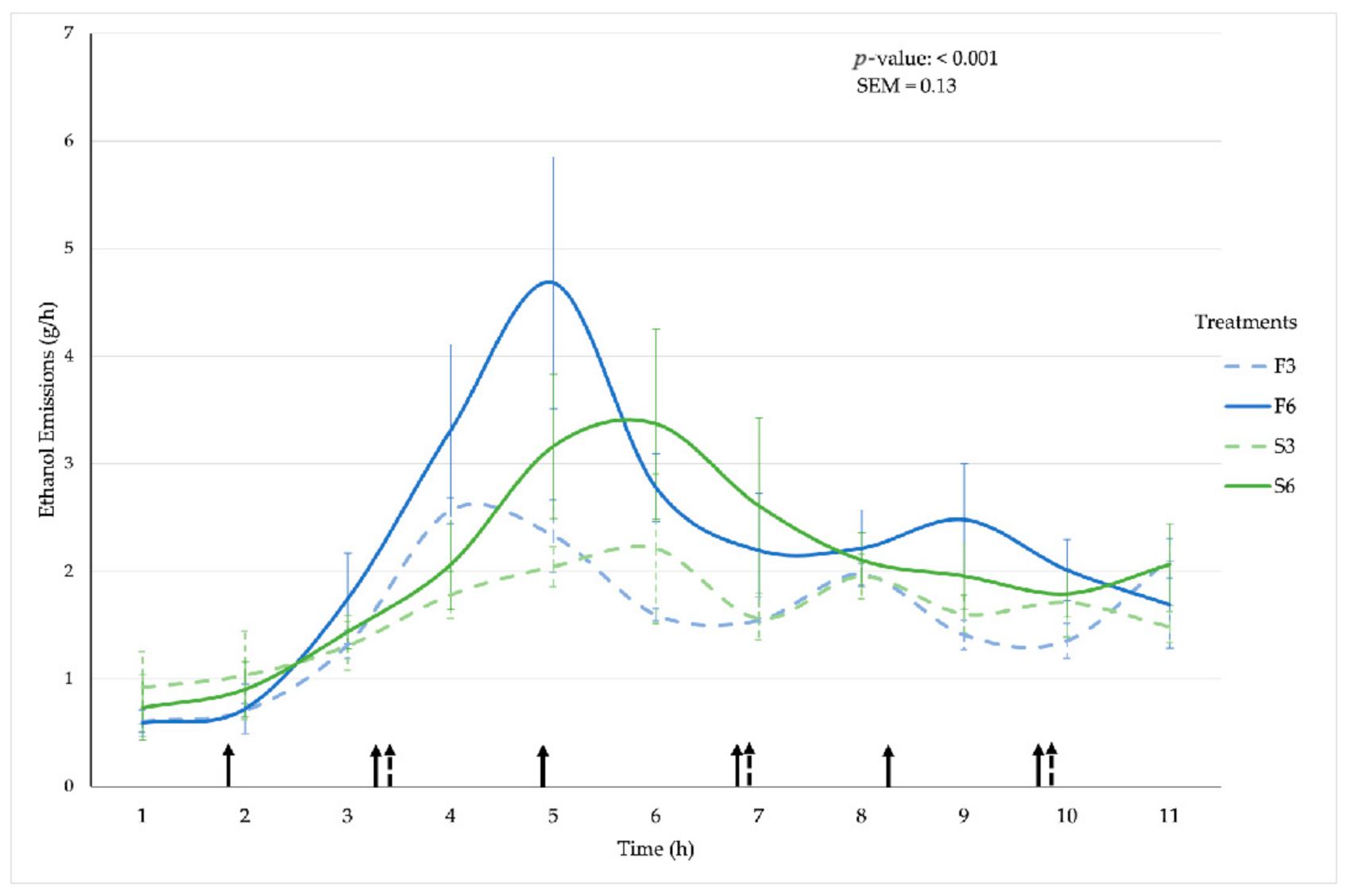
3.5. Ethanol Emissions
3.6. Carbon Dioxide Emissions
3.7. Relation to Manure Management
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pagliari, P.; Wilson, M.; He, Z. Animal manure production and utilization: Impact of modern concentrated animal feeding operations. Anim. Manure: Prod. Charact. Environ. Concerns Manag. 2020, 67, 1–14. [Google Scholar]
- Hatamiya, L. The Economic Importance of the California Dairy Quota Program. Available online: https://www.ams.usda.gov/sites/default/files/media/Exhibit%2054%20-%20Testimony%20of%20Lon%20Hatamiya.pdf (accessed on 26 February 2017).
- USDA. Dairy Situation at a Glance. 2021. Available online: https://www.ers.usda.gov/topics/animal-products/dairy/ (accessed on 1 January 2021).
- USDA. California Dairy Statistics and Trends Mid-Year Review January-June 2016 Data. Available online: https://www.cdfa.ca.gov/dairy/pdf/Annual/2016/MidYear2016.pdf (accessed on 27 April 2017).
- CARB. Ambient Air Qualtiy Standards (AAQS) for Particulate Matter. Available online: https://www.arb.ca.gov/research/aaqs/pm/pm.htm (accessed on 16 February 2017).
- CARB. Revised Proposed Short-Lived Climate Pollutant Reduction Strategy. 2017. Available online: https://ww2.arb.ca.gov/sites/default/files/2020-07/final_SLCP_strategy.pdf (accessed on 1 January 2021).
- Samet, J.M.; Dominici, F.; Curriero, F.C.; Coursac, I.; Zeger, S.L. Fine particulate air pollution and mortality in 20 US cities, 1987–1994. N. Engl. J. Med. 2000, 343, 1742–1749. [Google Scholar] [CrossRef] [PubMed]
- Aneja, V.P.; Blunden, J.; James, K.; Schlesinger, W.H.; Knighton, R.; Gilliam, W.; Jennings, G.; Niyogi, D.; Cole, S. Ammonia assessment from agriculture: US status and needs. J. Environ. Qual. 2008, 37, 515–520. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Air Actions in the San Joaquin Valley-PM2.5. 2015. Available online: https://19january2017snapshot.epa.gov/www3/region9/air/sjv-pm25/index.html (accessed on 1 January 2019).
- SJVDMTFAP. San Joaquin Valley Dairy Manure Technology Feasibility Assessment Panel. An Assessment of Technologies for Management and Treatment of Dairy Manure in California’s San Joaquin Valley. CARB. 2005. Available online: https://www.arb.ca.gov/ag/caf/dairypnl/dmtfaprprt.pdf (accessed on 1 January 2021).
- Sheppard, S.; Bittman, S.; Swift, M.; Tait, J. Modelling monthly NH3 emissions from dairy in 12 Ecoregions of Canada. Can. J. Anim. Sci. 2011, 91, 649–661. [Google Scholar] [CrossRef]
- Harper, L.A.; Flesch, T.K.; Powell, J.M.; Coblentz, W.K.; Jokela, W.E.; Martin, N.P. Ammonia emissions from dairy production in Wisconsin. J. Dairy Sci. 2009, 92, 2326–2337. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lemerTest: Tests in Linear Mixed Effects Models; R package version 2.0-30. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Lenth, R. Least-squares means: The R package lsmeans. J. Stat. Sofw. 2016, 69, 1–33. [Google Scholar]
- Graves, S.; Piepho, H.-P.; Selzer, L.; Dorai-Raj, S. Multcompview: Visualizations of Paired Comparisons; R Package version 0.1-7. 2017. Available online: https//github.com/rvlenth/emmeans/issues (accessed on 18 June 2018).
- Hassanat, F.; Gervais, R.; Benchaar, C. Methane production, ruminal fermentation characteristics, nutrient digestibility, nitrogen excretion, and milk production of dairy cows fed conventional or brown midrib corn silage. J. Dairy Sci. 2017, 100, 2625–2636. [Google Scholar] [CrossRef]
- Sutter, F.; Schwarm, A.; Kreuzer, M. Development of nitrogen and methane losses in the first eight weeks of lactation in Holstein cows subjected to deficiency of utilisable crude protein under restrictive feeding conditions. Arch. Anim. Nutr. 2017, 71, 1–20. [Google Scholar] [CrossRef]
- Bouwman, A.; Lee, D.; Asman, W.; Dentener, F.; Van Der Hoek, K.; Olivier, J. A global high-resolution emission inventory for ammonia. Glob. Biogeochem. Cycles 1997, 11, 561–587. [Google Scholar]
- Hristov, A.; Hanigan, M.; Cole, A.; Todd, R.; McAllister, T.; Ndegwa, P.; Rotz, A. Review: Ammonia emissions from dairy farms and beef feedlots 1. Can. J. Anim. Sci. 2011, 91, 1–35. [Google Scholar] [CrossRef]
- Sommer, S.G.; Petersen, S.O.; Søgaard, H.T. Greenhouse gas emission from stored livestock slurry. J. Environ. Qual. 2000, 29, 744–751. [Google Scholar] [CrossRef]
- Teye, F.K.; Hautala, M. A comparative assessment of four methods for estimating ammonia emissions at microclimatic locations in a dairy building. Int. J. Biometeorol. 2010, 54, 63–74. [Google Scholar] [CrossRef]
- Monteny, G.; Erisman, J. Ammonia emission from dairy cow buildings: A review of measurement techniques, influencing factors and possibilities for reduction. Njas Wagening. J. Life Sci. 1998, 46, 225–247. [Google Scholar] [CrossRef]
- Kroodsma, W.; in’t Veld, J.H.; Scholtens, R. Ammonia emission and its reduction from cubicle houses by flushing. Livest. Prod. Sci. 1993, 35, 293–302. [Google Scholar] [CrossRef]
- Elzing, A.; Monteny, G. Modeling and experimental determination of ammonia emissions rates from a scale model dairy-cow house. Trans. ASAE 1997, 40, 721–726. [Google Scholar] [CrossRef]
- Grant, R.H.; Boehm, M.T. Manure Ammonia and Hydrogen Sulfide Emissions from a Western Dairy Storage Basin. J. Environ. Qual. 2015, 44, 127–136. [Google Scholar] [CrossRef] [PubMed]
- McGinn, S.; Janzen, H.; Coates, T.; Beauchemin, K.; Flesch, T. Ammonia emission from a beef cattle feedlot and its local dry deposition and re-emission. J. Environ. Qual. 2016, 45, 1178–1185. [Google Scholar] [CrossRef]
- Rotz, C.A.; Montes, F.; Hafner, S.D.; Heber, A.J.; Grant, R.H. Ammonia Emission Model for Whole Farm Evaluation of Dairy Production Systems. J. Environ. Qual. 2014, 43, 1143–1158. [Google Scholar] [CrossRef]
- Sun, H.; Trabue, S.L.; Scoggin, K.; Jackson, W.A.; Pan, Y.; Zhao, Y.; Malkina, I.L.; Koziel, J.A.; Mitioehner, F.M. Alcohol, volatile fatty acid, phenol, and methane emissions from dairy cows and fresh manure. J. Environ. Qual. 2008, 37, 615–622. [Google Scholar] [CrossRef]
- USEPA. Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990–2003; EPA-430-R-05-003; USEPA: Washington, DC, USA, 2005.
- Hindrichsen, I.K.; Wettstein, H.R.; Machmüller, A.; Kreuzer, M. Methane emission, nutrient degradation and nitrogen turnover in dairy cows and their slurry at different milk production scenarios with and without concentrate supplementation. Agric. Ecosyst. Environ. 2006, 113, 150–161. [Google Scholar] [CrossRef]
- He, Z.; Pagliari, P.; Waldrip, H.M. Advances and outlook of manure production and management. Anim. Manure: Prod. Charact. Environ. Concerns Manag. 2020, 67, 373–383. [Google Scholar]
- Andriamanohiarisoamanana, F.J.; Sakamoto, Y.; Yamashiro, T.; Yasui, S.; Iwasaki, M.; Ihara, I.; Tsuji, O.; Umetsu, K. Effects of handling parameters on hydrogen sulfide emission from stored dairy manure. J. Environ. Manag. 2015, 154, 110–116. [Google Scholar] [CrossRef]
- Ni, J.-Q.; Heber, A.; Sutton, A.; Kelly, D. Mechanisms of gas releases from swine wastes. Trans. ASABE 2009, 52, 2013–2025. [Google Scholar]
- Maasikmets, M.; Teinemaa, E.; Kaasik, A.; Kimmel, V. Measurement and analysis of ammonia, hydrogen sulphide and odour emissions from the cattle farming in Estonia. Biosyst. Eng. 2015, 139, 48–59. [Google Scholar] [CrossRef]
- El-Mashad, H.M.; Zhang, R.; Rumsey, T.; Hafner, S.; Montes, F.; Rotz, C.A.; Arteaga, V.; Zhao, Y.; Mitloehner, F.M. A mass transfer model of ethanol emission from thin layers of corn silage. Trans. ASABE 2010, 53, 1903–1909. [Google Scholar] [CrossRef]
- Chung, M.Y.; Beene, M.; Ashkan, S.; Krauter, C.; Hasson, A.S. Evaluation of non-enteric sources of non-methane volatile organic compound (NMVOC) emissions from dairies. Atmos. Environ. 2010, 44, 786–794. [Google Scholar] [CrossRef]
- Bonifacio, H.; Rotz, C.; Hafner, S.; Montes, F.; Cohen, M.; Mitloehner, F. A process-based emission model of volatile organic compounds from silage sources on farms. Atmos. Environ. 2017, 152, 85–97. [Google Scholar] [CrossRef]
- USEPA. Emissions from Animal Feeding Operations. Contract No. 68-D6-0011. 2001. Available online: https://www.epa.gov/sites/production/files/2020-10/documents/draftanimalfeed.pdf (accessed on 4 January 2018).
- Petersen, S.O.; Sommer, S.G. Ammonia and nitrous oxide interactions: Roles of manure organic matter management. Anim. Feed Sci. Technol. 2011, 166, 503–513. [Google Scholar] [CrossRef]
- Bertora, C.; Alluvione, F.; Zavattaro, L.; van Groenigen, J.W.; Velthof, G.; Grignani, C. Pig slurry treatment modifies slurry composition, N2O, and CO2 emissions after soil incorporation. Soil Biol. Biochem. 2008, 40, 1999–2006. [Google Scholar] [CrossRef]
- Harrison, J.; Ndegwa, P. Anaerobic digestion of dairy and swine waste. Anim. Manure: Prod. Charact. Environ. Concerns Manag. 2020, 67, 115–127. [Google Scholar]
- Petersen, S.O. Nitrous Oxide Emissions from Manure and Inorganic Fertilizers Applied to Spring Barley. J. Environ. Qual. 1999, 28, 1610–1618. [Google Scholar] [CrossRef]
- Montes, F.; Meinen, R.; Dell, C.; Rotz, A.; Hristov, A.N.; Oh, J.; Waghorn, G.; Gerber, P.J.; Henderson, B.; Makkar, H. SPECIAL TOPICS—Mitigation of methane and nitrous oxide emissions from animal operations: II. A review of manure management mitigation options. J. Anim. Sci. 2013, 91, 5070–5094. [Google Scholar] [CrossRef] [PubMed]
| Measures | Total Mixed Ration (% DM) 1 |
|---|---|
| Crude Protein | 20.4 |
| Ash | 6.85 |
| Neutral Detergent Fiber | 31.8 |
| Acid Detergent Fiber | 23.7 |
| Feed Ingredients | As Fed (kg/d/cow) |
|---|---|
| Grain 1 | 11.91 |
| Alfalfa Hay | 11.34 |
| Whole Cotton Seed | 2.27 |
| Almond Hulls | 2.27 |
| Strata 2 | 0.1 |
| Milk Mineral | 0.34 |
| EnerGII 3 | 0.29 |
| Salt | 0.07 |
| Wheat Hay | 0.91 |
| Gas Analyzer | Gases 3 | Detection Limits | Upper Range |
|---|---|---|---|
| Thermo 17i NO/NOx/NH3 analyzer 1 | NO | 1.25 ng/L | 24.96 µg/L |
| NOx | 1.54 ng/L | 30.78 µg/L | |
| NH3 | 0.71 ng/L | 14.14 µg/L | |
| Thermo 55C CH4 analyzer 1 | CH4 | 13.31 ng/L | 665.56 µg/L |
| Thermo 450i SO2/H2S analyzer 1 | SO2 | 3.99 ng/L | 26.62 µg/L |
| H2S | 2.12 ng/L | 14.14 µg/L | |
| Thermo 46i N2O analyzer 1 | N2O | 0.04 µg/L | 36.61 µg/L |
| Innova 1412 photo-acoustic multi-gas analyzer 2 | CO2 | 2.75 µg/L | 1.83 g/L |
| EtOH | 0.15 µg/L | 1.91 g/L | |
| NH3 | 0.71 µg/L | 0.71 g/L | |
| MeOH | 0.11 µg/L | 1.33 g/L | |
| N2O | 0.05 µg/L | 1.83 g/L |
| Treatment LSM 1 | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| F3 2 | F6 | S3 | S6 | S vs. F 3 | 3 vs. 6 4 | Time | ||
| Ammonia | ||||||||
| Emission Rate (mg/h) | 622.42 a | 479.33 a | 1712.90 b | 1569.80 b | 154.70 | <0.001 | 0.12 | <0.001 |
| Reduction Potential (%) | 23% | −175% | −152% | |||||
| Methane | ||||||||
| Emission Rate (g/h) | 23.32 a | 26.29 b | 26.60 b | 29.56 c | 1.69 | <0.001 | <0.001 | 0.97 |
| Reduction Potential (%) | −13% | −14% | −27% | |||||
| Hydrogen Sulfide | ||||||||
| Emission Rate (mg/h) | 2.26 | 6.16 | 6.94 | 7.84 | 1.56 | 0.0496 | 0.29 | 0.13 |
| Reduction Potential (%) | −173% | −207% | −247% | |||||
| Ethanol | ||||||||
| Emission Rate (g/h) | 1.65 ab | 2.17c | 1.55 a | 2.07 bc | 0.13 | 0.426 | <0.001 | <0.001 |
| Reduction Potential (%) | −31% | 6% | −25% | |||||
| Carbon Dioxide | ||||||||
| Emission Rate (g/h) | 920.94 a | 1056.81 b | 1072.79 b | 1208.79 c | 58.42 | <0.001 | <0.001 | 0.98 |
| Reduction Potential (%) | −15% | −16% | −31% | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ross, E.G.; Peterson, C.B.; Zhao, Y.; Pan, Y.; Mitloehner, F.M. Manure Flushing vs. Scraping in Dairy Freestall Lanes Reduces Gaseous Emissions. Sustainability 2021, 13, 5363. https://doi.org/10.3390/su13105363
Ross EG, Peterson CB, Zhao Y, Pan Y, Mitloehner FM. Manure Flushing vs. Scraping in Dairy Freestall Lanes Reduces Gaseous Emissions. Sustainability. 2021; 13(10):5363. https://doi.org/10.3390/su13105363
Chicago/Turabian StyleRoss, Elizabeth G., Carlyn B. Peterson, Yongjing Zhao, Yuee Pan, and Frank M. Mitloehner. 2021. "Manure Flushing vs. Scraping in Dairy Freestall Lanes Reduces Gaseous Emissions" Sustainability 13, no. 10: 5363. https://doi.org/10.3390/su13105363
APA StyleRoss, E. G., Peterson, C. B., Zhao, Y., Pan, Y., & Mitloehner, F. M. (2021). Manure Flushing vs. Scraping in Dairy Freestall Lanes Reduces Gaseous Emissions. Sustainability, 13(10), 5363. https://doi.org/10.3390/su13105363






