The Influence of Hydrogen on Vaporization, Mixture Formation and Combustion of Diesel Fuel at an Automotive Diesel Engine
Abstract
1. Introduction
1.1. Pollutant Emissions of Diesel Engines
- whitish smoke (or cold smoke) which appears at engine cold start, is composed of liquid fuel particles in suspension. Its specific color appears due to droplet diameter over 0.8 μm, the wavelength corresponding to blue light, for which droplets appear in white color;
- blue smoke—the engine heats up and the smoke turns blue as a result of the reduction in the diameter of the droplets below 0.8 μm. The droplets appear colored blue, the smoke being visible at about one meter from the exhaust pipe;
- bluish-grey smoke—occurs when oil enters the combustion chamber because of leak tightness of the piston rings and advanced wear of the valve guides;
- black smoke (or hot smoke)—consists of carbon particles resulted from the incomplete combustion process. In the area with high temperatures, the cracking process occurs and forms carbon complexes, which together with aromatic hydrocarbons and tars, by agglomeration, produce flakes that form hot soot during a coagulation process; the black smoke occurs at high load, at acceleration, or at idle operating regime [4].
1.2. Hydrogen as Alternative Fuel for Diesel Engines
1.3. Aim of Research
2. Experimental Ant Theoretical Investigations Design
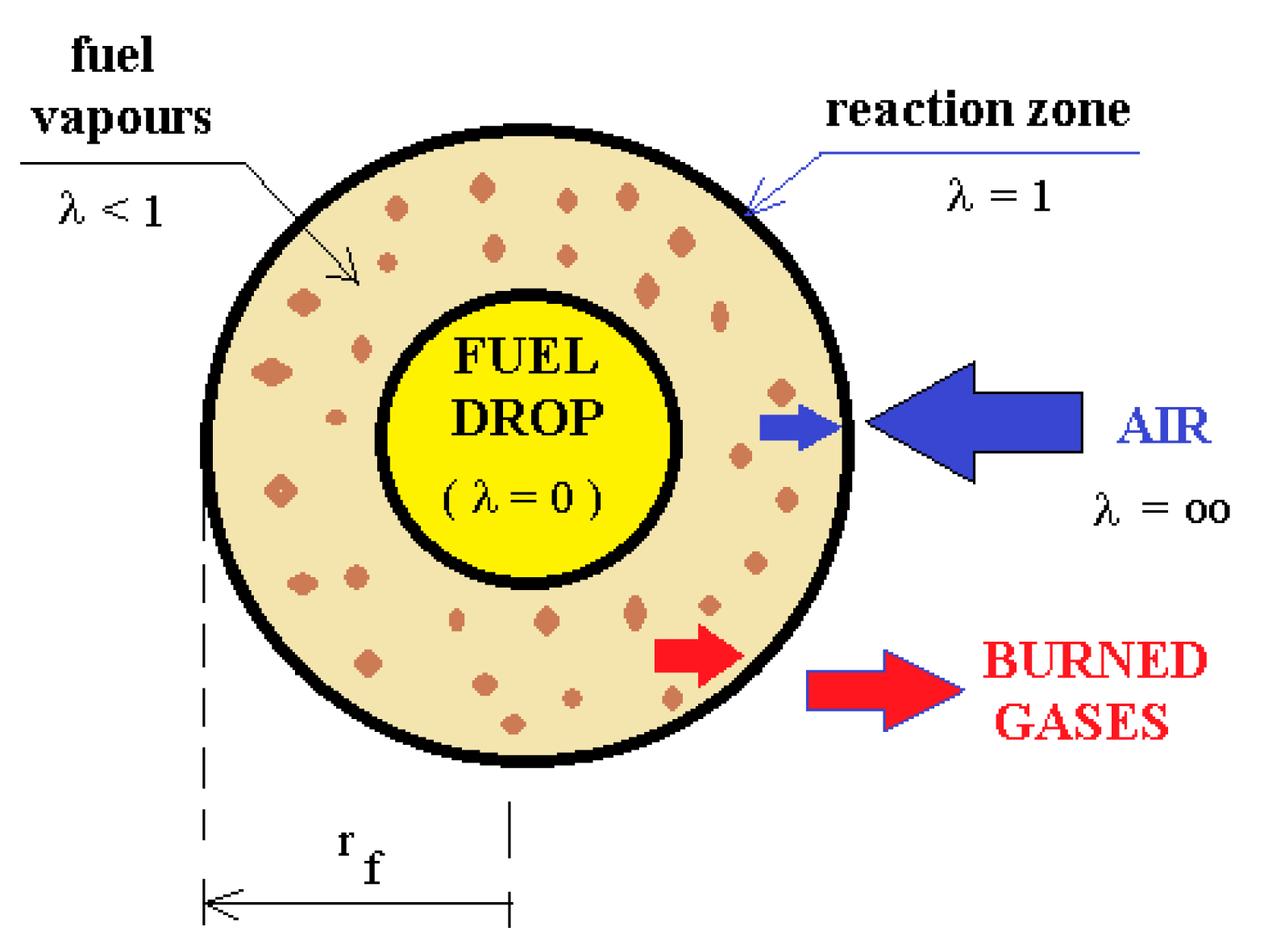
- The fuel droplet and its flame envelope are concentric spheres of constant radius;
- Not taken into consideration are convection and radiation of the hot gases;
- The permanent vaporization regime is an isobar;
- The temperature of the droplet is similar to the diesel fuel boiling point;
- The thermal conductivity coefficient is not influenced by the temperature;
- Specific heats of the perfect gases (air, fuel vapors) are not influenced by the temperature;
- The droplet radius influences concentration and temperature.
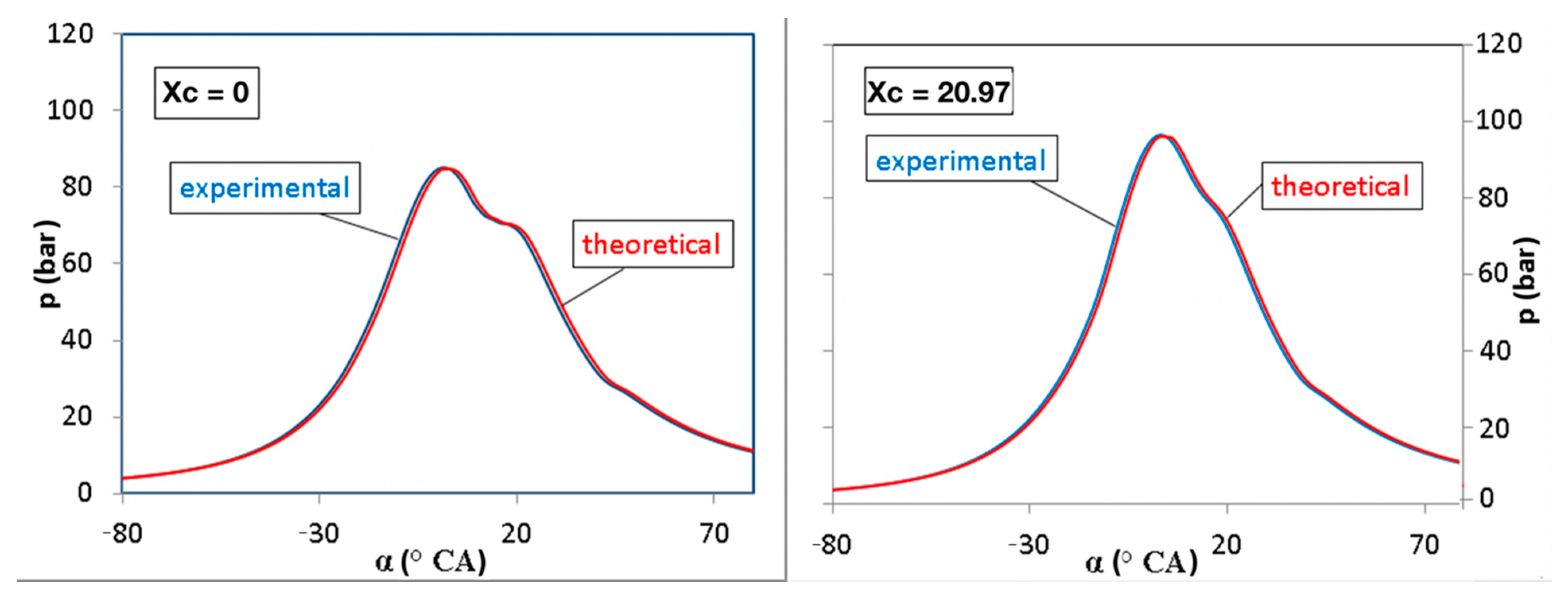
3. Results and Discussions
4. Conclusions
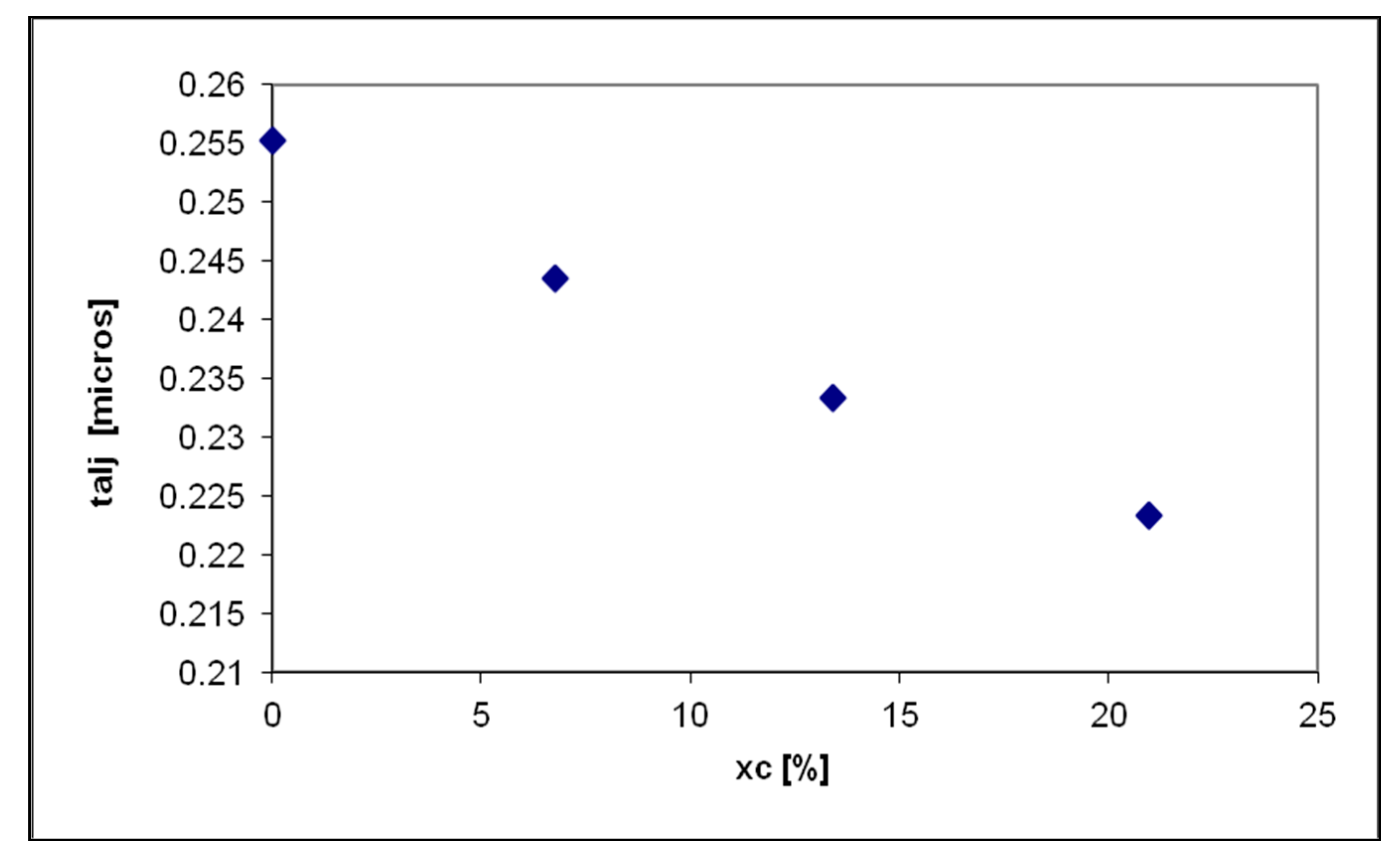
- Diesel fuel jets atomization and diesel fuel droplets vaporization of the diesel fuel are influenced by the presence of Hydrogen in the in-cylinder gaseous mixture. When Hydrogen content is increased, the duration of diesel fuel breakup starts to decrease with 12% at maximum xc versus classic fueling;
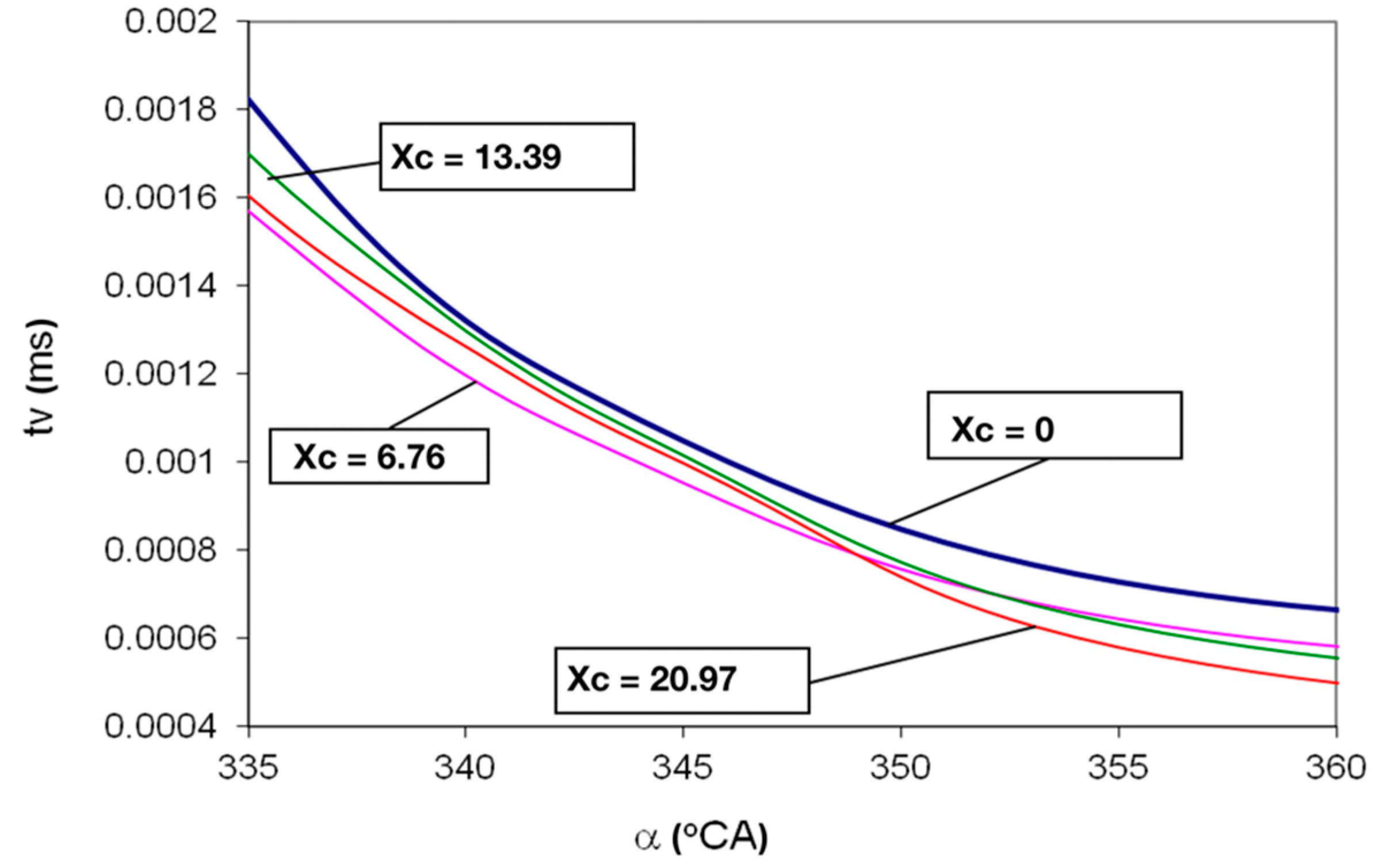
- The decrease of the evaporation time of the diesel fuel droplets by up to 28%, in the air-Hydrogen mixture combustion, ensures the increase of formation speed of the mixture between air-Hydrogen and diesel fuel droplets;
- The increase of mixture forming speed is related to the decrease of diesel fuel breakup duration at xc increase;
- The increase of combustion speed of diesel fuel droplets is in correlation with the acceleration of the vaporization process;
- Higher hydrogen heating value ensures the increase of heat released during combustion of the air-Hydrogen mixture and intensifies vaporization and combustion of the diesel fuel droplets which are situated in the vicinity of air-Hydrogen lean mixtures that burn at high speeds;
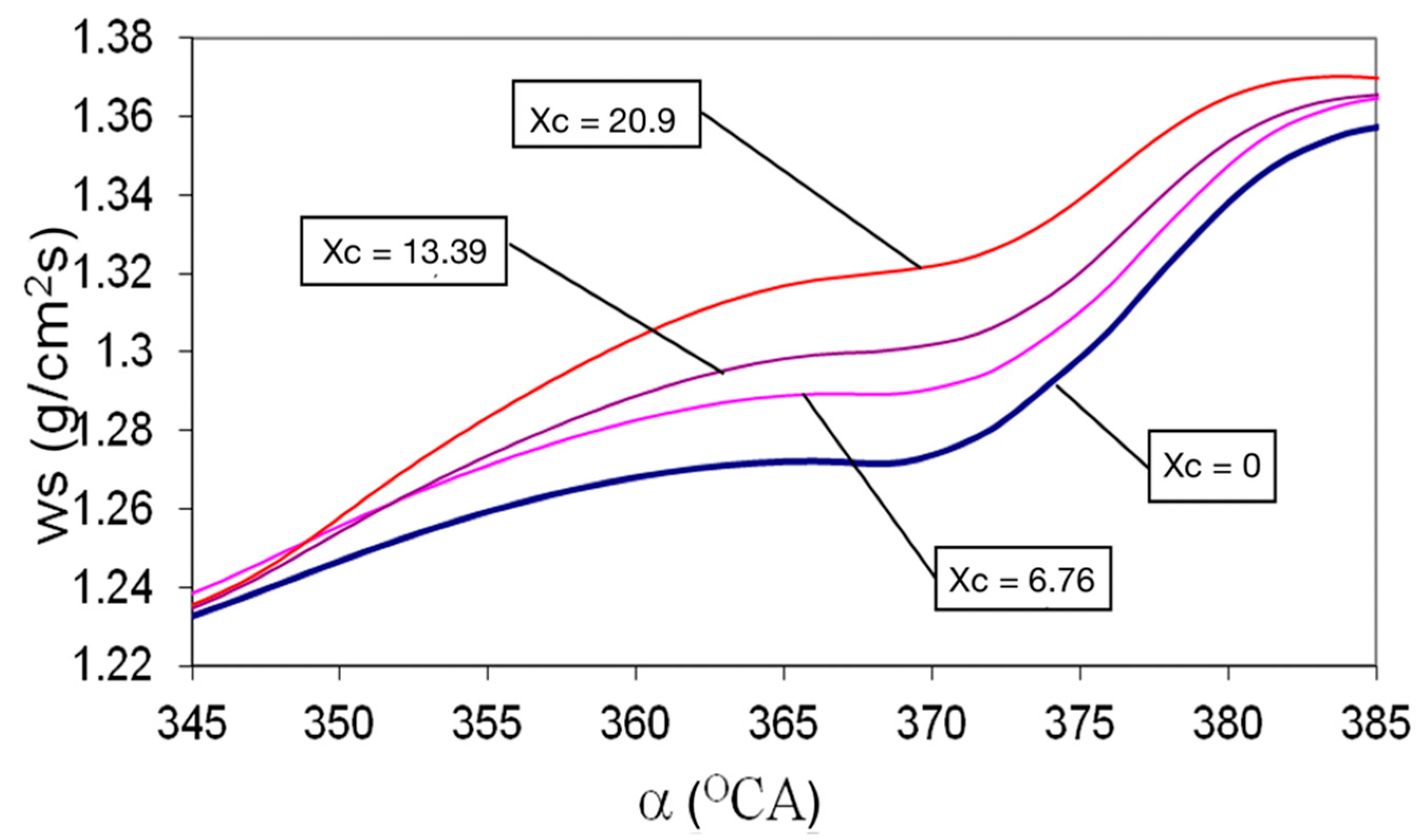
- The evaporated flow on the droplet surface increases by 3.9% and the maximum speed of droplets vaporization occurs sooner on cycle, with 5 °CA when comparing Hydrogen use versus classic fueling, xc = 0;
- Diesel fuel droplets autoignition decreases by 6% when Hydrogen is added into the inlet air
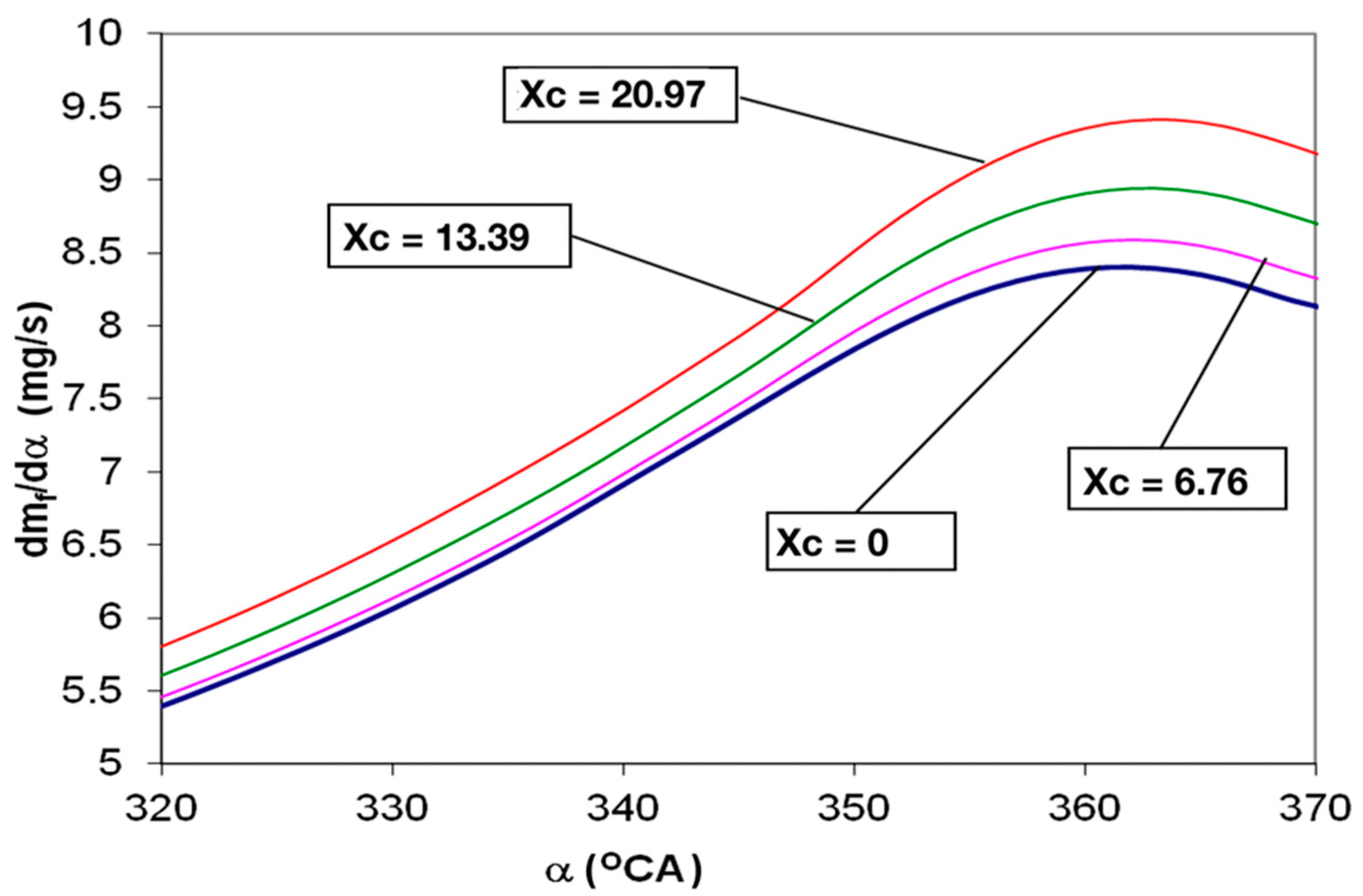
- The increase of the evaporated mass flow ensures the increase of burning speed and the decrease of droplet combustion time, in correlation with the tendency of autoignition decrease;
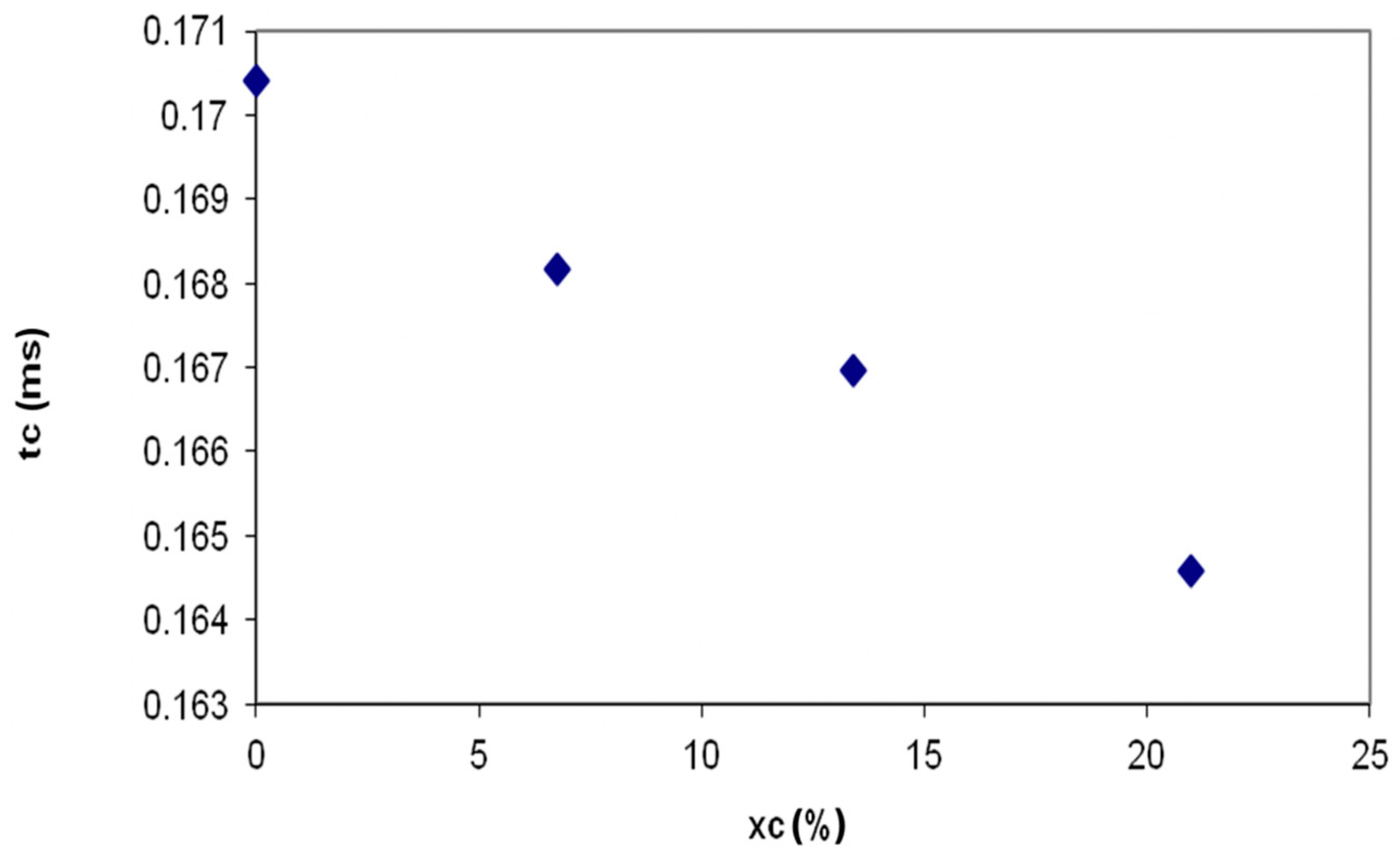
- Due to higher Hydrogen combustion rate, the combustion of the diesel droplet injected into an air-Hydrogen mixture is accelerated, the droplets combustion time being reduced by 3.6% versus classic fueling; the minimum combustion time of fuel droplets appears with 3 °CA sooner on cycle;
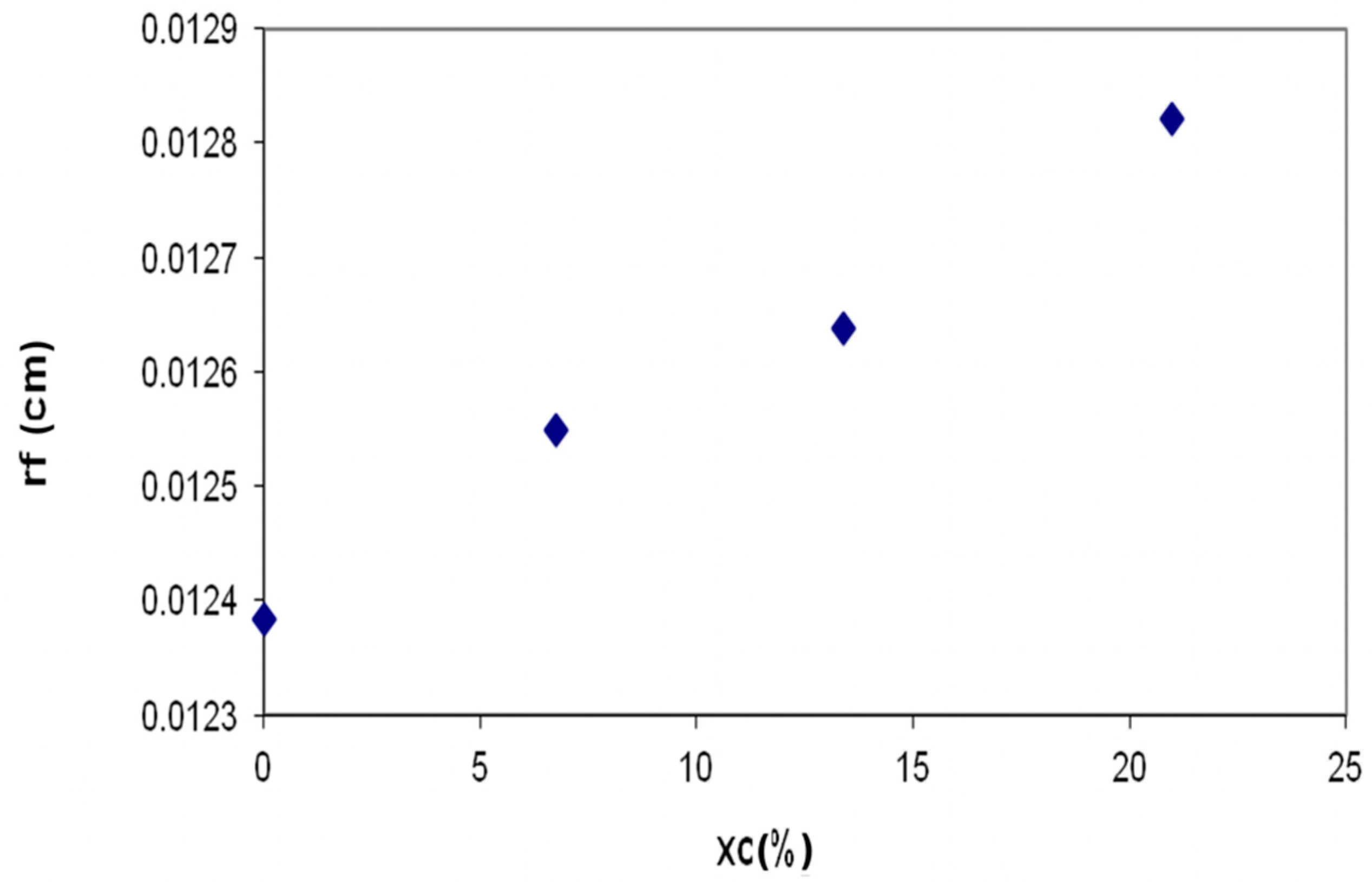
- The flame radius increases by 3.8% at maximum xc, in correlation with the increase of the combustion speed; Hydrogen combustion leads to the acceleration of the flame developing, the maximum radius of the flame developed around the fuel droplet being registered with 3 °CA sooner on combustion cycle comparative to xc = 0. In the presence of air-Hydrogen mixtures, the development of the flame around diesel fuel droplets is accelerated and the flames become larger at the increase of xc, the duration of the combustion process being shortened.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AVL | Anstalt für Verbrennungskraftmaschinen List GmbH—automotive research institute which fabricates the test bed equipments |
| BMW | Bayerische Motoren Werke GmbH—automotive company |
| °C | Celsius degree |
| °CA | crank angle degree |
| CIE | compression ignition engine |
| CO | carbon monoxide |
| CO2 | carbon dioxide |
| CH4 | methane |
| ECU | electronic control unit |
| EGR | exhaust gas recirculation |
| EU | European Union |
| FEV | Forschungsgesellschaft für Energietechnik und Verbrennungsmotoren |
| GHG | greenhouse gas |
| H2 | hydrogen |
| HCCI | homogeneous charge compression ignition |
| HHO | oxyhydrogen gas with a 2:1 ratio of hydrogen and oxygen |
| ICE | internal combustion engine |
| LHV | lower heating value |
| NH3 | ammonia |
| R&D | research and development |
| rpm | revolutions per minute |
| TDC | top dead centre |
| xc | diesel fuel substitute ratio with Hydrogen, % energetic |
| α | crankshaft angular position |
| l | air fuel ratio |
References
- Vision 2050. A Pathway for the Evolution of the Refining Industry and Liquid Fuels. Fuels Europe; European Union Communication: Brussels, Belgium, 2018. Available online: https://www.fuelseurope.eu/wp-content/uploads/2018/04/DEF_EN_FE_Vision2050_digital.pdf (accessed on 7 December 2020).
- DG MOVE-Expert Group Future Transport Fuels. State of Art on Alternative Fuels Transport Systems in the European Union; Final Report; European Union Communication: Brussels, Belgium, 2015. [Google Scholar]
- Bauen, A.; Gomez, I.; OudeNijeweme, D.; Paraschiv, M. European Commission, Directorate-General Research and Innovation, Smart, Green and Integrated Transport; Alternative Fuels; Expert Group Report; Studies and Reports; European Union Comunication: Brussels, Belgium, 2017. [Google Scholar]
- Popa, M.G.; Pana, C.; Negurescu, N. Diesel Engines. Processes; Matrixrom: Bucharest, Romania, 2003; pp. 1–706. [Google Scholar]
- Cernat, A.; Pana, C.; Negurescu, N.; Lazaroiu, G.; Nutu, C.; Fuiorescu, D. Hydrogen-As alternative fuel for diesel engines used in Transportation. Sustainability 2020, 12, 9321. [Google Scholar] [CrossRef]
- Lazaroiu, G.; Pană, C.; Mihaescu, L.; Cernat, A.; Negurescu, N.; Mocanu, R.; Negreanu, G. Solutions for energy recovery of animal waste from leather industry. Energy Convers. Manag. 2017, 149, 1085–1095. [Google Scholar] [CrossRef]
- Cernat, A.; Pana, C.; Negurescu, N.; Fuiorescu, D.; Nutu, C.; Mirica, I. Experimental Aspects of Hydrogen Use at Diesel Engine by Diesel Gas Method. Therm. Sci. 2018, 22, 1191–1200. [Google Scholar] [CrossRef]
- Hydrogen View. Available online: https://www.H2-view.com (accessed on 13 November 2020).
- Koch, D.; Ebert, T.; Sousa, A. Engine Adaptation from Diesel to H2 HP-EGR Lean Combustion Concept. MTZ Worldw. 2020, 81, 31–37. [Google Scholar] [CrossRef]
- Curry, P. Hydrogen: The Best Alternative to Internal Combustion? Auto Vista Group, 2019. Available online: https://autovistagroup.com/news-and-insights/hydrogen-best-alternative-internal-combustion (accessed on 13 November 2020).
- FEV Is Driving Forward Hydrogen Internal Combustion Engine Development. Available online: https://www.fev.com/uploads/media/PR_Hydrogen-Engine.pdf (accessed on 5 September 2020).
- Korn, T. The Hydrogen Engine is on its Way. MTZ Worldw. 2020, 81, 74. [Google Scholar] [CrossRef]
- Szwaja, S.; Grab-Rogalinski, K. Hydrogen combustion in a compression ignition diesel engine. Int. J. Hydrog. Energy 2009, 34, 4413–4421. [Google Scholar] [CrossRef]
- Karagöz, Y.; Sandalici, T.; Yuksek, L.; Wongwisess, S. Effect of hydrogen–diesel dual-fuel usage on performance, emissions and diesel combustion in diesel engines. Adv. Mech. Eng. 2016, 8. [Google Scholar] [CrossRef]
- Tutak, W.; Grab-Rogalinski, K.; Jamrozik, A. Combustion and Emission Characteristics of a Biodiesel-Hydrogen Dual-Fuel Engine. Appl. Sci. 2020, 10, 1082. [Google Scholar] [CrossRef]
- Qin, Z.; Yang, Z.; Jia, C.; Duan, J.; Wang, L. Experimental study on combustion characteristics of diesel–hydrogen dual-fuel engine. J. Therm. Anal. Calorim. 2020, 142, 1483–1491. [Google Scholar] [CrossRef]
- Temizer, I.; Cihan, O. Analysis of different combustion chamber geometries using hydrogen/diesel fuel in a diesel engine. Energy Sources Part A Recover. Utilization Environ. Effects 2020, 1–18. [Google Scholar] [CrossRef]
- Hoang, A.T.; Pham, V.V. A study on a solution to reduce emissions by using hydrogen as an alternative fuel for a diesel engine integrated exhaust gas recirculation. AIP Conf. Proc. 2020, 2235, 1. [Google Scholar] [CrossRef]
- Rimkus, A.; Pukalskas, S.; Juknelevičius, R.; Jonas Matijošius, J.; Kriaučiūnas, D. Evaluating Combustion, Performance and Emission Characteristics of CI Engine Operating on Diesel Fuel Enriched with HHO Gas. J. Kones 2019, 25, 2. [Google Scholar] [CrossRef]
- Vard, A.A. A Study on the Effects of Combined Diesel-Hydrogen Combustion on Diesel Engines Using Experimental and Simulation Techniques. Ph.D. Thesis, Brunel University School of Engineering and Design, London, UK, September 2018. [Google Scholar]
- Bika, A.S.; Franklin, L.; Kittelson, D.B. Hydrogen Assisted Combustion of Ethanol in Diesel Engines. University of Minnesota. 2020. Available online: http://dept.me.umn.edu/centers/cdr/reports/agexpoposter.pdf (accessed on 26 August 2020).
- Lhuillier, C.; Brequigny, P.; Contino, F.; Mounaim-Rousselle, C. Experimental study on ammonia/hydrogen/air combustion in spark ignition engine conditions. Fuel 2020, 269, 117448. [Google Scholar] [CrossRef]
- Rouhollah, A.; Mohammad, S.H. Numerical investigation on adding/substituting hydrogen in the CDC and RCCI combustion in a heavy duty engine. Appl. Energy 2018, 213, 450–468. [Google Scholar] [CrossRef]
- Şanlı, A.; Yılmaz, I.T.; Gümüş, M. Experimental Evaluation of Performance and Combustion Characteristics in a Hydrogen–Methane Port Fueled Diesel Engine at Different Compression Ratios. Energy Fuels 2020, 34, 2272–2283. [Google Scholar] [CrossRef]
- Saleh, H.E. Performance and emissions characteristics of direct injection diesel engine fueled by diesel-jojoba oil-butanol blends with hydrogen peroxide. Fuel 2020, 285, 119048. [Google Scholar] [CrossRef]
- Das, B.; Pai, A.; Kini, C.R. Oxy-hydrogen hybrid diesel engine. J. Eng. Appl. Sci. 2020, 15, 1063–1068. [Google Scholar]
- Asai, G. A Study of Gas-Diesel Dual Fuel Combustion for Higher Thermal Efficiency and Lower Emissions. Yanmar Tech. Rev. Res. Dev. Manag. Div. 2019. Available online: https://www.yanmar.com/nz/about/technology/technical_review/2019/1001_6.html (accessed on 3 September 2020).
- Shivaprasad, K.V.; Chitragar, P.R.; Kumar, G.N. Effect of Hydrogen Addition on Combustion and Emissions Performance of a High Speed Spark Ignited Engine at Idle Condition. Therm. Sci. 2018, 22, 1405–1413. [Google Scholar] [CrossRef]
- Pochet, M.; Jeanmart, H.; Contino, F. A 22:1 Compression Ratio Ammonia-Hydrogen HCCI Engine: Combustion, Load, and Emission Performances. Fundamental Characterization and performance of alterative fuels. Front. Mech. Eng. 2020, 6, 43. [Google Scholar] [CrossRef]
- Sathishkumar, S.; Mohamed Ibrahim, M. Effect of Hydrogen Energy Share on a Hydrogen Diesel Dual Fuel Mode using a Common Rail Direct Injection System. Int. J. Eng. Adv. Technol. 2019, 9, 1–7. [Google Scholar]
- Merker, G.; Schwarz, C.; Teichmann, R. Internal Combustion Engines Fundamentals: Running, Simulation, Measuring; Springer: Berlin, Germany, 2011. [Google Scholar] [CrossRef]
- Felis-Carrasco, F. Atomization and Dispersion of Liquid Jets: Numerical and Experimental Approaches. Ph.D. Thesis, Ecole Centrale Marseille, Marseille, France, March 2017. Available online: https://tel.archives-ouvertes.fr/tel-01558141 (accessed on 26 December 2020).
- Pimsner, V.; Vasilescu, C.; Radulescu, G. Energetics of Internal Combustion Turbo-Engines; Academy of RPR: Bucharest, Romania, 1964. [Google Scholar]
- Apostolescu, N.; Chiriac, R. Combustion Process in Internal Combustion Engines, Fuel Economy and Emissions Control; Tehnica: Bucharest, Romania, 1998. [Google Scholar]
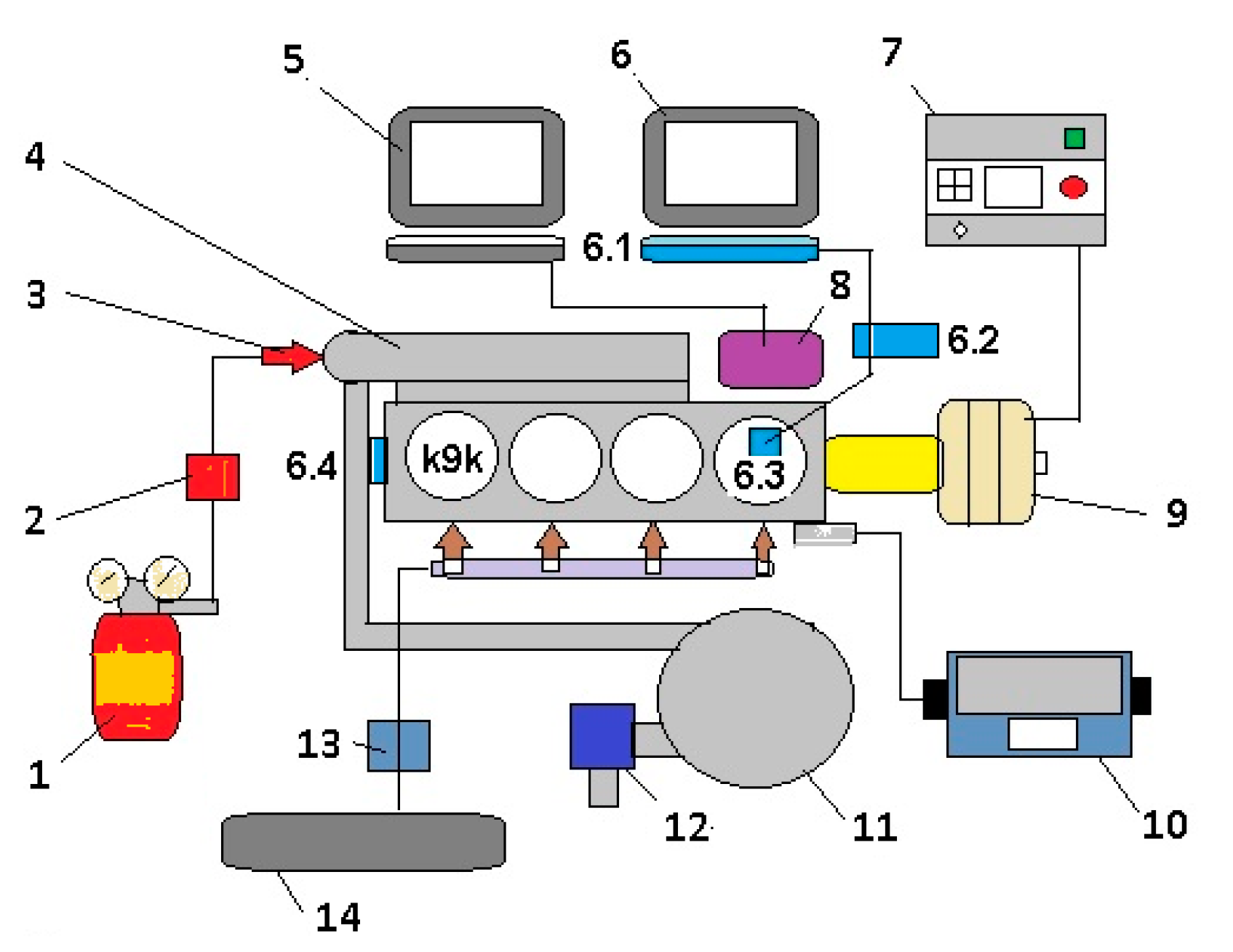
| Measured Parameter | Measurement Device | Unit | Uncertainties |
|---|---|---|---|
| Engine speed | Horiba Schenck E90 | rpm | ±1 rpm |
| Engine torque | Horiba Schenck E90 | Nm | ±0.2% |
| Diesel flow rate | Optimass 3050 C | kg/h | ±0.1% |
| Hydrogen flow rate | Alicat Scientific MCR | kg/h | ±0.4% |
| Inlet air flow rate | Krohne H 250 | m3/h | ±0.35% |
| In-cylinder pressure | AVL GU 12 P | bar | ±0.05% |
| Crank angle degree | AVL 365 CC | °CA | ±0.1% |
| Temperatures | Thermocouple Cromel–Alumel TTC | °C | ±1 °C |
| Thermoresistence P100 TTR | °C | ±2 °C | |
| Shimaden SR93 indicators | °C | ± 0.3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cernat, A.; Pana, C.; Negurescu, N.; Lazaroiu, G.; Nutu, C. The Influence of Hydrogen on Vaporization, Mixture Formation and Combustion of Diesel Fuel at an Automotive Diesel Engine. Sustainability 2021, 13, 202. https://doi.org/10.3390/su13010202
Cernat A, Pana C, Negurescu N, Lazaroiu G, Nutu C. The Influence of Hydrogen on Vaporization, Mixture Formation and Combustion of Diesel Fuel at an Automotive Diesel Engine. Sustainability. 2021; 13(1):202. https://doi.org/10.3390/su13010202
Chicago/Turabian StyleCernat, Alexandru, Constantin Pana, Niculae Negurescu, Gheorghe Lazaroiu, and Cristian Nutu. 2021. "The Influence of Hydrogen on Vaporization, Mixture Formation and Combustion of Diesel Fuel at an Automotive Diesel Engine" Sustainability 13, no. 1: 202. https://doi.org/10.3390/su13010202
APA StyleCernat, A., Pana, C., Negurescu, N., Lazaroiu, G., & Nutu, C. (2021). The Influence of Hydrogen on Vaporization, Mixture Formation and Combustion of Diesel Fuel at an Automotive Diesel Engine. Sustainability, 13(1), 202. https://doi.org/10.3390/su13010202