Pennisetum sinese: A Potential Phytoremediation Plant for Chromium Deletion from Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions
2.2. Analysis of Growth Parameters
2.3. Determination of Mineral Elements
2.4. Data Analysis and Statistics
3. Results
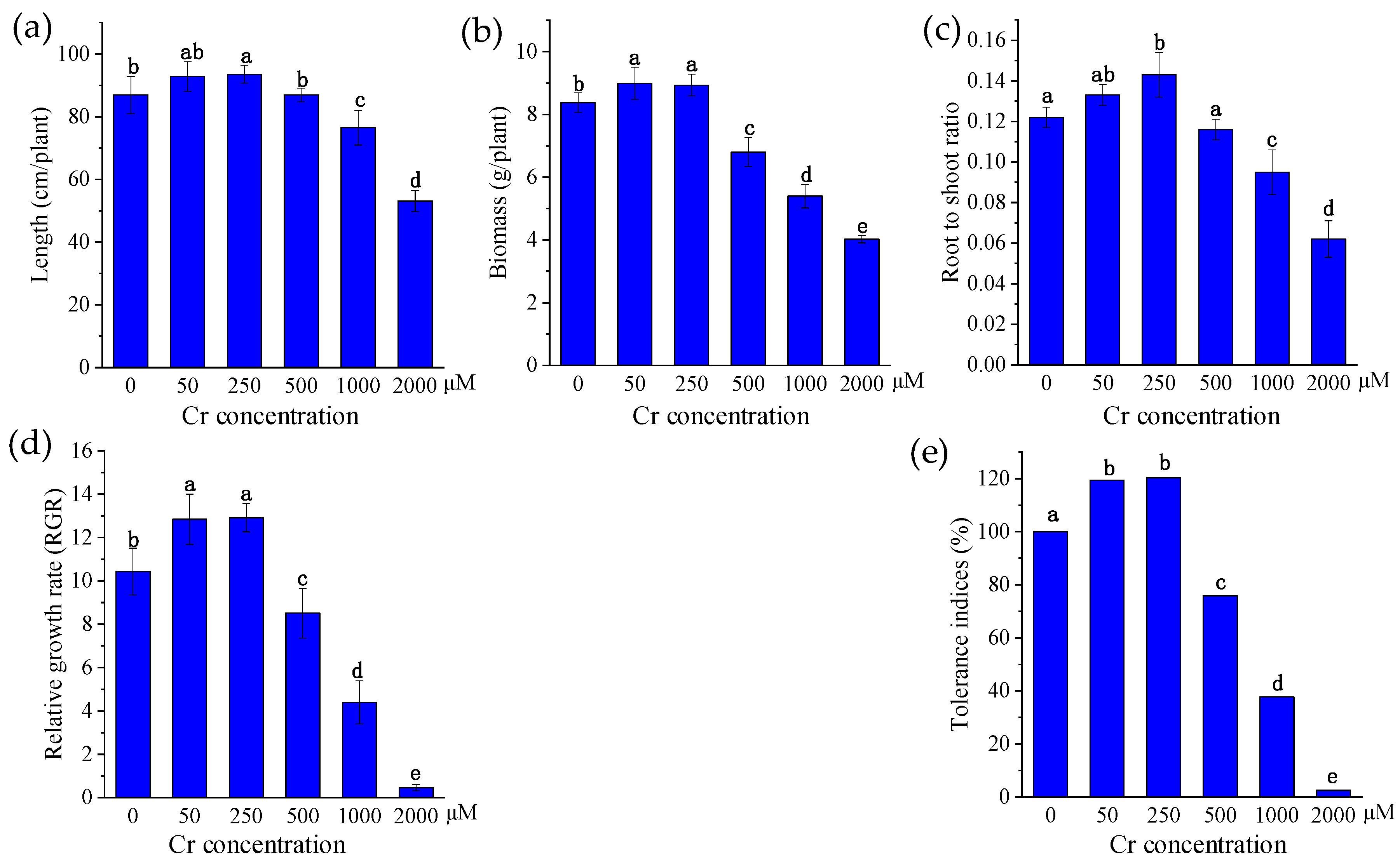
3.1. Growth Analysis of P. sinese
3.2. Effects of Cr on P. sinese Growth
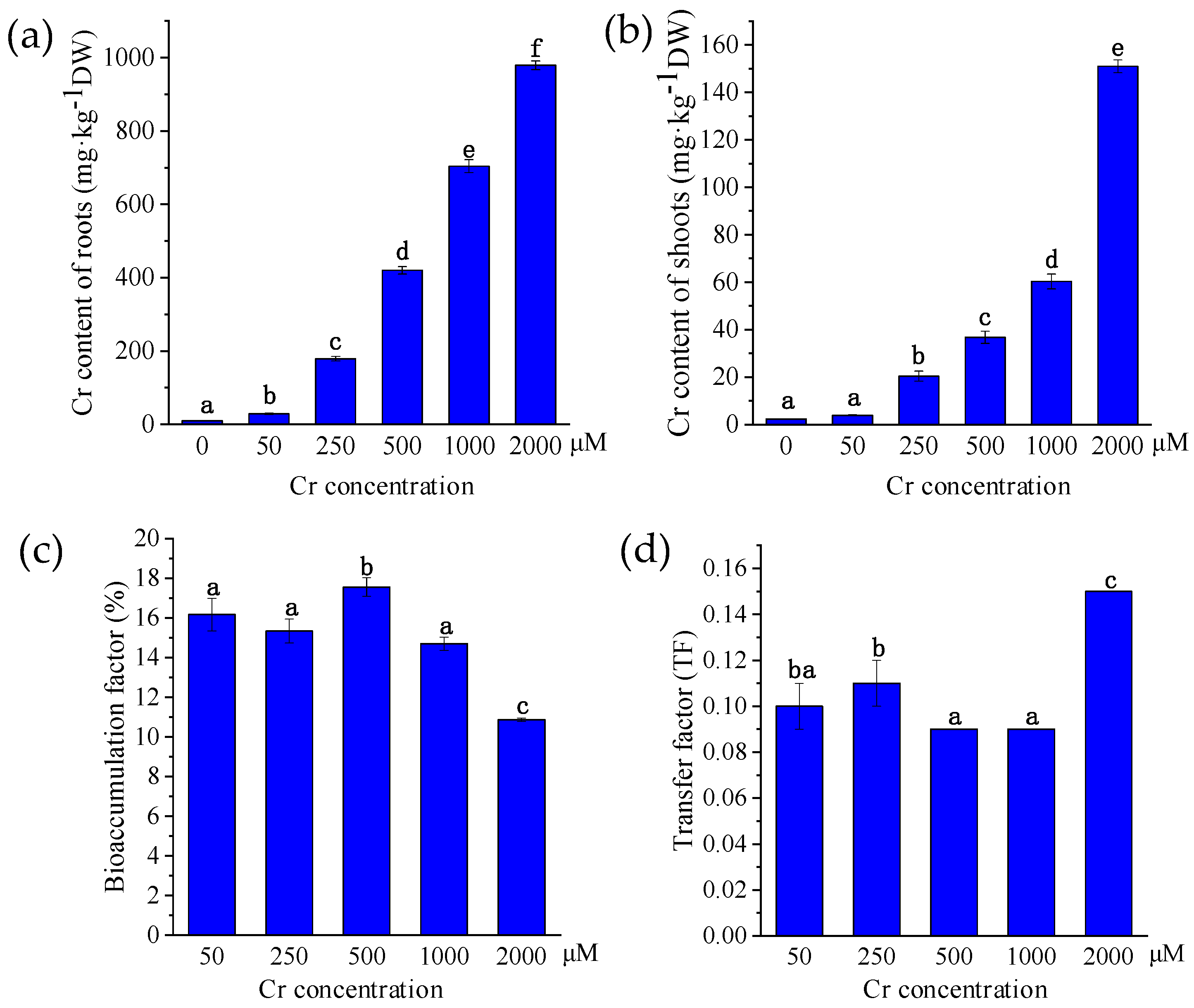
3.3. Cr Accumulation in P. sinese
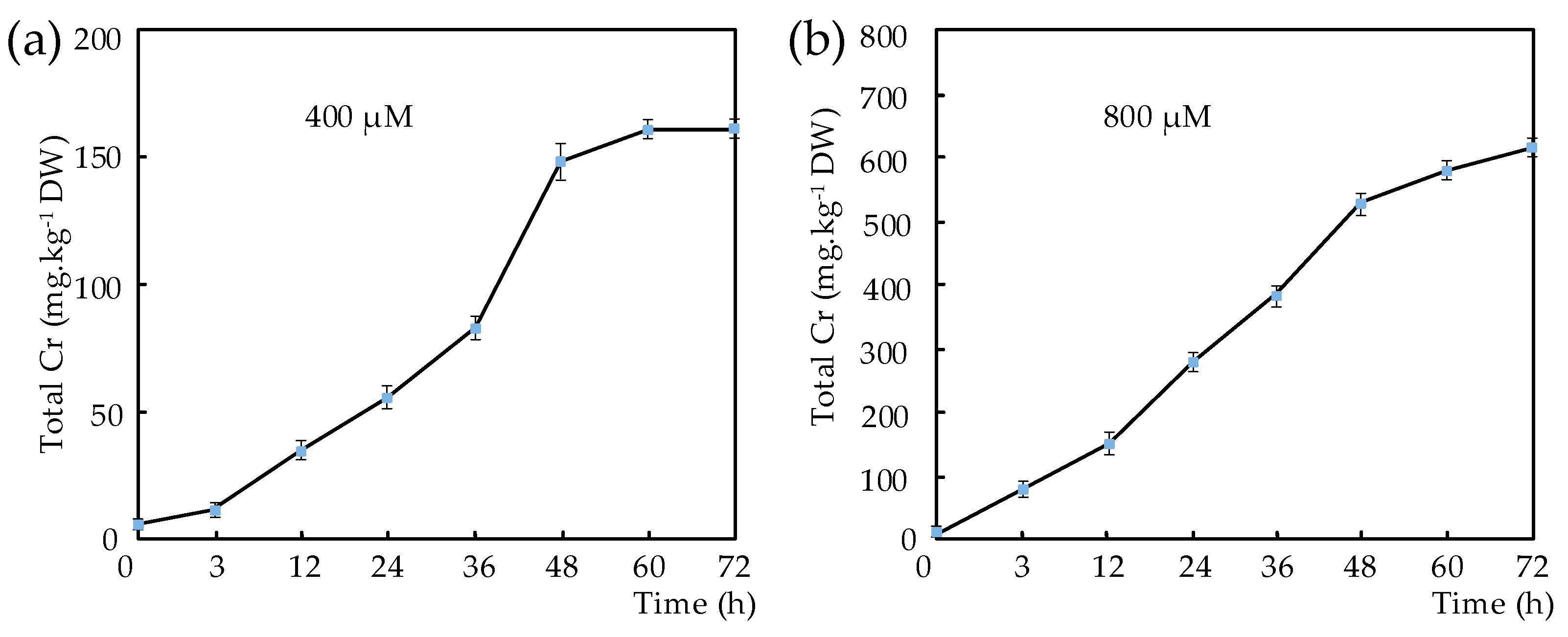
3.4. Dynamic of Cr Accumulation
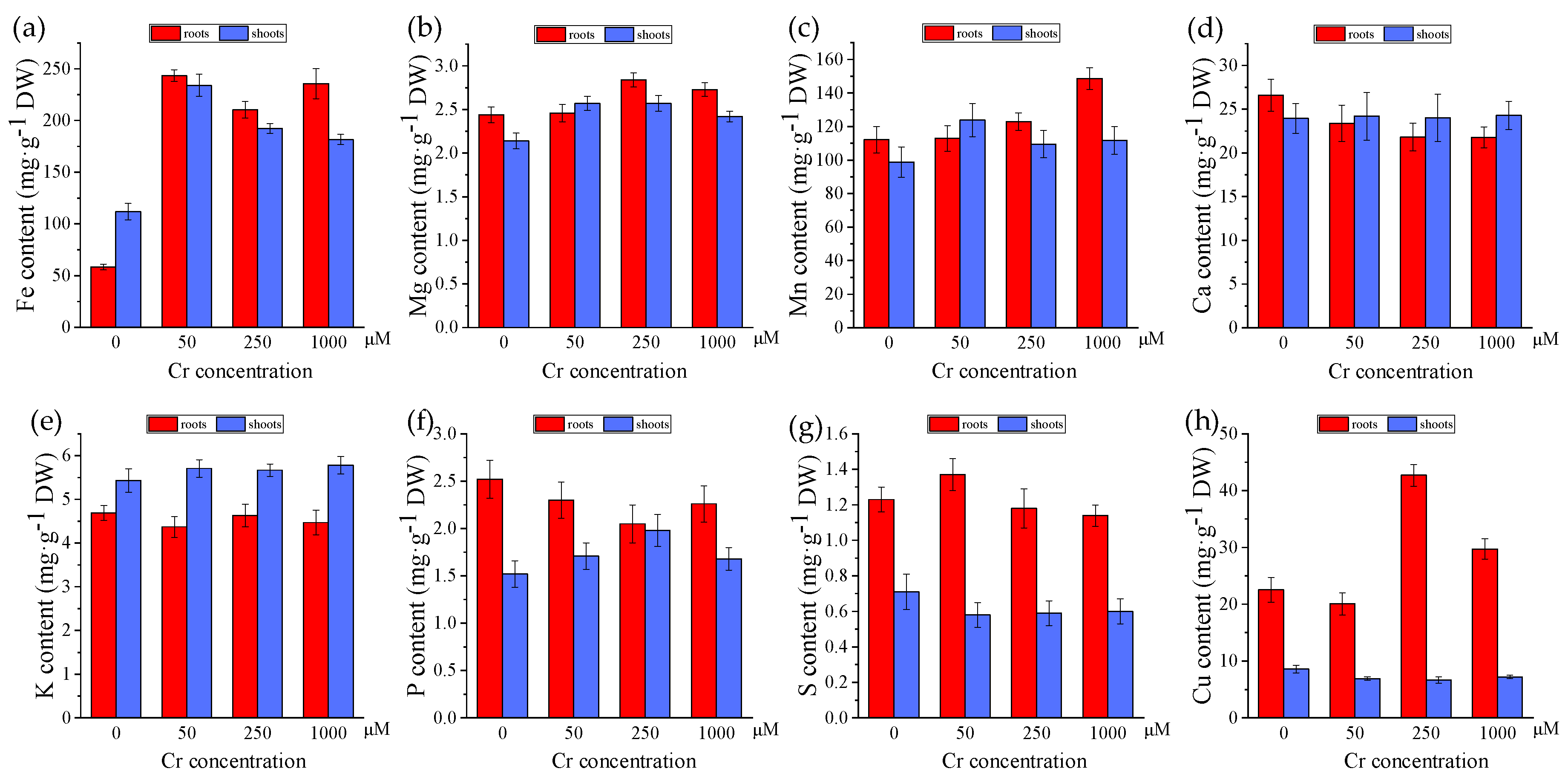
3.5. Effects of Cr on Element Accumulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dixit, R.; Malaviya, D.; Pandiyan, K.; Singh, U.B.; Sahu, A.; Shukla, R.; Singh, B.P.; Rai, J.P.; Sharma, P.K.; Lade, H.; et al. Bioremediation of heavy metals from soil and aquatic environment: An overview of principles and criteria of fundamental processes. Sustainability 2015, 7, 2189–2212. [Google Scholar] [CrossRef]
- Helena, O. Chromium as an environmental pollutant, insights on induced plant toxicity. J. Bot. 2012, 2012, 375843. [Google Scholar] [CrossRef]
- Kim, Y.; Roh, Y. Environmental Application of Biogenic Magnetite Nanoparticles to Remediate Chromium(III/VI)-Contaminated Water. Minerals 2019, 9, 260. [Google Scholar] [CrossRef]
- Ramos-Miras, J.J.; Gil, C.; Rodríguez-Martín, J.A.; Boluda, R. Ecological risk assessment of mercury and chromium in greenhouse soils. Environ. Geochem. Health 2019, 42. [Google Scholar] [CrossRef]
- Bosnir, J.; Puntaric, D.; Cvetkovic, Z.; Pollak, L.; Barusic, L.; Klaric, I.; Miskulin, M.; Puntarić, I.; Puntarić, E.; Milosević, M. Effects of magnesium, chromium, iron and zinc from food supplements on selected aquatic organisms. Coll. Antropol. 2013, 37, 965–971. [Google Scholar] [CrossRef]
- Kimbrough, D.E.; Cohen, Y.; Winer, A.M.; Chromiumeelman, L.; Mabuni, C. A critical assessment of chromium in the environment. Crit. Rev. Environ. Sci. Technol. 1999, 29, 1–46. [Google Scholar] [CrossRef]
- Janusz, B.; Joanna, K. A comparison of the in vitro genotoxocity of tri-hexavalent chromium. Mutat. Res. 2000, 469, 135–145. [Google Scholar] [CrossRef]
- Yuan, Z.; Cheng, X.; Zhong, L.; Wu, R.; Zheng, Y. Preparation, characterization and performance of an electrospun carbon nanofiber mat applied in hexavalent chromium removal from aqueous solution. J. Environ. Sci. 2019, 77, 78–87. [Google Scholar] [CrossRef]
- Peralta-Videa, J.R.; Lopez, M.L.; Narayan, M.; Saupe, G.; Gardea-Torresdey, J. The biochemistry of environmental heavy metal uptake by plants, implications for the food chain. Int. J. Biochem. Cell B 2009, 41, 1665–1677. [Google Scholar] [CrossRef]
- Paiva, L.B.; de Oliveira, J.G.; Azevedo, R.A.; Ribeiro, D.R.; da Silva, M.G.; Vitória, A.P. Ecophysiological responses of water hyacinth exposed to chromium3+ and chromium6+. Environ. Exp. Bot. 2009, 65, 403–409. [Google Scholar] [CrossRef]
- Islam, F.; Yasmeen, T.; Arif, M.S.; Riaz, M.; Shahzad, S.M.; Imran, Q.; Ali, I. Combined ability of chromium (Cr) tolerant plant growth promoting bacteria (PGPB) and stress alleviator (salicylic acid) in attenuation of chromium stress in maize plants. Plant Physiol. Biochem. 2016, 108, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Bavaresco, J.; Jessé, R.F.; Moraes, M.T.; Sánchez-Rodríguez, A.R.; Anghinoni, I. Chromium from hydrolyzed leather affects soybean growth and nodulation. Pedosphere 2017, 29, 95–101. [Google Scholar] [CrossRef]
- Diwan, H.; Ahmad, A.; Iqbal, M. Chromium-induced modulation in the antioxidant defense system during phenological growth stages of Indian mustard. Int. J. Phytoremediat. 2010, 12, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, S.S.; Goyal, D. Microbial and plant derived biomass for removal of heavy metals from waste water. Bioresour. Technol. 2007, 98, 2243–2257. [Google Scholar] [CrossRef] [PubMed]
- Hunce, S.Y.; Akgul, D.; Demir, G.; Mertoglu, B. Solidification/stabilization of landfillleachate concentrate using different aggregate materials. Waste Manag. 2012, 32, 1394–1400. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Tsang, D.C.; Li, J.S.; Yeung, T.L.; Ding, S.; Poon, C.S. Green remediation of contaminated sediment by stabilization/solidification with industrial byproducts and CO2 utilization. Sci. Total Environ. 2018, 631–632, 1321–1327. [Google Scholar] [CrossRef]
- Onireti, O.O.; Lin, C.; Qin, J. Combined effects of low-molecular-weight organic acids on mobilization of arsenic and lead from multi-contaminated soils. Chemosphere 2017, 170, 161–168. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef]
- Senneca, O.; Cortese, L.; Di-Martino, R.; Fabbricino, M.; Ferraro, A.; Race, M.; Scopino, A. Mechanisms affecting the delayed efficiency of cement based stabilization/solidification processes. J. Clean. Prod. 2020. [Google Scholar] [CrossRef]
- Sun, Y.; Guan, F.; Yang, W.; Wang, F. Removal of chromium from a contaminated soil using oxalic acid, citric acid, and hydrochloric acid: Dynamics, mechanisms, and concomitant removal of non-targeted metals. Int. J. Environ. Res. Public Health 2019, 16, 2771. [Google Scholar] [CrossRef]
- Hussein, H.; Farag, S.; Moawad, H. Isolation and characterization of Pseudomonas resistant toheavy metals contaminants. Arab. J. Biotechnol. 2004, 7, 13–22. [Google Scholar] [CrossRef]
- Kapoor, A.; Viraraghvan, T. Fungal biosorption—An alternative treatment option for heavy metal bearing wastewater: A review. Bioresour. Technol. 1995, 53, 195–206. [Google Scholar] [CrossRef]
- Malik, A. Metal bioremediation through growing cells. Environ. Int. 2004, 30, 261–278. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Lin, H.L. Remediation of soil contaminated with the heavy metal (Cd2+). J. Hazard. Mater. 2005, 122, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Abhisheka, A.; Saranyab, N.; Chandia, P.; Selvarajua, N. Studies on the remediation of chromium(VI) from simulated wastewater using novel biomass of Pinus kesiya cone. Desalin. Water Treat. 2018, 114, 192–204. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Chermaine Ong, S.M.; Chu, Z. Progress of phytoremediation focus on new plant and molecular mechanism. J. Plant Biol. Soil Health 2013, 1, 5. [Google Scholar]
- Zhang, X.H.; Liu, J.; Huang, H.T.; Chen, J.; Zhu, Y.N.; Wang, D.Q. Chromium accumulation by the hyperaccumulator plant Leersia hexandra Swartz. Chemosphere 2007, 67, 1138–1143. [Google Scholar] [CrossRef]
- Sampanpanish, P.; Khaodhiar, S.; Pongsapich, W.; Khan, E. Alternative for chromium removal, phytoremediation and biosorption with weed plant species in Thailand. Sci. Asia 2007, 33, 353–362. [Google Scholar] [CrossRef]
- Sampanpanish, P.; Ongsapich, P.W.; Khaodhiar, S.; Khan, E. Chromium removal from soil by phytoremediation with weed plant species in Thailand. Water Air Soil Pollut. 2006, 6, 191–206. [Google Scholar] [CrossRef]
- Xu, S.; Jaffé, P.R. Effects of plants on the removal of hexavalent chromium in wetland sediments. J. Environ. Qual. 2006, 35, 334–341. [Google Scholar] [CrossRef]
- Bareen, F.; Khilji, S. Bioaccumulation of metals from tannery sludge by Typha angustifolia L. Afr. J. Biotechnol. 2008, 7, 3314–3320. [Google Scholar] [CrossRef]
- Mangkoedihardjo, S.; Ratnawati, R.; Alfianti, N. Phytoremediation of hexavalent chromium polluted soil using Pterocarpus indicus and Jatropha curcas L. World Appl. Sci. J. 2008, 4, 338–342. [Google Scholar]
- Susana, R.G.; Enrique, M.N.; Inmaculada, V.B.; Susana, R.F. Accumulation and tolerance characteristics of chromium in a cordgrass chromium-hyperaccumulator Spartina argentinensis. J. Hazard. Mater. 2011, 185, 862–869. [Google Scholar] [CrossRef]
- Adki, V.S.; Jadhav, J.P.; Bapat, V.A. Nopalea cochenillifera, a potential chromium (VI) hyperaccumulator plant. Environ. Sci. Pollut. Res. 2013, 20, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, Z.; Zhang, Y.; Gao, Q. Introduction experiment of Pennisetum sp. in central and southern region of Hebei. J. Anhui Agric. Sci. 2015, 43, 78–80. [Google Scholar]
- Lin, X.S.; Lin, Z.X.; Lin, D.M.; Lin, H.; Luo, H.L.; Hu, Y.P.; Lin, C.M.; Zhu, C.Z. Effects of different years of planting Pennisetum sp. on the plant and insect diversity in Pennisetum sp. communities. Chin. J. Appl. Ecol. 2012, 23, 2849–2854. [Google Scholar]
- Lin, X.S.; Lin, Z.X.; Lin, H.; Lin, D.M.; Luo, H.L.; Hu, Y.P.; Lin, C.M.; Zhu, C.Z. Physiological responses and alkaline-tolerance evaluation on 5 species of Juncao under alkaline stress during seedling stage. Plant Physiol. J. 2013, 49, 167–174. [Google Scholar]
- Dhir, B.; Srivastava, S. Heavy metal tolerance in metal hyperaccumulator plant, Salvinia natans. Bull. Environ. Contam. Toxicol. 2013, 90, 720–724. [Google Scholar] [CrossRef]
- Cho, H.J.; Myung, S.W. Determination of cadmium, chromium and lead in polymers by ICP-OES using a high pressure asher (HPA). Bull. Korean Chem. Soc. 2011, 32, 489–497. [Google Scholar] [CrossRef]
- Lin, Z. Plants for Mushroom Culture, 3rd ed.; State Administration College Press: Beijing, China, 2013. [Google Scholar]
- Lasat, M.M. Phytoextraction of toxic metals: A review of biological mechanisms. J. Environ. Qual. 2002, 31, 109–120. [Google Scholar] [CrossRef]
- Pilon-Smits, E. Phytoremediation. Ann. Rev. Plant Biol. 2005, 56, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Samantaray, S.; Rout, G.R.; Das, P. Role of chromium on plant growth and metabolism. Acta Physiol. Plant. 1998, 20, 201–212. [Google Scholar] [CrossRef]
- Shanker, A.K.; Cervantes, C.; Loza-Tavera, H.; Avudainayagam, S. Chromium toxicity in plants. Environ. Int. 2005, 31, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lv, J.Y.; Zang, W. Effects of chromium6+ treatment on chromium accumulation and physiologyical characteristics of celery (Apium graveolens). J. Nucl. Agric. Sci. 2010, 24, 639–644. [Google Scholar] [CrossRef]
- López, M.L.; Peralta-Videa, J.R.; Benitez, T.; Gardea-Torresdey, J.L. Enhancement of lead uptake by alfalfa (Medicago sativa) using EDTA and a plant growth promoter. Chemosphere 2005, 61, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, I.; Marchal, G.; Corréal, E.; Zanuzzi, A.; Lutts, S. Variation in response to heavy metals during vegetative growth in Dorycnium pentaphyllum Scop. Plant Growth Regul. 2009, 59, 1–11. [Google Scholar] [CrossRef]
- Clabeaux, B.L.; Navarro, D.A.G.; Aga, D.S.; Bisson, M.A. Cd tolerance and accumulation in the aquatic macrophyte, Charaaustralis: Potential use for charophytes in phytoremediation. Environ. Sci. Technol. 2011, 45, 5332–5338. [Google Scholar] [CrossRef]
- Yin, G.; Bi, L.; Song, X.; Luo, H.; Ji, P.; Lin, Q.; Liu, Q.; Tang, G. Adsorption of Cd(II) from aqueous solution by Pennisetum sp. straw biochars derived from different modification methods. Environ. Sci. Pollut. Res. Int. 2019, 26, 7024–7032. [Google Scholar] [CrossRef]
- Zhang, S.R.; Tian, Y.K.; Yang, X.M.; Xu, X.X.; Li, T.; Huang, C.Y.; Pu, Y.L.; Li, Y. The Application of P. sinese in the Phytoremediation of Heavy Metal Cadmium Pollution in Soil. China Patent 102941219A, 13 April 2013. [Google Scholar]
- Chen, T.T.; Gao, J.; Liu, Z.F.; He, Y.H. Characteristics of chromium bioaccumulation of Hemarthria compressa. Environ. Sci. Technol. 2011, 34, 83–87. [Google Scholar] [CrossRef]
- Yang, D.; Lv, J.Y.; Cheng, Y.A.; Gao, J.F. Subcellular distribution of chromium and its effects on some enzyme activities in pumpkin. J. Agro-Environ. Sci. 2007, 4, 1352–1355. [Google Scholar]
- Choudhary, S.P.; Kanwar, M.; Bhardwaj, R.; Yu, J.Q.; Tran, L.S.P. Chromium stress mitigation by polyamine-brassinosteroid application involves phytohormonal and physiological strategies in Raphanus sativus L. PLoS ONE 2012, 7, e33210. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.R.; Ahmad, N.; Masood, K.R.; Zahid, D.M. The Influence of cadmium and chromium on the biomass production of Shisham (Dalbergia Sissoo Roxb.) seedlings. Pak. J. Bot. 2008, 40, 1341–1348. [Google Scholar] [CrossRef]
- Zeng, E.R.; Qiu, B.Y.; Ali, S.; Zhang, G.P. Genotypic differences in nutrient uptake and accumulation in rice under chromium stress. J. Plant Nutr. Soil 2010, 33, 518–528. [Google Scholar] [CrossRef]
- Kalve, S.; Sarangi, B.K.; Pandey, R.A.; Chakrabarti, T. Arsenic and chromium hyperaccumulation by an ecotype of Pteris vittata-prospective for phytoextraction from contaminated water and soil. Curr. Sci. India 2011, 100, 888–894. [Google Scholar] [CrossRef]

| Traits | Range | Average |
|---|---|---|
| Height (cm) | 198.7–372.8 | 335.7 |
| Length of internode (cm) | 6.4–14.3 | 10.7 |
| Effective tillers | 3–8 | 5.8 |
| Leaf length (cm) | 33.5–85.6 | 56.7 |
| Maximum stem circumference (cm) | 1.9–2.9 | 2.5 |
| Root/shoot ratio | 0.31–0.64 | 0.51 |
| Biomass of aerial part (kg/pot FW) | / | 1.68 |
| Theoretical yield (t/hm2 FW) | / | 168 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Tong, J.; Su, Y.; Xiao, L. Pennisetum sinese: A Potential Phytoremediation Plant for Chromium Deletion from Soil. Sustainability 2020, 12, 3651. https://doi.org/10.3390/su12093651
Chen X, Tong J, Su Y, Xiao L. Pennisetum sinese: A Potential Phytoremediation Plant for Chromium Deletion from Soil. Sustainability. 2020; 12(9):3651. https://doi.org/10.3390/su12093651
Chicago/Turabian StyleChen, Xiaofei, Jianhua Tong, Yi Su, and Langtao Xiao. 2020. "Pennisetum sinese: A Potential Phytoremediation Plant for Chromium Deletion from Soil" Sustainability 12, no. 9: 3651. https://doi.org/10.3390/su12093651
APA StyleChen, X., Tong, J., Su, Y., & Xiao, L. (2020). Pennisetum sinese: A Potential Phytoremediation Plant for Chromium Deletion from Soil. Sustainability, 12(9), 3651. https://doi.org/10.3390/su12093651





